Abstract
The genetic regulatory mechanisms that govern natural corolla senescence in petunia are not well understood. To identify key genes and pathways that regulate the process, we performed a transcriptome analysis in petunia corolla at four developmental stages, including corolla fully opening without anther dehiscence (D0), corolla expansion, 2 days after anthesis (D2), corolla with initial signs of senescence (D4), and wilting corolla (D7). We identified large numbers of differentially expressed genes (DEGs), ranging from 4626 between the transition from D0 and D2, 1116 between D2 and D4, a transition to the onset of flower senescence, and 327 between D4 and D7, a developmental stage representing flower senescence. KEGG analysis showed that the auxin- and ethylene-related hormone biosynthesis and signaling transduction pathways were significantly activated during the flower development and highly upregulated at onset of flower senescence. Ethylene emission was detected at the D2 to D4 transition, followed by a large eruption at the D4 to D7 transition. Furthermore, large numbers of transcription factors (TFs) were activated over the course of senescence. Functional analysis by virus-induced gene silencing (VIGS) experiments demonstrated that inhibition of the expression of TFs, such as ethylene-related ERF, auxin-related ARF, bHLH, HB, and MADS-box, significantly extended or shortened flower longevity. Our data suggest that hormonal interaction between auxin and ethylene may play critical regulatory roles in the onset of natural corolla senescence in petunia.
Flower development: When to wilt
How long flowers bloom is determined by genes that alter levels of the plant hormones ethylene and auxin. When flowers are no longer needed, plants signal them to die; the underlying genetic mechanisms are not well understood. Cai-Zhong Jiang at the University of California, Davis and co-workers used genetic analysis to determine how the signals for petal death are encoded. They studied petunia flowers at four stages: opening, pollen release, onset of wilting, and fully wilted, and identified over 5000 genes that were turned up or down. The master switches for petal death were found to be genes controlling ethylene and auxin levels. Using a virus to artificially switch off several genes, the researchers identified genes that shorten or extend flower life by up to three days. These results may be useful in plant breeding.
Introduction
Petal senescence is the irreversible and final stage of floral differentiation and development, associated with dynamic alterations once a flower has been successfully pollinated1,2. However, it is not clear how the process is regulated genetically. Homeostasis or alterations of plant hormones is involved in the onset of floral senescence3. In ethylene (ET)-sensitive flowers, the first sign of visible senescence is accompanied by a transient and sudden rise of ET production4. Other hormones such as cytokinin (CK)5, abscisic acid (ABA), auxin6, gibberellic acid (GA)7, and jasmonic acid (JA)8 are also involved in ET-sensitive petal senescence. ABA accelerates petal senescence9. Treatment with ABA promotes the large increase in ET production and hastens petal wilting in carnation flowers10. Pretreatments with silver thiosulfate (STS), a chemical that inhibits the perception of ET by the ET receptor, completely prevents the increase in ABA levels11. A negative relationship was observed between the level of CKs and petal senescence in petunia and carnation. In rose, the increase of CK content antagonized petal senescence prompted by ET12. Applying CKs delayed petunia petal senescence13. Auxin also plays a role in ET-sensitive petal senescence. Application of auxin prompted ET production and petal wilting in cut carnation flowers14. In addition, 2,4-dichlorophenoxyacetic acid (2,4-d), a synthetic auxin, induced the expression of 1-aminocyclopropane-1-carboxylic acid synthase (ACS) genes in petals15. In most research, these hormones are used as exogenous regulators to observe ET sensitivity and floral longevity in ET-sensitive species. Although the enrichment of ‘response to 1-aminocyclopropase-1-carboxylic acid (ACC) and auxin stimulus’ was observed 12 h after pollination in the petals of petunia16, the differential expression patterns of genes related to these hormones in petal senescence is unclear.
Petal senescence is regulated by transcription factors (TFs). On one hand, ethylene-insensitve-like (EIL) and ethylene response factors (ERFs) are correlated with the ET response signaling pathway2. EIL3, a homolog of ET-insensitive 3 (EIN3) in carnation, is a pivotal switch of ET-induced gene expression6,17. DAFSAG9, which is homologous to ERF2, was significantly upregulated in senescing daffodil petals18. On the other hand, a large group of other TFs, such as B-box zinc finger, bHLH DNA-binding, homeodomain-like (HD), MADS-box, MYB, and NAC, display differential expression when ET-insensitivity is induced in the etr1-1 transgenic petunia3. More than 20 members from the ERF, NAC, bZIP, HD-Zip, and WRKY TF families showed differential expression in petals at the early stage of pollination-induced senescence in petunia16. In addition, NAC, Aux/IAA, MYB, bZIP, and MADS-box are differentially expressed during carnation petal senescence6,19. These studies indicate that these TFs play regulating roles in ET-dependent petal senescence. However, the biological functions of these TFs are largely unknown.
High-throughput gene expression analysis using messenger RNA (mRNA) sequencing (RNA-Seq) represents the most powerful tool to elucidate the underlying regulatory mechanism of corolla senescence. Recently, pollination- and ET-induced corolla senescence in petunia has been studied through RNA-Seq analysis16,20, however, the regulatory mechanisms that govern the onset of natural corolla senescence from opening to wilting in petunia is unclear. Therefore, identifying the dynamic processes and regulatory factors in transcription is a crucial step in determining the master switches in corolla senescence. We employed RNA-seq technology to investigate the global and chronological sequence of transcriptional events during the initial corolla senescence in petunia. Furthermore, virus-induced gene silencing (VIGS) system was used to dissect biological functions of potential regulatory genes such as TFs. Our data suggest that hormonal interactions between auxin and ET may play a critical role in the regulation of onset of corolla senescence in petunia.
Materials and methods
Plant materials
All petunia plants were grown in a greenhouse at the University of California, Davis (USA), as described previously3,21.
Petunia hybrida ‘Mitchell Diploid’, a white flower cultivar, was used in the transcriptomic analysis. Corolla limbs were collected on day 0 (D0), when flowers were open but before the anthers dehisced, day 2 after anthesis (D2, corolla were fully expanded), day 4 (D4, corolla displayed a wilting sign at the tip edge, considered as the onset of flower senescence), and day 7 (D7, corolla showed wilting).
P. hybrida ‘Primetime Blue’, a purple flower hybrid cultivar, was used for VIGS analysis22.
RNA preparation and Illumina sequencing
At least five corolla limbs were collected from five plants at random on each replicate. Corolla limbs from two independent biological replicates were used to extract total RNA and to sequence separately. The total RNA was obtained using the TRIzol method (Invitrogen, USA), combined with Ambion RiboPure™ Kit (Ambion, USA) to remove the contaminant DNA3. The purity and concentration of RNA were verified using NanoDrop™ 3100 Spectrophotometer (Thermo Scientific, USA) and Qubit RNA Assay Kit 2.0 (Life Technologies, CA, USA). The RNA integrity was examined by RNA Nano 6000 Assay Kit using the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Eight complementray DNA (cDNA) libraries were prepared using purified mRNAs. An Illumina HiSeq2000 machine was used to perform 100 paired-end sequencing according to the Illumina protocols. The raw data were deposited to NCBI as project number SRP124540.
Sequence data processing and differential gene expression analysis
The quality of the raw reads was examined before and after trimming using FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Low-quality reads with a Phred quality score <20, sequences shorter than 40 bp, barcodes, polyA, polyT ends, and adapter sequences were removed. Clean reads from all samples were pooled and the read counts were normalized to the aligned FPKM (Fragments Per Kilobase of transcript per Million mapped reads) to quantify the gene expression level using cufflinks (version: 2.1.1)23. Differentially expressed genes (DEGs) were identified using Cuffdiff software (version: 2.1.1)23. The significance of DEGs was determined using fold change ≥2 or ≤0.5 as a cutoff24. False discovery rate (FDR) was adjusted across genes for significance levels (≤0.05) of all tests25.
Resulting sequences were mapped to the P. axillaris (one of P. hybrida’s parents) reference genome (https://solgenomics.net/organism/Petunia_axillaris/genome)26.
To obtain knowledge about expression profiles of DEGs throughout four development stages, the short time-series expression miner (STEM) was used to cluster DEGs27. The gene expression data and gene annotation files were uploaded to STEM. Expression profiles were analyzed using the STEM clustering algorithm27.
BiNGO 2.3 plugin tool in Cytoscape 3.2.128 was used to gain knowledge about gene ontology (GO) terms of DEGs in each cluster. Over-represented GO terms were identified using a hypergeometric test with a significance threshold of 0.05 after Benjamini and Hochberg FDR correction29 with the whole annotated genome as the reference set30,31.
Mapman visualizationwas performed as described previously32 to identify gene families that may play essential roles in regulating corolla senescence. Contigs were classified into a set of hierarchical functional categories (BINs), using Mercator with a blast cutoff of 5033. Because one unigene might have multiple contigs, a functional term of a unigene was derived from its representative contig that had the highest bit score34. Enrichment analysis was completed through Fisher’s test using Mefisto (http://www.usadellab.org/cms/index.php?page=mefisto) with Bonferroni correction. Gene expression changes were viewed in Mapman 3.5.1R232.
VIGS plasmid construction and Agrobacterium-mediated infection
Tobacco rattle virus (TRV)-based VIGS21 was used to analyze the role of TFs in corolla senescence. TRV is a bipartite positive sense RNA virus with the TRV-RNA1 (pTRV1) and TRV-RNA2 (pTRV2)35,36. pTRV1 can replicate and move systemically without RNA235,36. The pTRV2 was modified by introducing a chalcone synthase (CHS) fragment, which serves as a visual reporter for gene silencing to generate pTRV2/CHS vector for functional analysis of the genes-of-interest21. A gene specific fragment of each TF was amplified from petunia corolla cDNA using the corresponding specific primers listed in Supplementary Table S1, and then subcloned into the pTRV2/CHS vector to generate pTRV2/CHS-TF constructs. The constructs, pTRV1 and pTRV2/CHS (empty vector control) or pTRV2/CHS-TF were transformed into Agrobacterium tumefaciens strain GV3101 by electroporation22. The bacteria containing pTRV1 and pTRV2 (TRV2/CHS or TRV2/CHS-TF-) were mixed together in a 1:1 ratio immediately before inoculation21,22. The leaves of 4-week-old Primetime Blue seedlings were inoculated with the mixed bacterial culture using a 1 mL disposable syringe without a needle21,22. The inoculated seedlings were grown in a growth chamber under 16 h light/8 h dark (23/20 °C). The phenotypes of flowers were observed and monitored until silencing occurred, visualized as the purple anthocyanin pigment in the corollas turning white21,22. Flowers from wild-type, empty vector controls and TF-silenced petunias were used in longevity analysis.
qRT-PCR analysis
One microgram of total RNA was reverse-transcribed using PrimeScript RT reagent with gDNA Eraser Kit (TaKaRa, Japan), according to the manufacturer’s instructions. Specific primers were designed by the Primer 3 program and listed in Supplementary Table S1. Amplifications were performed in an Applied Biosystems 7300 system (Applied Biosystems, USA). Melting curve analysis was performed and the absence of non-specific products and primer dimers were verified. For data analysis, average threshold cycle (CT) values were calculated for each gene of interest, on the basis of three independent biological samples and were normalized and used to calculate relative transcript levels as described elsewhere37. 26S ribosomal RNA was used as an internal standard for normalization3.
ET measurement
ET emission was monitored using a laser-based ET detector (type ETD-300, Sense B·V., Nijmegen, The Netherlands) and a gas handling system (type VC-6, Sensor Sense B·V.) as described previously3. Briefly, flowers collected at D0 were placed into 70 ml sealed glass vials. The air was passed through a platinum-based catalyzer before entering the cuvettes in order to remove external ET and other hydrocarbons. A scrubber with KOH and CaCl2 was used to reduce the CO2 and the water content in the gas flow. ET emission was monitored and recorded in real time. Three biological replicates of every flowering stage were performed. Each experiment was repeated three times.
Floral longevity
To measure longevity of intact flowers, white flowers from pTRV/CHS-TFs inoculated petunia ‘Primetime Blue’ plants were tagged at D0. The time when the corollas wilted and the edges collapsed was recorded21. At least 20 flowers of three plants from each of the three independent biological replicates were monitored. Purple flowers from water-inoculated wild-type and white flowers from pTRV/CHS-silenced (empty vector) plants were used as controls. Statistical analyses were performed using the SPSS package (Version 16.0; SPSS Inc., Chicago, IL, USA). One-way analysis of variance was performed for experiments with one independent variable. Duncan’s test was used as the post hoc test if significant differences were found.
Results
Floral senescence and ET production
Flowers that were fully opened but anthers not yet dehisced were marked as D0. The corollas continued to expand for 2 days. Visible senescence symptoms, such as curving of the corolla edges, were observed at an average of 4 days. Corolla wilting was found at about 7–8 days (Fig. 1a). We measured ET production using a real-time ET detection system, EDT-300. An increase and decrease of ET emission was detected during D2–D7 stage. The level spiked around D4, reaching the maximum level at 5.5 days, and then decreasing sharply (Fig. 1b).
Fig. 1.
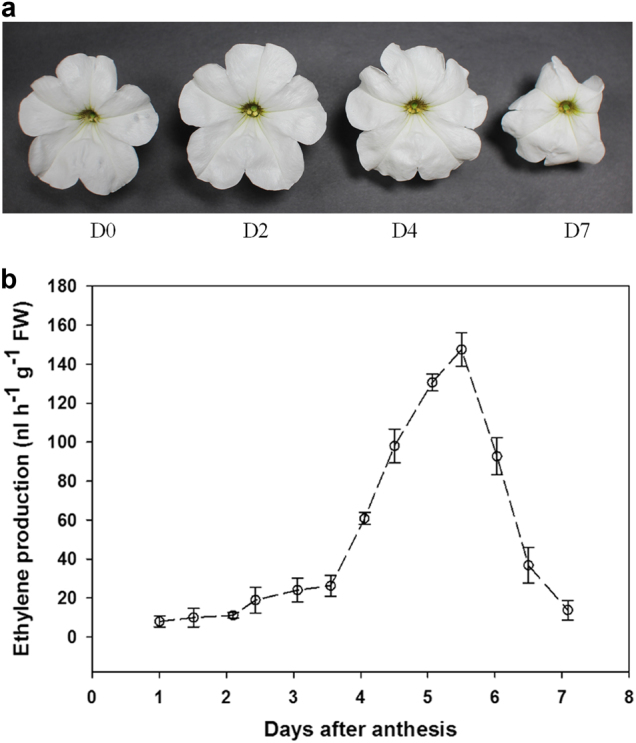
Natural senescence and ethylene production of wild-type (WT) Petunia × hybrida ‘Mitchell’ flowers. A representatives corolla phenotypes at different stages including corolla opening without anther dehiscence (D0), corolla expansion, 2 days after anthesis (D2), corolla with initial signs of senescence (D4), and wilting corolla (D7). B representatives ET production at various time points after corolla opening without anther dehiscence. ET emission was monitored using a laser-based ET detector (type ETD-300, Sense B.V., Nijmegen, The Netherlands) and a gas handling system (type VC-6, Sensor Sense B.V.). Three biological replicates of every flowering stage were performed. Values represent the average ± SD
Dynamic transcriptome profiles during corolla development
In order to determine the alteration in gene expression during corolla senescence, we generated cDNA libraries composed of the samples collected from four developmental stages (D0, D2, D4, and D7) with two biological replicates. RNA sequencing of these libraries produced 49,421,030, 52,985,600, 47,813,446, and 56,552,704 clean reads at D0, D2, D4, and D7, respectively (Table 1). The sequences were mapped to the P. axillaris reference genome26 for annotation of all unigenes. The mapping rate was over 93% for samples of each stage (Table 1). Differential expression analysis was conducted by comparing four different developmental stages. Analysis on all four stages generated 5167 unigenes that were significantly differentially expressed across these stages. The number of DEGs was decreased from 4626 between D0 and D2, to 1116 between D2 and D4, and to 327 between D4 and D7 (Fig. 2).
Table 1.
Statistics of annotation results for petunia unigenes
| Sample | Clean reads | Raw reads | Mapping rate (%) |
|---|---|---|---|
| D0 | 49,421,030 | 52,430,622 | 93.67 |
| D2 | 52,985,600 | 56,837,280 | 94.88 |
| D4 | 47,813,446 | 50,671,186 | 93.26 |
| D7 | 56,552,704 | 61,737,472 | 95.09 |
Fig. 2.
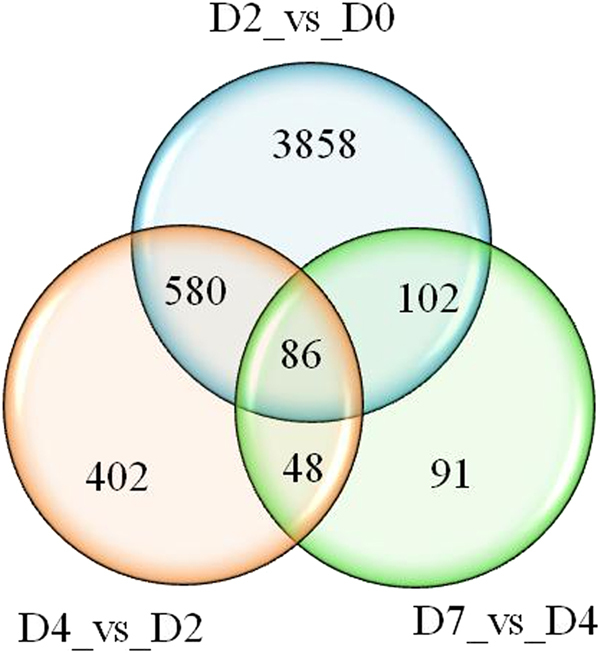
Unique and shared differential expression of unigenes in D0–D2, D2–D4, and D4–D7 pairwise analysis
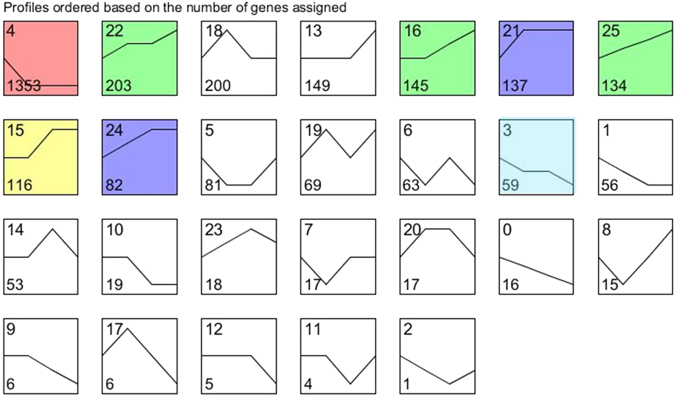
DEGs were clustered to generate expression patterns based on time series using the STEM software27. Cluster analysis of the data from four time points generated 26 clusters, including downregulated genes in clusters 0 through 12 and upregulated genes in clusters 13 through 25 (Fig. 3). A few clusters displayed a more complex pattern. For instance, clusters 2, 5, 7, 8, and 11 showed an initial decrease followed by upregulation. However, clusters 14 and 17 exhibited an initial increase followed by a decline (Fig. 3). In addition, the downregulated clusters 3 and 4 and the upregulated clusters 15, 16, 21, 24, and 25 were statistically significant (P ≤ 0.01) (Fig. 3).
Fig. 3.
Cluster and STEM analysis of DEGs. Twenty-six clusters were obtained using STEM software. The colored clusters represented a significant level (p value ≤ 0.05). The number on the top is a cluster number. The number at the bottom is gene number assigned in each cluster
Identification of up and downregulated gene ontology at distinct time points
In order to identify up and downregulated GO at each selected time point, seven gene clusters exhibiting either significantly decreased (clusters 3 and 4) or increased (clusters 15, 16, 21, 24, 25) expression were further analyzed using Cytoscape software with its GO enrichment tool BiNGO28.
At the transition from D0 to D2, the metabolic processes of major macronutrients including ‘carbohydrates, lipids, aromatic amino acids, and nitrogen compounds’ were downregulated (cluster 4, Supplementary Figure S2). In addition, ‘cell wall organization and biogenesis’, ‘S-adenosylmethionine biosynthesis’, and ‘negative regulation of transcription, DNA-dependent’ and ‘RNA metabolism’ were downregulated (cluster 4, Supplementary Figure S2). However, ‘CK pathway’, ‘RNA modification’, ‘macromolecule methylation’, ‘DNA metabolism’, ‘ATP activity’, and ‘S-adenosylmethionine-dependent methyltransferase activity’ were upregulated (cluster 22, 24, and 25). At the transition from D2 to D4, ‘monosaccharide metabolism (hexose and glucose)’, ‘polysaccharide metabolism (glucan)’, ‘lipid catabolism’, ‘amino acid metabolism (including glycine, l-serine, and methionine)’, ‘S-adenosylmethionine biosynthesis’, and ‘l-phenylalanine biosynthesis’ were significantly upregulated (clusters 22 and 24, Supplementary Figure S2). ‘Response to auxin stimulus’ was also significantly upregulated (cluster 25). Downregulated GO terms were mainly ‘nicotianamine metabolism and biosynthesis’ (cluster 1, Supplementary Figure S2). Biological regulation (‘anion channel activity’ and ‘ion transmembrane transporter activity’) was over-represented among downregulated GO terms (cluster 3, Supplementary Figure S2). At the transition from D4 to D7, 'iron ion binding' was significantly upregulated. The only downregulated biological process was the auxin-mediated signaling pathway (cluster 3). The over-representation of 'ribosome and cytosolic small ribosomal subunit' was also enriched in the downregulated GO group (Supplementary Figure S2).
Differential gene expression in hormone biosynthesis and signaling pathways
Hormone and transcriptional regulation pathways were found to be enriched in the significant cluster groups (cluster 16, 22, and 25). In order to further understand the key DEGs regulating corolla senescence, all DEGs related to hormone signaling and TFs across these three transitions were further analyzed with Mapman.
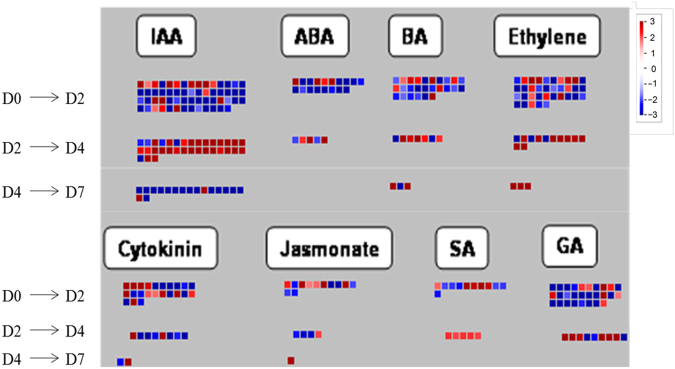
Within the hormonal function term, we identified DEGs related to hormone biosynthesis and signaling pathways. Among these genes, the largest numbers of DEGs were involved in auxin and ET biosynthesis and signaling pathways, followed by DEGs in the GA pathway (Fig. 4). DEGs related to auxin were predominantly downregulated through the D0 to D2 (24 out of 27 DEGs) and D4 to D7 transitions (21 out of 27), but upregulated in the D2 and D4 transition (26 out of 27 DEGs) (Fig. 4). Among these DEGs, 17 SAUR and SAUR-like genes, three auxin-induced genes (Peaxi162scf01044g00226, Peaxi162scf00000g43013, and Peaxi162scf01133g00016), and two IAA-amido synthases were upregulated through the D2 to D4 transition (Supplementary Table S2). For the ET pathway, 37.5% of the DEGs (9/24) were upregulated through the D0 to D2 transition, and 45.8% of the DEGs (11/24) were upregulated through the D2 to D4 transition. DEGs relating to ET biosynthesis pathway such as ACS (Peaxi162scf00074g01725) and ACO1 (Peaxi162scf00294g00812 and peaxi162scf00712g00513) were upregulated through the D2 to D4 transition, while ACS10 (Peaxi162scf00620g00121) and ACO (Peaxi162scf00047g01927) were upregulated through the D0 to D2 as well as the D4 to D7 transitions (Supplementary Table S2).
Fig. 4.
Display of gene expression involved in hormone biosynthesis and signaling pathway. Significantly DEGs (log2 fold changes (FC) ≥1, FDR ≤0.05) were visualized using Mapman software and organized into functional categories (BINs)32,33. Blue indicates a decrease and red an increase in gene expression (see color set scale on top right corner). Detailed information on each gene and its expression level is listed in Supplementary Table S2
DEGs related with GA pathway were upregulated in both the D0 to D2 and D2 to D4 transitions. Among these DEGs, GA2OX8 (Peaxi162scf00082g02010 and Peaxi162scf00111g00920) and GA3OX1 (Peaxi162scf00015g00525) were upregulated during petunia flower senescence (Fig. 4 and Supplementary Table S2). In addition, DEGs related to CK, ABA, JA, salicylic acid (SA), and 6-benzylaminopurine (6-BA) were obtained (Fig. 4 and Supplementary Table S2). Three genes related to the CK pathway were identified, including CK oxidase 3, cytokinin-independent 1 (CKI1), and CK response regulator 12 (RR12). These three genes were upregulated in the D0 to D2 transition (Fig. 4 and Supplementary Table S2). For the ABA biosynthesis pathway, AAO3 (Peaxi162scf00217g00412) was upregulated at the D0 to D2 transition. NCED4 (Peaxi162scf00045g00726) transcript was accumulated at the D2 to D4 transition. ABA 8’-hydroxylase (Peaxi162scf00045g00725) transcript was accumulated in the D2 to D4 and D4 to D7 transitions. Among those JA-related genes, AOS (Peaxi162scf00684g00578), OPDA (Peaxi162scf00688g00362), LOX2 (Peaxi162scf00895g00019 and Peaxi162scf00895g00113), and LOX3 (Peaxi162scf00038g02025) were upregulated at the D0 to D2 transition. No upregulated DEGs related to JA biosynthesis were detected at the D2 to D4 transition (Supplementary Table S2). For the SA pathway, two UDP-glucosyltransferase genes, UGT74E2 (Peaxi162scf00883g00811) and UGT74F1 (Peaxi162scf00303g00048), were upregulated at the D0 to D2 transition. SABATH methyltransferase was upregulated at the D2 to D4 transition. No DEGs involved in the SA pathway were detected at the D4 to D7 transition (Fig. 4 and Table Supplementary S2). In the 6-BA pathway, identified DEGs were mostly members of cytochrome P450 family (Supplementary Table S2).
Differential expression related to transcription factors
The differential expression of specific TFs at specific time points during corolla senescence was analyzed with Mapman (Fig. 5). A total of 409 DEGs encoding TFs was observed at D2, with 175 upregulated DEGs. Major TF families included AP2-EREBP, ARF, bHLH, HSF, MADS-box, MYB, WRKY and C2C2-CO-like (Fig. 5 and Table S3). Among all the differentially expressed TFs, ERF was the predominant family with 25 DEGs, including 14 upregulated genes at the D2 to D4 transition, followed by the zing-finger (15/24), ARF (9/15), MYB (9/26), bHLH (8/18), and HD-ZIP (5/11) families (Fig. 5 and Supplementary Table S3).
Fig. 5.
Display of gene expression of TFs. Significantly DEGs (log2 fold changes (FC) ≥1, FDR ≤0.05) were visualized using Mapman software and organized into functional categories (BINs)32,33. Blue indicates a decrease and red an increase gene expression (see color set scale on top right corner). Detailed information on each gene and its expression level is listed in Supplementary Table S3
Verification of RNA-seq results with qRT-PCR
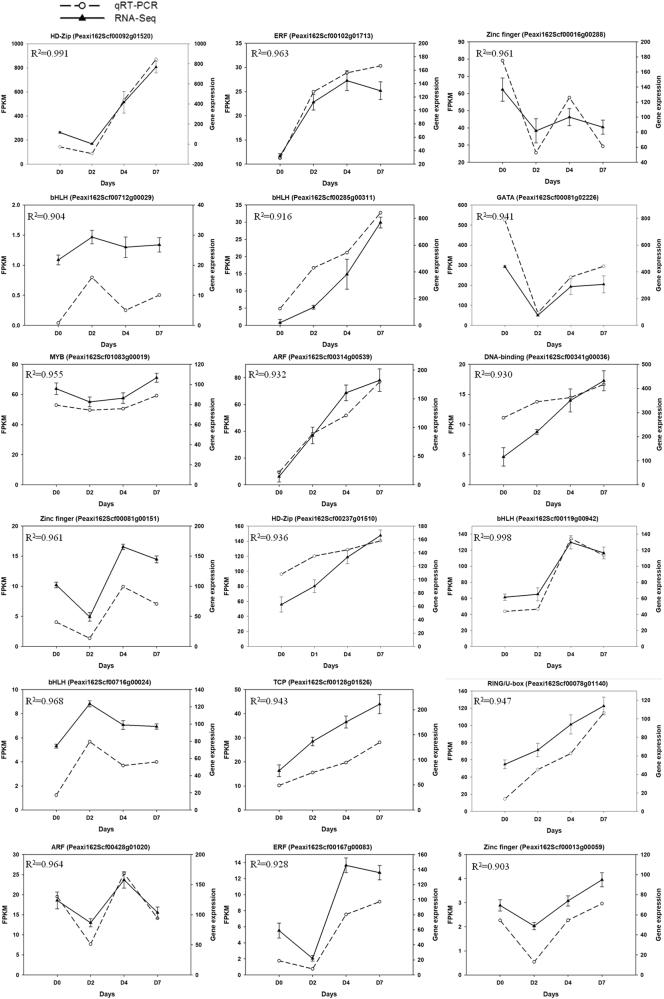
In order to verify our RNA-seq data, 18 differentially expressed TFs were randomly selected for qRT-PCR (Fig. 6). The correlation coefficients between RNA-seq and qRT-PCR were calculated. Overall, the qRT-PCR data were in agreement with the DEGs results. The pairwise correlation coefficient is higher than 0.90. The results showed that RNA-seq for counting transcripts reflects transcript abundance and can be used for gene expression analysis.
Fig. 6.
Validation of RNA-seq data by qRT-PCR. Eighteen genes were selected and their time-course expression profiles were evaluated at specific time points. cDNA analysis was performed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) amplification with specific primers designed by PRIMER 3 (Table S1). Transcript levels were normalized to 26S rRNA. Data represent three independent replicates (SD, n = 5). The correlation coefficient between RNA-seq and qRT-PCR was listed on the left corner of each gene expression figure
Silencing TFs affects floral longevity
Although many TFs show differential expression during corolla senescence, few experiments employing silencing or over-expression have been conducted to confirm the regulatory function of TFs. We used TRV-based VIGS technology to examine the role of TFs in corolla senescence (Table 2). The results showed that the inhibition of some TFs prolonged the longevity of corolla in silenced flowers compared with wild-type (WT) and empty vector controls. Among these selected TFs, plants with the silenced gene encoding MADS-box (Peaxi162Scf01084g00119) significantly extended floral longevity by >3 days on unpollinated intact flowers (Table 2, experiment 4). Silencing ERF (Peaxi162Scf00167g00083) and bHLH (Peaxi162Scf00285g00311) extended longevity by >2 days. These increases in longevity were substantial compared with WT flowers and empty vector controls. Moreover, suppressed expression of Zinc finger (Peaxi162Scf00081g00151, Peaxi162Scf00105g01114, Peaxi162Scf00016g00288, and Peaxi162Scf00013g00059), ERF (Peaxi162Scf00024g00271), HD-Zip (Peaxi162Scf00092g01520), bHLH (Peaxi162Scf00285g00311), and ACBF (Peaxi162Scf01105g00218) TFs extended floral longevity by >1 day (Table 2, experiment 1). On the other hand, silencing of some TFs shortened the corolla longevity compared with WT and empty vector controls, including ARF (Peaxi162Scf00314g00539) (Table 2, experiment 1), bZIP (Peaxi162Scf00285g00011) (Table 2, experiment 3), and several members of the zinc finger family (Peaxi162scf00013g00084 and Peaxi162Scf00060g00211) (Table 2, experiment 1).
Table 2.
Longevity of unpollinated TFs silenced flowers on petunia
| Gene ID | Flower longevity (days ± SD) | Description | Increased/decreased days compared to WT | Increased/decreased rate of longevity compared to WT (%) | Reference |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Peaxi162Scf00081g00151 | 9.89 ± 1.43c | Zinc finger | 1.47* | 17.46 | |
| Peaxi162scf00013g00084 | 7.04 ± 1.08b | Zinc finger | −1.38 | −16.39 | |
| Peaxi162Scf00060g00211 | 7.93 ± 1.48b | Zinc finger | −0.49 | −5.82 | |
| Peaxi162Scf00105g01114 | 9.5 ± 1.57c | Zinc finger | 1.08* | 12.83 | |
| Peaxi162Scf00207g01365 | 9.17 ± 0.75c | Zinc finger | 0.75 | 8.91 | |
| Peaxi162Scf00003g00267 | 8.5 ± 1.06a | Zinc finger | 0.08 | 0.95 | |
| Peaxi162Scf00016g00288 | 9.95 ± 1.29c | Zinc finger | 1.53* | 18.17 | |
| Peaxi162Scf00013g00059 | 9.63 ± 0.69c | Zinc finger | 1.21* | 14.37 | |
| Peaxi162Scf00167g00083 | 10.7 ± 1.19d | ERF | 2.28** | 27.08 | |
| Peaxi162Scf00024g00271 | 9.64 ± 0.55b | ERF | 1.22* | 14.49 | |
| Peaxi162Scf00102g01713 | 8 ± 0.87a | ERF | −0.42 | −4.99 | |
| Peaxi162Scf00092g01520 | 9.67 ± 1.68c | Homeobox | 1.25* | 14.85 | |
| Peaxi162Scf00237g01510 | 10.15 ± 1.55d | Homeobox | 1.73* | 20.55 | [22] |
| Peaxi162Scf00285g00311 | 11.36 ± 1.23d | bHLH | 2.94** | 34.92 | [51] |
| Peaxi162Scf00712g00029 | 9.42 ± 1.46c | bHLH | 1* | 11.88 | |
| Peaxi162Scf00119g00942 | 8.41 ± 1.22a | bHLH | −0.01 | −0.12 | |
| Peaxi162Scf00428g01020 | 8.35 ± 0.77a | bHLH | −0.07 | −0.83 | |
| Peaxi162Scf00081g02226 | 8.79 ± 1.07a | LIM | 0.37 | 4.39 | |
| Peaxi162Scf01083g00019 | 10.25 ± 1.6d | MYB | 1.83* | 21.73 | |
| Peaxi162Scf00420g00728 | 8.4 ± 1.17a | NAM | −0.02 | −0.24 | |
| Peaxi162Scf00045g01824 | 8.73 ± 1.49a | CO-like | 0.31 | 3.68 | |
| Peaxi162Scf00314g00539 | 7.27 ± 0.88b | ARF2 | −1.15 | −13.66 | |
| Peaxi162Scf00341g00036 | 9.7 ± 1.46c | DNA binding | 1.28* | 15.20 | |
| TRV CHS white flowers | 8.68 ± 1.19a | Control 1 | |||
| WT purple flowers | 8.42 ± 1.14a | Control 2 | |||
| Experiment 2 | |||||
| Peaxi162Scf01105g00218 | 9.6 ± 0.77a | NAM | 0.1 | 1.05 | |
| Peaxi162Scf00051g01410 | 10 ± 1.15b | NAM | 0.5 | 5.26 | |
| TRV CHS white flowers | 9.35 ± 0.75a | Control 1 | |||
| WT purple flowers | 9.5 ± 0.79a | Control 2 | |||
| Experiment 3 | |||||
| Peaxi162Scf00998g00127 | 7.96 ± 0.96a | NAM | 0.75 | 10.40 | [3] |
| Peaxi162Scf00287g00193 | 8.5 ± 1.89a | ARF19 | 1.29* | 17.89 | |
| Peaxi162Scf00285g00011 | 5.6 ± 0.68b | bZIP | −1.61 | −22.33 | [54] |
| TRV CHS white flowers | 7.79 ± 0.92a | Control 1 | |||
| WT purple flowers | 7.21 ± 0.95a | Control 2 | |||
| Experiment 4 | |||||
| Peaxi162Scf00002g00037 | 9.8 ± 1.13a | MYB | 0.75 | 8.29 | |
| Peaxi162Scf00716g00024 | 9.07 ± 1.28a | bHLH | 0.02 | 0.22 | |
| Peaxi162Scf00128g01526 | 9.88 ± 1.38a | TCP | 0.83 | 9.17 | |
| Peaxi162Scf01084g00119 | 12.8 ± 2.9b | MADS | 3.75*** | 41.44 | |
| TRV CHS white flowers | 9.15 ± 1.55a | Control 1 | |||
| WT purple flowers | 9.05 ± 1.23a | Control 2 | |||
Petunia plants were infected with Agrobacterium transformed with pTRV2/CHS as an empty vector control (named TRV CHS white flowers) or pTRV2/CHS-TF vector (displayed as Gene ID). WT purple flowers as an uninfected control. For each line, 10 corollas from each of three individual plants were used for testing flower longevity. The recorded longevity represents the time from flower full open but before anthers dehiscing to complete wilting of the corolla. Flower longevity data (means ± SD, n = 10) followed by different letters are significantly different at P = 0.05 within a given experiment
Notes: *: extending 1 day more than WT; **: extending 2 days more than WT; ***: extending 3 days more than WT; −: days and % of WT decreased
Discussion
Onset and execution of corolla senescence in ET-sensitive petunia
ET is a key flower senescence promoting hormone in ET-sensitive species1,3. In this study, transcriptional dynamics at four distinct developmental stages of corolla in petunia were monitored. The ‘S-adenosylmethionine biosynthesis’ GO term was significantly upregulated at the D2 and D4 transition (Supplementary Figure S2). Expression of ACS and ACO genes was upregulated through D2 to D4 and D4 to D7 transitions. The increase of ET emission was initially detected at the D2 to D4 transition, while the spike of ET emission occurred at the D4 to D7 transition (Fig. 1b). These data suggest that early onset of corolla senescence may occur in the transition from D2 to D4, and execution of senescence takes place in the transition from D4 to D7.
Roles of the auxin signaling pathway during corolla senescence
Notably, large alterations in abundances of auxin-related transcripts occurred throughout the four developmental stages, especially through the transition from D2 to D4. Although, at present, the role of auxin in plant senescence remain poorly defined, and contrasting observations have been obtained from different species. Several studies have reported an involvement of auxin in the process of senescence, especially in petal senescence. For example, in cut carnation flowers, exogenous application of IAA hastened the rise in ET production and flower wilting14. 2,4-dichlorophenoxyacetic acid (2,4-d), a synthetic auxin, induced the expression of ACC synthase genes in the styles, ovaries, and petals15. It was reported that in the corollas of pollinated petunias, ‘response to auxin stimulus’ and ‘response to ACC’ were significantly enriched at 12 hap16. Interaction between auxin and ET occurred at the early stage of pollination16. Furthermore, the interaction between ET and auxin was also reported in ET-induced corolla senescence in petunia20. Interestingly, during pear ripening, the auxin-associated transcripts are significantly upregulated in the S2 to S3 transition (106–113 days after full bloom, when fruit gained the ability to soften after ET treatment) before pear ripening and downregulated in the S3 to S4 transition (113–120 days after full bloom, when fruit developed the capacity to soften without ET treatment)34. In addition, auxin level declined prior to ripening in tomato, grape, and strawberry fruit38,39. Moreover, the largest number of DEGs related to auxin were observed in the abscission process of rose petal40. Downregulation of RhIAA16 by VIGS in rose promoted petal abscission40. In our transcriptome data, DEGs in the auxin pathway, including auxin-responsive genes (SAUR-like genes), auxin-induced genes (IAA13), and auxin efflux carrier were all induced at the D2 to D4 transition, where ET production was increased. However, those auxin-related genes were downregulated in the D4 to D7 transition (Supplementary Table S2), while expression of ACO and ACS genes was upregulated (Supplementary Table S2) and ET production reached a peak at 5.5 days (Fig. 1b). Taken together, we postulate that auxin might play common and vital positive roles in activating ET production and regulating developmental process that lead to subsequent attainment of ripening, senescence, and abscission capacity.
Other hormone changes in petunia corolla senescence
Changes in ABA levels during petal senescence depend on the species. For example, during petal senescence in cut carnations, there is a small increase in ABA level41. In P. × hybrida cv. Ultra (Blue) and cv. Primetime (purple), the ABA level was decreased at the initial senescence stage and then increased at the late stage. However, in P. × hybrida cv. Dreams Appleblossom (pink), the ABA level increased as senescence progressed9. Proteomic analysis for ET-treated petunia corolla suggested that the external ET increased the protein level of PP2C and SnRK2A20. In our experiments, the upregulated expression of genes related to ABA biosynthesis suggests that the ABA biosynthesis pathway is involved in ET-dependent petal senescence.
In ET-dependent floral senescence, the role of CK is still unclear. The endogenous CK level in petal senescence varies with the species. CK levels are decreased during corolla development and senescence in carnation, roses and cosmos1. However, CK levels showed different patterns in petunia, such as an increase during floral development and a decrease at the late stages of senescence42,43. Exogenous application of 6-BA, prolonged flower life and repressed ET biosynthesis and perception gene expressions43. In Hibiscus rosa-sinensis, the CK-related transcripts had a higher abundance in an earlier stage than a later stage of flower senescence44. Our results also showed that three identified DEGs related to CK biosynthesis were induced at the earlier stage of corolla development. Therefore, the CK signaling pathway might play a role in an earlier development stage of corolla. In the SA pathway, identified DEGs included one UGT74E2 (Peaxi162scf00883g00811), two UGT74F1 (Peaxi162scf00303g00048 and Peaxi162scf00045g01732), and a TPR-like gene (Peaxi162scf00265g00033). UGT74E2 are strongly induced by H2O2 and may allow integration of ROS and auxin signaling45.
Transcription factors regulate corolla senescence in petunia
Expression changes of TFs have profound effects on flower longevity. Many members of ERFs, ARF, NAC, bZIP, HD-Zip, and bHLH families were identified during pollination-induced16 and ET-induced corolla senescence3. However, there is little research on the roles of TFs in flower longevity. Our VIGS experiments provided valuable data for further investigation of the functional role and interactions of these TFs for regulating flower senescence. ERF2 in the ET signaling pathway is highly upregulated in senescing daffodil corollas18. In our experiments, two upregulated ERFs (Peaxi162Scf00024g00271 and Peaxi162Scf00050g00086) were silenced using TRV-based VIGS system, resulting in significantly extended flower longevity (Table 2). Notably, expression of most auxin-related genes, such as ARF genes, was upregulated at the D2 to D4 transition. Interestingly, Small Auxin Up RNA 36 (SAUR36) is upregulated during leaf senescence and acts as a positive regulator of leaf senescence in Arabidopsis46. Auxin Response Factor 2 (ARF2) positively regulated leaf senescence. Transcript abundance of ARF2, together with ARF7/NPH4 and ARF19, is highly accumulated in senescing Arabidopsis leaves47. In Arabidopsis, arf2 mutant displays delayed leaf senescence47. Our VIGS experiments showed that silencing ARF19 (Peaxi162Scf00287g00193) prolonged flower longevity, but silencing ARF2 (Peaxi162Scf00314g00539) caused premature corolla senescence. Taken all together, we postulate that genes related to auxin play either positive or negative roles in regulating corolla senescence. A recent study has illustrated that AINTEGUMENTA (ANT), a member of the AP2/ERF TF family functioning downstream of ARF2 in Arabidopsis, negatively regulated leaf senescence48. The underlying mechanisms how ARFs regulate onset of corolla senescence require further studies in the future. With the exception of ARF, the MADS-box gene (Peaxi162Scf01084g00119) was upregulated in the corolla senescence process in petunia. Silencing this gene substantially extended the flower longevity (Table 2). It would be very interesting to investigate how the MADS-box protein regulates flower senescence although it is beyond the scope in this study.
In addition, significant upregulation of TFs, including bHLH, HD-Zip, and C2C2-CO-like, was observed. Recent reports have demonstrated that bHLHs are involved in petunia petal senescence. For example, a bHLH TF ANTHOCYANIN1 (AN1) has been showed to regulate petal senescence in petunia49. In our experiments, four bHLHs were silenced by VIGS (Peaxi162Scf00119g00942, Peaxi162Scf00285g00311, and Peaxi162Scf00712g00029), resulting in extended flower longevity (Table 2, experiment 1). Interestingly, over-expression of a bHLH (Peaxi162Scf00285g00311, PhFBH4) hastened petal senescence by modulating the expression of the ET biosynthesis gene ACO50. HD-Zip TFs have been reported to be involved in ABA- and ET-induced senescence in rose petals51. Chang et al.22 demonstrated that silencing PhHD-Zip reduced the expression of ET biosynthesis-related genes (ACO1, ACO4, and ACS) and ET production. In our study, petunia plants with VIGS-silenced HD-Zip TFs (Peaxi162Scf00092g01520 and Peaxi162Scf00237g01510) extended flower longevity (Table 2, experiment 1 and 2). Furthermore, ET treatment induced the transcript abundance of PhHD-Zip [22]. These results suggest that HD-Zip TFs might be involved in petal senescence through regulating ET biosynthesis. NAC TFs were upregulated during senescence of petunia petal. Our previous study demonstrated that multiple NAC TFs were downregulated in ET-insensitive petunia petals3. In Arabidopsis, a NAC TF, AtNAC092, is induced through ETHYLENE INSENSITIVE 2 (EIN2) and EIN352 during leaf senescence. However, silencing three selected NAC TFs (Peaxi162Scf00051g01410, Peaxi162Scf01105g00218, and Peaxi162Scf00998g00127), lightly increased flower longevity (Table 2, Experiment 2). Interaction of senescence-associated NAC TFs expression and ET signals needs to be further clarified in ET-sensitive petal senescence.
Notably, some members of other putative TFs that share high similarity with MYB-DNA-binding proteins, bZIPs, and zinc fingers were downregulated. The petunia with VIGS-silenced zinc finger TF (Peaxi162scf00013g00084) had shortened flower longevity (Table 2, experiment 1). SUF4, encoding a zinc finger superfamily protein, was downregulated in the transgenic ET-insensitivity etr1-1 petunia3. The differential expression of bZIP was identified in pollinated petal senescence16. Flowers with overexpressed bZIP (Peaxi162Scf00285g00011) have been shown to have delayed senescence while flowers with silenced bZIP have shortened longevity53. The relationship between these TFs and ET needs to be clarified in the future.
We would like to point out that there were many downregulated DEGs that may play critical roles in the regulation of flower senescence. Study on the downregulated DEGs using upregulation approaches or CRISPR/Cas9 technology will be valuable and informative in the future.
At present, most research has applied heterologous genes to modify traits of ornamental plants54. Modifying the ET pathway both directly and indirectly extended flower longevity55. In summary, hormone pathways, especially the crosstalk between auxin and ET, and transcriptional regulation play a vital role in ET-sensitive corolla senescence. Silencing or overexpressing several genes associated with senescence simultaneously is needed to further manipulate flower longevity in the future. Combined with the RNA-seq data, functional analysis using a relative high-throughput VIGS system provides valuable data and generates a list of promising targets for further investigation and future plant breeding in the flower industry.
Electronic supplementary material
Acknowledgements
We thank Ayla Norris for careful editing of this manuscript and Linda Donnelly for assisting in VIGS experiments. We thank Chuan-Bei Jiang (Genepioneer Biotechnologies, Nanjing 210014, China) for providing bioinformatics analysis. This work was partially supported by National Natural Science Foundation of China (grant nos. 31540052; 31300469), Jiangsu Natural Science Foundation of China (grant no. BK 20141383), and United States Department of Agriculture (USDA) CRIS project 5306-21000-019-00D. The funders had no role in study design, data collection and analysis. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author contributions
Conceived and designed the experiments: H.W., J.L., M.S.R., and C.-Z.J. Performed the experiments: H.W., X.C., and J.-C.C. Analyzed the data: H.W. and C.-Z.J. Contributed reagents/materials/analysis tools: C.-Z.J., M.S.R., J.L., and Y.C. Wrote the paper: H.W. and C.-Z.J.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41438-018-0018-1.
Contributor Information
Jing Lin, Email: lj84390224@126.com.
Cai-Zhong Jiang, Email: caizhong.jiang@ars.usda.gov, Email: cjiang@ucdavis.edu.
References
- 1.Jones, M., Stead, A. & Clark, D. in Petunia Evolutionary, Developmental and Physiological Genetics, Vol. 2 (eds Gerats, T. & Strommer, J.) Ch 14. (Springer, New York, 2009).
- 2.van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. J. Exp. Bot. 2008;59:453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, et al. Transcriptome changes associated with delayed flower senescence on transgenic petunia by inducing expression of etr1-1, a mutant ethylene receptor. PLoS ONE. 2013;8:e65800. doi: 10.1371/journal.pone.0065800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill SD. Pollination regulation of flower development. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:547–574. doi: 10.1146/annurev.arplant.48.1.547. [DOI] [PubMed] [Google Scholar]
- 5.Eisinger W. Role of cytokinins in carnation flower senescence. Plant Physiol. 1977;59:707–709. doi: 10.1104/pp.59.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoeberichts FA, van Doorn WG, Vorst O, Hall RD, van Wordragen MF. Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J. Exp. Bot. 2007;58:2873–2885. doi: 10.1093/jxb/erm076. [DOI] [PubMed] [Google Scholar]
- 7.Saks Y, Van Staden J. Evidence for the involvement of gibberellins in developmental phenomena associated with carnation flower senescence. Plant Growth Regul. 1993;12:105–110. doi: 10.1007/BF00144590. [DOI] [Google Scholar]
- 8.Xu Y, Ishida H, Reisen D, Hanson MR. Upregulation of a tonoplast-localized cytochrome P450 during petal senescence in Petunia inflata. BMC Plant Biol. 2006;6:8. doi: 10.1186/1471-2229-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante A, Vernieri P, Tognoni F, Serra G. Changes in abscisic acid and flower pigments during floral senescence of petunia. Biol. Plant. 2006;50:581. doi: 10.1007/s10535-006-0091-4. [DOI] [Google Scholar]
- 10.Mayak S, Dilley DR. Regulation of senescence in carnation (Dianthus caryophyllus): effect of abscisic acid and carbon dioxide on ethylene production. Plant Physiol. 1976;58:663–665. doi: 10.1104/pp.58.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak J, Veen H. Effects of silver thiosulfate on abscisic acid content in cut carnations as related to flower senescence. J. Plant Growth Regul. 1982;1:153–159. [Google Scholar]
- 12.Wu L, et al. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 2016;174:01064. doi: 10.1104/pp.16.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trivellini A, Cocetta G, Vernieri P, Mensuali-Sodi A, Ferrante A. Effect of cytokinins on delaying petunia flower senescence: a transcriptome study approach. Plant Mol. Biol. 2015;87:169–180. doi: 10.1007/s11103-014-0268-8. [DOI] [PubMed] [Google Scholar]
- 14.van Staden, J. Hormonal control of carnation flower senescence. In VI International Symposium Postharvest Physiology of Ornamental Plants, Vol. 405, 232–239 (1995).
- 15.Jones ML, Woodson WR. Differential expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in carnation. Plant Physiol. 1999;119:755–764. doi: 10.1104/pp.119.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick SR, et al. RNA-sequencing reveals early, dynamic transcriptome changes in the corollas of pollinated petunias. BMC Plant Biol. 2014;14:307. doi: 10.1186/s12870-014-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iordachescu M, Verlinden S. Transcriptional regulation of three EIN3-like genes of carnation (Dianthus caryophyllus L. cv. improved white sim) during flower development and upon wounding, pollination, and ethylene exposure. J. Exp. Bot. 2005;56:2011–2018. doi: 10.1093/jxb/eri199. [DOI] [PubMed] [Google Scholar]
- 18.Hunter DA, Steele BC, Reid MS. Identification of genes associated with perianth senescence in Daffodil (Narcissus pseudonarcissus L. ‘Dutch Master’) Plant Sci. 2002;163:13–21. doi: 10.1016/S0168-9452(02)00068-7. [DOI] [Google Scholar]
- 19.van Doorn WG, et al. Gene expression during anthesis and senescence in Iris flowers. Plant Mol. Biol. 2003;53:845–863. doi: 10.1023/B:PLAN.0000023670.61059.1d. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, et al. Proteomes and ubiquitylomes analysis reveals the involvement of ubiquitination in protein degradation in petunias. Plant Physiol. 2017;173:668–687. doi: 10.1104/pp.16.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JC, et al. Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol. Biol. 2004;55:521–530. doi: 10.1007/s11103-004-0590-7. [DOI] [PubMed] [Google Scholar]
- 22.Chang X, et al. A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS ONE. 2014;9:e88320. doi: 10.1371/journal.pone.0088320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Lin J, Chang Y, Jiang CZ. Comparative transcriptomic analysis reveals that ethylene/H2O2-mediated hypersensitive response and programmed cell death determine the compatible interaction of sand pear and Alternaria alternata. Front. Plant Sci. 2017;8:195. doi: 10.3389/fpls.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Liu W. A step-down multiple hypotheses testing procedure that controls the false discovery rate under independence. J. Stat. Plan. Inference. 1999;82:163–170. doi: 10.1016/S0378-3758(99)00040-3. [DOI] [Google Scholar]
- 26.Bombarely A, et al. Insight into the evolution of the Solanaceae from the parental genomes of Petunia hybrida. Nat. Plants. 2016;2:16074. doi: 10.1038/nplants.2016.74. [DOI] [PubMed] [Google Scholar]
- 27.Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics. 2006;7:1–11. doi: 10.1186/1471-2105-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maere S, Heymans K, Kuiper M. BiNGO: a cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57:289–300. [Google Scholar]
- 30.Breeze E, et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23:873–894. doi: 10.1105/tpc.111.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliver W, et al. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell. 2012;24:3530–3557. doi: 10.1105/tpc.112.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimm O, et al. Mapman: a user‐driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313X.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- 33.Lohse M, et al. Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. Plant Cell Environ. 2013;37:1–9. doi: 10.1111/pce.12231. [DOI] [PubMed] [Google Scholar]
- 34.Nham NT, et al. A transcriptome approach towards understanding the development of ripening capacity in ‘Bartlett’ pears (Pyrus communis L.) BMC Genomics. 2015;16:762. doi: 10.1186/s12864-015-1939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacFarlane SA. Molecular biology of the tobraviruses. J. Gen. Virol. 1999;88:2799–2807. doi: 10.1099/0022-1317-80-11-2799. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–786. doi: 10.1046/j.1365-313X.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottcher C, Keyzers RA, Boss PK, Davies C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J. Exp. Bot. 2010;61:3615–3625. doi: 10.1093/jxb/erq174. [DOI] [PubMed] [Google Scholar]
- 39.Given NK, Venis MA, Grierson D. Hormonal-regulation of ripening in the strawberry, a non-climacteric fruit. Planta. 1988;174:402–406. doi: 10.1007/BF00959527. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, et al. Transcriptome profiling of petal abscission zone and functional analysis of an AUX/IAA family gene rhiaa16 involved in petal shedding in rose. Front. Plant Sci. 2016;7:1375. doi: 10.3389/fpls.2016.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onoue T, Mikami M, Yoshioka T, Hashiba T, Satoh S. Characteristics of the inhibitory action of 1, 1-dimethyl-4-(phenylsulfonyl) semicarbazide (DPSS) on ethylene production in carnation (Dianthus caryophyllus L.) flowers. Plant Growth Regul. 2000;30:201–207. doi: 10.1023/A:1006324715438. [DOI] [Google Scholar]
- 42.Hsiang C, Jones ML, Banowetz GM, Clark DG. Overproduction of cytokinins in petunia flowers transformed with P(SAG12)-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 2003;132:2174–2183. doi: 10.1104/pp.103.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taverner E, Wang JE, Willcocks D, Letham D. Influence of ethylene on cytokinin metabolism in relation to petunia corolla senescence. Phytochemistry. 1999;51:341–347. doi: 10.1016/S0031-9422(98)00757-2. [DOI] [Google Scholar]
- 44.Trivellini A, Cocetta G, Hunter DA, Vernieri P, Ferrante A. Spatial and temporal transcriptome changes occurring during flower opening and senescence of the ephemeral hibiscus flower, Hibiscus rosa-sinensis. J. Exp. Bot. 2016;67:5919–5931. doi: 10.1093/jxb/erw295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tognetti VB, et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell. 2010;22:2660–2679. doi: 10.1105/tpc.109.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou K, Wu W, Gan SS. SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013;161:1002–1009. doi: 10.1104/pp.112.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis CM, et al. Auxin Response Factor1 and Auxin Response Factor2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development. 2005;132:4563–4574. doi: 10.1242/dev.02012. [DOI] [PubMed] [Google Scholar]
- 48.Feng G, Xu Q, Wang Z, Zhuoma Q. AINTEGUMENTA negatively regulates age-dependent leaf senescence downstream of AUXIN RESPONSE FACTOR 2 in Arabidopsis thaliana. Plant Biotech. 2016;33:71–76. doi: 10.5511/plantbiotechnology.16.0222a. [DOI] [Google Scholar]
- 49.Prinsi B, Negri AS, Quattrocchio FM, Koes RE, Espen L. Proteomics of red and white corolla limbs in petunia reveals a novel function of the anthocyanin regulator ANTHOCYANIN1 in determining flower longevity. J. Proteomics. 2016;131:38–47. doi: 10.1016/j.jprot.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Yin J, et al. A basic helix-loop-helix transcription factor, PhFBH4, regulates flower senescence by modulating ethylene biosynthesis pathway in petunia. Hortic. Res. 2015;2:15059. doi: 10.1038/hortres.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lü P, et al. RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J. 2014;78:578–590. doi: 10.1111/tpj.12494. [DOI] [PubMed] [Google Scholar]
- 52.Kim HJ, et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J. Exp. Bot. 2014;65:4023–4036. doi: 10.1093/jxb/eru112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun D, et al. PhOBF1, a petunia ocs element binding factor, plays an important role in antiviral RNA silencing. J. Exp. Bot. 2017;68:erw490. doi: 10.1093/jxb/erw490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasajima I, Ohtsubo N, Sasaki K. Combination of Cyclamen persicum Mill. floral gene promoters and chimeric repressors for the modification of ornamental traits in Torenia fournieri Lind. Hortic. Res. 2017;4:17008. doi: 10.1038/hortres.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsen A, Lütken H, Hegelund JN, Müller R. Ethylene resistance in flowering ornamental plants-improvements and future perspectives. Hortic. Res. 2015;2:15038. doi: 10.1038/hortres.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.