Abstract
Background
Parkinson’s disease (PD) can cause severe dysphagia, especially later in disease progression. Early identification of swallowing dysfunction may lead to earlier intervention. Pharyngeal high-resolution manometry (HRM) provides complementary information to videofluoroscopy, with advantages of being quantitative and objective. Artificial neural network (ANN) classification can examine nonlinear relationships among multiple variables with relatively low bias. We evaluated if ANN techniques could differentiate between patients with PD and healthy controls.
Methods
Simultaneous videofluoroscopy and pharyngeal HRM were performed on 31 patients with early to mid-stage PD and 31 age- and sex-matched controls during thin-liquid swallows of 2cc, 10cc, and comfortable sip volume. We performed multilayer-perceptron analyses on only videofluoroscopic data, only HRM data, or a combination of the two. We also evaluated variability-based parameters, representing variability in manometric parameters across multiple swallows. We hypothesized that patients with PD and controls would be classified with at least 80% accuracy, and that combined videofluoroscopic and HRM data would classify participants better than either alone.
Key Results
Classification rates were highest with all parameters considered. Maximum classification rate was 82.3±5.2%, recorded for 2 cc swallows. Inclusion of variability-based parameters improved classification rates. Classification rates using only manometric parameters were similar to those using all parameters, and rates were substantially lower for the comfortable sip volumes.
Conclusions & Inferences
Results from these classifications highlight the differences between swallowing function in patients with early and mid-stage PD and healthy controls. Early identification of swallowing dysfunction is key to developing preventative swallowing treatments for those with PD.
Keywords: high resolution manometry, pharyngeal swallow, pattern recognition, artificial neural network, Parkinson’s disease
Abbreviated abstract
We evaluated if pattern recognition of pharyngeal high-resolution manometry with or without videofluoroscopy could be used to identify patients with early- and mid-stage Parkinson’s disease. Classification accuracy exceeded 80%, and was similar when considering manometric parameters with and without videofluoroscopic parameters. Use of high-resolution manometry coupled with pattern recognition-based analysis may help clinicians identify early changes in swallowing function in patients with Parkinson’s disease that could lead to earlier and potentially more effective treatment.
INTRODUCTION
Parkinson’s disease (PD) is a progressive, neurodegenerative disease that impacts 1% of the world population over the age of 60.1 Aside from the classic signs of tremor, rigidity, bradykinesia, hypokinesia, and posture and gait instability, PD also impacts cranial sensorimotor function, including swallowing. Patients with PD-associated dysphagia can have difficulty with bolus propulsion, a delayed pharyngeal swallowing response, reduced range of motion of pharyngeal structures, and reduced opening of the upper esophageal sphincter.2–6 The resulting inefficient swallow, residue, and airway invasion can lead to malnutrition, pneumonia, reduced quality of life, and death.7,8 Aspiration pneumonia is the most common cause of death in this population,9 and can add over $10,000 on top of other hospitalization costs.10
Typically, patients with PD do not report changes in swallowing function until the later stages of disease progression.11,12 At this point, however, the dysphagia may be beyond the point of meaningful improvement with swallow rehabilitation. Early identification of changes in swallowing function in patients with PD has the potential to introduce the patient into swallowing rehabilitation at an earlier stage, with a goal of prolonging function and improving health and quality of life.13
Videofluorsocopy is the most common instrumental technique used to evaluate patients with PD, but may lack the sensitivity needed to detect subtle deviations in swallowing function occurring early in disease progression.14 Pharyngeal high-resolution manometry (HRM) is a technique by which a small-diameter catheter is placed through the nose and pharynx into the esophagus, and pressure is measured with a high spatial and temporal resolution. Pharyngeal HRM gives complementary information to videofluoroscopy or other swallowing visualization techniques, and has the benefits of being quantitative and objective. The sensitivity and objectivity of pressures measured by HRM make it a good candidate for determining subtle, even sub-clinical changes to swallowing function.15
Previously, we have performed pharyngeal high-resolution manometry and videofluoroscopy to evaluate swallowing function in patients in the early and mid-stages of PD. Logistic regression was used to compare patients with early and mid-stage PD to healthy, age- and sex-matched controls.16 Logistic regression is popular in clinical research due to ease of interpretation, but it is inherently open to bias in the process of model evaluation (i.e., determining which model fits the data best) and cannot address complex relationships among parameters. Artificial neural network (ANN) classification, on the other hand, removes a portion of this bias by performing analysis based on few assumptions about data distribution. ANN classifications can thus explore more complex and non-linear relationships among the predictor variables. Classification results in a weighted set of criteria that can be used to classify new cases. ANN performance is then calculated by the number of cases correctly classified.17
ANN classification has been used for pharyngeal high-resolution manometry data in order to classify presence/absence of dysphagia, level of penetration/aspiration, amount of residue, and to predict overall swallowing risk.18–23 Most published ANN studies evaluating swallowing function have used a single swallow as a case for training, testing, and validation. However, this lacks a degree of ecological validity. In order to determine differences between healthy individuals and those with a particular disease process, many aspects of that one individual’s performance need to be considered, for example, within-individual performance variability. Additionally, if classification accuracy is not hampered by using an individual as a case, this puts less emphasis on performing swallowing evaluations simultaneously, e.g., videofluoroscopy and HRM.
The purpose of this study was to determine if artificial neural network techniques could differentiate between patients with early to mid-stage PD and healthy controls with each participant serving as a case, rather than each swallow. We performed separate ANN classifications using only HRM data, only videofluoroscopic data, or a combination of the two. We hypothesized that data from individuals as cases would be sufficient to determine differences in health status (healthy vs. PD) with at least 80% accuracy, and that a combination of videofluoroscopic and HRM data would classify participants better than HRM only or videofluoroscopy only.
MATERIALS AND METHODS
Participants
Sixty-two subjects participated in this study with the approval of the University of Wisconsin Health Sciences Institutional Review Board, thirty-one with early Parkinson’s disease and thirty-one age-matched and sex-matched control subjects with no history of swallowing, gastrointestinal, or neurologic conditions. For the subjects with Parkinson’s disease, there were 17 males and 14 females with average age of 68.7±9.9 years. Hoehn and Yahr score ranged from 1–3 with an average of 1.9±0.9 A score of 1 indicates unilateral limb involvement only, 2 indicates bilateral limb involvement with no balance impairment, and 3 indicates bilateral limb involvement with mild postural instability. Additional patient scores on the scale which were not included in this study are 4 (severely disabling but able to walk or stand unassisted) and 5 (confined to bed or wheelchair unless aided).24 PD diagnosis was confirmed by neurologist report, and disease staging was performed by a physician member of the study team. For the control subjects, there were 17 males and 14 females with average age of 69.6±10.1 years. Subjects completed a Sydney Swallow Questionnaire,25 with average total scores of 80±107 (interquartile range: 20 – 101.5) for the control participants and 319±260 (interquartile range: 116 – 424) for the participants with PD. Fifteen participants with PD (48%) reported SSQ scores above the upper limit reported for typical healthy swallowers of 234.26 Sydney Swallow Questionnaire data were not used in the classification analyses.
Data collection
All participants swallowed ten 2 cc, 10 cc, and self-selected sip thin liquid barium swallows (40% water by volume; Varibar, Bracco Diagnostics, Monroe Township, NJ) with the head in the neutral position. The 2 and 10 cc boluses were delivered to the oral cavity via syringe; subjects sipped from a straw for the comfortable-volume swallows. Simultaneous videofluoroscopic and HRM data were collected. Continuous videofluoroscopy (OEC 9900, General Electric, Fairfield, CT) was captured in the lateral plane at a rate of 30 frames per second. Video was digitized and saved to a DVD+RW for offline analysis. The videofluoroscopic frame included the incisors, cervical vertebrae, superior border of the soft palate, and cervical esophagus.
A solid-state high-resolution manometer with 36 sensors and outer diameter of 2.75 mm was used for manometry (ManoScan360, Medtronic, GI Solutions, Duluth, GA). The catheter was calibrated before each use according to manufacturer specifications. Data were collected at a sampling rate of 50 Hz (ManoScan Data Acquisition, Medtronic, GI Solutions).
Videofluoroscopic analysis
Manometric and videofluoroscopic data were aligned temporally with a time stamp embedded into the videofluoroscopy signal (UTG-50, Horita, Mission Viejo, CA) and recorded by the manometric system (ManoScan 2.1, Medtronic, GI Solutions). Videofluoroscopic data were analyzed using a modified version of the MBSImP,27 an ordinal rating system designed to evaluate multiple physiologic components observed on videofluoroscopy. Score ranges fall between 0 – 2, 0 – 3, or 0 – 4. The following parameters were included in the present analysis: initiation of pharyngeal swallow (0 – 4), hyoid movement (0 – 2), epiglottic movement (0 – 2), pharyngeal stripping wave (0 – 2), pharyngo-esophageal segment opening (0 – 3), tongue base retraction (0 – 4), residue score (0 – 4), and number of locations with residue (0 – 5). The Penetration-Aspiration Scale was also used, an ordinal scale ranging from 1–8 that describes laryngeal penetration and aspiration as well as the patient’s response.28 Each swallow was evaluated by two raters who completed the MBSImP training (Northern Speech Services, Gaylord, MI), with a third rater to resolve rating disagreements.
Manometric analysis
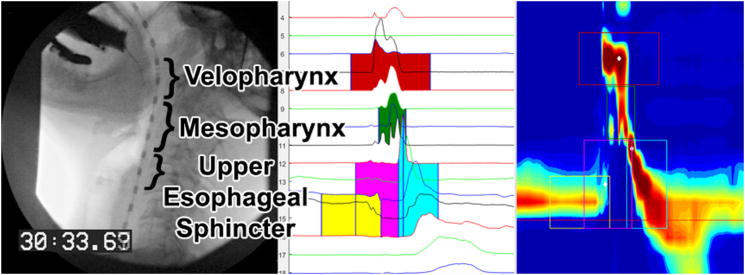
Manometric data were extracted using a custom MATLAB program as described previously.19,29 Five regions are identified for a swallow: velopharynx, mesopharynx, upper esophageal sphincter UES), pre-opening UES pressure peak, and post-closure UES pressure peak (Figure 1). The velopharynx, pre-opening UES pressure peak, and post-closure UES pressure peaks are selected by the user and the mesopharynx and UES are identified automatically.
Figure 1.
Videofluoroscopic still image (left panel) prior to 10cc swallow and equivalent high-resolution manometry waveform (middle panel) and spatiotemporal (right panel) plots in the HRM analysis software.29 Velopharynx pressures are highlighted in red, mesopharynx sensors in green, pre-opening UES pressures in yellow, UES opening pressures in pink, and post-closure UES pressures in light blue.
The box corresponding to the velopharynx has a duration of 1 second (shorter duration of actual pressure activity does not adversely affect measurements). The mesopharynx consists of all sensors between the velopharynx and post-closure UES pressure peak and is identified automatically following user identification of those two regions. Duration of pressure events in the mesopharynx is automatically determined by the program using a 2-second test window and a 2-second background window. The mean and standard deviation of the background noise are calculated in the background window and the mesopharynx is defined as the area with pressure higher than background. The starting and ending time are the first and last points with pressure above baseline. The pre-opening UES pressure peak is identified by the user and represents the maximum pressure occurring in the UES prior to relaxation. This region spans three sensors with duration of 0.5 seconds. The post-closure UES pressure peak is the last user-identified region and represents the maximum UES pressure during closure; the spatial boundary is variable depending on the trial and subject pharynx length, and extends from the point of maximum pressure (selected by user) to the inferior boundary of the pre-opening UES pressure peak region. It has a duration of 1 second. The last region is the UES during relaxation, with temporal constraints of the pre-opening and post-closure pressure peaks, and spatial constraints of the superior-most UES-related sensor and the pre-opening UES pressure peak. This region is identified automatically. Several parameters are calculated for each region. Maximum pressure, rise time, rise rate, fall time, and duration of pressure above baseline are calculated for the velopharynx and mesopharynx. For the pre-opening and post-closure UES pressure peak regions, maximum pressure is calculated; for the UES, minimum pressure, and nadir pressure duration (bolus passage time) are calculated. Total swallow duration is calculated and defined as the time lapse between onset of velopharyngeal pressure and the post-swallow UES pressure peak. For all five regions, two- (total pressure generated in region of one sensor corresponding to maximum pressure in that region) and three-dimensional integrals (defined as the total pressure generated in the entire area spanning all sensors) are also calculated. See Figure 1 for a graphical representation of the analysis software.
Manometric variability analysis
We included variability analysis parameters for regions of interest, consisting of coefficient of variation parameters for the velopharynx, tongue base, hypopharynx, tongue base with hypopharynx, and UES. To calculate variability, the coefficient of variation of pressure measurement over the course of the swallow-related pressure change is calculated for each sensor in a given region of interest. The mean is then calculated by averaging the coefficient of variation across all sensors in that region. We also calculated a total coefficient of variation parameter, determined by summing the four individual coefficient of variation parameters.16
Artificial neural network (ANN) analysis
ANN analysis using MATLAB and the Neural Network Toolbox (The MathWorks, Inc.) was performed to determine if HRM, videofluoroscopic, or HRM and videofluoroscopic data could be used to identify patients with early Parkinson’s disease. In prior studies,19,20,29 we treated each swallow as a data point and included multiple swallows per swallower. In this study, we treated each swallower as a data point. For the videofluoroscopic parameters, the median score for all swallows at a particular volume was used as the input to the neural network. Thus, 62 data points (31 subjects with Parkinson’s disease and 31 control subjects) were used per parameter. Classification status (Parkinson’s or control) was attached to each data point and machine learning techniques were employed to model the relationship between the data and swallow status. Data were randomly divided into training (60%), testing (20%), and validation (20%) sets. A multi-layer perceptron ANN was created with the number of hidden nodes varied from 10 to 20 to 30 (maximum number of hidden nodes being approximately one-half the number of data points to ensure algorithm generalizability) to achieve optimal performance. Ten replicates were performed at each to determine classification accuracy and reliability.
For each bolus volume (2 cc, 10 cc, or self-selected sip), four different classifications were performed: only manometric parameters, only videofluoroscopic parameters, only manometric variability parameters, or all parameters. Classifications using only manometric parameters included the manometric variability parameters. To evaluate what parameters contributed most significantly to the multiparametric neural network classification accuracy, single parameter classification accuracies were also determined for the volume and number of hidden nodes corresponding to the highest classification accuracy. Ten classifications were performed for each parameter and the average classification accuracy was determined.
Statistical analysis
Total classification accuracy, sensitivity, and specificity were determined. Receiver operating characteristic (ROC) curves were generated.
RESULTS
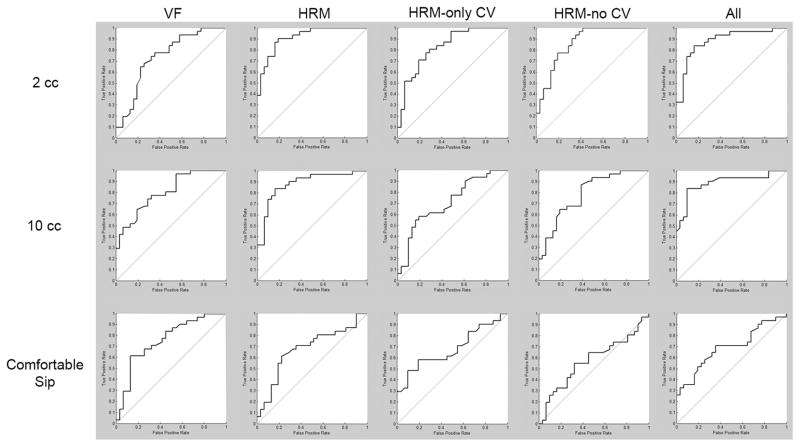
Summary data are presented in Table 1. Classification rates, sensitivity, and specificity are presented in Table 2 and ROC curves are presented in Figure 2. Classification rates generally improved with higher levels of hidden nodes. Classification rates when using only HRM parameters were higher than when using only videofluoroscopic parameters, but using all parameters led to the highest rates. Maximum classification rate was 82.3 ± 5.2%, recorded for the 2 cc swallows when using all parameters. Classification rates when using only manometric parameters were similar to those when using all parameters.
Table 1.
Summary data for subjects with Parkinson’s disease (PD) and control subjects at each bolus volume. Values are presented as mean ± standard deviation (range). PES = pharyngo-esophageal segment; TB = tongue base; PAS = penetration-aspiration scale (minimum score is 1); 2D = two-dimensional; 3D = three-dimensional; UES = upper esophageal sphincter; maxP = maximum pressure; CV = coefficient of variation (unitless). Group differences at individual bolus volumes for each parameter were not calculated to avoid Type I error inherent in performing multiple statistical comparisons.
| Parameter |
|
|||||
|---|---|---|---|---|---|---|
| 2 cc | 10 cc | Sip | ||||
|
| ||||||
| PD | Control | PD | Control | PD | Control | |
| Videofluoroscopic | ||||||
| Swallow initiation | 0.1±1.2 (0 – 3) | 1.1±1.1 (0 – 3) | 1.0±1.1 (0 – 3) | 0.9±1.1 (0 – 3) | 1.2±1.2 (0 – 3) | 1.5±1.2 (0 – 3) |
| Laryngeal movement | 0.0±0.0 (0) | 0.1±0.3 (0 – 1) | 0.1±0.3 (0 – 1) | 0.2±0.4 (0 – 1) | 0.0±0.2 (0 – 1) | 0.2±0.4 (0 – 1) |
| Hyoid movement | 0.2±0.4 (0 – 1) | 0.2±0.4 (0 – 1) | 0.3±0.5 (0 – 1) | 0.2±0.4 (0 – 1) | 0.3±0.5 (0 – 1) | 0.2±0.4 (0 – 1) |
| Epiglottic movement | 0.3±0.5 (0 – 2) | 0.2±0.4 (0 – 1) | 0.2±0.5 (0 – 2) | 0.1±0.3 (0 – 1) | 0.3±0.5 (0 – 2) | 0.1±0.3 (0 – 1) |
| Pharyngeal stripping wave | 0.3±0.4 (0 – 1) | 0.2±0.4 (0 – 1) | 0.2±0.4 (0 – 1) | 0.2±0.4 (0 – 1) | 0.3±0.5 (0 – 1) | 0.1±0.4 (0 – 1) |
| PES opening | 0.1±0.3 (0 – 1) | 0.1±0.3 (0 – 1) | 0.3±0.4 (0 – 1) | 0.2±0.4 (0 – 1) | 0.1±0.3 (0 – 1) | 0.2±0.4 (0 – 1) |
| TB retraction | 0.9±0.5 (0 – 2) | 0.9±0.4 (0 – 2) | 1.1±0.7 (0 – 2) | 0.8±0.6 (0 – 2) | 1.3±0.6 (0 – 2) | 1.2±0.7 (0 – 3) |
| Residue score | 1.3±0.6 (0 – 2) | 1.1±0.4 (0 – 2) | 1.5±0.6 (0 – 3) | 1.3±0.5 (0 – 2) | 1.6±0.6 (0 – 3) | 1.7±0.5 (1 – 2) |
| Locations with residue | 2.2±1.3 (0 – 4) | 2.4±1.2 (0 – 4) | 2.2±1.2 (0 – 4) | 2.5±1.1 (0 – 4) | 2.9±1.3 (0 – 4) | 3.2±1.1 (1 – 4) |
| PAS | 1.3±0.6 (1 – 3) | 1.2±0.4 (1 – 2) | 1.7±1.4 (1 – 8) | 1.3±0.5 (1 – 3) | 1.6±0.7 (1 – 4) | 1.7±1.1 (1 – 6) |
| Manometric | ||||||
| Velopharynx | ||||||
| Duration (s) | 0.87±0.24 | 0.84±0.16 | 0.89±0.27 | 0.88±0.23 | 0.89±0.26 | 0.94±0.22 |
| Rise time (s) | 0.32±0.22 | 0.31±0.13 | 0.34±0.22 | 0.36±0.22 | 0.33±0.22 | 0.42±0.20 |
| Rise rate (mmHg/s) | 734±346 | 732±277 | 685±331 | 737±388 | 708±495 | 616±343 |
| Fall time (s) | 0.55±0.12 | 0.53±0.14 | 0.55±0.14 | 0.52±0.17 | 0.56±0.18 | 0.52±0.17 |
| Max pressure (mmHg) | 158±60 | 176±53 | 161±57 | 184±47 | 163±57 | 181±42 |
| 2D integral (mmHg*s) | 66±44 | 74±34 | 75±42 | 81±34 | 81±46 | 87±33 |
| 3D integral (mmHg*s) | 129±101 | 134±77 | 151±93 | 152±74 | 160±101 | 171±83 |
| Mesopharynx | ||||||
| Duration (s) | 0.85±0.26 | 0.73±0.19 | 0.82±0.29 | 0.76±0.22 | 0.78±0.21 | 0.76±0.19 |
| Rise time (s) | 0.48±0.19 | 0.45±0.12 | 0.48±0.20 | 0.47±0.16 | 0.46±0.14 | 0.48±0.15 |
| Rise rate (mmHg/s) | 379±149 | 462±162 | 447±344 | 420±139 | 404±273 | 459±265 |
| Fall time (s) | 0.37±0.15 | 0.29±0.14 | 0.34±0.20 | 0.29±0.13 | 0.32±0.18 | 0.28±0.13 |
| Max pressure (mmHg) | 162±67 | 184±45 | 157±67 | 174±50 | 147±46 | 178±47 |
| 2D integral (mmHg*s) | 43±25 | 42±16 | 40±26 | 41±18 | 40±27 | 41±18 |
| 3D integral (mmHg*s) | 155±93 | 160±70 | 153±116 | 145±63 | 156±108 | 146±58 |
| UES | ||||||
| Pre-UES maxP (mmHg) | 118±41 | 114±43 | 97±33 | 102±40 | 91±37 | 95±42 |
| Post-UES maxP (mmHg) | 188±44 | 218±65 | 205±42 | 223±64 | 209±52 | 229±64 |
| Min pressure (mmHg) | −6±10 | −8±6 | −3±5 | −6±5 | −3±10 | −5±4 |
| Nadir duration (s) | 0.52±0.14 | 0.51±0.18 | 0.55±0.15 | 0.52±0.19 | 0.51±0.14 | 0.53±0.19 |
| 2D integral (mmHg*s) | 36±19 | 43±27 | 50±26 | 50±27 | 54±32 | 57±33 |
| 3D integral (mmHg*s) | 97±60 | 111±87 | 145±91 | 122±88 | 147±83 | 136±85 |
| Swallow duration (s) | 1.05±0.22 | 0.98±0.20 | 1.11±0.23 | 1.06±0.19 | 1.14±0.24 | 1.13±0.20 |
| Variability | ||||||
| CV velopharynx | 0.78±0.77 | 0.53±0.28 | 1.01±1.02 | 0.62±0.51 | 1.10±1.33 | 0.57±0.28 |
| CV tongue base | 0.87±0.54 | 0.79±0.62 | 1.55±1.39 | 0.91±0.68 | 0.97±0.49 | 0.86±0.48 |
| CV hypopharynx | 1.08±0.78 | 0.88±0.62 | 1.90±2.28 | 1.18±1.00 | 1.28±1.13 | 0.83±0.45 |
| CV mesopharynx | 4.03±2.16 | 6.17±3.73 | 1.73±1.42 | 1.03±0.68 | 1.11±0.58 | 0.86±0.37 |
| CV UES | 0.82±0.60 | 0.80±0.48 | 0.98±1.03 | 0.95±1.58 | 2.15±5.43 | 1.06±0.92 |
| CV total | 3.55±1.70 | 3.00±1.25 | 5.43±3.89 | 3.66±1.93 | 5.50±6.76 | 3.31±1.39 |
Table 2.
Classification rates for each set of parameters at each set of hidden nodes, for each bolus volume. VF = only videofluoroscopic parameters included; HRM = only high-resolution manometry parameters included; HRM-only CV = only the high-resolution manometry coefficient of variation parameters included; HRM-no CV = only high-resolution manometry parameters without the coefficient of variation parameters included; All = all parameters included. All values represent percent correctly classified and are presented as mean ± standard deviation.
|
|
|||||
|---|---|---|---|---|---|
| Bolus volume | |||||
|
| |||||
| Parameters used | Nodes | 2 cc | 10 cc | Sip | |
| VF | 10 | Classification accuracy | 65.2 ± 6.9 | 67.8 ± 6.0 | 63.7 ± 6.9 |
| Sensitivity | 70.3 ± 8.3 | 63.9 ± 9.7 | 63.3 ± 8.7 | ||
| Specificity | 60.0 ± 11.0 | 71.6 ± 9.0 | 65.2 ± 8.2 | ||
|
| |||||
| 20 | Classification accuracy | 67.6 ± 8.3 | 71.0 ± 5.5 | 61.8 ± 4.6 | |
| Sensitivity | 63.2 ± 10.7 | 71.0 ± 6.6 | 62.6 ± 7.3 | ||
| Specificity | 71.9 ± 9.1 | 71.0 ± 5.5 | 61.0 ± 8.4 | ||
|
| |||||
| 30 | Classification accuracy | 70.7 ± 4.4 | 69.2 ± 3.3 | 69.2 ± 3.4 | |
| Sensitivity | 70.6 ± 3.9 | 62.9 ± 5.9 | 67.8 ± 8.0 | ||
| Specificity | 70.6 ± 9.2 | 75.4 ± 5.8 | 70.6 ± 7.4 | ||
|
| |||||
| HRM | 10 | Classification accuracy | 74.4 ± 5.4 | 75.8 ± 6.3 | 57.3 ± 3.5 |
| Sensitivity | 75.8 ±9.6 | 72.9 ± 9.0 | 62.6 ± 17.6 | ||
| Specificity | 73.9 ± 9.4 | 78.7 ± 10.9 | 51.9 ± 18.8 | ||
|
| |||||
| 20 | Classification accuracy | 78.1 ± 6.1 | 80.6 ± 6.3 | 59.0 ± 4.6 | |
| Sensitivity | 78.4 ± 7.1 | 79.4 ± 6.8 | 66.1 ± 20.9 | ||
| Specificity | 77.7 ± 9.2 | 81.9 ± 8.1 | 51.9 ± 22.5 | ||
|
| |||||
| 30 | Classification accuracy | 81.5 ± 6.1 | 80.0 ± 4.8 | 65.2 ± 6.1 | |
| Sensitivity | 81.6 ± 8.3 | 78.4 ± 6.3 | 58.4 ± 11.4 | ||
| Specificity | 81.3 ± 7.7 | 81.6 ± 8.3 | 67.1 ± 11.6 | ||
|
| |||||
| HRM-only CV | 10 | Classification accuracy | 71.1 ± 6.5 | 63.1 ± 2.9 | 62.6 ± 2.1 |
| Sensitivity | 74.8 ± 9.0 | 53.5 ± 5.7 | 57.7 ± 6.5 | ||
| Specificity | 67.4 ± 8.9 | 72.6 ± 9.3 | 67.7 ± 8.0 | ||
|
| |||||
| 20 | Classification accuracy | 72.6 ± 5.0 | 64.3 ± 2.2 | 62.1 ± 3.7 | |
| Sensitivity | 74.5 ± 6.9 | 58.7 ± 6.6 | 58.7 ± 4.2 | ||
| Specificity | 70.7 ± 7.5 | 70.0 ± 6.5 | 65.7 ± 9.0 | ||
|
| |||||
| 30 | Classification accuracy | 70.7 ± 3.7 | 64.3 ± 2.2 | 64.3 ± 2.0 | |
| Sensitivity | 73.2 ± 5.9 | 58.1 ± 9.0 | 58.4 ± 6.9 | ||
| Specificity | 68.1 ± 6.5 | 70.7 ± 8.4 | 70.3 ± 7.9 | ||
|
| |||||
| HRM-no CV | 10 | Classification accuracy | 71.3 ± 3.9 | 68.1 ± 4.4 | 56.5 ± 5.3 |
| Sensitivity | 72.3 ± 11.0 | 68.1 ± 12.4 | 55.8 ± 18.7 | ||
| Specificity | 70.3 ± 6.8 | 68.1 ± 8.4 | 57.1 ± 14.7 | ||
|
| |||||
| 20 | Classification accuracy | 72.3 ± 6.7 | 68.9 ± 6.6 | 51.8 ± 3.1 | |
| Sensitivity | 69.4 ± 9.5 | 67.7 ± 12.6 | 51.0 ± 15.7 | ||
| Specificity | 75.2 ± 6.6 | 70.0 ± 12.8 | 52.6 ± 15.7 | ||
|
| |||||
| 30 | Classification accuracy | 76.5 ± 9.1 | 69.2 ± 6.6 | 54.2 ± 4.7 | |
| Sensitivity | 76.8 ± 10.2 | 67.7 ± 7.5 | 55.8 ± 23.0 | ||
| Specificity | 76.1 ± 11.8 | 70.7 ± 6.5 | 52.6 ± 23.5 | ||
|
| |||||
| All | 10 | Classification accuracy | 79.7 ± 4.1 | 76.8 ± 7.8 | 56.0 ± 3.7 |
| Sensitivity | 78.4 ± 9.1 | 71.3 ± 8.5 | 59.0 ± 13.1 | ||
| Specificity | 81.0 ± 7.2 | 82.2 ± 13.1 | 52.9 ± 14.1 | ||
|
| |||||
| 20 | Classification accuracy | 75.5 ± 3.3 | 79.8 ± 8.7 | 52.9 ± 4.7 | |
| Sensitivity | 76.8 ± 10.1 | 79.4 ± 9.4 | 54.2 ± 9.4 | ||
| Specificity | 74.2 ± 9.0 | 80.3 ± 12.8 | 51.6 ± 8.0 | ||
|
| |||||
| 30 | Classification accuracy | 82.3 ± 5.2 | 82.1 ± 4.2 | 58.1 ± 4.2 | |
| Sensitivity | 83.9 ± 9.4 | 79.7 ± 8.7 | 59.7 ± 18.1 | ||
| Specificity | 80.6 ± 8.9 | 84.5 ± 6.9 | 56.5 ± 15.2 | ||
Figure 2.
Receiver operator characteristic (ROC) curves for 30-node ANN classifications. VF = only videofluoroscopic parameters included; HRM = only high-resolution manometry parameters included; HRM-only CV = only the high-resolution manometry coefficient of variation parameters included; HRM-no CV = only high-resolution manometry parameters without the coefficient of variation parameters included; All = all parameters included.
Classification rates were substantially lower for the comfortable-volume sip swallows. Highest classification for this bolus type occurred with videofluoroscopic parameters, with lower rates for the manometric parameters. Looking at the value of the variability parameters, classification rates when excluding the coefficient of variation (CV) parameters from the HRM set resulted in decreased classification rates.
Single parameter classification analyses were performed for 2 cc swallows at 30 hidden nodes, which had the highest overall classification rate. In reviewing the single parameter classification rates, classification rates were generally higher for the manometric parameters compared to the videofluoroscopic parameters (average of 60.5±4.3% versus 53.1±4.1%). Highest rates were observed for duration of velopharyngeal pressure (69.0 ± 5.5%), post-UES closure maximum pressure (68.7 ± 8.5%), and coefficient of variation for the mesopharynx (65.8 ± 4.5%) and UES (66.3 ± 3.2%).
DISCUSSION
Classification accuracies for swallows of 2 cc and 10 cc were greatest when all HRM and videofluoroscopic parameters were included. However, these accuracies closely matched those with only HRM parameters, suggesting that the full HRM dataset substantially contributed to differentiating patients with early and mid-stage PD from healthy controls.
For data from sips of a comfortable volume, classification accuracies were comparably lower than those using 2 cc or 10 cc swallows, except for the classifications performed with videofluoroscopic data only. This suggests that the group differences seen on videofluoroscopy are relatively stable across volumes, with maximum classification accuracies of 70.7±4.4% for 2 cc swallows (30 hidden nodes), 71.0±5.5% for 10 cc swallows (20 hidden nodes), and 69.2±3.4% for sip swallows (30 hidden nodes). It is well-documented that pharyngeal swallowing dynamics change with different measured bolus volumes, including hyolaryngeal excursion,30–32 submental muscle activation,33,34 pharyngeal movement sequencing,34–36 and select pharyngeal pressure parameters, including mainly velopharyngeal and UES pressures.34,37–40 While not directly investigated in the present study, the relatively stable classification accuracies across 2 cc and 10 cc swallows may reflect adequate compensation for bolus volume changes in these two groups. Classification accuracies using only HRM variability data, however, were greater for the 2 cc than the other volumes, which may reflect an increased difficulty handling a small sip size in our patient group. When given the opportunity to select a comfortable volume, the differences between patients with early and mid-stage PD and healthy controls become less apparent. It could be that either patients with early and mid-stage PD are choosing volumes of liquid that normalize their pharyngeal swallowing pressure parameters, or it could be that taking a sip at a comfortable volume increases the between-individual variability in swallowing pressure values, so that group differences are no longer as discernable. Bennett and colleagues41 reported variation in sip size depending on instruction method. Given reported variations in sip size and decrease classification accuracies, measured volumes may be more sensitive to subtle changes in swallowing physiology.
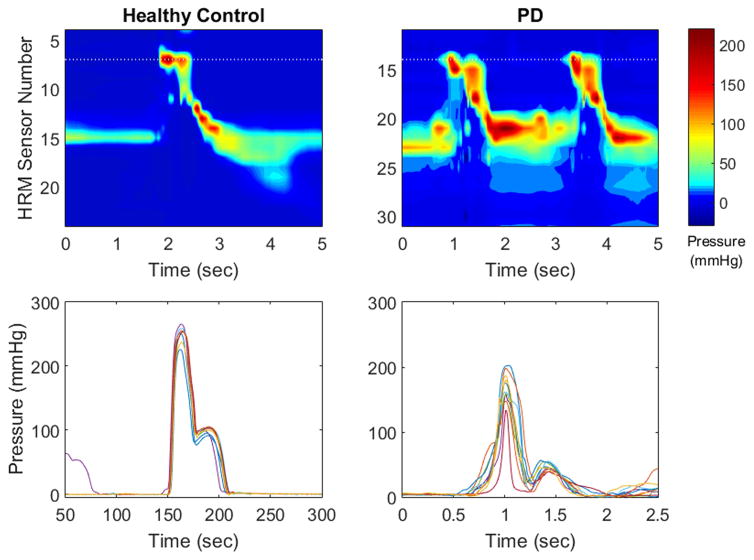
Patients with PD have been shown to have increased within-individual variability in several motor domains, such as gait,42 reach-grasp,43 fine motor control,44 speech45, and swallowing.16 Using only the variability parameters did not reach classification accuracy of 80% for any volume, and had accuracies similar to those of videofluoroscopy. While we have shown that patients with PD have increased swallowing pressure variability to age- and sex-matched healthy controls,16 the present study suggests that classification between patients and controls is greater than chance with variability alone but best when combined and weighted with other variables. Indeed, classification accuracies improved by up to 11% when adding variability measures onto other pressure data, demonstrating the value in exploring swallowing pressure variability in this population (Figure 3). Further investigations are warranted to determine if these differences are specific to PD or rather a reflection of a more general neurologic disease process impacting swallowing function.
Figure 3.
High-resolution manometry spatiotemporal plots (top row) and single sensor pressure waveforms (bottom row) from a healthy control participant (left column) and a participant with Parkinson’s disease (right column). Pressure waveforms display pressure variability from one sensor in the velopharynx (marked with a dotted line on the spatiotemporal plot) over 10 swallows. Variability is higher in the participant with PD. HRM = high resolution manometry; PD = Parkinson’s disease.
The single parameter analyses highlight several points. First, they provide information on what parameters are most important for the pattern recognition algorithm. The manometric parameters typically had higher classification accuracies, with the videofluoroscopic parameters performing around the level of chance when considered in isolation. This is in accordance with the analyses including all manometric parameters demonstrating improved accuracy over those using all videofluoroscopic parameters. Second, two of the highest-performing parameters were coefficient of variation for the mesopharynx and for the UES, reflecting the potential importance of variability-based parameters in evaluating patients with PD. Third, no parameter in isolation reached an accuracy of 70%, providing further support for the importance of a multiparameter analysis when assessing the pharyngeal swallow.
Compared to other ANN classification analyses of pharyngeal swallowing pressures, the classification accuracies in the present study are lower in most, but not all, cases.18–23 This is likely due to our use of the individual as a data point, instead of the swallow, and a relatively low number of participants. The low n restricted the number of hidden nodes we could use without risking over-fitting, and may have limited the between-parameter relationships able to be explored. As some parameters entered into ANN analysis are inherently tied to overall performance by an individual, such as swallowing pressure variability, this limitation in our study could be remedied in the future with a greater number of participants. In addition to recruiting more participants, classification may have been improved with additional complementary swallowing physiology parameters, such as sensory function, tongue pressure, or electromyography. An additional factor that may have contributed to our classification rates was our investigation of a population in earlier stages of PD and with relatively mild dysphagia. A larger study with more representation from all stages of the disease would be able highlight relationships both between and within groups of patients with PD and healthy controls. As dysphagia progresses in PD, so may our ability to classify patients based on pharyngeal swallowing pressures and videofluoroscopic findings. The goal of the present analysis was to classify patients with PD from healthy controls, which inherently limits the immediate clinical applicability of the study. Further work evaluating longitudinal changes to swallowing function in PD and comparing patients with PD to those with other causes of dysphagia will improve the clinical use of ANN-type modelling. However, this work confirms the power of an ANN approach at a conceptual level.
Results from these classifications highlight the differences in swallowing function in patients with early and mid-stage PD and healthy, age- and sex-matched controls. Early identification of deviation in swallowing function, especially in pressure patterns, is key to determining whether an individual’s swallowing function is impacted by PD. As treatments continue to develop for improving swallowing function in patients with PD,46,47 early identification of patients with physiologic changes may lead to preserved swallowing function, improved quality of life, and a prolonged the lifespan for these individuals.
Table 3.
Individual parameter classification results. Values represent percentage of subjects classified correctly using only the parameter given, and are presented as mean ± standard deviation. 2D = two-dimensional; 3D = three-dimensional; UES = upper esophageal sphincter.
| Parameter | Classification accuracy |
|---|---|
| Videofluoroscopic | |
| Initiation of pharyngeal swallow | 60.3 ± 6.0 |
| Laryngeal elevation | 53.9 ± 4.5 |
| Anterior hyoid excursion | 50.0 ± 0.0 |
| Epiglottic movement | 50.0 ±1.7 |
| Pharyngeal stripping wave | 51.9 ± 2.9 |
| Pharyngoesophageal segment closing | 50.6 ± 0.8 |
| Tongue base retraction | 49.4 ± 4.5 |
| Pharyngeal residue | 58.7 ± 6.6 |
| Manometric | |
| Velopharynx duration | 69.0 ± 5.5 |
| Velopharynx rise time | 65.2 ± 4.6 |
| Velopharynx rise rate | 55.3 ± 4.0 |
| Velopharynx fall time | 55.3 ± 6.6 |
| Velopharynx maximum pressure | 61.1 ± 6.6 |
| Velopharynx 2D integral | 61.9 ± 5.1 |
| Velopharynx 3D integral | 56.1 ± 5.0 |
| Mesopharynx duration | 57.1 ± 4.0 |
| Mesopharynx rise time | 58.7 ± 5.8 |
| Mesopharynx rise rate | 60.6 ± 5.2 |
| Mesopharynx fall time | 61.0 ± 5.4 |
| Mesopharynx maximum pressure | 66.1 ± 6.4 |
| Mesopharynx 2D integral | 57.7 ± 8.0 |
| Mesopharynx 3D integral | 60.3 ± 5.7 |
| Pre-UES opening maximum pressure | 62.9 ± 5.8 |
| Post-UES opening maximum pressure | 68.7 ± 8.5 |
| UES minimum pressure | 63.1 ± 3.9 |
| UES nadir pressure duration | 56.3 ± 3.9 |
| UES 2D integral | 61.8 ± 4.4 |
| UES 3D integral | 56.8 ± 3.7 |
| Swallow duration | 56.1 ± 6.3 |
| Velopharynx coefficient of variation | 56.5 ± 5.8 |
| Tongue base coefficient of variation | 55.0 ± 4.3 |
| Hypopharynx coefficient of variation | 57.9 ± 3.1 |
| Mesopharynx coefficient of variation | 65.8 ± 4.5 |
| UES coefficient of variation | 66.3 ± 3.2 |
KEY POINTS.
Parkinson’s disease can cause swallowing disorders; early identification is challenging but could lead to earlier treatment and better patient outcomes. We evaluated if pattern recognition of pharyngeal high-resolution manometry could be used to identify early- and mid-stage Parkinson’s disease.
Pattern recognition of high-resolution manometry can be used effectively to differentiate between patients with Parkinson’s disease and healthy controls. Classification is better when high-resolution manometry is used with videofluoroscopy.
Use of these techniques may help clinicians identify early changes in swallowing function in patients with Parkinson’s disease that could lead to earlier and thus more effective treatment.
Acknowledgments
Grant Support: This research was supported by NIH grant number R33 DC011130A from the National Institute on Deafness and other Communicative Disorders and a grant from the University of Wisconsin Institute for Clinical and Translational Research.
FUNDING, AND DISCLOSURES
This study was funded by NIH grant number R33 DC011130. Three authors (MRH, JJJ, and TMM) have applied for a patent related to some of the algorithms used in this study.
CAJ designed the study, collected the data, analyzed the videofluoroscopic data, wrote the manuscript, and approved the final version of the manuscript.
MRH designed the study, performed the pattern recognition analysis, wrote the manuscript, and approved the final version of the manuscript.
LL analyzed the manometric data, performed the pattern recognition analysis, and approved the final version of the manuscript.
SA collected the data, analyzed the videofluoroscopic data, and approved the final version of the manuscript.
JJJ designed the study and approved the final version of the manuscript.
TMM designed the study and approved the final version of the manuscript.
Footnotes
Conflicts of interest: three of the authors (MRH, JJJ, TMM) have applied for a patent related to some of the algorithms used in this study.
References
- 1.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63(7):1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 2.Fuh JL, Lee RC, Wang SJ, et al. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99(2):106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 3.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11(1):14–22. doi: 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 4.Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. Journal of neurology, neurosurgery, and psychiatry. 2009;80(9):1047–1049. doi: 10.1136/jnnp.2008.157701. [DOI] [PubMed] [Google Scholar]
- 5.Potulska A, Friedman A, Krolicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism & related disorders. 2003;9(6):349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 6.Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. The British journal of radiology. 1991;64(766):890–893. doi: 10.1259/0007-1285-64-766-890. [DOI] [PubMed] [Google Scholar]
- 7.Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13:69–81. doi: 10.1007/PL00009559. [DOI] [PubMed] [Google Scholar]
- 8.Plowman-Prine EK, Sapienza CM, Okun MS, et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24(9):1352–1358. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennington S, Snell K, Lee M, Walker R. The cause of death in idiopathic Parkinson’s disease. Parkinsonism & related disorders. 2010;16(7):434–437. doi: 10.1016/j.parkreldis.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Fuller RL, McCullough EC, Bao MZ, Averill RF. Estimating the costs of potentially preventable hospital acquired complications. Health Care Financing Review. 2009;30(4):17–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Müller J, Wenning GK, Verny M, et al. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Archives of neurology. 2001;58(2):259–264. doi: 10.1001/archneur.58.2.259. [DOI] [PubMed] [Google Scholar]
- 12.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Current opinion in otolaryngology & head and neck surgery. 2008;16(3):205–210. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 13.Ciucci MR, Grant LM, Rajamanickam ES, et al. Early identification and treatment of communication and swallowing deficits in Parkinson disease. Seminars in Speech and Language. 2013;34(3):185–202. doi: 10.1055/s-0033-1358367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baijens LW, Speyer R, Passos VL, Pilz W, Roodenburg N, Clave P. Swallowing in Parkinson Patients versus Healthy Controls: Reliability of Measurements in Videofluoroscopy. Gastroenterology research and practice. 2011;2011:380682. doi: 10.1155/2011/380682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57(3):405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]
- 16.Jones CA, Ciucci MR. Multimodal Swallowing Evaluation with High-Resolution Manometry Reveals Subtle Swallowing Changes in Early and Mid-Stage Parkinson Disease. Journal of Parkinsons Disease. 2016;6(1):197–208. doi: 10.3233/JPD-150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Yuan Z, Ji J, Li H, Xue F. Network or regression-based methods for disease discrimination: a comparison study. BMC medical research methodology. 2016;16:100. doi: 10.1186/s12874-016-0207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman MR, Mielens JD, Omari TI, Rommel N, Jiang JJ, McCulloch TM. Artificial neural network classification of pharyngeal high-resolution manometry with impedance data. Laryngoscope. 2013;123(3):713–720. doi: 10.1002/lary.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman MR, Jones CA, Geng Z, et al. Classification of high-resolution manometry data according to videofluoroscopic parameters using pattern recognition. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;149(1):126–133. doi: 10.1177/0194599813489506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielens JD, Hoffman MR, Ciucci MR, McCulloch TM, Jiang JJ. Application of classification models to pharyngeal high-resolution manometry. Journal of Speech Language and Hearing Research. 2012 doi: 10.1044/1092-4388(2011/11-0088). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omari TI, Papathanasopoulos A, Dejaeger E, et al. Reproducibility and agreement of pharyngeal automated impedance manometry with videofluoroscopy. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(10):862–867. doi: 10.1016/j.cgh.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Kritas S, Dejaeger E, Tack J, Omari T, Rommel N. Objective prediction of pharyngeal swallow dysfunction in dysphagia through artificial neural network modeling. Neurogastroenterology and Motility. 2016;28(3):336–344. doi: 10.1111/nmo.12730. [DOI] [PubMed] [Google Scholar]
- 23.Lee TH, Lee JS, Park JW, et al. High-resolution impedance manometry facilitates assessment of pharyngeal residue and oropharyngeal dysphagic mechanisms. Dis Esophagus. 2014;27(3):220–229. doi: 10.1111/dote.12101. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 25.Wallace KL, Middleton S, Cook IJ. Development and validation of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118(4):678–687. doi: 10.1016/s0016-5085(00)70137-5. [DOI] [PubMed] [Google Scholar]
- 26.Szczesniak MM, Maclean J, Zhang T, Liu R, Cook IJ. The normative range for and age and gender effects on the Sydney Swallow Questionnaire (SSQ) Dysphagia. 2014;29(5):535–538. doi: 10.1007/s00455-014-9541-x. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS Measurement Tool for Swallow Impairment-MBSImp: Establishing a Standard. Dysphagia. 2008;23(4):392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 29.Geng Z, Hoffman MR, Jones CA, McCulloch TM, Jiang JJ. Three-dimensional analysis of pharyngeal high-resolution manometry data. The Laryngoscope. 2013;123(7):1746–1753. doi: 10.1002/lary.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barikroo A, Carnaby G, Crary M. Effects of Age and Bolus Volume on Velocity of Hyolaryngeal Excursion in Healthy Adults. Dysphagia. 2015 doi: 10.1007/s00455-015-9637-y. [DOI] [PubMed] [Google Scholar]
- 31.Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17(4):278–287. doi: 10.1007/s00455-002-0070-7. [DOI] [PubMed] [Google Scholar]
- 32.Dodds WJ, Man KM, Cook IJ, Kahrilas PJ, Stewart ET, Kern MK. Influence of bolus volume on swallow-induced hyoid movement in normal subjects. AJR American journal of roentgenology. 1988;150(6):1307–1309. doi: 10.2214/ajr.150.6.1307. [DOI] [PubMed] [Google Scholar]
- 33.Alfonsi E, Cosentino G, Mainardi L, et al. Electrophysiological Investigations of Shape and Reproducibility of Oropharyngeal Swallowing: Interaction with Bolus Volume and Age. Dysphagia. 2015;30(5):540–550. doi: 10.1007/s00455-015-9634-1. [DOI] [PubMed] [Google Scholar]
- 34.Cock C, Besanko L, Kritas S, et al. Maximum upper esophageal sphincter (UES) admittance: a non-specific marker of UES dysfunction. Neurogastroenterology and Motility. 2016;28(2):225–233. doi: 10.1111/nmo.12714. [DOI] [PubMed] [Google Scholar]
- 35.Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. Journal of Speech and Hearing Research. 1994;37(5):1041–1059. doi: 10.1044/jshr.3705.1041. [DOI] [PubMed] [Google Scholar]
- 36.Hiss SG, Strauss M, Treole K, Stuart A, Boutilier S. Effects of age, gender, bolus volume, bolus viscosity, and gustation on swallowing apnea onset relative to lingual bolus propulsion onset in normal adults. Journal of Speech, Language, and Hearing Resesarch. 2004;47(3):572–583. doi: 10.1044/1092-4388(2004/044). [DOI] [PubMed] [Google Scholar]
- 37.Butler SG, Stuart A, Castell D, Russell GB, Koch K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. Journal of Speech, Language, and Hearing Resesarch. 2009;52(1):240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 38.Butler SG, Stuart A, Castell DRG, Koch K, Kemp S. Effects of age, gender, bolus volume, condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. Dysphagia. 2007;22(4):367–367. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 39.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high resolution manometry. The Laryngoscope. 2010;120:2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungheim M, Kallusky J, Ptok M. Effect of Bolus Volume on Pharyngeal Swallowing Dynamics Evaluated with Small High-Resolution Manometry Catheters. Laryngo- rhino- otologie. 2017;96(2):112–117. doi: 10.1055/s-0042-118231. [DOI] [PubMed] [Google Scholar]
- 41.Bennett JW, Van Lieshout PH, Pelletier CA, Steele CM. Sip-sizing behaviors in natural drinking conditions compared to instructed experimental conditions. Dysphagia. 2009;24(2):152–158. doi: 10.1007/s00455-008-9183-y. [DOI] [PubMed] [Google Scholar]
- 42.Hausdorff JM. Gait variability: methods, modeling and meaning. Journal of Neuroengineering and Rehabilitation. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tresilian JR, Stelmach GE, Adler CH. Stability of reach-to-grasp movement patterns in Parkinson’s disease. Brain. 1997;120( Pt 11):2093–2111. doi: 10.1093/brain/120.11.2093. [DOI] [PubMed] [Google Scholar]
- 44.Dounskaia N, Van Gemmert AW, Leis BC, Stelmach GE. Biased wrist and finger coordination in Parkinsonian patients during performance of graphical tasks. Neuropsychologia. 2009;47(12):2504–2514. doi: 10.1016/j.neuropsychologia.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson’s disease. Journal of the Acoustical Society of America. 2011;129(1):350–367. doi: 10.1121/1.3514381. [DOI] [PubMed] [Google Scholar]
- 46.van Hooren MR, Baijens LW, Voskuilen S, Oosterloo M, Kremer B. Treatment effects for dysphagia in Parkinson’s disease: A systematic review. Parkinsonism and Related Disorders. 2014 doi: 10.1016/j.parkreldis.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Athukorala RP, Jones RD, Sella O, Huckabee ML. Skill Training for Swallowing Rehabilitation in Patients With Parkinson’s Disease. Arch Phys Med Rehabil. 2014 doi: 10.1016/j.apmr.2014.03.001. [DOI] [PubMed] [Google Scholar]