Abstract
Advanced glycation end-products (AGEs) are a category of post translational modification associated with the degradation of the structural properties of multiple different types of tissues. Typically, AGEs are the result of a series of post-translational modification reactions between sugars and proteins through a process known as non-enzymatic glycation (NEG). Increases in the rate of NEG of bone tissue is associated with type 2 diabetes and skeletal fragility. Current methods of assessing NEG and its impact on bone fracture risk involve measurement of pentosidine or total fluorescent AGEs (fAGEs). However, pentosidine represents only a small fraction of possible fAGEs present in bone, and neither pentosidine nor total fAGE measurement accounts for non-fluorescent AGEs, which are known to form in significant amounts in skin and other collagenous tissues. Carboxymethyl-lysine (CML) is a non-fluorescent AGE that is often measured and has been shown to accumulate in tissues such as skin, heart, arteries, and intervertebral disks, but is currently not assessed in bone. Here we show the localization of CML to collagen I using mass spectrometry for the first time in human bone. We then present a new method using demineralization followed by heating and trypsin digestion to measure CML content in human bone and demonstrate that CML in bone is 40–100 times greater than pentosidine (the current most commonly used marker of AGEs in bone). We then establish the viability of CML as a measurable AGE in bone by showing that levels of CML, obtained from bone using this technique, increase with age (p<0.05) and are correlated with previously reported measures of bone toughness. Thus, CML is a viable non-fluorescent AGE target to assess AGE accumulation and fragility in bone. The method developed here to extract and measure CML from human bone could facilitate the development of a new diagnostic assay to evaluate fracture risk and potentially lead to new therapeutic approaches to address bone fragility.
Keywords: carboxymethyl-lysine, pentosidine, bone, glycation, bone matrix
Introduction
The accumulation of advanced glycation end products (AGEs) on proteins and lipids is known to negatively affect the biophysical properties and function of many different kinds of tissue including skin, bone, and blood vessels [1–6]. AGEs are the result of a series of varying reactions, many driven by oxidation, following the spontaneous post-translational addition of a sugar to a protein. This set of reactions, collectively referred to as the Maillard reaction, occurs by a process known as non-enzymatic glycation (NEG). The rate of NEG modification is increased with hyperglycemia and higher oxidative stress [2]. Increased amounts of the AGE pentosidine in urine has been associated with bone fragility in cases of type 2 diabetes (T2D) [7], although increased pentosidine content in T2D bone has yet to be confirmed [8, 9]. In bone, NEG has been shown to increase with age and in vitro studies have shown that NEG causes bone fragility [4, 10]. In vivo, AGEs in bone have been shown to be associated with reduced bone turnover and decreased post-yield deformation [5, 11, 12].
Bone AGEs are assessed though measurement of pentosidine, a fluorescent AGE (fAGE) crosslink formed between lysine and arginine, or from total fluorescent AGE content [3, 4, 10, 11, 13–16]. However, pentosidine is only one of many possible fAGEs, and fAGEs represent only a fraction of all the AGEs that form during NEG [17]. In addition, depending on the type of bone, pentosidine in particular has been shown to be present in varying ratios to total fluorescent AGEs. This implies that pentosidine alone may not be an adequate assessment of AGE content of bone [13]. More importantly, unlike other tissues, there has been very limited assessment of non-fluorescent AGEs in bone.
Carboxymethyl-lysine (CML), the structure of which is shown in Figure 1, is an abundant and well characterized non-crosslinking, non-fluorescent AGE that is commonly known as a biomarker of oxidative stress and long term protein damage [18–20]. CML has been shown to accumulate in serum as well as in tissues such as skin, heart, arteries, and intervertebral disks; this accumulation increases in cases of uremia or diabetes [1, 21–23]. CML is known to be involved in receptor for AGE (RAGE) signaling and thus pro-inflammatory effects [24, 25]. Assessment of AGE content, including CML, to gauge tissue biomechanical deterioration is also ubiquitous for most tissues and for serum, and the levels of CML detected are higher than that of pentosidine [17]. Evidence of a possible relationship between CML and bone in particular was implied by studies showing increased levels of circulating CML correlated with increased risk of hip fracture in older patients, independent of bone mineral density, and an increase in circulating CML levels in osteoporotic patients [26]. However, neither of these studies suggests a mechanism for this association as AGEs in serum are not specific to bone. Thus, it is necessary to determine if CML forms in bone and whether the relationship between CML and bone fragility can be found in the bone tissue itself.
Figure 1.
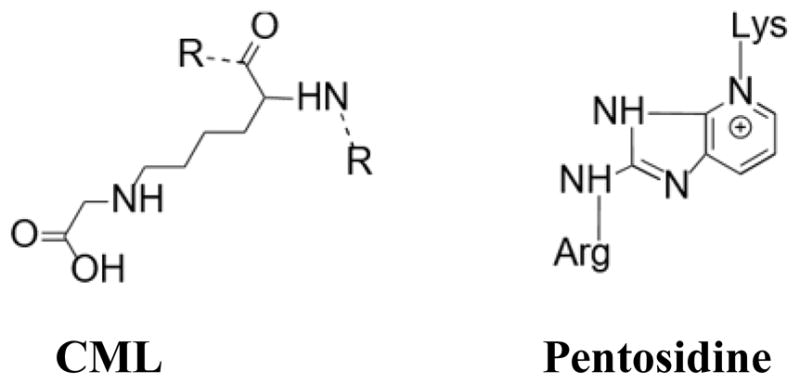
The chemical structure of commonly measured AGEs: CML, a side-chain modification, and pentosidine, a crosslink. Both AGEs result from multiple pathways of glycation and oxidation reactions.
A previous study has shown preliminary evidence, based on immunostaining, to support the presence of CML in bone; however, CML from bone has not been isolated, characterized or quantified [27]. This is because of the inherent difficulty in extracting protein from bone. In particular, bone proteins are embedded in hydroxyapatite mineral, and must be separated from the mineral and solubilized in order to be analyzed. There are many methods for extracting protein from bone, and each method favors the extraction of a different set of bone proteins, based on the characteristics of the interaction [28, 29]. Current extraction methods have not been optimized for the extraction of CML, and thus there is an absence of measured CML content in bone.
This study used mass spectrometry to show the presence of CML extracted from human bone tissue and presents a method to extract and quantify the CML content of human bone. This method was then used in concert with pentosidine quantified using ultra-high performance liquid chromatography (UPLC) and bone fragility measurements from the same donors to evaluate the proportion of CML relative to pentosidine and to determine the viability of CML as a potential predictor of bone fragility.
Materials and Methods
Bone Samples
This study utilized unmodified human bone samples cut from transverse sections of cortical bone from the distal section of the diaphysis of tibiae from donors over an age range of 19–97 and from both sexes, all Caucasian (23F, 25M, 50M, 61M, 79M, 97F). All donors were diagnosed as without metabolic bone diseases, diabetes, HIV, and hepatitis B (National Disease Research Interchange and International Institute for the Advancement of Medicine). Variety was chosen to ensure results encompassed a range of donors. Bone samples were taken from storage at −80°C and ~2 mm wide slices were cleaned and defatted with three 15-minute washes submerged in diisopropyl ether. The slices then were cut into 50–80 mg pieces for protein extraction by two different procedures described below.
Protein Extraction – Demineralization
A demineralization and denaturation approach to bone extraction was chosen to target crosslinked collagen for extraction. Demineralization with EDTA was used to dissolve the hydroxyapatite mineral, leaving the organic matrix. To accomplish this, bone was homogenized using the Omni BeadRuptor 24 in a buffer of 0.5 M EDTA, followed by demineralization in the same buffer at 4°C for 6 days. Of the organic matrix left over after demineralization, crosslinked collagen is insoluble without further processing and was separated by centrifugation at 13,000 rpm for 15 minutes. The supernatant was discarded and the pellet, consisting primarily of crosslinked collagen, can be solubilized using heat denaturation and trypsin digestion. The solubilization was performed using a protocol described by Terajima et al, with a temperature of 85°C used for the denaturation steps instead of the published 65°C [30]. The protocol used first a period of heating in 100 μL Hanks buffer, followed by digestion using an added 100 μL 1 mg/mL trypsin solution, another period of heating, then a second trypsin digestion using an added 50 μL 1mg/mL trypsin solution. The protein content of the sample was assessed using a Bradford assay with a bovine serum albumin (BSA) standard.
Protein Extraction – Non-Demineralization
A non-demineralization protocol was tested based on a consistently high yield of non-collagenous bone protein from previous experiments [31]. In this protocol, bone proteins are decoupled from the bone mineral, hydroxyapatite, using mechanical separation in 600 μL of extraction buffer, the composition of which was determined from the principles of hydroxyapatite chromatography. This method was developed for use in mass spectrometry and is capable of extracting both collagenous and non-collagenous proteins [31]. For this method, samples were homogenized using an Omni BeadRuptor 24 (Omni International, Atlanta, GA) in 600 μL 1M ammonium phosphate, 200 mM ammonium bicarbonate, and 4 M guanidine hydrochloride. The samples were then immediately centrifuged at 13,000 rpm for 15 minutes, and the supernatant dialyzed against water for two days. The resulting dialysate was concentrated using spin filters (Corning Spin-X UF 500, 5k MWKO). The protein content of the sample was assessed using a Bradford assay with a bovine serum albumin (BSA) standard.
Mass Spectrometry
Mass spectrometric analysis was performed on the 79M donor bone to demonstrate the presence of CML in bone. To this end, bone proteins were extracted using the non-demineralization protocol and mass spectrometry appropriate reagents that required no further purification. Prior to analysis with mass spectrometry, proteins must be digested into smaller peptides in order to fall within the mass range of the instrument. Protein extract was alkylated with iodoacetamide (IAA), reduced with dithiothreitol (DTT), and digested overnight at 37°C with Promega Trypsin Gold at a 1:100 ratio. The digest was desalted by stage tipping with Empore C18 resin [32]. The resultant peptides were separated using an Agilent 1200 series HPLC and micro-electrosprayed into an LTQ-Orbitrap XL. Peptide ions were fragmented using collision induced dissociation and identified by searching against a human database in Mascot 2.3 (Matrix Sciences). Mascot database searches were performed using SwissProt 57.15 Human taxonomy, with variable modifications carbamidomethyl (C), carboxy (E), oxidation (KMP), deamidated (NQ), and carboxymethyl (K). The database searches were run with a peptide mass tolerance of 10 ppm, a fragment tolerance of 0.5 Da, and allowing for 2 missed cleavages by trypsin. Due to glycations frequently occurring on residues cleaved by trypsin, glycation may block the activity of trypsin and lead to more missed cleavages.
CML Quantification
All CML content was measured for both demineralized and non-demineralized samples with Cell BioLabs CML Competitive ELISA kit (STA-816) following manufacturer’s instructions. The bone protein extract was diluted with kit-provided diluent to fall within the measurable concentration range of the kit, and measured in triplicate. The CML ELISA kit is a colorimetric immunoassay comparing samples to a standard curve of CML-modified BSA. The protein content of the final sample was assessed using a Bradford assay with a BSA standard.
Measurement of Pentosidine by UPLC
Pentosidine (PEN) was measured on the 23F, 25M, 50M, 61M, and 97F donors using ultra-high performance liquid chromatography. Bone hydrolysates were prepared from separate bone slices from the same donor. The slices were defatted with isopropyl ether, dried, and hydrolysis was performed by submerging the samples in 6M hydrochloric acid at 110°C overnight. Two analyses were performed on each bone hydrolysate, one to measure pentosidine content, and a second to determine hydroxyproline content that was further used to calculate collagen concentration.
Before the UPLC analysis, each hydrolysate was dissolved in 1% n-heptafluorobutyric acid (HFBA). PEN was separated using an Acquity UPLC machine (Waters Corp., Milford, MA, USA) equipped with the reverse-phase Acquity UPLC HSS T3 column (1.8 μm; 2.1 x 100 mm). The column flow rate and temperature were 0.400 ml/min and 40°C, respectively. Solvent A contained 0.06% HBFA in 18 megaohms pure water, and solvent B was composed of 50 : 50 (v : v) mixture of solvent A : acetonitrile. Prior the use, the column was equilibrated using 10% solvent B. Gradient of 10 to 50% of solvent B (from 8 to 20 min) was used for the separation of PEN. The elution of PEN was monitored for fluorescence emission at 385 nm after excitation at 335 nm. PEN was quantified using a standard curve.
Measurement of Hydroxyproline by UPLC
Hydroxyproline was quantified after overnight hydrolysis of all types of samples (demineralized bone, non-demineralized bone, protein extracts containing different types of collagen) after overnight hydrolysis in 6N HCL at 110°C. Under these conditions, both soluble and insoluble collagens are fully hydrolyzed to their amino acid components, and this includes the quantitative release of hydroxyproline. Considering extracellular bone matrix, practically all measured hydroxyproline originates from collagen [33]. Thus, the determined CML and PEN levels can be expressed either per hydroxyproline or collagen. Hydroxyproline content was determined using reagents from the HPLC assay kit (Bio-Rad Labratories GmbH, Müchen, Germany), but the mobile phase solvents and conditions were developed specifically for the UPLC separation. The column flow rate and temperature were 0.400 ml/min and 60°C, respectively. The 0 to 50% gradient of acetonitrile was achieved by mixing 100% acetonitrile (solvent B) with a buffer composed of 0.3% acetic acid and 0.6% triethylamine, pH 4.50 (solvent A). The elution of the derivatized hydroxyproline was monitored at 471 nm. The amount of hydroxyproline was determined using a standard curve. The amount of collagen was calculated assuming 300 nmol of hydroxyproline in 1 mol of collagen (e.g., PEN [μmol PEN/mmol Col]) [34, 35].
Linear Regression and Statistical Comparisons
Data was tested for normality prior to linear regression and statistical analysis of correlations comparing CML to PEN amounts. All statistical tests were performed using IBM SPSS Version 24.
Results
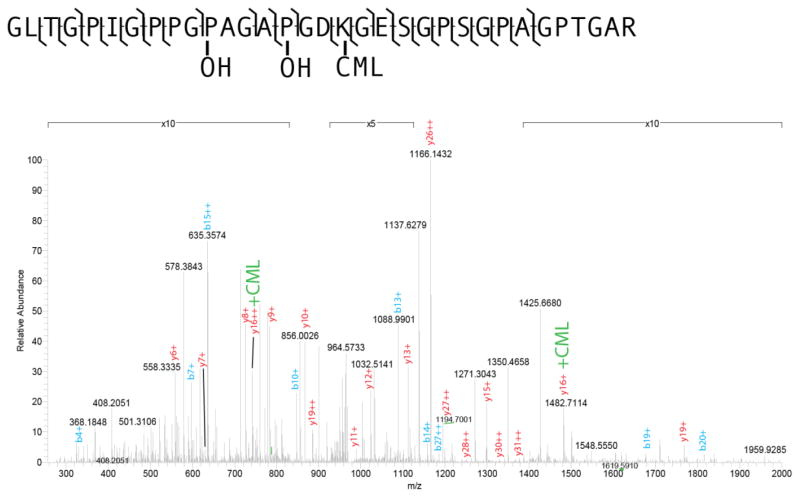
The presence of CML in bone was confirmed by mass spectrometric analysis, and is demonstrated by the spectrum shown in Figure 2. This figure shows an example of an MS/MS spectrum of a tryptic peptide of collagen, with several peaks showing the CML mass shift. This indicates the presence of CML on protein extracted from human cortical bone tissue. Peptides with CML modifications often showed a missed cleavage at the lysine residue, consistent with trypsin’s inability to cleave at modified arginine and lysine residues, further supporting the presence of this modification on the detected peptides.
Figure 2.
Mass spectrum of the Collagen I alpha 1 peptide (GLTGPIGPPGP(OH)AGAP(OH)-GDK(CML)GESGPPSGPAGPTGAR) from donor 79M; 42% of Collagen I alpha 1 was sequenced. This spectrum shows the presence of CML on residue 18 and hydroxylation of proline (OH) on residues 11 and 15. Labeled peaks show b- and y- series ions with + denoting charge.
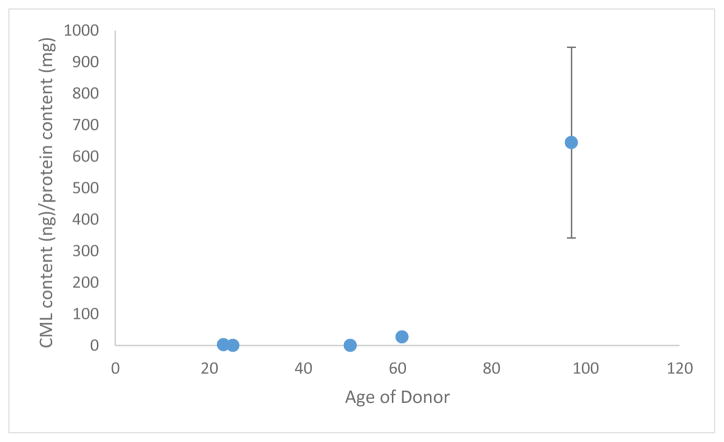
The protein extraction utilizing non-demineralized samples, expected to measure the CML content of NCPs and soluble collagen, showed no CML content for younger donors but significant CML content for the oldest donor. This indicates either inherent limitations in measuring small amounts of CML in this fraction of organic matrix in young donors or it provides an indication of a non-linear trend in the accumulation of CML in soluble collagen. The values obtained through the measurement of CML, normalized to the protein content of the extract tested, is shown in Figure 3.
Figure 3.
Ammonium bicarbonate/ammonium phosphate/guanidine hydrochloride extraction CML content measured by ELISA, normalized to total protein extracted.
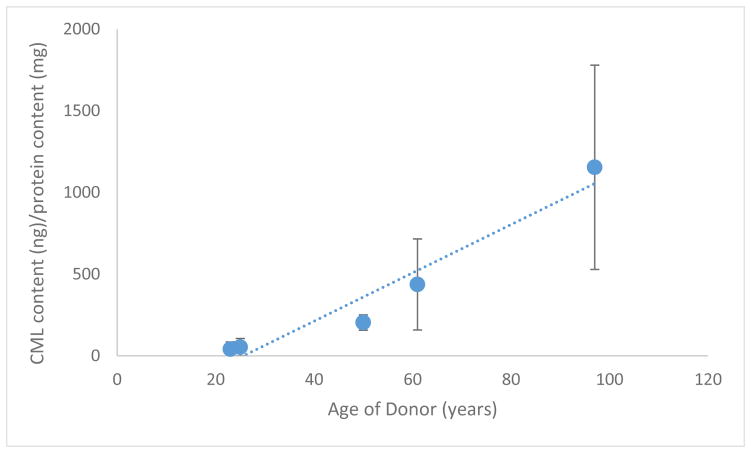
In contrast to the above, the protein extraction utilizing demineralized samples showed steadily increasing values for CML content as normalized to the protein content of the digested pellet. A plot of these measurements can be found in Figure 4. The values of CML content ranged from 41–1154 μg CML/mg collagen, and correlated with age with an R2 of 0.94, p<0.05.
Figure 4.
Demineralized Pellet CML content measured by ELISA, showing a trend line given by (CML content) = 14.788 (Donor Age) – 379.096 with an R2 = 0.939.
Pentosidine measurements obtained from bone tissue yielded pentosidine concentrations in the range of 2–22 μmol pentosidine per mmol total collagen, as assessed by hydroxyproline assay. Previous studies in this lab have shown that the amount of insoluble collagen isolated from a human donor is much higher than the soluble fraction [34], making the addition of soluble collagen negligible, and thus allowing the measurements of CML taken from insoluble collagen and PEN measurements taken from hydrolysates to be compared directly. This comparison showed the same correlation as that of the normalization to protein content, showing an increase of CML with age (R2=0.88; p<0.05). The comparison, detailed along with both measurements in Table 1, also shows that significantly more CML than PEN is present in bone (p<0.05) and CML is correlated to PEN concentration (R2=0.87; p<0.05). CML content was also compared to crack propagation toughness reported previously in Poundarik et al, 2015 [5], and correlated with an R2=0.90, at p<0.05.
Table 1.
Normalization of CML measurements (by ELISA) to collagen content, and comparison of pentosidine concentration to CML concentration.
| Age (yrs.) | CML (μmol/mmol coll) | PEN (μmol/mmol coll) | CML/PEN Ratio |
|---|---|---|---|
| 23 | 116.69 | 3.06 | 38 |
| 25 | 130.33 | 2.09 | 62 |
| 50 | 871.27 | 9.50 | 92 |
| 61 | 521.00 | 12.37 | 42 |
| 97 | 2528.00 | 21.70 | 117 |
Discussion
This study shows the confirmation of the presence of CML on human bone and presents the first example of its spectroscopic characterization and quantification from human bone tissue.
The CML content of demineralized samples show a significantly stronger correlation with age. The correlation of CML content to donor age is consistent with literature reports of AGE accumulation with age. In particular, the accumulation of CML on collagen is likely the result of the inability of collagen crosslinked through NEG to be resorbed during bone turnover [4, 10, 11]. This leads to the increased longevity of crosslinked collagen, and therefore to its increased exposure to sugar over time. Once CML has formed on a protein, it is stable and is not known to undergo any reactions that would reduce its abundance [36].
In contrast to the bone demineralization method, non-demineralization extractions of bone protein show no signal for the younger bone, and a large signal for older bone. The low yield of CML using this method was reflected in the mass spectrometric analysis of the non-demineralized samples, which did detect CML, albeit in low abundance. Previously performed solid state nuclear magnetic resonance (NMR) spectroscopic analysis of glycated bone suggests that NEG decreases the collagen-mineral interface distance, implying a stronger interaction between the two [37]. The non-demineralization method used is based on the use of hydroxyapatite chromatography solvents to detach protein from bone mineral [31], and altered interactions between protein and mineral may cause the technique to be less effective at extracting NEG modified protein and therefore make the method unsuitable for glycation quantification.
The success of the demineralization protocol over the non-demineralization protocol shows that in order to get a measureable concentration of CML, the extraction process needs to focus on solubilizing otherwise insoluble collagen fibers, which are likely to contain glycation products. Results showed that the amount of CML detected on insoluble collagen increased when normalized to both total collagen content and insoluble collagen content of the sample. This implies that the rate at which CML forms in vivo on insoluble collagen significantly exceeds the rate at which collagen is rendered insoluble by further NEG of non-NEG crosslinked collagen. The presence of CML in the older bones detected by both of these protocols implies that there is either some overlap between the two methods, or that while concentrations of CML in soluble collagen and protein remain undetectable for healthier bones it becomes detectable above a certain threshold, as evidenced by the detectable amount from the 97 year old donor. The CML content obtained from the non-demineralization technique showed a maximum of 644 ng CML/mg protein, or 3.15 nmol/mg protein. This is an order of magnitude more than previously measured values of pentosidine in bone, which has been measured at a maximum of approximately 120 pmol/mg collagen [38]. Furthermore, comparing the concentration of CML found via demineralization to collagen content from different bone samples taken from the same donor bone resulted in CML values ranging from 100–2500 μmol CML/mmol total collagen content, again, orders of magnitude higher than those found for pentosidine, which ranged from 2–22 umol pentosidine/mmol total collagen. This is consistent with previous experimental results showing the abundance of CML over pentosidine in tissues other than bone [17].
The increased abundance of CML relative to pentosidine provides an alternative, and potentially more sensitive, method for assessing AGE accumulation in bone tissue. In addition, the relative formation of certain types of AGEs over others and the importance of this to bone fragility, or other types of tissue degradation, has yet to be extensively studied. While pentosidine, in its role as a crosslink, is primarily linked to stiffening of the extracellular matrix [4, 39], CML as a non-crosslink still can be related to other potential functions of AGEs such as RAGE (Receptor for Advanced Glycation End-products) signaling [6, 40, 41]. In other tissues and in circulation, CML-RAGE interactions have been connected with inflammatory response and insulin resistance [24, 25, 41]. In bone, AGE-RAGE interactions have been shown to affect bone material properties through alterations to the behavior of osteoblasts, osteoclasts, and bone marrow cells [40, 42–44]. Furthermore, our previous work has shown that the presence of AGEs alter the physical interactions between collagen and mineral in particular the collagen-HA distance [37]. Consequently, CML may affect bone fracture through alterations in collagen-mineral interactions.
Indeed, we find that CML content was also highly correlated (R2=0.90, p<0.05) with fracture properties of bone, specifically with measurements of crack propagation toughness reported previously in Poundarik et al, 2015 [5]. Comparing the rates of formation of different types of AGEs, such as between pentosidine and CML, would allow for the further study of the effects of each type of interaction on bone fragility. This method could also be used to determine differences in the rates of formation of different types of AGEs; while CML content correlates with pentosidine content in this study, this may not be the case for metabolic bone diseases.
Taken together, the results of this study demonstrate the use and potential importance of CML as a marker of bone NEG. The comparison of multiple extraction methods show that while CML is present in multiple different forms of collagen, it is most consistently found on insoluble collagen. The demineralization protocol, which focuses on this type of collagen, received the best signal and showed a correlated relationship with age and bone fragility. Its comparison with pentosidine levels of the same bone show that it may correlate with pentosidine levels in some cases, but more importantly, that CML is present in levels an order of magnitude higher than that of pentosidine. This establishes CML as a viable non-fluorescent AGE target to assess bone AGE accumulation. This protocol can be used to measure CML levels in concert with pentosidine levels or fAGE content to elucidate the effects of different types of NEG on bone matrix quality and fragility.
Highlights.
Carboxymethyl-lysine (CML), a non-fluorescent marker of Advanced Glycation End-products (AGEs), was measured in bone for the first time using a newly developed extraction method and commercially available ELISA.
Measurements showed that CML was present in bone in quantities 40–100 times greater than pentosidine, the commonly measured AGEs marker and a fluorescent AGE.
The amount of CML in bone increased with age and correlated to a decrease in crack propagation toughness.
Acknowledgments
We would like to acknowledge the RPI CBIS Proteomics core facility for use of their equipment and National Institute of Health, AG20618 and AR49635 for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–23. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 3.Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26:93–100. doi: 10.1007/s00774-007-0784-6. [DOI] [PubMed] [Google Scholar]
- 4.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 5.Poundarik AA, Wu PC, Evis Z, Sroga GE, Ural A, Rubin M, Vashishth D. A direct role of collagen glycation in bone fracture. J Mech Behav Biomed Mater. 2015;52:120–30. doi: 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, Health A Body Composition S. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:2380–6. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oren TW, Botolin S, Williams A, Bucknell A, King KB. Arthroplasty in veterans: Analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. J Rehabil Res Dev. 2011;48:1195–1210. doi: 10.1682/jrrd.2010.09.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pritchard JM, Papaioannou A, Schwarcz HP, Adachi JD, DeBeer J, Winemaker M, Avram V, Willett T. A Comparison of Collagen Crosslink Content in Bone Specimens from Elective Total Hip Arthroplasty Patients with and without Type 2 Diabetes. Journal of Bone Reports & Recommendations. 2016:2. [Google Scholar]
- 10.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40:1144–51. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ural A, Janeiro C, Karim L, Diab T, Vashishth D. Association between non-enzymatic glycation, resorption, and microdamage in human tibial cortices. Osteoporos Int. 2015;26:865–873. doi: 10.1007/s00198-014-2938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24:2441–7. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karim L, Poundarik AA, Vashishth D. Non-collagenous matrix proteins and post-translational modifications explain bone toughness loss in an aging mouse model [abstract]. Transactions of the 58th Annual Meeting of the Orthopaedic Research Society; Long Beach, CA: Orthopaedic Research Society; 2012. p. 116. [Google Scholar]
- 15.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–9. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Grandhee SK, Monnier VM. Mechanism of formation of the maillard protein cross-link pentosidine. J Biol Chem. 1991;266:11649–11653. [PubMed] [Google Scholar]
- 17.Verzijl N, DeGroot J, Ben Zaken C, Braun-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JWJ, Lafeber FPJG, Tekoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage. Arthritis Rheumatol. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N′-(Carboxymethy1)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10852–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 19.Fu M-X, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Ne-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 20.Nowotny K, Jung T, Hohn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schalkwijk CG, Baidoshvili A, Stehouwer CDA, van Hinsbergh VWM, Niessen HWM. Increased accumulation of the glycoxidation product Nε-(carboxymethyl)lysine in hearts of diabetic patients: generation and characterisation of a monoclonal anti-CML antibody. Biochim Biophys Acta. 2004;1636:82–89. doi: 10.1016/j.bbalip.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1996;99:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57:2571–85. doi: 10.1046/j.1523-1755.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 24.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, de Caterina R. Advanced Glycation End Products Activate Endothelium Through Signal-Transduction Receptor RAGE: A Mechanism for Amplification of Inflammatory Responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 25.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–92. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Hein G, Wiegand R, Lehmann G, Stein G, Franke S. Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology (Oxford) 2003;42:1242–6. doi: 10.1093/rheumatology/keg324. [DOI] [PubMed] [Google Scholar]
- 27.Hein G, Weiss C, Lehmann G, Niwa T, Stein G, Franke S. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Ann Rheum Dis. 2006;65:101–104. doi: 10.1136/ard.2004.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleland TP, Voegele K, Schweitzer MH. Empirical evaluation of bone extraction protocols. PLoS One. 2012;7:e31443. doi: 10.1371/journal.pone.0031443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroeter ER, DeHart CJ, Schweitzer MH, Thomas PM, Kelleher NL. Bone protein “extractomics”: comparing the efficiency of bone protein extractions of Gallus gallus in tandem mass spectrometry, with an eye towards paleoproteomics. PeerJ. 2016;4:e2603. doi: 10.7717/peerj.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terajima M, Perdivara I, Sricholpech M, Deguchi Y, Pleshko N, Tomer KB, Yamauchi M. Glycosylation and cross-linking in bone type I collagen. J Biol Chem. 2014;289:22636–47. doi: 10.1074/jbc.M113.528513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleland TP, Vashishth D. Bone protein extraction without demineralization using principles from hydroxyapatite chromatography. Anal Biochem. 2015;472:62–66. doi: 10.1016/j.ab.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 33.Sroga GE, Vashishth D. UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:379–85. doi: 10.1016/j.jchromb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sroga GE, Karim L, Colon W, Vashishth D. Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol Cell Proteomics. 2011:10. doi: 10.1074/mcp.M110.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sroga GE, Siddula A, Vashishth D. Glycation of human cortical and cancellous bone captures differences in the formation of Maillard reaction products between glucose and ribose. PLoS One. 2015;10:e0117240. doi: 10.1371/journal.pone.0117240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed MU, Thorpe SR, Baynes JW. Identification of N-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261:4889–4894. [PubMed] [Google Scholar]
- 37.Nikel O, Laurencin D, Bonhomme C, Sroga GE, Besdo S, Lorenz A, Vashishth D. Solid state NMR investigation of intact human bone quality: balancing issues and insight into the structure at the organic-mineral interface. J Phys Chem C Nanomater Interfaces. 2012;116:6320–6331. doi: 10.1021/jp2125312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–7. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 39.Vashishth D. Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J Biomech. 2004;37:943–6. doi: 10.1016/j.jbiomech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ding KH, Wang ZZ, Hamrick MW, Deng ZB, Zhou L, Kang B, Yan SL, She JX, Stern DM, Isales CM, Mi QS. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes related bone loss. Biochem Biophys Res Commun. 2006;340:1091–7. doi: 10.1016/j.bbrc.2005.12.107. [DOI] [PubMed] [Google Scholar]
- 41.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CD, Schalkwijk CG. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 42.Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes. 2003;52:1502–1510. doi: 10.2337/diabetes.52.6.1502. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto E, Mihara C, Ikuta T, Inagaki Y, Kido J, Nagata T. Inhibitory effects of advanced glycation end-products and Porphyromonas gingivalis lipopolysaccharide on the expression of osteoblastic markers of rat bone marrow cells in culture. J Periodontal Res. 2016;51:313–20. doi: 10.1111/jre.12310. [DOI] [PubMed] [Google Scholar]
- 44.Aikawa E, Fujita R, Asai M, Kaneda Y, Tamai K. Receptor for advanced glycation end products-mediated signaling impairs the maintenance of bone marrow mesenchymal stromal cells in diabetic model mice. Stem Cells Dev. 2016;25:1721–1732. doi: 10.1089/scd.2016.0067. [DOI] [PubMed] [Google Scholar]