Abstract
Objective
Supplementing lactating mothers with high doses of vitamin D3 can adequately meet vitamin D requirements of the breastfed infant. We compared the effect of bolus versus daily vitamin D3 dosing in lactating mothers on vitamin D3 catabolism. We hypothesized that catabolism of 25(OH)D3 to 24,25(OH)2D3 would be greater in the bolus than in the daily dose group.
Design, Setting and Patients
Randomized controlled trial (clinicaltrials.gov NCT01240265) in 40 lactating women.
Interventions
Subjects were randomized to receive vitamin D3 orally, either a single dose of 150,000 IU or 5000 IU daily for 28 days. Vitamin D metabolites were measured in serum and breast milk at baseline, 1, 3, 7, 14 and 28 days.
Main Outcome Measure
Temporal changes in the serum 24,25(OH)2D3/25(OH)D3 ratio.
Results
The concentration of serum 24,25(OH)2D3 was directly related to that of 25(OH)D in both groups (r2=0.63; p<0.001). The mean (±SD) 24,25(OH)2D3/25(OH)D3 ratio remained lower at all time points than baseline values in the daily dose group (0.093±0.024, 0.084±0.025, 0.083±0.024, 0.080±0.020, 0.081±0.023, 0.083±0.018 at baseline, 1, 3, 7, 14, and 28 days, respectively). In the single dose group, the increase in 24,25(OH)2D3 lagged behind that of 25(OH)D, but the 24,25(OH)2D3/25(OH)D3 values (0.098±0.032, 0.067±0.019, 0.081±0.017, 0.092±0.024, 0.103±0.020, 0.106±0.024, respectively) exceeded baseline values at 14 and 28 days and were greater than the daily dose group at 14 and 28 days (P=0.003). The 24,25(OH)2D3/25(OH)D3 ratio remained in the normal range with both dosing regimens. Greater breast milk vitamin D3 values in the single dose group were inversely associated with the 24,25(OH)2D3/25(OH)D3 ratio (r2 = 0.14, p<0.001), but not with daily dosing.
Conclusions
After a 14-day lag, a single high dose of vitamin D led to greater production of 24,25(OH)2D3, presumably via induction of the 24-hydroxylase enzyme (CYP24A1), relative to the 25(OH)D3 value than did daily vitamin D supplementation, and this effect persisted for at least 28 days after vitamin D administration. A daily dose of vitamin D may have more lasting effectiveness in increasing 25(OH)D3 with lesser diversion of 25(OH)D3 to 24,25(OH)2D3 than does larger bolus dosing.
Keywords: vitamin D metabolism, catabolism, nutrition, LC-MS/MS, lactation, safety
1. Introduction
Low levels of vitamin D in breast milk (20–80 IU/L)1, 2 increase the risk of nutritional rickets in exclusively breast fed infants compared with infants who are formula fed, supplemented with vitamin D or who are breastfed by mothers taking high dose vitamin D supplements.3, 4 Supplemental vitamin D 400 IU/d has been recommended for breastfed infants by the American Academy of Pediatrics and Institute of Medicine.5 Several studies have investigated the optimal maternal vitamin D dose as an alternative to direct supplementation of the infant. However, vitamin D supplementation of lactating mothers with doses of 4000–6400 IU/day effectively enriches the breast milk with appropriate levels of vitamin D to satisfy the breastfed infant’s requirements,3, 4, 6 and this approach is often preferred by mothers over infant supplementation.7 Cholecalciferol (not 25(OH)D or 1,25(OH)2D), which crosses readily from maternal circulation into breast milk, is the form available to the infant.
Vitamin D differs in its side chain substitution based on the vitamin’s source wherein the plant derived form is called vitamin D2 and mammalian form is called vitamin D3. Metabolism of both forms are follow the same biochemical route in humans.8 Vitamin D and parathyroid hormone (PTH) are essential for normal calcium and phosphorus homeostasis.9 Vitamin D3 is synthesized in the skin by ultraviolet-light mediated photo-isomerization of 7-hydrocholesterol 9, 10 and is metabolized in the liver to 25(OH)D3 by the 25-hydroxylase CYP2R1. Calcium and phosphorus demands regulate the synthesis of 1,25-dihydroxyvitamin D3 (1α,25(OH)2D3) by the 25(OH)D3-1α-hydroxylase CYP27A1. The 25(OH)D3 metabolite can alternatively be catabolized to an inactive metabolite, 24,25-dihydroxyvitamin D3 (24,25(OH)2D3), by the 25(OH)D-24-hydroxylase CYP24A1.8, 9, 11–14 A dose dependent linear correlation exists between serum 25(OH)D3 and 24,25(OH)D3 at normal 25(OH)D3 levels. The effect of a single large dose vs daily low dose vitamin D3 on temporal changes in the vitamin metabolites including 25(OH)D3, 1,25(OH)2D3 in the supplemented mother, in the breast milk and the exclusively breast fed infant has been studied.4 While the 24,25(OH)2D3/25(OH)D3 ratio has been proposed as an indicator of response to vitamin D3 supplementation,15 changes in 24,25(OH)2D3/25(OH)D3 with different vitamin D3 dosing regimens in breast feeding mothers have not been reported. Our study aimed to investigate the effect of bolus versus daily vitamin D3 dosing regimens on temporal changes in 25(OH)D3/24,25(OH)2D3 ratio in a group of breast feeding mothers who were randomized to receive either a single large dose (150,000 IU) or a daily dose (5000 IU) of vitamin D over 28 days. We sought to investigate if catabolism of 25(OH)D3 to 24,25(OH)2D3 would be greater in the bolus than the daily dose group, its correlation with breast milk vitamin D3 concentration, and whether it can be used as a safety marker for high dose vitamin D3 supplementation in lactating women.
2. Methods and Materials
Subjects and samples
The study was a randomized controlled trial in 40 lactating females, ages 24–40 years, with a singleton infant between the ages of 1 and 6 months. Subjects were randomized to receive vitamin D3 as a single oral dose of 150,000 IU (N = 20), or 5000 IU daily (N = 20) for 28 days. Additional details regarding the study design have been previously described.4 The Mayo Clinic Institutional Review Board approved the study.
We measured serum vitamin D3, 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3, and breast milk vitamin D3 at baseline and 1, 3, 7, 14 and 28 days following vitamin D3 administration. All vitamin D3 metabolites were measured at Mayo Clinic by previously established liquid chromatography mass spectrometry based methods.16–18 The Mayo 25(OH)D test is calibrated against NIST and has been successfully passing the DEQAS-PT program.
24,25(OH)2D3 Measurement
Briefly, 500 μL of calibrator, quality control or patient serum was placed in a glass test tube to which 50 μL acetonitrile solution of the internal standard (1.25 ng deuterated 24,25(OH)2D3-d6, Toronto Research Chemicals, Canada) was added. The contents of the test tube were mixed using a vortex mixer, and the samples were incubated at room temperature for 15 minutes. Then, 500 μL aqueous hydrochloric acid solution (0.2 M) was added. The samples were vortex mixed and incubated at room temperature for 15 minutes. The contents of the test tube were transferred into a solid phase extraction cartridge (Bond-Elut (C18, 250 mg, 6 mL), Varian Instruments). The solid phase extraction cartridges containing the sample were placed on a positive pressure manifold, and the contents of the cartridge were passed through the resin by applying 3–5 psi pressure. The cartridge was washed once with 2 mL of 70:30 methanol/water (vol/vol) and once with 2 mL of 90:10 hexane/methylene chloride (vol/vol). Vitamin D metabolites were eluted with 2 mL of 90:10 hexane/isopropyl alcohol (vol/vol). Eluents were dried and derivatized with 4-phenyl-1,2,4,-triazoline-3,5-dione (PTAD) (Sigma; 250 μL of 200 μg/mL solution in acetonitrile). The derivatized vitamin D metabolites were separated by liquid chromatography at a on an Agilent XDB-C8, 2.1×50-mm column with a methanol-H2O-ammonium formate (1 mM) linear gradient (60%–95%) and analyzed on a AB Sciex 5500 mass spectrometer with Analyst 1.6.2 software (AB Sciex) for data acquisition and analysis.
For 25(OH)D quantification, to a 100 μL serum sample 2.5 ng deuterated internal standard solution (25(OH)D3-d6) was added, and the sample was equilibrated on an orbital shaker for 15 min at room temperature. 100μL acetone, followed by 450 μL ethyl acetate were added. After the solutions had settled, the organic top layer was transferred to a different sample tube. The extraction was repeated 4 times. The samples were subjected to PTAD derivatization as described above. Reaction mixtures were dried under a N2 stream, and the residue was reconstituted in 150 μL of 50:50 methanol: water mixture. The reconstituted residue was then separated on an analytical LC column and analyzed on an AB Sciex 4500 mass spectrometer.
The normal range for serum 24,25(OH)2D3/25(OH)D3 ratio was determined using serum samples from 91 healthy individuals were collected between May and August 2012, and 24,25(OH)2D3 and 25(OH)D were measured by the methods described above.17 All patients included in the reference range study were screened by a detailed chart review to ascertain that none of the individuals from whom samples were obtained were taking drugs known to affect mineral metabolism or had systemic conditions known to affect mineral metabolism. They were not taking vitamin D or calcium supplements, or if they were, were taking no more than 1000 mg calcium per day and 1000 IU vitamin D3 per day.
Statistical analysis
Data were entered in and analyzed with Excel 2010 (Microsoft Corp., Redmond, WA). The Student t test was used to compare continuous variables between the two treatment groups. P values less than 0.05 were considered significant.
3. Results
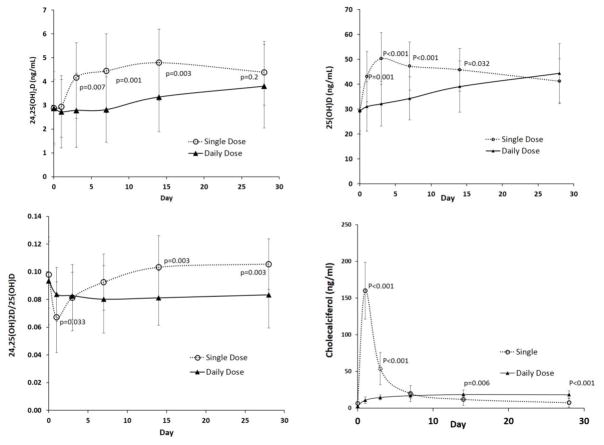
Baseline characteristics of the study participants are shown in Table 1. The two study groups had no statistically significant differences. Alterations in serum concentration of 24,25(OH)2D3 and 24,25(OH)2D3/25(OH)D3 ratio in the single dose and daily dose are shown in Figure 1. Following a single oral dose of 150,000 IU of vitamin D3, serum 24,25(OH)2D3 increased by day 3 to 4.17 ± 1.46 ng/mL (45% increase) from a basal value of 2.88 ± 1.20 ng/mL (p<0.001) and remained elevated for the subsequent 28 days compared to the baseline values. On day 28, while the serum 24,25(OH)2D3 concentration was not significantly different between the two groups (p = 0.20), the 24,25(OH)2D3/25(OH)D3 ratio was significantly greater in the single dose group than the daily dosing group. Serum 25(OH)D3 increased by day 1 to 43.05 ± 10.15 ng/mL (48 % increase) from a basal value of 29.06 ± 7.75 ng/mL in the single dose group. With daily administration of vitamin D3 (5000 IU/day), no increase in serum 24,25(OH)2D3 was noted until day 14 (Figure 1a), with a value at day 14 of 3.34 ± 1.35 ng/mL compared with a baseline value of 2.87 ± 1.40 ng/mL (p = 0.03). In the daily dose group, serum 24,25(OH)2D3 did not reach the same final concentration as the single dose group. Compared with the corresponding basal value, the day 28 serum 24,25(OH)2D3 concentration was 52% higher in the single dose group and 32% greater in the daily dosing group.
Table 1.
Baseline Characteristics of Study Subjects
| Characteristic | Daily Dose 5000 IU (n=20) | Single Dose 150,000 IU (n=20) |
|---|---|---|
| Maternal age (years) | 30.3 ± 2.9 | 30.1 ± 4.0 |
| Infant age (weeks) | 13.7 ± 7.3 | 11.0 ± 5.6 |
| Infant gestation at birth (weeks) | 39.9 ± 1.3 | 39.5 ± 0.9 |
| Maternal race (% white) | 95% | 95% |
| Maternal weight (kg) | 72.7 ± 10.6 | 67.6 ± 12.1 |
| Maternal height (cm) | 165.6 ± 5.5 | 163.8 ± 4.2 |
| Maternal BMI (kg/m2) | 26.5 ± 4.0 | 25.2 ± 4.7 |
| Maternal serum 25(OH)D (ng/mL) | 28.8 ± 9.2 | 29.3 ± 7.5 |
| Maternal serum 24,25(OH)2D3 (ng/mL) | 2.87 ± 1.40 | 2.88 ± 1.20 |
| Maternal serum 1,25(OH) 2D3 (pg/mL) | 60.4 ± 24.9 | 52.0 ± 10.7 |
| Enrollment date | ||
| January–March | 9 (45%) | 8 (40%) |
| April–July | 11 (55%) | 12 (60%) |
Figure 1.
Alterations in (a) serum concentration of 24,25(OH)2D3, (b) serum concentration of 25(OH)D3, (c) ratio of 25(OH)D3/24,25(OH)2D3, and (d) serum concentration of cholecalciferol in single dose (open circle) and daily dose (black triangles) groups. The error bars represent standard deviations. P values are for comparison of values in daily and single dosing groups at corresponding time points.
In the single dose group, the rise in concentration of 25(OH)D3 preceded the increase in 24,25(OH)2D3, accounting for a decline in the 24,25(OH)2D3/25(OH)D3 ratio on day 1. The 24,25(OH)2D3/25(OH)D3 ratio in the single dose group gradually attained a significantly greater value than the daily dose group after day 7 (Figure 1c), and the final value was greater than the baseline value in the single dose group. In contrast, the 24,25(OH)2D3/25(OH)D3 ratio in the daily dose group initially declined and remained relatively stable and lower than the baseline value throughout the duration of treatment in the daily dosing group.
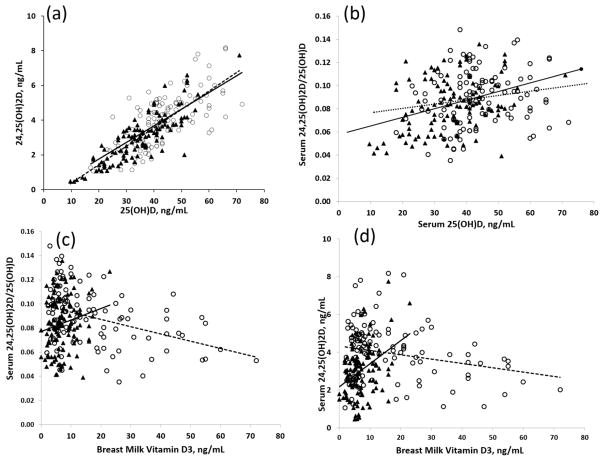
Serum 25(OH)D3 and 24,25(OH) 2D3 over all time points were strongly correlated (r2=0.63; p < 0.0001) in both groups (Figure 2a). Serum 24,25(OH)2D3/25(OH)D3 and serum 25(OH)D3 showed a significant direct correlation in the single dose group (r2=0.13, p<0.001) but not in the single dose group (r2=0.025; p=0.09; Figure 2b). Serum 24,25(OH)2D3/25(OH)D3 and breast milk vitamin D3 were inversely correlated in the single dose group (r2 = 0.14, p<0.001) but not in daily dose group (r2= 0.03, p=0.07; Figure 2c). An inverse relationship was observed between breast milk vitamin D3 and 24,25(OH)2D3 in the single dose group (r2=0.05, p=0.01) whereas an opposite (direct) correlation (r2=0.13, p<0.001; Figure 2d) was observed in the daily dose group. We did not observe a significant reciprocal relationship between 24,25(OH)2D3 and 1,25(OH)2D3.
Figure 2.
Correlations between serum concentration of (a) 25(OH)D3 and 24,25(OH)D3 (r2=0.63; p < 0.0001), (b) serum 24,25(OH)2D3/25(OH)D3 and serum 25(OH)D3 (p<0.001), (c) serum 24,25(OH)2D/25(OH)D3 and breast milk vitamin D3 and (d) serum 24,25(OH)2D and breast milk vitamin D3 in single dose (open circle) and daily dose (black triangles) groups. The solid line represents the linear trend in the daily dose group and the dotted line the linear trend in single dose group.
4. Discussion
We found evidence of greater production of 24,25(OH)2D3 resulting from a single, high dose bolus of vitamin D than with a daily dose of vitamin D over the course of 28 days. The concentration of serum 24,25(OH)2D3 was directly related to that of 25(OH)D in both groups, but the increase in 24,25(OH)2D3 lagged behind that of 25(OH)D in the single dose group. The 24,25(OH)2D3/25(OH)D3 ratio was significantly correlated with the serum 25(OH)D3 in the daily dose group but not in single dose group, which likely reflects the lag time in activation of the CYP24A1 enzyme.
Use of a high-dose bolus vitamin D was historically used for treatment of rickets in the setting of vitamin D deficiency. Contemporary studies have evaluated bolus cholecalciferol and ergocalciferol dosing in several clinical settings where potential benefit of modulating the vitamin D metabolic pathway is hypothesized including osteoporosis, cystic fibrosis, chronic kidney disease and inflammatory diseases. Results from studies on the effect of high-dose vitamin D supplementation in populations with high risk of fracture have been mixed. A paradoxical increase in risk of fracture found in some studies has been hypothesized to be caused by an up-regulation of CYP24A1 with high bolus doses of ergocalciferol or cholecalciferol, causing increased catabolism of 1,25(OH)2D. Turner et al. reported an increase in serum 1,25(OH)2D2 concentration three months after a bolus dose of 300,000 IU of ergocalciferol was administered to vitamin D deficient patients.19 In our study, while we were unable to determine if the greater production of 24,25(OH)2D3 in the single dose group was due to induction of the CYP24A1 gene or due to increased substrate concentrations, the data raises an important point of whether an assessment of serum vitamin D metabolites over a longer time-frame might have shown differences in serum metabolic patterns consistent with other reports. Of note, 1,25(OH)2D3 concentrations were not significantly different in the two dosing groups in our study.4
Whereas the ratio of 24,25(OH)2D3/25(OH)D3 initially declined and remained relatively constant below the baseline value with daily supplementation, this ratio was significantly greater in the single dose group and greater than the baseline value after 7 days. This suggests that the single high dose of vitamin D led to greater induction of the 24-hydroxylase CYP24A1 relative to the 25(OH)D3 value than daily vitamin D supplementation, and this effect persisted for at least 28 days after vitamin D administration. The high single dose may produce more prolonged catabolic activation of CYP24A1 than the smaller daily dose. The implication of this is that smaller daily doses of vitamin D would have a more favorable effect of increasing 25(OH)D with lesser activation of CYP24A1 catabolism.
The 24,25(OH)2D3/25(OH)D3 ratio remained in the normal range with both dosing regimens and supports the safety of high dose vitamin D3 supplementation used in this study, irrespective of the lag time to reach catabolic activity. Sub-optimal CYP24A1 activity may exacerbate the risk of toxicity in mothers receiving a high dose vitamin D3 supplement. The correlation between breast milk vitamin D3 content and maternal serum 24,25(OH)2D3/25(OH)D3 differed between the single and daily dose groups. The inverse relationship between breast milk vitamin D3 and 24,25(OH)2D3 after the single large dose of vitamin D3 likely reflects the fact that initially high concentrations of vitamin D3 in breast milk in the first 3 days preceded the delayed increase in 24,25(OH)2D3 concentrations. Because of the 14-day lag in the increase of the 24,25(OH)2D3/25(OH)D3 ratio, higher values of breast milk vitamin D3 were associated with a lower 24,25(OH)2D3/25(OH)D3 ratio. The direct relationship in the daily dose group reflects breast milk vitamin D3 increasing over the 28 days of supplementation, while the 24,25(OH)2D3/25(OH)D3 ratio remained relatively unchanged. Given the nearly identical increment of serum 25(OH)D in breast fed infants of these two groups of women,4 it is unlikely that differences in 25(OH)D catabolism between these two dosing groups, reflected in the 24,25(OH)2D3/25(OH)D3 ratio, have an impact on vitamin D3 availability in breast milk.
One limitation of our study is that we did not measure 3-epi-25(OH)D3 in mother’s serum or breast milk. In adults, the concentration of 3-epi-25(OH)D3 is generally very low, but production of 3-epi-25(OH)D3 might be predicted to be higher in the high dose group. If this were the case, the changes in 24,25(OH)2D3/25(OH)D3 ratios could be even more extreme or the level of 25(OH)D3 at the end of the study might differ between groups.
An average fetus requires approximately 30 g of calcium for bone mineralization.20 An increase in serum 1,25(OH)2D3 in pregnant females has been demonstrated.21 In mouse models of high bone density, a change in CYP24A1 activity lower 24,25(OH)2D3/25(OH)D compared to wild type mice has been demonstrated as the cause of increased calcium accretion.22 However, the effect of increased calcium demand during lactation on CYP24A1 activity and 24,25(OH)2D3/25(OH)D3 is largely unexplored. 24,25(OH)2D3 is a major catabolic product of 25(OH)D3. While its biological role is debated, its ratio with its precursor, 25(OH)D3 is well established as a biomarker of abnormal CYP24A1 enzyme function.17, 23–27 Additionally, 24,25(OH)2D3/25(OH)D3 functions as an index of 25(OH)D3 clearance in healthy adult male and female subjects.15 During 8 weeks of 28,000 IU/wk vitamin D3 supplementation, 24,25(OH)2D3 was directly correlated to 25(OH)D3. Evidence of a lag in 24-hydroxylation was similarly found during the early phase of supplementation. This was attributed to the slower reaction kinetics of CYP24A1 (24-hydroxylase) compared to CYP27A1 (25-hydroxylase).28 The effect of a greater 24,25(OH)2D3/25(OH)D3 ratio was to blunt the increase in 25(OH)D3 with vitamin D3 supplementation. In this sense, the 24,25(OH)2D3/25(OH)D3 ratio could be considered a functional marker of vitamin D sufficiency, reflecting increased catabolism of 25(OH)D as the concentration of 25(OH)D increases.
Our data in vitamin D3 supplemented breast feeding mothers, in whom the calcium demands are high, confirms the dependence of serum concentrations of 24,25(OH)2D3 upon the concentrations of its precursor, 25(OH)D3. While changes in serum 24,25(OH)2D3 occur in parallel with changes in serum 25(OH)D3, concentrations of 1,25(OH)2D3 did not change,4 demonstrating that the production of 1,25(OH)2D3 is substrate independent, and is regulated by other factors, such as serum calcium, parathyroid hormone, and phosphate. With low 25(OH)D3 concentrations (<20 ng/mL), the 24,25(OH)2D3/25(OH)D3 ratio declines to low normal ranges in healthy subjects,17, 26 likely due to favored biosynthesis of the bioactive 1,25(OH)2D3 during stages of 25(OH)D3 depletion.29
Conclusions
A single large dose of vitamin D3 produced evidence of greater production of the catabolic product of 24-hydroxylase CYP24A1 activity than daily dosing of vitamin D. Evidence of increased 24,25(OH)2D3 production relative to serum 25(OH)D3 values persisted for at least 28 days following a single large dose of vitamin D3, but remained within the normal range. Daily vitamin D3 supplementation may provide more predictable effects on vitamin D status due to the greater stability of the 24,25(OH)2D3/25(OH)D3 ratio compared with bolus dosing.
Highlights.
Bolus high-dose vitamin D produced more 24,25(OH)2D than daily supplementation
After bolus vitamin D, the increase in 24,25(OH)2D lagged behind that of 25(OH)D
Daily vitamin D may increase 25(OH)D more effectively than larger bolus dosing
Acknowledgments
Funding
This research was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additionally small grants funding from the authors’ institution supported the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reeve LE, Chesney RW, DeLuca HF. Vitamin D of human milk: identification of biologically active forms. Am J Clin Nutr. 1982;36:122–6. doi: 10.1093/ajcn/36.1.122. [DOI] [PubMed] [Google Scholar]
- 2.Hollis BW, Roos BA, Draper HH, Lambert PW. Vitamin D and its metabolites in human and bovine milk. J Nutr. 1981;111:1240–8. doi: 10.1093/jn/111.7.1240. [DOI] [PubMed] [Google Scholar]
- 3.Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG, Taylor SN, Morella K, Lawrence RA, Hulsey TC. Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics. 2015;136:625–34. doi: 10.1542/peds.2015-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberhelman SS, Meekins ME, Fischer PR, Lee BR, Singh RJ, Cha SS, Gardner BM, Pettifor JM, Croghan IT, Thacher TD. Maternal vitamin D supplementation to improve the vitamin D status of breast-fed infants: a randomized controlled trial. Mayo Clin Proc. 2013;88:1378–87. doi: 10.1016/j.mayocp.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 6.Wagner CL, Hulsey TC, Fanning D, Ebeling M, Hollis BW. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeeding medicine: the official journal of the Academy of Breastfeeding Medicine. 2006;1:59–70. doi: 10.1089/bfm.2006.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Umaretiya PJ, Oberhelman SS, Cozine EW, Maxson JA, Quigg SM, Thacher TD. Maternal Preferences for Vitamin D Supplementation in Breastfed Infants. Ann Fam Med. 2017;15:68–70. doi: 10.1370/afm.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. Historical Perspective. New York: Acedemic Press/Elsevier; 2011. [Google Scholar]
- 10.DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–39. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF, Garabedian M, DeLuca HF. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972;176:1146–7. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- 12.Kumar R. The metabolism and mechanism of action of 1,25-dihydroxyvitamin D3. Kidney Int. 1986;30:793–803. doi: 10.1038/ki.1986.258. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972;11:4251–5. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64:478–504. doi: 10.1152/physrev.1984.64.2.478. [DOI] [PubMed] [Google Scholar]
- 15.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126:72–7. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Netzel BC, Cradic KW, Bro ET, Girtman AB, Cyr RC, Singh RJ, Grebe SK. Increasing liquid chromatography-tandem mass spectrometry throughput by mass tagging: a sample-multiplexed high-throughput assay for 25-hydroxyvitamin D2 and D3. Clin Chem. 2011;57:431–40. doi: 10.1373/clinchem.2010.157115. [DOI] [PubMed] [Google Scholar]
- 17.Ketha H, Kumar R, Singh RJ. LC-MS/MS for Identifying Patients with CYP24A1 Mutations. Clin Chem. 2016;62:236–42. doi: 10.1373/clinchem.2015.244459. [DOI] [PubMed] [Google Scholar]
- 18.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:1279–85. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner C, Dalton N, Inaoui R, Fogelman I, Fraser WD, Hampson G. Effect of a 300 000-IU Loading Dose of Ergocalciferol (Vitamin D2) on Circulating 1,25(OH)2-Vitamin D and Fibroblast Growth Factor-23 (FGF-23) in Vitamin D Insufficiency. The Journal of Clinical Endocrinology & Metabolism. 2013;98:550–6. doi: 10.1210/jc.2012-2790. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev. 2016;96:449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Cohen WR, Epstein FH. Vitamin D and calcium hormones in pregnancy. N Engl J Med. 1980;302:1143–5. doi: 10.1056/NEJM198005153022010. [DOI] [PubMed] [Google Scholar]
- 22.Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A. 2013;110:6199–204. doi: 10.1073/pnas.1221255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Keeffe DT, Tebben PJ, Kumar R, Singh RJ, Wu Y, Wermers RA. Clinical and biochemical phenotypes of adults with monoallelic and biallelic CYP24A1 mutations: evidence of gene dose effect. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3615-6. [DOI] [PubMed] [Google Scholar]
- 24.Tebben PJ, Milliner DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97:E423–7. doi: 10.1210/jc.2011-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs TP, Kaufman M, Jones G, Kumar R, Schlingmann KP, Shapses S, Bilezikian JP. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-3802. jc20133802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann M, Gallagher JC, Peacock M, Schlingmann K-P, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G. Clinical Utility of Simultaneous Quantitation of 25-Hydroxyvitamin D and 24,25- Dihydroxyvitamin D by LC-MS/MS Involving Derivatization With DMEQ-TAD. The Journal of Clinical Endocrinology & Metabolism. 2014;99:2567–74. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365:410–21. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 28.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–34. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 29.Hoogenboezem T, Degenhart HJ, de Muinck Keizer-Schrama SM, Bouillon R, Grose WF, Hackeng WH, Visser HK. Vitamin D metabolism in breast-fed infants and their mothers. Pediatric research. 1989;25:623–8. doi: 10.1203/00006450-198906000-00014. [DOI] [PubMed] [Google Scholar]