Abstract
There are some predictable patterns of trauma in captive rhesus macaque (Macaca mulatta) social groups. Several factors have been documented to contribute to these patterns, including group formation of unrelated animals and the establishment of dominance ranks. Here, we report on how socially induced trauma in groups of rhesus monkeys is influenced by the breeding season, numbers of matrilines per group and matriline size. We analyzed three years of data collected from veterinary admittance logs for four groups in our specific pathogen free (SPF) breeding colony. Since the groups differed in time from formation, both the numbers of matrilines and the composition of those matrilines were different. Across the four groups, trauma rates were significantly higher during the fall breeding season than the spring and summer months when births occur. The group that was formed most recently, comprised of the greatest number of matrilines but fewest related animals, showed significantly higher rates of trauma than the older social groups. Further, the middle and lowest ranking families received signifincantly higher rates of trauma than the highest ranking families, suggesting a rank–related phenomenon. Additionally, there was a significant negative correlation between numbers of adult females in a matriline and rates of trauma observed in each matriline, but the numbers of adult females are significantly higher in the top ranked families compared to all of the other matrilines. These findings suggest that trauma rates increase during the breeding season and may be exacerbated in recently formed breeding groups that have smaller matrilines and reduced opportunities for social support to mitigate rank-related aggression. Management practices should be devised to ensure adequate matrilineal size to decrease rates of trauma in captive rhesus macaque groups.
Keywords: trauma, rhesus macaque, social stability, colony management, seasonality
Introduction
The NIH-funded National Primate Research Centers began the conversion of their conventional breeding colonies to Specific Pathogen Free (SPF) status in the 1990s in response to National Center for Research Resources (NCRR) contracts released in 1988 to reduce risk of exposure of workers to potential harmful pathogens, in particular, Cercopithecine Herpesvirus 1 (CHV-1). Because endemic simian retrovirus infection in rhesus monkeys was widespread and in an experimental setting impacted Human Immunodeficiency Virus (HIV) research in macaques, simian immunodeficiency virus [SIV], Type D simian retrovirus [SRV] and simian T-lymphotrophic virus 1 [STLV-1] were also targeted for exclusion in the SPF breeding groups. Like HIV, simian retroviruses can cause neoplasia, leukemias, neurological disease, and immunodeficiency syndrome in nonhuman primates (Buchl et al., 1997;, Daniel et al., 1984, 1985; Lerche et al., 1991; Lerche et al., 1994; Letvin et al., 1985; Lowenstine et al,1986; Murphey-Corb et al., 1986; Chakrabarti et al., 1987) which can negatively impact the HIV/AIDS research in macaque species.
The conversion of the Yerkes National Primate Research Center (YNPRC) colony to SPF status was accomplished by removing juvenile animals from their natal conventional groups and creating small, peer-reared groups that were isolated from the conventional animals and underwent rigorous viral testing to achieve and maintain a pathogen-free state (Goin and Gust, 1998). Due to this animal selection process, the newly formed social groups of SPF monkeys were usually composed of multiple peer-groups of unrelated animals, resulting in social groups with many small sized matrilines. These new social groups did not reflect the large and multigenerational matrilines that characterize both free-ranging and captive, long-established conventional social groups of rhesus monkeys (Maestripieri and Hoffman, 2012). For example, one study in Cayo Santiago (a semi free ranging population of rhesus macaques) found that the average number of individuals in matrilines ranged from 7 to 35 with the number of matrilines ranging from two to six across four social groups (Olivier et al., 1981). Further, Maestripieri and Hoffman (2012) found that between 2005–2010, the Cayo Santiago population consisted of 900–1000 individuals divided into six groups, ranging in size from 70 to 300 individuals. These groups contained only a few, very large matrilines (e.g., group R, N= 280, contained only three matrilines) that likely functioned to facilitate the influence of kinship on grooming and agonistic support within the group (Maestripieri and Hoffman, 2012). These large social groups can also exhibit very high rates of aggression and fighting (Bernstein, 1976).
There are multiple causes of aggression in captive rhesus monkeys. For example, it is well documented that formation of new groups contributes to extensive aggressive behavior (Bernstein and Mason, 1963; Southwick, 1967; Bernstein, 1969). This is due to the behaviors associated with the establishment of a dominance hierarchy, with lower-ranking animals generally receiving disproportionately more aggression (Rose, et al., 1974; Gust, et al., 1991). The dominance structure is maintained by aggression (Bernstein et al., 1974) and aggression is known to primarily occur over competition for resources for which in captive groups can include preferred food items, space and mates (Bernstein et al., 1974; Deutsch and Lee, 1991; Meishvili et al., 2009). While it has been suggested that social groups stabilize following initial aggression, outbreaks of severe aggression do occur in these seemingly stable social groups (Samuels and Henrickson, 1983). One factor which contributes to these increased levels of aggression is the simultaneous maturation of adolescents, either a larger cohort or a few individuals (Samuels and Henrickson, 1983). Additionally, while infrequent, high ranking matrilines can be displaced by lower ranking group mates through matriline “overthrows”. These extremely aggressive interactions generally result in injury and sometimes death to the members of the deposed matriline.
Rhesus macaques are seasonal breeders with the majority of breeding occurring between September and December in North America with subsequent births occurring in the spring and summer months (Lindburg, 1971; Loy, 1971; Drickamer, 1974). The breeding season is associated with increases in the gonadal hormones that produce fertility and increase socially motivated behaviors such as mate seeking. Specifically testosterone rises in males with the onset of the breeding season and estradiol increases in females during the periovulatory period (Baulu, 1976). Both the rise of the gonadal hormones and the associated behaviors can result in an increase in both affiliative and aggressive behaviors (Wilson and Boelkins, 1970; Michael and Zumpe, 1993). As observed in free-ranging rhesus macaque groups, it is not unexpected that aggression resulting in trauma would increase during the breeding season in captive macaques as well (Drickamer, 1975, Walker et al., 1983).
Successful management of captive rhesus macaque colonies includes maintaining the social and physical health of the animals. Thus, by identifying and understanding the hierarchical ranking of individual animals, patterns and interactions that deviate from expectations can indicate unrest in the group, possibly affecting the overall health of that social group. The colony management department at YNPRC performs routine behavioral observations on all social groups to gather information on rank hierarchy. Agonistic interactions as well as affiliative behaviors are recorded and if animals are not directing their behavior appropriately, for example, if subordinate animals are able to threaten or displace dominant animals, this might indicate instability within a single matriline within the group, or overall group instability. These agonistic events might result in trauma events, ranging in severity. Recognizing that trauma rates differ across seasons is also valuable information when evaluating social stability and social health of a breeding group. Our objective for this study was to investigate the relationship between the numbers and size of matrilines with the rates of trauma in a subset of our SPF rhesus macaque breeding groups. Since the formation of our SPF groups included small numbers of related adult females and multiple unrelated peers, we hypothesize that this composition of groups could potentially contribute to increased aggression and long-standing social instability. Furthermore, we predict that the increase in size of individual matrilines and/or reduction of the number of small matrilines over time should be correlated with reduced aggression.
Methods
All procedures described in this report adhere to the American Society of Primatologists Principles for the Ethical Treatment of Primates, the NIH Guide for the Care and Use of Laboratory Animals, and the Emory Institutional Animal Care and Use Committee (IACUC) standards. Yerkes National Primate Research Center is an AAALAC International accredited facility.
Study Subjects and Housing
This study focuses on four groups of rhesus macaques, ranging in size from 100 – 180 individuals, living at the Yerkes National Primate Research Center, Field Station (Lawrenceville, GA) between 2012-2014. The four study groups, housed in semi-natural, indoor/outdoor compounds, were comprised of breeding aged males (BAM), breeding aged females (BAF) and juveniles and infants of both sexes. At the time of the study, no females had reached an age that has been associated with perimenopause or menopause in macques. (Walker & Herndon, 2008). Matriline size and overall number of animals within each group were fairly stable over the entire study period (see Table 1). Matriline size was determined by the number of adult females (> 3 years old). All groups lived in similarily-size areas with the large, outdoor, open-top enclosure comprising 16,128 sqft with an adjacent heated inside area comprising 388.5 sqft. The large outdoor portion has natural environmental stimulation and includes multiple climbing and perching apparatus for physical enrichment, to permit social privacy and to elicit natural behaviors. Animals were provisioned with ad libitum water, monkey chow (Lab Diets, 5038) and regular produce. Standard toys and destructible or edible enrichment were offered on a rotating, daily basis.
Table 1. Group Demographics and Formation Data.
| Social Groups | |||||
|---|---|---|---|---|---|
| A | D* | B | C | ||
| # Animals | 150 | 119 | 105 | 181 | |
| #BAMa | 2-3 | 2-1 | 2 | 2 | |
| # Matrilines | 10 | 8 | 18 | 13 | |
| # BAFb | 64 | 43 | 40 | 68 | |
| BAF per matriline | Average | 6.4 | 5.38 | 2.22 | 5.23 |
| Midpoint | 5.5 | 4 | 1 | 5 | |
| Range | 2-12 | 1-13 | 1-12 | 1-10 | |
| YEAR FORMED | 1996, split 2003 | 2009 | 2001** | ||
| Founding Members | # Peer Groups | 6 | 1 | 5 | |
| # Females per Peer Group | 1-6 | 4 | 3-12 | ||
| # Matrilines | 29 | 24 | 34(14) | ||
Table 1 depicts the demographics of each social group (A-D) during the study period 2012-2014 (top) as well as the historical components during original group formation (bottom).
Data from group D includes only years 2013 and 2014 due to a temporary group split in 2012 for management purposes.
A subset of animals was removed from group C in 2002 and the number of matrilines was reduced from 34 to 14.
Breeding aged males – The number of BAM fluctuated during study period.
Breeding aged females
Formation of SPF Groups
The groups were designated as A, B, C, D. All groups studied in this analysis were originally formed in a similar manner. Briefly, young male and female rhesus macaques, aged 9-12 months, were removed from different non-SPF groups across the breeding colony based on the absence of Herpes B, SIV, SRV and STLV-1. These SPF candidates lived in peer groups and completed at least one year of viral screening to ensure pathogen-free status. Older females identified in the initial SPF cohort served as maternal role models for the juvenile peer groups and eventually developed into the alpha matrilines within each group. With the addition of breeder males, these newly formed groups began to expand as females reached reproductive age and produced offspring. Each group averaged two breeder males throughout the study period as well. Table 1 summarizes original group formations and current demographics.
Data Collection
Social and clinical observations were carried out on a daily basis for all animals. The behavioral data collected by Colony Management personnel include all occurrences of dyadic agonistic and submissive behaviors as well as affiliative and sexual interactions as defined by departmental ethograms. Agonistic and submissive data are plotted in a dominance matrix to determine rank.
Technicians who were trained in both animal identification and behavioral observations performed daily behavioral assessments on the rhesus colony. Animals exhibiting changes in behavior that indicated signs of clinical illness or trauma were triaged by the veterinary technicians in consultation with the attending clinicians. Traumatic injuries included digit and tail trauma, skin lacerations or puncture wounds, skin tears and lameness. Based upon a set of YNPRC veterinary guidelines, these categories were further defined as mild, moderate or severe; however, for the purposes of this study, there was no differentiation in the severity or type of trauma. Generally, animals that exhibited injuries that included bone exposure, muscle involvement, continued or severe lameness or degloving would be transported to the veterinary clinic for evaluation and recorded in the clinic admittance log. For these analyses, these clinic admittance log data from each of the four groups (A, B, C, D) between 2012 and 2014 were used, however only data from 2013-2014 were used for group D since the group was temporarily split in 2012. Table 1 provides a summary of the groups included in the study.
Statistical Analyses
For the purposes of this paper, only animals that were removed from the social group, requiring veterinary treatment were included in analyses. To account for varying number of animals per group and matriline, trauma frequencies were standardized to reflect rates of trauma per matriline, except for the analysis of seasonal effect since we were simply describing the seasonal pattern of trauma rather than rates of trauma. In this standardization, trauma frequencies were adjusted by numbers of females in each matriline. For example, if the alpha matriline in Group A received 15 instances of trauma, that number was adjusted by dividing by the number of females in that matriline (N=12). This adjustment allowed the comparison of rates of trauma in groups with varying numbers of animals, specifically adult females. In the analysis of seasonal effect, actual trauma frequencies were used and thus the larger groups with more animals had higher frequencies of trauma. Each of the four social groups were also divided into three dominance tiers: High (top three families), low (bottom three families) and middle (all remaining families). The middle ranking families were combined even if there were more than three total families because many of these middle-ranking matrilines were from the same peer group and composed of a few numbers of adult females. Finally, to evaluate the effect of season on instances of trauma, we grouped months into Winter (Jan, Feb, Mar), Spring (Apr, May, Jun), Summer (Jul, Aug, Sep) and Fall (Oct, Nov, Dec). One-way and Two-way ANOVA tests, as well as correlations were analyzed using SPSS Statistics v. 24 (IBM). For all tests, α value was set at 0.05 for significance and all tests were two-tailed. After an overall significant One-way ANOVA result, Bonferroni's multiple comparison tests were explored, while Tukey's test was used for the Two-way ANOVA results.
Results
The population size and number of matrilines differed across the four social groups. Due to the removal of animals from each social group for research study assignments (normally mid or low ranking animals which did not impact the social stability of the group), the population of each group and size and number of matriline remained fairly consistent over the study period, in spite of annual births (see Table 1). The number of breeding aged females differed between matrilines, ranging from one to 13 adult females. The older social groups (Groups A and D formed in 1996 and subsequently split in 2003) had the fewest number of matrilines but largest number of adult females per matriline, while the newest social group (Group B - established in 2009) had the greatest number of matrilines with the smallest number of adult females per matriline. Generally, the alpha matrilines were the largest in number for each social group. Group D was the exception, with two families (rank 5/8 and 6/8) containing more adult females than the alpha family in that group. Trauma patterns were consistent over the three-year study period in each group; therefore, the data have been combined for all years included in the study. There appeared to be a trend of increasing frequencies of traumas from 2012–2014, but this did not impact the seasonal pattern of trauma over each year.
One-way ANOVA demonstrated that both the categories of High rank and Middle rank had significantly larger families than the Low ranked families F=10.6, df=2,46, P<0.001. Overall however, One-way ANOVA demonstrated that the age distribution of females in the groups was not significantly different across the four study groups (P=0.827). There was a significant difference in frequencies of trauma across all groups by season (F=7.856, df=3,12 P=0.004). In this population, most of the births occur during the Spring months, while breeding and conception is largely restricted to the Fall months. Post-hoc analyses indicate that trauma frequencies were significantly higher during the breeding season (October, November, December) than the other seasons Winter (P=0.029), Spring (P=0.004), and Summer (P=0.031). (Figure 1)
Figure 1. Seasonal Patterns of Trauma.
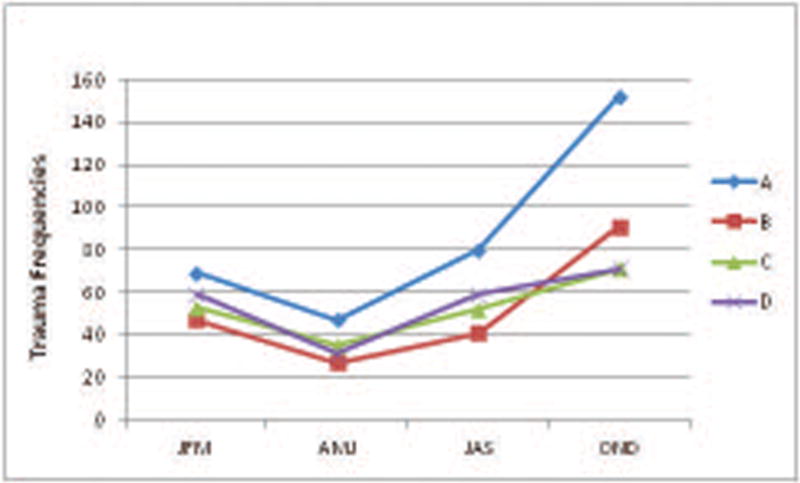
Pattern of trauma frequencies (2012-2014) across season for each group. These data are not adjusted by numbers of females in the groups, therefore the higher incidences of trauma exhibited by the larger social groups is evident here. *Group D data was from 2013-2014 due to temporary group split in 2012.
There was a significant difference in trauma rates across the four groups (F=3.398, df=3,37, P=0.028). Tukey's post-hoc analyses indicate that Group B, which had the highest number of unrelated matrilines, had significantly higher rates of trauma than Group A (P=0.029) Group C (P=0.037) and Group D (P=0.006). None of the other groups exhibited trauma rates that were significantly different from one another (P > 0.353); Figure 2.
Figure 2. Trauma Rates in each Social Group.
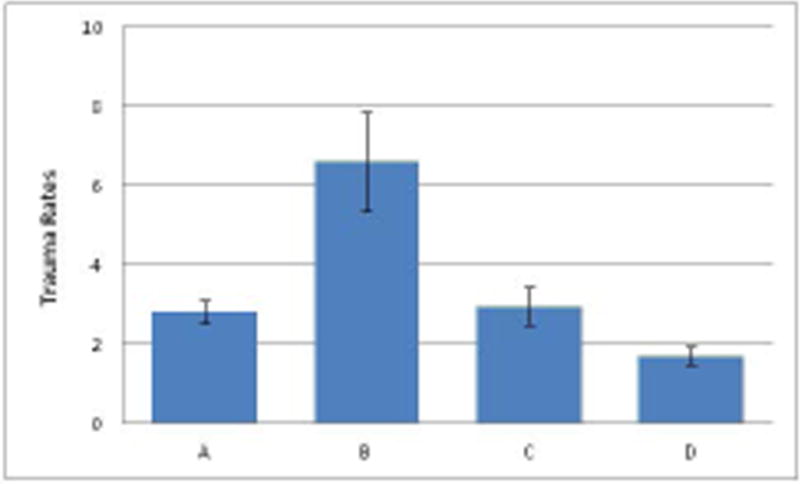
Overall rates of trauma in groups A-D. These data are adjusted by numbers of females in each family/group.
Rank played a significant role in rates of traumas across all of the social groups (F=4.019, df=2,37, P=0.026). The highest ranking families (the top three in each social group) received significantly lower rates of trauma than either of the other two social rank categories; middle rank (p=0.05) or low rank (p=0.009) (Figure 3). There was no difference in rates of trauma in the middle or lower ranking matrilines (P=0.272).
Figure 3. Trauma Rates by Rank.
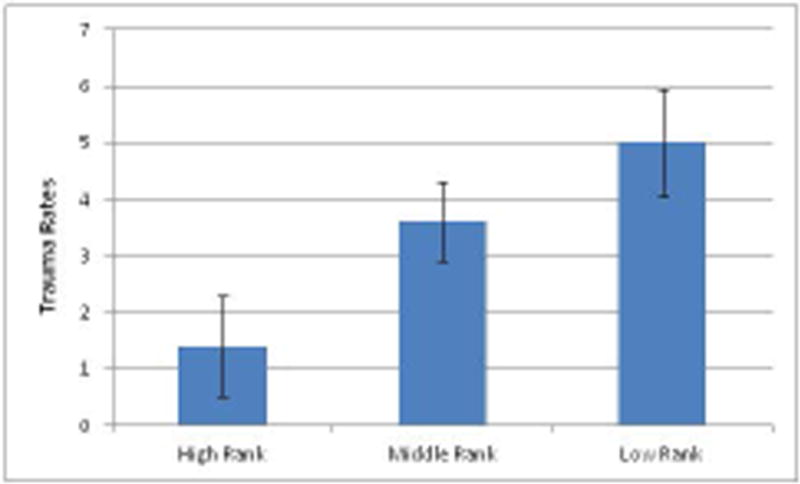
Trauma rates were averaged for the high, middle and low ranking families. High ranking contained the three top families in each group, low ranking contained the bottom three families. The middle ranking families were comprised of the remaining matrilines *Group D had only two bottom ranking families since the group was comprised of only eight matrilines.
Finally, there was a significant negative correlation between numbers of adult females in a matriline and rates of observed trauma in each matriline across the four groups (R= -0.383, P=0.007, N=49) (Figure 4).
Figure 4. Correlation of Trauma Event and Number of Females in a Matriline.
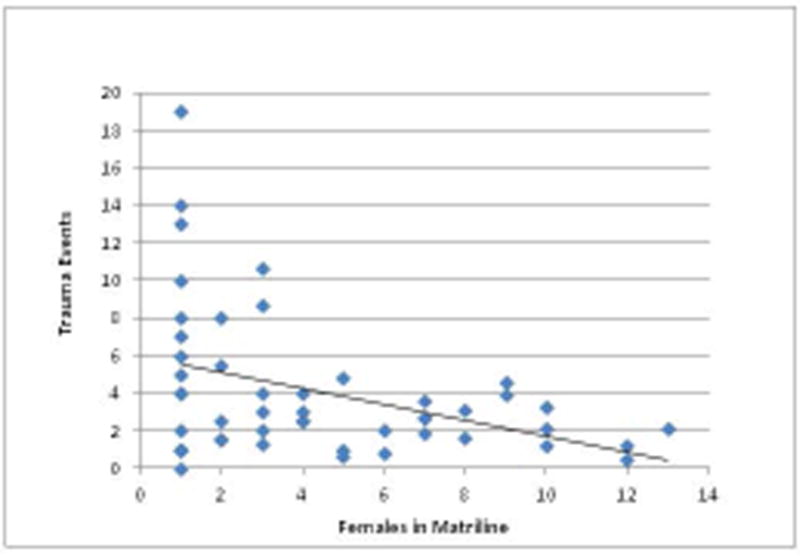
Regression line depicting the relationship between the number of females in each matriline across the social groups and incidences of trauma experienced by those matrilines.
Discussion
Social group formation and population growth of captive breeding colonies provides an opportunity to examine and evaluate the dynamics of group structure and stability. Social instability and an unstable dominance hierarchy can result in the decreased reproductive success (Dettmer et al., 2015, Wilson et al., 1978), therefore the goal of Colony Management at YNPRC is to maintain the social stability of our rhesus monkey groups in order to reduce aggression and trauma for animal welfare and increase reproductive success. The stability of the matrilineal social structure of rhesus monkeys is a key factor in maintaining overall social health and production. The results of this study demonstrate that variables, such as the number of adult females in a matriline and number of matrilines within a social group, have a significant impact on trauma events. Other studies have also demonstrated that the number of females in a matriline may play a role in group stability. Specifically, the larger, more dominant matrilines, may provide support and social stability to a social group (Oates-O'Brien et al., 2010). The Colony Management strategy at YNPRC is to generally keep the Alpha and Beta ranked matrilines in each group larger than the remaining matrilines to encourage group stability and to reduce the risk of matrline overthrows. Subsequently, higher ranked animals receive significantly lower rates of trauma than middle or low ranking animals. It's likely that this is due to establishment and maintenance of the dominance hierarchy (Rose, et al., 1974; Gust, et al., 1991) as well as having fewer family members to offer support in the typically smaller lower ranked families in the colony. Further, as described in the results, we noted that trauma frequencies changed in the groups over the 3 year study period, however there were consistent seasonal patterns each year associated with the breeding season. Since rhesus macaques are seasonal breeders (Lindburg, 1971; Loy, 1971; Drickamer, 1974) the competition for mates, as well as the rise in gonadal steroids may be related to the observed increases in trauma that occur during the peak breeding season (Wilson and Boelkins, 1970; Michael and Zumpe, 1993). While we have yet to identify the primary reasons for these increases in trauma, it has been observed that duration of breeder male tenure is correlated with changes in social group trauma (Stavisky et al., ms in prep). Specifically, it appears that trauma frequencies increase significantly when new breeder males are introduced; however, these levels significantly decrease by the second breeding season. Data from this study covered a three year period, thus there may have been an effect of breeder male tenure on the change in trauma frequencies. The influence of males on social group trauma can also be seen through social network analyses. Specifically, the ratio of adult males to adult breeding age females is a critical factor for group stability (Beisner et al., 2012). Non-natal, adult males function to “police” breeding groups, which maintains social stability and therefore reduces trauma, while a lower ratio of adult males to adult females contributes to social instability and likely higher levels of trauma or more severe trauma (Backström and Windberg; 2013; Beisner et al., 2011; Beisner and McCowan, 2013; Flack et al., 2005; Flack et al., 2006; McCowan et al., 2008).
The formation of these groups produced a matrilineal dominance hierarchy similar to groups in the wild but the multigenerational composition of these matrilines was very different. In typical multigenerational rhesus monkey groups, animals are part of distinct matrilines and each individual has a significant social and familial relationship with other animals in its group. The formation of our SPF groups eliminated these familial bonds, by housing peers together in small groups and subsequently introducing multiple peer groups, and adult mentors, to eventually form the basis of larger breeding groups. We have anecdotal evidence that peer group members preferentially affiliate with each other and tend to act like a family or a matriline in conflicts with other group members. However, it has also been observed that when there is a major stressor in the group, the true families act independently and peer group membership becomes less important. This phenomenon was most evident during an overthrow of a social group unrelated to this study that had been formed in the ways described here with multiple peer groups and small matrilines.
These findings suggest that an evaluation and manipulation of group composition may reduce rates of trauma in captive rhesus macaque groups. In particular, manipulation of matrilines, either decreasing numbers of matrilines and/or increasing the number of matriline members could be effective strategies. Based on the data from the present analysis, we are currently removing some small matrilines from social groups in the SPF colony at Yerkes in an attempt to mitigate trauma. Generally, the candidates for removal are either families that are the recipients of repeated trauma, or matrilines that are represented by only one or two adult females. We postulate that small matrilines are particularly vulnerable to trauma because of the lack of family support. The removal of these animals should reduce overall group aggression and is appropriate as long as there is sufficient genetic diversity in the population. A second strategy could be to focus on increasing the size of these small families. Since there is removal of animals from social groups for research studies, it is possible to allow the small families to increase in size over time by not removing females born into those matrilines. Continued manipulations of our groups should result in reduced levels of trauma if these strategies are successful and future analyses will investigate the impact of both scenarios.
Acknowledgments
This research was supported by the Office of Research Infrastructure Programs, NIH, OD P51OD11132. No authors held conflicts of interest. The authors would like to thank the Division of Animals Resources including the Colony Management Unit, Veterinary Department, Behavioral Management and Animal Care. Thanks also to Dr. Mark Wilson for his thoughtful comments on the manuscript.
References
- Backström T, Winberg S. Central corticotropin releasing factor and social stress. Frontiers in Neuroscience. 2013;7:117. doi: 10.3389/fnins.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulu J. Seasonal Sex Skin Coloration and Hormonal Fluctuations in Free-Ranging and Captive Monkeys. Hormones and Behavior. 1976;7:481–494. doi: 10.1016/0018-506X(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A, McCowan B. Effects of natal male alliances on aggression and power dynamics in rhesus macaques. American Journal of Primatology. 2011;73:790–801. doi: 10.1002/ajp.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A, McCowan B. Sex ratio, conflict dynamics, and wounding in rhesus macaques (Macaca mulatta) Applied Animal Behaviour Science. 2012;137:137–147. doi: 10.1016/j.applanim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, McCowan B. Policing in nonhuman primates: partial interventions serve a prosocial conflict management function in rhesus macaques. PLoS ONE. 2013;8:e77369. doi: 10.1371/journal.pone.0077369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS. Introductory techniques in the formation of pigtailed monkey troops. Folia Primatologica. 1969;10:1–19. doi: 10.1159/000155186. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. Journal of Theoretical Biology. 1976;60(2):459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Mason WA. Group formation by rhesus monkeys. Animal Behaviour. 1963;11:28–31. doi: 10.1016/0003-3472(63)90004-6. [DOI] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and Social Controls in Rhesus Monkey (Macaca mulatta) Groups Revealed in Group Formation Studies. Folia Primatologica. 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Buchl SJ, Keeling ME, Voss WR. Establishing Specific Pathogen-Free (SPF) Nonhuman Primate Colonies ILAR Journal. 1997;38:22–27. doi: 10.1093/ilar.38.1.22. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Guyader M, Alizon M, Daniel MD, Desrosiers RC, Tiollais P, Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987;328:543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Daniel MD, King NW, Letvin NL, Hunt RD, Sehgal PK, Desrosiers RC. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984;223:602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Woodward RA, Suomi SJ. Reproductive Consequences of a Matrilineal Overthrow in Rhesus Monkeys. American Journal of Primatology. 2015;77:346–352. doi: 10.1002/ajp.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JC, Lee PC. Dominance and feeding competition in captive rhesus monkeys. International Journal of Primatology. 1991;12:615–628. doi: 10.1007/BF02547673. [DOI] [Google Scholar]
- Drickamer LC. Social rank, observability, and sexual behavior of rhesus monkeys (Macaca mulatta) Journal of Reproduction and Fertility. 1974;37:117–120. doi: 10.1530/jrf.0.0370117. [DOI] [PubMed] [Google Scholar]
- Drickamer LC. Quantitative observation of behavior in free-ranging Macaca mulatta: Methodology and aggression. Behaviour. 1975;55:209–236. doi: 10.1163/156853975X00470. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proceedings of the Royal Society B. 2005;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- Goin DL, Gust DA. Peer-rearing Influences Subsequent Maternal Behavior and Infant Survival in a Newly Formed Herpes B-Virus Negative Rhesus Macaque Group. Primates. 1998;39:539–543. doi: 10.1007/BF02557574. [DOI] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, Behavior and Immunity. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Lerche NW, Max PA, Gardner MB. Elimination of type D retrovirus infection from group housed rhesus monkeys using serial testing and removal. Laboratory Animal Science. 1991;41:123–127. [PubMed] [Google Scholar]
- Lerche NW, Yee JL, Jennings MB. Establishing specific retrovirus-free breeding colonies of macaques: an approach to primary screening and surveillance. Laboratory animal science. 1994;44(3):217–221. [PubMed] [Google Scholar]
- Letvin NL, Daniel MD, Sehgal PK, Desrosiers RC, Hunt RD, Waldron LM, MacKey JJ, Schmidt DK, Chalifoux LV, King NW. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lindburg DG. The rhesus monkey in North India: an ecological and behavioral study. Primate behavior: developments in field and laboratory research. 1971;2:1–106. [Google Scholar]
- Lowenstine LJ, Pedersen NC, Higgins J, Pallis KC, Uyeda A, Marx P, Lerche NW, Munn RJ, Gardner MB. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys) International Journal of Cancer. 1986;38:563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Loy J. Estrous behavior of free-ranging rhesus monkeys (Macaca mulatta) Primates. 1971;12:1–31. doi: 10.1007/BF01730379. [DOI] [Google Scholar]
- Maestripieri D, Hoffman CL. Bones, genetics, and behavior of rhesus macaques. Springer; New York: 2012. Behavior and social dynamics of rhesus macaques on Cayo Santiago; pp. 247–262. [Google Scholar]
- Meishvili NV, Chalyan VG, Rozhkova YY. The Causes of Intragroup Aggression In Rhesus Macaques. Neuroscience and Behavioral Physiology. 2009;39:147–151. doi: 10.1007/s11055-009-9115-9. [DOI] [PubMed] [Google Scholar]
- Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macques. American Journal of Primatology. 1993;30:213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M, Martin LN, Ranga SR, Basking GB, Gormus BJ, Wolf RH, Andes WA, West M, Montelaro RC. Isolation of an HTLV-Ill related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of Matrilineal Overthrows in Large Captive Breeding Groups of Rhesus Macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2010;49:196–201. [PMC free article] [PubMed] [Google Scholar]
- Olivier TJ, Ober C, Buettner-Janusch J, Sade DS. Genetic Differentiation among Matrilines in Social Groups of Rhesus Monkeys. Behavioral Ecology and Sociobiology. 1981;8:279–285. [Google Scholar]
- Rose RM, Bernstein IS, Gordon TP, Catlin SF. Primate Aggression, Territoriality, and Xenophobia. Academic Press; New York: 1974. Androgens and aggression: a review and recent findings in primates; pp. 275–304. [Google Scholar]
- Samuels A, Henrickson RV. Brief report: outbreak of severe aggression in captive Macaca mulatta. American Journal of Primatology. 1983;3:277–281. doi: 10.1002/ajp.1350050314. [DOI] [PubMed] [Google Scholar]
- Southwick CH. An experimental study of intragroup agonistic behavior in rhesus monkeys (Macaca mulatta) Behaviour. 1967;28:182–209. doi: 10.1163/156853967X00235. [DOI] [PubMed] [Google Scholar]
- Walker ML, Wilson ME, Gordon TP. Female rhesus-monkey aggression during the menstrual-cycle. Animal Behaviour. 1983;31(83):1047–1054. 80011–6. doi: 10.1016/S0003-3472. [DOI] [Google Scholar]
- Walker ML, Herndon JG. Menopause in nonhuman primates? Biology of reproduction. 2008;79(3):398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AP, Boelkins RC. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Animal Behavior. 1970;18:719–724. doi: 10.1016/0003-3472(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Bernstein IS. Timing of Births and Reproductive Success in Rhesus Monkey Social Groups. Journal of Medical Primatology. 1978;7:202–212. doi: 10.1159/000459880. [DOI] [PubMed] [Google Scholar]


