Abstract
Diets given for 30 days with various mono-(MUFA) and poly-(PUFA) unsaturated fatty acid contents were evaluated for brain protection in magnesium-deficient mice: a commercial and three synthetic diets (n-6PUFA, n-3PUFA and MUFA-based chows enriched with 5% corn/sunflower oils 1:3, with 5% rapeseed oil and with 5% high oleic acid sunflower oil/sunflower oil 7:3, respectively). Unlike magnesium deprivation, they induced significant differences in brain and erythrocyte membrane phospholipid fatty acid compositions. n-3PUFA but not other diets protected magnesium-deficient mice against hyperactivity and moderately towards maximal electroshock- and NMDA-induced seizures. This diet also inhibited audiogenic seizures by 50%, preventing animal deaths. Because, like n-6PUFA diet, matched control MUFA diet failed to induce brain protections, alpha-linolenate (ALA) rather than reduced n-6 PUFA diet content is concluded to cause n-3PUFA neuroprotection. Present in vivo data also corroborate literature in vitro inhibition of T type calcium channels by n-3 PUFA, adding basis to ALA supplementation in human anti-epileptic/neuroprotective strategies.
Keywords: Polyunsaturated fatty acids, Alpha-linolenic acid (ALA), Eicosapentaenoic acid (EPA), Rapeseed oil, Brain protection, Magnesium deficiency, Hyperexcitability, Chemical seizures, Electrical seizures, Audiogenic seizures, Anticonvulsant, Anti-inflammatory
1. Introduction
Long-chain polyunsaturated fatty acids (PUFA) are essential lipid components of the central nervous system and are supplied exclusively by the diet [1]. In the last decade, in vivo and in vitro studies have documented beneficial effect of PUFAs of the n-3 family towards cardiovascular [2] and neurological [3–8] disorders. Supplementations with n-3 PUFAs and notably with alpha-linolenic acid (ALA (18:3 n-3)) have been proposed in cerebral protection after focal brain ischemia, and against epileptic seizures [2,9]. Inversely, chronic low intake of n-3 PUFA predisposes to brain toxicity through weak anti-inflammatory on strong pro-inflammatory signal imbalance [10]. The abundance of ALA is by far higher (10-fold and more) in vegetable, including rapeseed, soya and walnut oils, than in some other oils such as corn and sunflower oils [11]. ALA represents 9% of rapeseed oil which contains also 60% of monounsaturated fatty acids (18:1 fatty acid) and 20% linoleic acid [LA] (α n-6 PUFA). Its n-6/n-3 ratio is low (close to 3) whereas that of corn:sunflower oils is by far higher (more than 80).
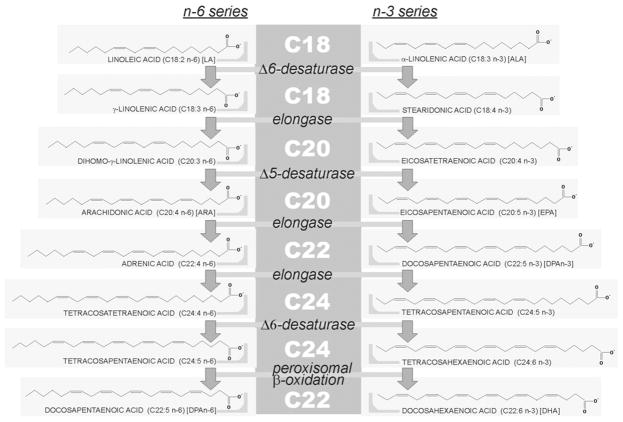
PUFAs generate oxygenated lipid-derived eicosanoids which modulate the inflammatory response [12,13]. n-6 PUFAs and notably arachidonate (ARA; 20:4n-6) are the precursors of various pro-inflammatory eicosanoids (prostaglandins of the 2-series and leukotrienes of the 4-series) which are incriminated in adverse inflammatory processes. However, one of these eicosanoids, PGE2, may restrict the inflammatory response by inhibiting the production of inflammatory leukotrienes and by inducing the inflammation resolving lipoxin A4 [12]. On the other hand, production of ARA-derived-eicosanoids is lowered by n-3 fatty acids, eicopentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), which are incorporated partly at the expense of ARA and other n-6 PUFAs into cell membrane phospholipids [1,12]. This reduced incorporation of ARA into cell membrane phospholipids lowers the amount of substrate available for release of ARA and hence for synthesis of ARA-derived-eicosanoids. In addition, EPA is a substrate for both cyclooxygenase and 5-lipooxygenase, giving rise to an alternative family of eicosanoids with anti-inflammatory properties (prostaglandins of the 3-series and leukotrienes of the 5-series) [12,14]. Finally, a class of EPA and DHA-derived lipid mediators with potent anti-inflammatory properties called resolvins E and D, respectively, has been recently described [12,14]. DHA can generate also a dihydroxy derivative, neuroprotectin D1, a docosanoid with also potent anti-inflammatory properties [12,15,16]. As a whole, derivatives of the n-3 series essentially mediate major anti-inflammatory action, justifying their dietary supplementation to control disorders in which inflammation contributes to the pathogenesis. Fig. 1 illustrates the metabolic pathway (elongation, desaturase) involved in the synthesis of PUFAs from the n-6 and n-3 series.
Fig. 1.
PUFAs of the n-6 and n-3 series. Linear structure representation of PUFAs is illustrated and their names are given between parentheses. Abbreviations used throughout the text are further given between brackets. C20 PUFAs are the reservoir for synthesis of eicosanoids and C22 PUFAs obtained by peroxisomal β-oxidation-driven retroconversion of C24 precursors are the source for docosanoids. Other comments are the text.
A particular condition associated with a general inflammatory and/or oxidant state is severe magnesium deficiency [17–19]. It can alter brain function, leading to central nervous hyperexcitability (CNHE) and to decreased protection against experimental seizures [20,21]. In this respect, experimental magnesium deficiency in mice has been shown to lower seizure threshold to N-methyl-D-aspartate (NMDA) [22] and to induce susceptibility to audiogenic seizures [23]. Magnesium deficiency-dependent audiogenic seizures (MDDAS) represent a useful murine experimental in vivo tool to identify a large spectrum of brain protective drugs and mechanisms including anti-inflammatory, anti-oxidant, neuroprotective and/or anti-epileptic compound properties [22–28]. The pro-convulsant status induced by magnesium deficiency may be the result of a loss of protective effects normally provided by magnesium. Regarding these protective effects, magnesium is endowed with anti-NMDA, anti-inflammatory and anti-oxidant properties. In a previous work, anti-NMDA properties of magnesium were shown to take place in vivo [22]. On the other hand, magnesium is a cofactor for the enzymes in glutathione biosynthesis (γ-glutamylcysteine synthetase and glutathione synthetase) [29,30] and NADPH-producing pentose phosphate pathway (6-phosphogluconate dehydrogenase and transketolase) [31,32], explaining why magnesium deficiency may affect reduced glutathione biosynthesis and recycling. In normal diet conditions, the physiological cellular loss of reduced glutathione is counterbalanced by intracellular glutathione biosynthesis. In magnesium deficiency, cellular loss is maintained, whereas biosynthesis decreases, resulting in a drop of cell reduced glutathione content and anti-oxidant potential [33]. Anti-inflammatory properties of magnesium are not fully elucidated and have been proposed to result from different mechanisms including inhibition of calcium-based processes (priming of phagocytic cells, NMDA receptor activation), prevention of the release of neuropeptides (e.g. substance P) and of the activation of NF-κB signaling [17]. Magnesium deficiency-driven pro-convulsant susceptibilities in mice including MDDAS were here challenged by chronic diets with distinct n-6/n-3 PUFA ratio contents of magnesium-deficient chows. Erythrocyte and brain phospholipid fatty acid compositions under the various diet conditions were also studied. Part of data appearing in this manuscript was the topic of an invited lecture with a subsequent written account [34] at the Journées Chevreul 2007 symposium (AFECG and DGF Joint Meeting, Lipids and Brain: PUFA Metabolism, Function and Protection Against Diseases, Paris (France), 13 and 14th March 2007).
2. Materials and methods
2.1. Chemicals
Pentylenetetrazol (Ptz) and N-methyl-D-aspartate (NMDA) were purchased from Sigma-Aldrich Fine Chemicals (Saint Quentin Fallavier, France). Fresh commercial oils were from Lesieur (Asnières-sur Seine, France).
2.2. Animals
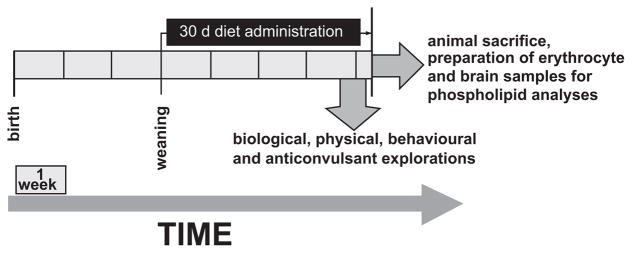
Female Swiss OF1 mice, 3-weeks old, weighing 20–22 g, were purchased from Janvier (Le Genest-St-Isle, France) and were assigned at random to the various experimental groups. They were placed eight per cage and maintained on a 12:12 h light-dark schedule at 21 ± 1 °C. They had free access to food and to distilled water, which avoids additional magnesium input. In current practice, in order to avoid food oxidation, fresh diets were lyophilized and frozen at −20 °C. Diets were given daily to mice in sufficient amounts for a period of 30 days. An overview of the present study design devoted to explore the effects of a 30 days diet administration on seizures tests, brain and erythrocyte phospholipid fatty acid compositions is given in Fig. 2.
Fig. 2.
Design of studying the effects of a 30 days diet administration on seizures tests, brain and erythrocyte phospholipid fatty acid compositions. The various diet conditions and seizures tests are explained in the text. For a same diet condition, various groups of eight mice were used for each seizure test and for each of the analyses of fatty acid compositions of both brain and erythrocyte phospholipids.
2.3. Diets
Various diets determining the different animal groups were designed.
Four control magnesium diets were prepared with a normal magnesium contents (900 and 1300 ppm for synthetic and commercial diets, respectively) and with distinct oil contents. The commercial diet consisted of industrial pellets containing soya oil (UAR, Villemoisson-sur-Orge, France). Three other diets were prepared as previously described [22,35]; these synthetic diets containing 5% of vegetable oils: either a mix of corn and sunflower oils (3:1) (n-6PUFA diet), a mix of high oleic sunflower oil and sunflower oil (7:3) (MUFA diet), or rapeseed oil alone (n-3PUFA diet).
Three synthetic diets impoverished in magnesium were designed by restricting the magnesium content to 40 ± 5 ppm as described previously [22,35] and by including 5% vegetable oils as indicated in the preceding paragraph.
2.4. Physical and biological measurements
At the end of diet administration, body weight, serum magnesium concentrations and erythrocyte and brain phospholipid composition in fatty acids were measured. Magnesium concentrations were determined by atomic spectrophotometry in plasma and expressed in mg/mL [36]. Fatty acid compositions of phospholipids were measured according to a procedure based on classical methods of lipid extraction and analysis by fast GLC chromatography as described elsewhere [37].
2.5. Behavioral tests
Locomotor activity and equilibrium on a rotating rod (rotarod test) were monitored as previously described [26]. More particularly for measurement of locomotor activity, mice were transferred individually in an Apelex type 01-1668B actimeter (Bagneux, France). They were allowed to explore for a 2 min period. Their spontaneous activity was measured for another 3 min by the crossing of the photocell activity meter and automatically recorded.
2.6. Seizures tests
2.6.1. MDDAS test
Magnesium deficiency-dependent audiogenic seizures (MDDAS) test was performed as described previously [23]. Individual animals were placed in a 9 dm3-volume test chamber (30, 20 and 15 cm for length, width and height, respectively) and exposed for 15 s to an acoustic signal of 10 ± 0.1 kHz frequency and 100 ± 1 dBA intensity. This acoustic stimulus signal was produced by a signal generator and projected via a high frequency speaker mounted on the roof of the chamber. The noise level was measured close to the animal’s ear by an external decibel-meter probe. Each animal was subjected to single audiogenic stimulation. The test measured the capacity of a test compound to provide complete protection against seizures induced by the acoustic stimulus, and the audiogenic seizure phase pattern indicated the underlying mechanisms exhibited by tested compounds. Audiogenic seizures were videotaped and the durations in seconds of the four successive phases (latency, wild running, tonic seizure and recovery) were recorded.
2.6.2. Maximal electroshock-induced seizures (MES test)
MES was induced via a pair of auricular clip electrodes by means of an electroshock stimulator (Karl Kolbe, Scientific Technical Supplies, Frankfurt, Germany) in order to obtain the lowest convulsant intensity induced by increasing from 4 to 7 mA, 0.2 s duration, 50 Hz, sine wave form. Classically, this test easily responds to drugs acting on Na+ voltage-dependent channels.
2.6.3. Chemical seizure tests
Chemical seizure tests were induced by increasing subcutaneous doses of Ptz and NMDA in order to detect the capacity of the various diets to provide protection against threshold seizures allowing the determination of the lethal dose 100 (LD100, minimal dose inducing death of 100% tested animals). Ptz test is classically used to detect potential GABAergic properties and NMDA seizure test was introduced to reveal anti-glutamatergic properties.
2.7. Ethics
Present studies were in agreement with our institutional guidelines, which comply with current national and international laws and recommendations (Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH, No 85-23, revised 1996)). Animal experimentation protocols were approved by the French Ministère de l’Alimentation, de l’Agriculture et de la Pêche.
2.8. Statistical analysis
Data were expressed as mean ± SEM and analyzed by Student’s t-test. Normal distribution was assessed according to Shapiro Wilk’s test. Statistical significance was calculated by analysis of variance (ANOVA).
3. Results
3.1. Fatty acid contents of the various oil-containing diets
The contents in major classes of (saturated, mono (MUFA)- and polyunsaturated (PUFA)) fatty acids of experimental diets as well as their n-6/n-3 fatty acid content ratios are given in Table 1. These diets are referred throughout the present text to as commercial diet (commercial soya oil-based animal chow), and to n-3PUFA (5% rapeseed oil-enriched), n-6PUFA (5% sunflower oil/corn oil (1:3) -enriched) and MUFA (5% high oleic sunflower oil (HOSO)/sunflower oil (7:3) enriched) synthetic diets. Compared to commercial diet, n-6PUFA and n-3PUFA diets distinguished by higher and lower n-6/n-3 ratios, respectively; their monounsaturated fatty acid content were little (n-6PUFA diet) and 2.4-fold (n-3PUFA) higher than in commercial diet. The MUFA diet was designed as a matched dietary “blank” for n-3PUFA diet ALA, and compared to rapeseed-enriched diet was impoverished in the n-3 fatty alpha-linolenic acid (ALA) and shared in common with this diet the same n-9 MUFA and n-6 PUFA contents.
Table 1.
Oil enrichment and fatty acid content of the diets.
| DIETS | ||||
| Oil enriching the diet | Soya oil | Rapeseed oil | SUN oil/Corn oil 1:3 | HOSO/SUN oil 7:3 |
| Percentage of oil enrichment (%) | ± 5 | 5 | 5 | 5 |
| Diet appellation throughout the text | Commercial diet | n-3PUFA diet | n-6PUFA diet | MUFA diet |
| FATTY ACID CONTENT (in %) OF OIL ENRICHMENT | ||||
| Saturated fatty acids | 15 | 11 | 15 | 13 |
| Monounsaturated fatty acids Oleic acid (18:1n-9) | 25 | 60 | 28 | 66 |
| n-6 Polyunsaturated fatty acids Linoleic acid (LA) (18:2n-6) | 53 | 21 | 56 | 21 |
| n-3 Polyunsaturated fatty acids Alpha-linolenic acid (ALA) (18:3n-3) | 7 | 8.3 | 0.6 | 0.1 |
| RATIO BETWEEN PUFA SERIES CONTENT OF THE DIET | ||||
| n6/n3 ratio | 7.5 | 2.6 | 90 | 4100 |
Note: Relative fatty acid contents of diets are expressed as percentage values. For the three synthetic diets, taking into account that the only source of fatty acids was the added oil, these values also apply to the oil fatty acid composition. Abbreviations are HOSO, high oleic sunflower oil; SUN, sunflower.
3.2. Effects of the various oil-containing diets on fatty acid composition of erythrocyte membrane phospholipids
Contents of erythrocyte membrane phospholipids in unsaturated fatty acids as well as the arachidonic acid (ARA) (n-6) on docosahexaenoic acid (DHA) (n-3) ratio were diet dependent (Table 2). Regarding the n-9 series, phospholipid composition in oleic acid was not significantly different in erythrocytes from animals given n-6PUFA diet in comparison with those from animal receiving commercial diet, whereas MUFA and n-3PUFA diets were associated with significantly higher oleic acid contents (Table 2). In the fatty acid n-6 series, erythrocyte phospholipid content in linoleic acid (LA) was significantly increased in animals given either n-6PUFA or commercial diet in comparison with those supplied with either MUFA or n-3PUFA diets (Table 2). Arachidonic acid (ARA) content was significantly highest upon n-6PUFA diet and similar in the other diet conditions whereas n-6 docosapentaenoic acid (DPAn-6) contents were upon n-3PUFA diet lower than upon commercial diet and highest in n-6PUFA and MUFA diet conditions (Table 2). In the n-3 series, erythrocyte membrane contents in ALA, eicopentaenoic acid (EPA), n-3 docosapentaenoic acid (DPAn-3) and DHA were lowest in n-6PUFA diet conditions and to a lesser extent upon MUFA diet (Table 2). Erythrocyte membrane contents in fatty acids from the n-3 series were in animals given n-3PUFA diet higher than in those with the commercial diet except for DHA contents which were similar in the latter two diet conditions (Table 2). The n-6/n-3 (ARA/DHA) erythrocyte phospholipid fatty acid ratio was highest in animals supplied with the n-6PUFA diet, and lower in n-3PUFA diet and commercial diet compared to MUFA diet conditions (Table 2).
Table 2.
Fatty acid contents of erythrocyte membrane phospholipids under the various diet conditions.
| Commercial diet | n-3PUFA diet | n-6PUFA diet | MUFA diet | |
|---|---|---|---|---|
| n-9 series | ||||
| Oleic Acid | 17.5 ± 3.9a | 23 ± 3.3b | 19.6 ± 3.1a | 26.1 ± 3.2b |
| n-6 series | ||||
| LA | 11 ± 0.65a | 9.2 ± 1.2c | 11.7 ± 0.9a | 9.5 ± 1.3b |
| ARA | 14.3 ± 2.1a | 14.2 ± 1.1a | 19.4 ± 1.3b | 15.8 ± 1.2a |
| DPAn-6 | 0.56 ± 0.07b | 0.41 ± 0.08a | 1.22 ± 0.2c | 1.03 ± 0.2c |
| n-3 series | ||||
| ALA | 0.17 ± 0.04a | 0.37 ± 0.16b | 0.04 ± 0.02c | 0.04 ± 0.02c |
| EPA | 0.36 ± 0.02a | 0.63 ± 0.07b | 0.06 ± 0.02d | 0.08 ± 0.02c |
| DPAn-3 | 0.53 ± 0.2a | 0.75 ± 0.1b | 0.19 ± 0.03d | 0.33 ± 0.04c |
| DHA | 5.79 ± 0.9a | 5.79 ± 0.9a | 3.79 ± 0.6b | 4.06 ± 0.7b |
| n-6/n-3 ratio | ||||
| ARA/DHA | 2.57 ± 0.6a | 2.45 ± 0.2a | 5.14 ± 0.3c | 3.92 ± 0.3b |
Note: Results are means ± SEM and are expressed as percents of the total fatty acid content of erythrocyte membrane phospholipids. Distinct and similar superscript letters (a, b, c and/or d) within a same horizontal line indicate significant (p<0.01) and not significant differences between dietary groups towards the considered fatty acid, respectively. Abbreviations are: LA, linoleic acid; ARA, arachidonic acid; DPAn-6, docosapentaenoic acid of the n-6 series; ALA, alpha-linolenic acid; EPA, eicosapentanoic acid; DPAn-3, docosapentaenoic acid of the n3 series, DHA, docosahexaenoic acid. Diets have been defined in the text and in Table 1 and have been given to mice for a period 30 days before analyses of erythrocyte membrane phospholipids. Contents in phospholipid saturated fatty acids are 100% from which are withdrawn percentages of phospholipid unsaturated fatty acids given in the table.
3.3. Effects of the various oil-containing diets on fatty acid composition of brain phospholipids
Contents of brain phospholipids in unsaturated fatty acids were diet independent for oleic acid and for the ARA/DHA ratio (Table 3). In contrast, significant differences were induced by the various diets on the brain phospholipid contents in individual fatty acids of the n-6 and n-3 series (Table 3). Regarding the n-6 series, phospholipid contents in LA were similar in n-6PUFA, MUFA and commercial diet conditions, being significantly highest under n-3PUFA diet. ARA contents were similar under n-6PUFA, n-3PUFA and MUFA diets, being significantly lowest under commercial diet. DPAn-6 contents were under n-6PUFA and MUFA diets higher than under n-3PUFA and commercial diets. Regarding brain phospholipid contents in n3 PUFA, those in ALA were similar in n-6PUFA, MUFA and commercial diet conditions, being significantly highest under n-3PUFA diet whereas contents in DHA were similar in n-3PUFA, MUFA and commercial diet conditions, being significantly lowest under n-6PUFA diet. For brain phospholipid contents in EPA, highest levels were observed under n-3PUFA diet and lowest levels under n-6PUFA and MUFA diets whereas intermediary levels occurred upon commercial diet conditions. Each diet conditions induced a significantly different brain phospholipid content in DPAn-3 with n-3PUFA diet > commercial diet > MUFA diet > n-6PUFA diet.
Table 3.
Fatty acid contents of brain phospholipids under the various diet conditions.
| Commercial diet | n-3PUFA diet | n-6PUFA diet | MUFA diet | |
|---|---|---|---|---|
| n-9 series | ||||
| Oleic Acid | 15.5 ± 0.5 | 15.2 ± 0.9 | 15.4 ± 0.5 | 15.3 ± 0.7 |
| n-6 series | ||||
| LA | 0.53 ± 0.03a | 0.59 ± 0.03b | 0.49 ± 0.04a | 0.52 ± 0.04a |
| ARA | 9.2 ± 0.9a | 9.8 ± 0.7b | 9.6 ± 0.4b | 9.75 ± 0.6b |
| DPAn-6 | 0.40 ± 0.05a | 0.37 ± 0.06a | 0.67 ± 0.1b | 0.63 ± 0.1b |
| n-3 series | ||||
| ALA | 0.001 ± 0.001a | 0.004 ± 0.003b | 0.000 ± 0.0000a | 0.000 ± 0.000a |
| EPA | 0.019 ± 0.003a | 0.03 ± 0.004b | 0.006 ± 0.001c | 0.006 ± 0.002c |
| DPAn-3 | 0.166 ± 0.008a | 0.185 ± 0.01b | 0.102 ± 0.01d | 0.119 ± 0.01c |
| DHA | 16.4 ± 0.7a | 16.7 ± 0.7a | 15.6 ± 0.5b | 15.9 ± 0.8a |
| n-6/n-3 ratio | ||||
| ARA/DHA | 0.56 ± 0.03 | 0.59 ± 0.03 | 0.60 ± 0.03 | 0.61 ± 0.03 |
Note: Results are means ± SEM and are expressed as percents of the total fatty acid content of brain phospholipids. Distinct and similar superscript letters (a, b and/or c) within a same horizontal line indicate significant (p<0.01) and not significant differences between dietary groups towards the considered fatty acid, respectively. The absence of a superscript letter throughout a horizontal line means that no significant difference between the four diet groups occurs in the levels of the considered fatty acid. Abbreviations are : LA, linoleic acid; ARA, arachidonic acid; DPAn-6, docosapentaenoic acid of the n-6 series; ALA, alpha-linolenic acid; EPA, eicosapentanoic acid; DPAn-3, docosapentaenoic acid of the n-3 series, DHA, docosahexaenoic acid. Diets have been defined in the text and in Table 1 and have been given to mice for a period 30 days before analyses of brain phospholipids. Contents in phospholipid saturated fatty acids are 100% from which are withdrawn percentages of phospholipid unsaturated fatty acids given in the table.
3.4. Effects of magnesium deficiency on the erythrocyte and brain phospholipid contents under the various diet contents in unsaturated fatty acids
No significant changes were induced here by magnesium deficiency in erythrocyte and brain phospholipid contents in fatty acids, irrespective to the oil content of the diets (data not shown).
3.5. Influence of the diets on the effects of magnesium deficiency produced on behavior in actimeter and rotarod tests
The ability of magnesium-deficient diets with various n-6/n-3 ratio contents to induce a lowering of serum magnesium levels was measured, indicating a similar drop in serum magnesium levels, irrespective to the diet fatty acid content (Table 4). Body weights did not differ from the group fed a normal magnesium diet and the successful ability of mice to stay in equilibrium for 2 min in the rotarod test was maintained (Table 4). A significant propensity to develop hyperactivity was noticed in the groups of animals given magnesium-deficient MUFA and n-6PUFA diets (versus commercial diet). In contrast, activity significantly decreased in animals receiving the magnesium-deficient n-3PUFA diet (versus commercial diet and versus other magnesium-deficient diets; Table 4).
Table 4.
Effect of 30 days diet administrations on serum magnesium levels, body weight and behavioral activities.
| Normal magnesium diet | Diets rendered deficient in magnesium content | |||
|---|---|---|---|---|
|
|
||||
| NM commercial diet | MD MUFA diet | MD n-6PUFA diet | MD n-3PUFA diet | |
| Serum magnesium (mg/ml) | 21.52 ± 1.26 | 5.72 ± 0.4* | 5.70 ± 0.51* | 5.55 ± 0.43* |
| Body weight (g) | 25.3 ± 2.02 | 25.7 ± 1.58 | 26.1 ± 1.18 | 26.6 ± 0.88 |
| Actimetry (number of crossings) | 115.0 ± 28.6 | 134.3 ± 32.1* | 152.7 ± 37.9* | 97.0 ± 22.5*,** |
| Rotaroda | + | + | + | + |
Note: abbreviations are NM, normal magnesium; MD, magnesium-deficient.
Rotarod: + means that the mice stood in equilibrium on the rotarod for 120 s in three separate trials.
p<0.05 (MD diet groups versus NM diet group).
p<0.01 (MD n-3PUFA diet groups versus MD MUFA and MDn-6PUFA diet groups).
3.6. Influence of the diets on the pro-convulsant properties of magnesium deficiency
Previous works from our group have extensively documented pro-convulsant properties of magnesium deficiency including susceptibilities to audiogenic seizures and to NMDA-induced seizures [20–28,34]. Table 5 accounts for pro-convulsant state of magnesium deficiency in MES, Ptz and NMDA seizure tests. The extent to which animal responses in these electric and chemical seizure tests were modulated by the fatty acid content of their magnesium-deficient diets was inconsiderable, with under n-3PUFA diet; however, a slight reversion of reduced seizure threshold to MES and NMDA seizures. Fatty acid contents of magnesium-deficient diet influenced, in contrast, substantially the development of audiogenic seizures and deaths, which were dramatically inhibited under n-3PUFA diet (Table 5). The audiogenic seizure phase profile (sequential durations of each of the four phases [latency, wild running, convulsions and recovery] of audiogenic seizures) was also partly diet dependent (Table 6). Compared to the n-6PUFA diet impoverished in magnesium, n-3PUFA and MUFA diets induced a significant increase in the duration of latency and wild running phases (Table 6). Importantly, the comparison (performed to emphasize out ALA-driven effects) between animals given n-3PUFA diet (considered as ALA-treated animals) and MUFA diet (considered as controls, i.e. ALA-untreated animals) indicated that, in contrast, MDDAS phase modulations by n-3PUFA diet ED50 (anticonvulsant dose or diet protecting 50% tested animals) did not modify durations of audiogenic seizure phases (Table 6).
Table 5.
Pro-convulsant properties of nutritional magnesium deficiency and their modulation under various diet designs.
| Diet design and magnesium content | |||||
|---|---|---|---|---|---|
|
| |||||
| NM commercial diet | NM MUFA diet | MD n-6PUFA diet | MD MUFA diet | MD n-3PUFA diet | |
| MES test (current amperage needed to induce seizures in 33% of mice) (mA) | 7 | 7 | 4 | 4 | 4.5 |
| Ptz seizure test (LD100) (mg/kg) | 85 | 87 | 55 | 57 | 57 |
| NMDA seizure test (LD100) (mg/kg) | 137 | 135 | 51 | 52 | 58 |
| MDDAS test | |||||
| Percentage of convulsing mice (%) | 0 | 0 | 100 | 100 | 50 |
| Percentage of deaths (%) | 0 | 0 | 50 | 50 | 0 |
Note: Results are means of seizure test condition parameters including percentage of convulsing mice and of animal deaths, and dose of the chemical (Ptz, pentylenetetrazole, NMDA, N-methyl-D-aspartate) needed to induce lethal seizures in 100% of tested mice (LD100, lethal dose 100). NM and MD indicate normal and deficient magnesium contents of the diets, respectively. Data collected for other NM diet conditions did not distinguish from those appearing in this Table. Abbreviations are MES, maximal electroshock-induced seizures; MDDAS, magnesium deficiency-dependent audiogenic seizures.
Table 6.
Modulation of MDDAS phases by various diet designs of the nutritional magnesium deficiency in mice.
| Magnesium-deficient diets | |||
|---|---|---|---|
|
| |||
| MD n-6PUFA diet | MD MUFA diet | MD n-3PUFA diet | |
| n | 8 | 8 | 8 |
| Convulsing mice (%) | 100 | 100 | 50 |
| Duration of the successive phases (seconds) | |||
| Latency | 4.0 ± 1.4 | 6.5 ± 0.6* | 6.7 ± 0.5* |
| Wild running | 2.3 ± 0.4 | 3.5 ± 0.4* | 3.7 ± 0.5* |
| Convulsions | 1.8 ± 0.5 | 1.6 ± 0.4 | 1.6 ± 0.4 |
| Recovery | 43.3 ± 4.1 | 44.5 ± 5.1 | 44.5 ± 6.7 |
Note: Abbreviations and superscript are n, number of mice submitted to the MDDAS test; MD, magnesium-deficient, MDDAS, magnesium deficiency-dependent audiogenic seizures,
significant at p<0.05 (versus MD n-6PUFA diet). Other comments are in the text.
4. Discussion
4.1. Magnesium deficiency as a model for evaluation of brain protective mechanisms
Anticonvulsant properties of magnesium salts have been largely documented to be effective via diverse routes of administration in the human [20,38] and in the animals pre-supplied [39,40] or not [41] with a magnesium-deficient diet. Magnesium suppressive action on the NMDA receptor calcium channel complex results from a block in ion permeation, occurs in a voltage-dependent manner, and this effect has been supported to take place in vitro [42,43], ex vivo [44] and in vivo [22]. Other mechanisms cited to contribute to magnesium central anticonvulsant protection include competition of Mg2+ and Ca2+ for similar pre-synaptic sites, lowering local calcium-dependent neurotransmitter release [45] and ubiquitous propensity of magnesium to act as a physiological calcium blocker [46]. These relationships between magnesium and brain excitability have been previously exploited to develop a nutritional tool for the successful identification of anti-epileptic, anti-oxidant and/or anti-inflammatory properties of compounds with distinct modes of action, notably a synthetic ovothiol analog (endowed with glutathione peroxidase-like properties) [27], FMOC leucine (a synthetic PPARγ agonist) [25], melatonin (an anti-oxidant and physiological ligand of melatonin receptors) and WEB 2170 (a synthetic PAF receptor antagonist) [23].
4.2. Brain protective mechanisms as a function of fatty acid contents of diets
Previous work [34] has emphasized permissive-like effects towards CNHE and seizures by n-6PUFA diet and protective-like effects provided by n-3PUFA diet. The present work further explores whether protection given by n-3PUFA diet essentially results from a gain in protective-like properties of dietary n-3PUFAs or a loss in permissive-like properties of dietary n-6PUFAs. This issue is addressed by design and study of MUFA diet. Data obtained on this diet suggest that brain protection by n-3PUFA diet is not the result of dietary n-6PUFA and/or oleic acid contents. Indeed, n-3PUFA and MUFA diets, despite their similar n-6PUFA and oleic acid contents, differ in their capacities to protect brain against CNHE and seizure development. This difference in brain protections, in contrast, appears to coincide with ALA contents by far higher in n-3PUFA than in n-6PUFA and MUFA diet conditions.
4.3. Brain protective mechanisms assessed by brain phospholipid fatty acid contents
Phospholipid fatty acid composition is a resultant of dietary intake in essential fatty acids and of their lengthening mainly by liver metabolism [1]. This composition also accounts for local availability of precursors for eicosanoid and/or docosanoid biosyntheses, being aware of metabolite exchange between cells of similar or different origins, and in the case of brain, of sparing mechanisms for PUFAs such as DHA. Taking into account these general principles, how might n-3PUFA diet-driven changes in phospholipid fatty acid compositions especially in brain provide protective mechanisms? A pragmatic approach is to individualize changes in brain phospholipid unsaturated fatty acids, which are specific to n-3PUFA diet and to its content in ALA. Identifying changes specifically induced by ALA may be helped by a comparison of brain phospholipid compositions under n-3PUFA and MUFA diets (Table 3). The list of significant differences in brain fatty acid compositions of these lipids between the two diet groups includes for the n-3PUFA (versus MUFA) diet conditions, a reduction in DPAn-6 and an increase in LA, ALA, EPA and DPAn-3. These results indicate that among known precursors of eicosanoids and docosanoids, ARA and DHA were in similar and EPA was in different relative amounts in the two diet groups. Brain phospholipid levels in EPA were higher under n-3PUFA diet. In view of protective metabolites (prostaglandins of the 3-series, leukotrienes of the 5-series, and resolvin E) that EPA may generate [12,14], enhanced brain availability of EPA, along with other changes including rise in brain ALA content, might offer a missing link between n-3PUFA diet and brain protection. The significant increase noticed in brain LA under n-3PUFA diet was apparently paradoxical. However, a similar observation was reported in the liver of animals given diets with similar LA and increasing ALA contents [47]. Substrate competition might be incriminated [47], this eventuality might account for reduced DPAn-6 content here observed in brain phospholipids. In this respect, while part of the discussion relative to brain protective mechanisms as a function of diet fatty acid contents concludes that brain protective mechanisms of n-3PUFA diet essentially results from diet ALA, brain changes in phospholipid fatty acids associated with protection might suggest, in addition, competitive regulatory mechanisms between ALA and LA metabolic pathways. Importantly, the brain contains relatively low levels of LA, and very little LA is converted into ARA within the brain [48]. Thus the brain relies on a steady supply of ARA from the plasma. Within the brain, ARA is released from the sn-2 position of neural phospholipids by cytosolic phospholipase A2 (c-PLA2) activation coupled to dopaminergic, cholinergic, glutamatergic and serotonergic stimulation, via G-proteins or calcium [49]. Although the signals that ARA and its derivates relay are not completely understood, with regard to the bipolar disorder [50], they have been reported to play a role notably in neuroinflammation [10,51], and excitotoxicity [52], attenuation of which may also contribute to brain protection.
4.4. Brain protective mechanisms assessed by development of MDDAS
Like study of phospholipid contents in fatty acids, study of the pharmacological-like effects of the diets, notably those assessed by anticonvulsant evaluation, may provide insight into the mechanisms of brain protection in the magnesium-deficient animals. Interestingly, among magnesium-deficient diets here developed, the n-3PUFA diet, compared to the n-6PUFA and MUFA diets, halved the number of mice convulsing during the MDDAS test and prevented deaths of animals. In this type of study, testing the modulation of the MDDAS phases, by a dose close to the ED50 (efficacious dose 50, dose protecting 50% tested animals) of a compound, here mimicked using a diet condition protecting 50% of tested animals, may provide information on its mode of action [23]. More specifically here, the modulation of MDDAS phases by the n-3PUFA diet (in conditions protecting 50% animals) to MDDAS occurring under the matched control MUFA diet, was characterized by the absence of significance changes in the durations of each of the four successive MDDAS (latency, wild running, convulsion and recovery) phases. This MDDAS phase modulation pattern distinguished from those expressed on one hand by GABAergic compounds and on the other hand by sodium channel blockers (for an extensive consideration on these relationships between modulations of MDDAS phases and respective anticonvulsant modes of action, see Ref. [23]). This modulation pattern also distinguished from those exhibited by compounds more recently identified to be active in the MDDAS test such as 1-methyl-2-[3–trifluoromethylphenyl]-4-mercapto-imidazole (a synthetic ovothiol analog) [27], FMOC-L-leucine (a PPARγ agonist) [25], 1,2-ethane bis-1-amino-4-benzamidine and piperazine-N1, N4-bis-benzamidine (bis-benzamidine compounds) [28]. In fact, MDDAS phase modulation pattern exhibited by the n-3PUFA diet was exactly similar to that expressed by the prototype anti-epileptic drug ethosuccimide [23], indicating similar mechanisms for diet and drug modes of action.
4.5. Brain protective mechanisms common to ethosuccimide and n-3PUFAs
Ethosuccimide is a drug that exerts many of these brain effects via inhibition of the T type calcium channels, and for this reason is classically referred to as an antagonist of these channels [53–55]. Interestingly, n-3PUFAs have been also described as potent inhibitors of the T type calcium channels, with the following order of potency DHA > ALA > EPA [56]. These inhibitions occur at micromolar concentrations and hence have been previously considered to be of physiological relevance [56]. Since DHA levels were unaffected under n-3PUFA diet, ALA and EPA-mediated inhibitions of the T type calcium channels might contribute to the brain protective mechanisms here observed. In the presently reported brain protective properties of n-3PUFA diet, the two, ALA and EPA, might contribute equally because if ALA levels are lower than EPA levels in brain phospholipids under this diet, ALA is more effective than EPA to inhibit T type calcium channels [56]. Along with the generation of EPA-derived anti-inflammatory eicosanoids, inhibitions of T type calcium channels by ALA and EPA represent other n-3PUFA diet-driven brain protection mechanisms supported by the present work to occur in vivo.
5. Conclusion
By combining diet designs modulating on one hand magnesium and on the other hand PUFA (especially n-6/n-3 PUFA ratio) contents, and by including the MUFA diet as a control for n-3PUFA diet, the present study validates n-3PUFAs and especially ALA diet supplementation as an effective brain protective dietary measure. Comparison of brain phospholipid compositions in unsaturated fatty acids further indicates that ALA and EPA rather than DHA might mediate locally these protective mechanisms. Testing pharmacological-like properties of the diets in magnesium deficiency-driven pro-convulsant state conditions, including MDDAS test further gets insight in the underlying protective mechanisms, notably by establishing a parallelism between n-3PUFA diet and ethosuccimide drug profile and mode of action, providing elegant in vivo evidence attesting to T type calcium inhibition known to be shared by the two, diet and drug, conditions. For n-3PUFAs, there is, to our knowledge, the first in vivo demonstration, which validates previously documented in vitro inhibition of T type calcium channel as an effective brain protective mechanism.
Acknowledgments
This work was supported by the French Inserm (BD, PM and JV). The research conducted by S.I. Rapoport was supported entirely by the Intramural Program of the National Institute of Aging, Bethesda, Maryland, USA.
References
- 1.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally poly-unsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukotrienes Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heurteaux C, Laigle C, Blondeau N, Jarretou G, Ladzunski M. Alpha linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience. 2006;137:241–251. doi: 10.1016/j.neuroscience.2005.08.083. [DOI] [PubMed] [Google Scholar]
- 3.Blondeau N, Nguemeni C, Debruyne DN, Piens M, Wu X, Pan H, Hu X, Gandin C, Lipsky RH, Plumier JC, Marini AM, Heurteaux C. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology. 2009;34:2548–2559. doi: 10.1038/npp.2009.84. [DOI] [PubMed] [Google Scholar]
- 4.Delattre AM, Kiss A, Szawka RE, Anselmo-Franci JA, Bagatini PB, Xavier LL, Rigon P, Achaval M, Iagher F, de David C, Marroni NA, Ferraz AC. Evaluation of chronic omega-3 fatty acids supplementation on behavioral and neurochemical alterations in 6-hydroxydopamine-lesion model of Parkinson’s disease. Neurosci Res. 2010;66:256–264. doi: 10.1016/j.neures.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim HY, Akbar M, Kim KY. Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J Mol Neurosci. 2001;16:223–227. doi: 10.1385/JMN:16:2-3:223. [DOI] [PubMed] [Google Scholar]
- 6.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Ladzunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ. Polyunsaturated acids modulate sodium and calcium currents in CA1 neurons. Proc Nat Acad Sci USA. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao YF, Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal hyperexcitability during excitotoxic or convulsant stimulation. Brain Res. 1999;846:112–121. doi: 10.1016/s0006-8993(99)01997-6. [DOI] [PubMed] [Google Scholar]
- 9.Taha AY, Filo E, Ma DW, Burnham WM. Dose-dependent anticonvulsant effects of linoleic and alpha-linolenic polyunsaturated fatty acids on pentylenetetrazol induced seizures in rats. Epilepsia. 2009;50:72–82. doi: 10.1111/j.1528-1167.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- 10.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008;9:735–743. [PubMed] [Google Scholar]
- 11.Darmon N, Darmo M, Ferguson E. Identification of nutritionally adequate mixtures of vegetable oils by linear programming. J Hum Nutr Diet. 2006;19:59–69. doi: 10.1111/j.1365-277X.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Stanke-Labesque F, Molière P, Bessard J, Laville M, Véricel E, Lagarde M. Effect of dietary supplementation with increasing doses of docosahexaenoic acid on neutrophil lipid composition and leukotriene production in human healthy volunteers. Br J Nutr. 2008;100:829–833. doi: 10.1017/S0007114508923692. [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediator. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann NY Acad Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- 17.Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y. Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Weglicki WB, Krammer JH, Mak IT, Dickens BF, Kamarov AM. Pro-oxidant and pro-inflammatory neuropeptides in magnesium deficiency. In: Rayssiguier Y, Mazur A, Durlach J, editors. Advances in Magnesium research: Nutrition and Health. J. Libbey; 2001. pp. 285–289. [Google Scholar]
- 19.Weglicki WB, Phillips TM. Pathobiology of magnesium deficiency: a cytokine/neurogenic inflammation hypothesis. Am J Physiol Regul Integr Comp Physiol. 1992;263:R734–R737. doi: 10.1152/ajpregu.1992.263.3.R734. [DOI] [PubMed] [Google Scholar]
- 20.Durlach J, Bac P, Bara M, Guiet-Bara A. Physiopathology of symptomatic and latent forms of central nervous system hyperexcitability due to magnesium deficiency: a current general scheme. Magnesium Res. 2000;13:293–302. [PubMed] [Google Scholar]
- 21.Durlach J, Pages N, Bac P, Bara M, Guiet-Bara A. Biorhythms and possible central regulation of magnesium status, phototherapy, darkness therapy and chronopathological forms of magnesium depletion. Magnesium Res. 2002;15:49–66. [PubMed] [Google Scholar]
- 22.Maurois P, Pages N, Bac P, German-Fattal M, Agnani G, Delplanque B, Durlach J, Poupaert J, Vamecq J. Threshold to NMDA-induced seizures in mice undergoing chronic nutritional magnesium deprivation is lowered in a way partly responsive to acute magnesium and antioxidant administrations. Brit J Nutr. 2009;101:317–321. doi: 10.1017/S0007114508006752. [DOI] [PubMed] [Google Scholar]
- 23.Bac P, Maurois P, Dupont C, Pagès N, Stables J, Gressens P, Evrard P, Vamecq J. Magnesium-deficiency-dependent audiogenic seizures (MDDASs) in adult mice: a nutritional model for discriminatory screening of anticonvulsant drugs and original assessment of neuroprotection properties. J Neurosci. 1998;18:4363–4373. doi: 10.1523/JNEUROSCI.18-11-04363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurois P, Bailly F, Pagès N, Bac P, Fourniat J, Dupont C, Bernier JL, Catteau JP, Stables JP, Vamecq J. The multiple facets of the MDDAS test: application to the detection of neurotoxic molecules or neuroprotective (anti-inflammatory and/or antioxidant) molecules in central nervous hyper-excitability induced by severe magnesium deficiency. In: Rayssiguier J, Mazur A, Durlach J, editors. Nutrition and Health. John Libbey and Co; Londres: 2001. pp. 434–437. [Google Scholar]
- 25.Maurois P, Rocchi S, Pages N, Bac P, Auwerx J, Stables JP, Gressens P, Vamecq J. The PPAR gamma-agonist FMOC-L-Leucine protects both mature and immature brain. Biomed Pharmacother. 2008;62:259–263. doi: 10.1016/j.biopha.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Pages N, Maurois P, Delplanque B, Bac P, Stables JP, Tamariz J, Chamorro G, Vamecq J. Activities of α-asarone in various animal seizure models and in biochemical assays might be essentially accounted for by antioxidant properties. Neurosci Res. 2010;68:337–344. doi: 10.1016/j.neures.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Vamecq J, Maurois P, Bac P, Bailly F, Bernier JL, Stables JP, Husson I, Gressens P. Potent mammalian cerebroprotection and neuronal cell death inhibition are afforded by a synthetic antioxidant analogue of marine invertebrate cell protectant ovothiols. Eur J Neurosci. 2003;18:1110–1120. doi: 10.1046/j.1460-9568.2003.02846.x. [DOI] [PubMed] [Google Scholar]
- 28.Vamecq J, Maurois P, Pages N, Bac P, Stables JP, Gressens P, Stanicki D, Vanden Eynde JJ. 1,2-ethane bis-1-amino-4-benzamidine is active against several brain insult and seizure challenges through anti-NMDA mechanisms targeting the 3H-TCP binding site and antioxidant action. Eur J Med Chem. 2010;45:3101–3110. doi: 10.1016/j.ejmech.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JM, Rubenstein B, Paleker AG. Role of magnesium in glutathione metabolism of rat erythrocytes. J Nutr. 1982;112:488–496. doi: 10.1093/jn/112.3.488. [DOI] [PubMed] [Google Scholar]
- 30.Mills BJ, Lindeman RD, Lang CA. Magnesium deficiency inhibits biosynthesis of blood glutathione and tumor growth in the rat. Proc Soc Exp Biol Med. 1986;181:326–332. doi: 10.3181/00379727-181-42260. [DOI] [PubMed] [Google Scholar]
- 31.Heinrich PC, Steffen H, Jauser P, Wiss O. Studies on the reconstitution of apotransketolase with thiamin pyrophosphate and analogs of the coenzyme. Eur J Biochem. 1972;30:533–541. doi: 10.1111/j.1432-1033.1972.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 32.Veronese FM, Boccu E, Fontana A, Benassi CA, Scoffone E. Isolation and some properties of 6-phosphogluconate dehydrogenase from Bacillus stearothermophilus. Biochim Biophys Acta. 1974;334:31–44. [Google Scholar]
- 33.Regan RF, Guo Y. Magnesium deprivation decreases cellular reduced glutathione and causes oxidative neuronal death in murine cortical cultures. Brain Res. 2001;890:177–183. doi: 10.1016/s0006-8993(00)03156-5. [DOI] [PubMed] [Google Scholar]
- 34.Pages N, Maurois P, Agnani G, Vamecq J, Bac P, Delplanque B. Is chronic rapeseed oil more neuroprotective than chronic corn/sunflower diet? Evaluation by audiogenic seizure test in magnesium-deficient mice (MDDAS), OCL (Oléagineux, Corps gras, Lipides) 2007;14:214–215. [Google Scholar]
- 35.Maurois P, Gueux E, Rayssiguier Y. Protective effect of severe magnesium deficiency on Plasmodium chabaudi infection. Magnesium Res. 1989;2:183–187. [PubMed] [Google Scholar]
- 36.Rousselet F, Durlach J. SGEMV, editor. Techniques analytiques et explorations pratiques du métabolisme magnésique en clinique humaine. In: Duralch J, editor. 1er Symposium International sur le Déficit Magnésique en Pathologie Humaine. I. Vol. des rapports. Vittel: 1971. pp. 65–90. [Google Scholar]
- 37.Morise A, Combe N, Boué C, Legrand P, Catheline D, Delplanque B, Fénart E, Weill P, Hermier D. Dose effect of alpha-linolenic acid on PUFA conversion, bioavailability, and storage in the hamster. Lipids. 2004;39:325–334. doi: 10.1007/s11745-004-1236-0. [DOI] [PubMed] [Google Scholar]
- 38.Gupta SK, Manhas AS, Gupta VK, Bhatt R. Serum magnesium levels in idiopathic epilepsy. J Assoc Physicians India. 1994;42:456–457. [PubMed] [Google Scholar]
- 39.Bac P, Pages N, Herrenknecht C, Teste JF. Inhibition of mouse-killing behaviour in magnesium-deficient rats: effect of pharmacological doses of magnesium pidolate, magnesium aspartate, magnesium lactate, magnesium gluconate and magnesium chloride. Magnesium Res. 1995;8:37–45. [PubMed] [Google Scholar]
- 40.Morris ME, Brain CSF. magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnesium Res. 1992;5:303–313. [PubMed] [Google Scholar]
- 41.Dhande PP, Ranade RS, Ghongane BB. Effect of magnesium oxide on the activity of standard anti-epileptic drugs against experimental seizures in rats. Indian J Pharmacol. 2009;41:268–272. doi: 10.4103/0253-7613.59926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cotton DB, Hallak M, Janusz C, Irtenkauf SM, Berman RF. Central anticonvulsant effects of magnesium sulfate on N-methyl-D-aspartate_induced seizures. Am J Obstet Gynecol. 1993;168:974–978. doi: 10.1016/s0002-9378(12)90855-8. [DOI] [PubMed] [Google Scholar]
- 43.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 44.Nowak L, Bregestrovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:432–435. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 45.Clark S, Wilson W. Mechanisms of epileptogenesis and the expression of epileptiform activity. In: Wyllie, editor. The treatment of epilepsy: principles and practice. 2. Williams and Wilkins; Baltimore: 1996. pp. 53–81. [Google Scholar]
- 46.Iseri LT, French JH. Magnesium: nature’s physiologic calcium blocker. Am Heart J. 1989;4:229–234. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 47.Tu WC, Cook-Johnson RJ, James MJ, Mühlhäusler BS, Gibson RA. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukotrienes Essent Fatty Acids. 2010;83:61–68. doi: 10.1016/j.plefa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Demar JC, Jr, Lee HJ, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990;18:503–507. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- 50.Bazinet RP. Is the brain arachidonic acid cascade a common target of drugs used to manage bipolar disorder? Biochem Soc Trans. 2009;37:1104–1109. doi: 10.1042/BST0371104. [DOI] [PubMed] [Google Scholar]
- 51.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 52.Barbour B, Szatkowski M, Ingledew N, Attwell D. Arachidonic acid induces a prolonged inhibition of glutamate uptake into glial cells. Nature. 1989;342:918–920. doi: 10.1038/342918a0. [DOI] [PubMed] [Google Scholar]
- 53.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 54.Gomora JC, Daud AN, Weiergräber M, Perez-Reyes E. Block of cloned human T-type calcium channels by succinimide antiepileptic drugs. Mol Pharmacol. 2001;60:1121–1132. [PubMed] [Google Scholar]
- 55.Patsalos PN. Properties of antiepileptic drugs in the treatment of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):140–148. doi: 10.1111/j.1528-1167.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 56.Danthi SJ, Enyeart JA, Enyeart JJ. Modulation of native T-type calcium channels by omega-3 fatty acids. Biochem Biophys Res Commun. 2005;327:485–493. doi: 10.1016/j.bbrc.2004.12.033. [DOI] [PubMed] [Google Scholar]