Abstract
Blastocystis subtype 3 (ST3) is a parasitic protist found in the digestive tract of symptomatic and asymptomatic humans around the world. While this parasite exhibits a high prevalence in the human population, its true geographic distribution and global genetic diversity are still unknown. This gap in knowledge limits the understanding of the spread mechanisms, epidemiology, and impact that this parasite has on human populations. Herein, we provided new data on the geographical distribution and genetic diversity of Blastocystis ST3 from a rural human population in Mexico. To do so, we collected and targeted the SSU-rDNA region in fecal samples from this population and further compared its genetic diversity and structure with that previously observed in populations of Blastocystis ST3 from other regions of the planet. Our analyses reveled that diversity of Blastocystis ST3 showed a high haplotype diversity and genetic structure to the world level; however, they were low in the Morelos population. The haplotype network revealed a common widespread haplotype from which the others were generated recently. Finally, our results suggested a recent expansion of the diversity of Blastocystis ST3 worldwide.
1. Introduction
Blastocystis (Heterokonta, Stramenopiles) is a genus comprising parasitic protists that inhabit the digestive tract of several metazoans, such as fishes, amphibians, birds, reptiles, rodents, and humans [1–3]. Blastocystis is globally distributed, showing a high rate of infection from underdeveloped to developed countries [4, 5].
This parasite is often transmitted via the oral-fecal route to people who work directly with animals, such as those involved in intensive animal farming or industrial livestock production [6]. In humans, the signs and symptoms associated with Blastocystis infection range from diarrhea to flatulence, bloating, and abdominal discomfort [7, 8], with the “irritable bowel syndrome” (IBS) being the most frequent clinical manifestation [8–11].
Molecular evidence based on the small subunit ribosomal RNA (SSU-rDNA) gene suggests that, at least, 17 genetic subtypes can be recognized within Blastocystis [12]. Nine of these subtypes are found in humans, with subtype 3 (ST3 hereafter) being the most common in epidemiological studies worldwide [12–18]. ST3 has been regarded to trigger IBS in humans [8], and recent research also suggests an association between this subtype and colorectal cancer [19]. Other studies, however, suggest a lack of association between the ST3 and some type of symptomatology in humans [7, 20]. While Blastocystis ST3 has medical importance and high prevalence in humans, the real magnitude of its genetic diversity and geographical distribution remains so far unknown [5, 17]. Apparently, the ST3 exhibits a broader geographical distribution and higher genetic diversity than other genetic subtypes of Blastocystis [16, 21], but this hypothesis still needs to be tested using genetic data and clinical cases from both well-studied and undersampled geographical areas. In this context, there are very few geographical and genetic data on Blastocystis ST3 from Mexico.
Herein, we aimed to provide new data on the geographical distribution and genetic diversity of Blastocystis ST3 from a rural and asymptomatic human population in Mexico. To do so, we collected and targeted the SSU-rDNA region in fecal samples from this population and further compared its genetic diversity and structure with those previously observed in populations of Blastocystis ST3 from other regions of the planet.
2. Materials and Methods
2.1. Ethical Considerations
The protocol used in this study was conducted under the ethical principles and approval of both the Mexican Commission on Ethics and Research of the Health Ministry of the State of Morelos (Comisiones de Ética y de Investigación del Ministerio de Salud del Estado de Morelos) and the Commission on Ethics in Research of the Facultad de Medicina of the Universidad Nacional Autónoma de México (UNAM) (Comité de Ética de Investigación de la Facultad de Medicina de la Universidad Nacional Autónoma de México). The guidelines of the committees are based on the Mexican Official Norm (Norma Oficial Mexicana NOM-012-SSA3-2007), which regulates the ethical principles of every research on humans and on laboratory animals, as well as on the Declaration of Helsinki, which set ethical principles regarding human experimentation developed by the World Health Organization (WHO).
Based on the abovementioned guidelines, our study only used samples from volunteers, who were respectively informed about the objectives of this research, the potential risks (if any), and the sampling procedures. We obtained an informed consent letter from all the participants.
2.2. Sampling and Analysis
Between May and November 2015, fecal samples were collected from 182 volunteers (86 males and 96 females) from Puente de Ixtla in the community of Xoxocotla, State of Morelos (Mexico), ranging in age from 2 to 51 years old. The asymptomatic status was defined according to the ROME III criteria. Three fecal samples were collected from each volunteer on three consecutive days. The samples were maintained at 4°C and transported to the laboratory in Mexico City on the same day of collection. A subsample of each fecal sample was smeared, stained with 4% Lugol's iodine solution, and examined under a light microscope at 10x and 40x magnifications [22].
2.3. Amplification and Sequencing of SSU-rDNA
DNA was extracted from fresh fecal samples using QIAamp DNA stool kit (QIAGEN, Hilden, Germany) and following the manufacturer's instructions. PCR protocol targeting the SSU-rDNA was conducted according to Scicluna et al. [23]. In brief, we used a total mixture of 20 μl : 20 μM of primers RD5 (5′-ATC TGG TTG ATC CTG CCAG T-3′) and BhRDr (5′-GAG CTT TTT AAC TGC AAC AAC G-3′) [23], as well as 0.025 U of polymerase (AmpliTaq Platinum Polymerase, Invitrogen). To verify the presence of a single band and the size of the amplified products (approximately 600 bp), the PCR products were separated by electrophoresis in agarose gel (1.5%) in the presence of ethidium bromide, visualized by ultraviolet transillumination, and photographed. The amplification product of a 600 bp fragment of the Blastocystis SSU-rDNA was purified and sequenced using a dideoxynucleotide-terminal method. Sequencing was carried out in a capillary sequencer (ABI-Avant 100, University of Washington). The sequences obtained were edited and/or analyzed with BioEdit, MEGA 5.0 software [24, 25], and ad hoc scripts from Python. These sequences were compared to sequences available in GenBank, employing BLAST to establish their identity. The final sequences were deposited in GenBank under accession numbers MF539962–MF540015.
2.4. Global Genetic Diversity and Haplotype Network for Blastocystis ST3
We investigated the global genetic diversity (i.e., Latin America, Europe, and Asia) within Blastocystis ST3 using the novel SSU-rDNA sequences reported in the present study and those previously reported within the literature. We provided an exhaustive list of the latter sequences (n = 169) and sources in the Supplementary Material (available here). We investigated the following descriptive statistics of genetic diversity using the software DnaSP ver. 5.10.01 [26]: number of segregating sites (S), number of haplotypes (h), haplotype diversity (Hd), and nucleotide diversity (π) for each set of sequences according to their geographical region of origin. We built a global haplotype network using TCS network inference method [27] implemented in the PopART program ver. 1.7 (http://popart.otago.ac.nz/downloads.shtml) to investigate the global genealogical relationship between the different haplotypes of Blastocystis ST3. We also ran Tajima's D test in the software DnaSP [26] to investigate possible events of global population expansion on Blastocystis ST3. We finally estimated pairwise FST statistics in the software Arlequin ver. 3.11 (http://cmpg.unibe.ch/software/arlequin3) to investigate whether the geographical populations of Blastocystis ST3 were genetically structured.
3. Results
3.1. Frequency of Blastocystis ST3 in Morelos, Mexico
A microscopic analysis revealed that 148 (81.32%) of the 182 fecal samples collected in Morelos (Mexico) exhibited at least some type of intestinal parasite. These 148 samples (positive samples hereafter) harbored different parasites, including representatives of Blastocystis, Chilomastix mesnili, Entamoeba coli, Hymenolepis nana, Iodamoeba bütschlii, Endolimax nana, the Entamoeba histolytica/Entamoeba dispar complex, and Giardia lamblia. Among the abovementioned parasites, Blastocystis had the greatest frequency, occurring in 109 (74%) out of 148 positive samples. It was also the unique parasite in 99 (67%) out of 148 positive samples, and 7% of the samples (10/148) were coinfected with other parasites (Figure 1).
Figure 1.
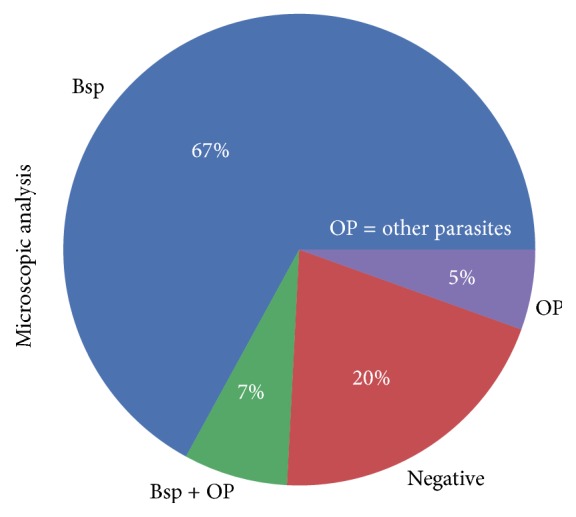
Frequency of intestinal parasites based on microscopic analysis of the fecal samples taken in Morelos, Mexico. Blastocystis was the only parasitic infection found in 67% of individuals and in 7% in coinfection with other parasites. Bsp: Blastocystis; OP: parasites other than Blastocystis; BSP + OP: coinfection of Blastocystis and other parasites; Negative: no parasite found. Among OP: Chm, Chilomastix mesnili; Ec, Entamoeba coli; En, Endolimax nana; Hn, Hymenolepis nana; Gl, Giardia lamblia; Ib, Iodamoeba bütschlii.
Further PCR and sequencing procedures successfully confirmed the presence of three different Blastocystis subtypes in 72 of the 148 positive samples collected in Morelos. These three Blastocystis subtypes (ST) were recorded according to the following frequencies: Blastocystis ST1, 9.7% (n = 7 samples); ST2, 15.3% (n = 11 samples); and ST3, 75% (n = 54 samples) (Figure 2).
Figure 2.
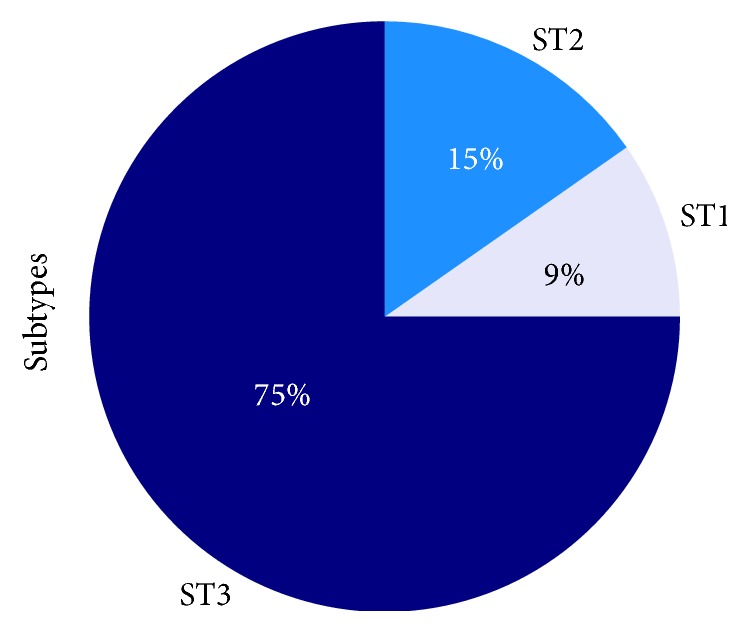
Frequency of Blastocystis subtypes in the study population. Targeting the SSU-rDNA according to DNA-barcoding, Three Blastocystis subtypes (ST) were recorded according to the following frequencies: Blastocystis ST1, 9.7% (n = 7 samples); ST2, 15.3% (n = 11 samples); and ST3, 75% (n = 54 samples).
3.2. Genetic Diversity of ST3 and Haplotype Network
Genetic diversity indices revealed a total of 44 segregating sites (S) and 20 haplotypes (h), as well as a total haplotype diversity (Hd) of 0.563 and nucleotide diversity (π) of 0.019. Tajima's D test provided values ranging between −1.303 and −2.363 (Table 1). A pairwise Fst analysis revealed that there is very low genetic differentiation between all geographical populations of Blastocystis ST3 (Table 2).
Table 1.
Statistics data of genetic diversity observed within different geographical populations of Blastocystis ST3 around the world.
| Populations | N | S | h | Hd | π | ±SD | Tajima's D |
|---|---|---|---|---|---|---|---|
| Morelos | 54 | 1 | 3 | 0.14186 | 0.00107 | 0.068 | −1.68258ns |
| Latin America | 69 | 25 | 15 | 0.67775 | 0.01334 | 0.058 | −2.36261∗∗ |
| Eurasia | 46 | 35 | 11 | 0.74010 | 0.04473 | 0.052 | −1.30307ns |
| All populations | 169 | 44 | 20 | 0.56276 | 0.01886 | 0.044 | −2.20000∗∗ |
N: number of sequences; S: number of segregating sites; h: number of haplotypes; Hd: haplotype diversity; π: nucleotide diversity; ns: not significant. ∗∗p < 0.01. Latin America: Blastocystis populations of North and South America (i.e., Mexico, Colombia, Brazil, Ecuador, Bolivia, Peru, and Argentina), except that of Morelos. Eurasia: Blastocystis populations of Europa and Asia (i.e., Nepal, Switzerland, Iraq, Italy, and France).
Table 2.
Estimates of FST based on the SSU-rDNA variation observed between different geographical populations of the parasite Blastocystis ST3.
| Population | Morelos | Latin America | Eurasia |
|---|---|---|---|
| Morelos | ------ | ||
| Latin America | 0.04165ns | ------ | |
| Eurasia | 0.09164ns | 0.05975ns | ------ |
Latin America: Blastocystis populations of North and South America (i.e., Mexico, Colombia, Brazil, Ecuador, Bolivia, Peru, and Argentina), except that of Morelos. Eurasia: Blastocystis populations of Europe and Asia (i.e., Nepal, Switzerland, Iraq, Italy, and France). Probability obtained by a permutation test with 50,000 replicates. ns: not significant.
The number of haplotypes ranged from 3 to 15 between human populations, the number of segregating sites ranged between 1 and 35, haplotype diversity ranged between 0.142 and 0.740, and nucleotide diversity ranged between 0.001 and 0.045 (Table 1). The ST3 genetic diversity of Latin American populations (except Morelos's population) and Eurasia exhibited the highest values of genetic diversity indices in contrast to Morelos's population, where low haplotype diversity (three haplotypes) was detected (Table 1).
The haplotype network showed the haplotype distribution of the ST3 (Figure 3). In general, the worldwide haplotype network evidenced large levels of diversity, with a total of 20 haplotypes, and haplotype 1 was the dominant. The network showed a star topology radial distribution (Figure 3). Also, haplotype 1 was the most frequently found in Morelos's population and this haplotype is commonly distributed in American populations.
Figure 3.
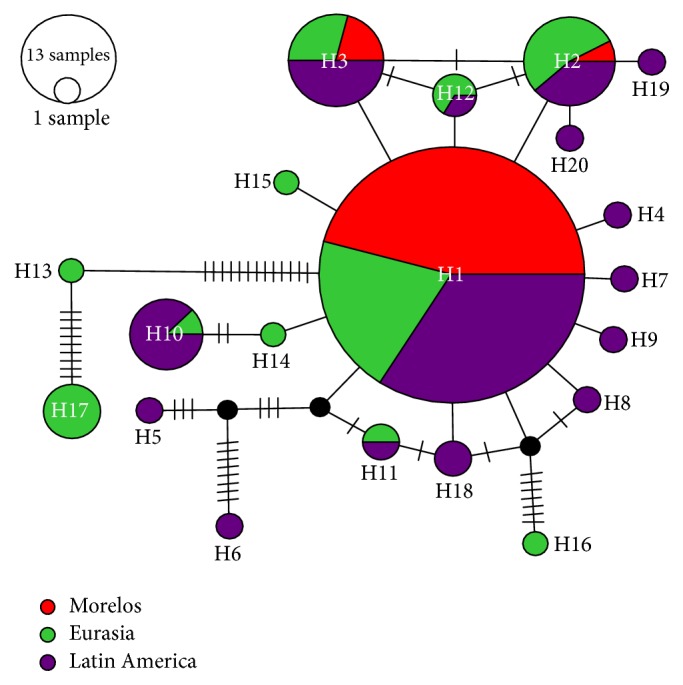
Haplotype network of Blastocystis ST3 of human populations at different regions from Latin America, Europe, and Asia. Each circle represents a haplotype, and each color represents the place where it was obtained. The size of each circle is proportional to the frequency of the haplotype in each population, where it was found. The circles in black stand for missing haplotypes and the short lines show the mutational steps.
4. Discussion
In the present study, we analyzed the frequency and distribution of Blastocystis subtypes in an asymptomatic rural population. The results revealed a great frequency of Blastocystis from 74%, above the national average, as the frequency of this parasite varies from 23% to 61% in Mexico [20, 28, 29]. Recent studies in South America have described similar frequency of Blastocystis in the human population (21% to 67%) [30–32]. Around the world, Blastocystis exhibits a frequency range of 0.5% to 62% [4]. The higher prevalence of Blastocystis has been linked to hygiene factors including the consumption of food/water contaminated with Blastocystis and exposure to domestic and peridomestic animals infected too with this parasite [4, 33].
Many years ago, Blastocystis was considered as saprophytic yeast of the digestive tract, innocuous for the host [34]. Nowadays, we can observe that this parasite is widely distributed in the human population in the world and it is similarly distributed in symptomatic and asymptomatic individuals. For instance, Morelos's population (Mexico) showed a high infection frequency of Blastocystis although the participants were asymptomatic, suggesting tolerance to this parasite, as reported elsewhere [20, 35, 36].
Blastocystis has a high worldwide genetic diversity, represented by 17 subtypes (ST1–ST17) [5]. It is possible that there are other subtypes capable of infecting humans and other vertebrates [4, 5]. Regarding the distribution of subtypes in the present study, ST1 (9.7%), ST2 (15.3%), and ST3 (75%) were identified among 72 human isolates successfully genotyped. Globally, Blastocystis ST3 is the most prevalent subtype in humans found in different geographic areas [5, 37]. In our study, the frequency of ST3 was 75%, high compared to other populations of Mexico as the state of Michoacan, where the frequency of this subtype was 21%, and in Mexico City it was 42% [38, 39].
In Latin America, the frequency of ST3 is high [4, 37], with the following frequencies reported for Blastocystis ST3 in this political region: 14% in Colombia, 36% in Brazil, 84% in Ecuador, 30% in Bolivia, 92% in Peru, and 63% in Argentina [40]. While ST3 is amply distributed worldwide, it is more prevalent in Latin America [37], which opens the possibility that this subtype was generated in this geographic area and spread to the rest of the continents.
To improve our knowledge on the magnitude of the genetic diversity of Blastocystis ST3 on the planet, we analyzed sequences of Mexico City, South America, France, Switzerland, Italy, Nepal, and Iraq and the sequences obtained in the present study (169 total sequences, 54 from Morelos and 115 from the NCBI database). The genetic parameters calculated for these sequences suggested a recent population increase or directional/purifying selection. These results are supported by the haplotype network, which showed a star topology with the haplotypes distributed in a radial way supporting inferences of a recent geographical expansion in Blastocystis ST3. This behavior is similar to reporting by other parasites, such as Plasmodium falciparum [41].
A total of 20 haplotypes were found on all sequences analyzed. Haplotype 1 was the most abundant and widely distributed, being detected in the majority of the studied countries, mainly in Latin America. These results are showing that haplotype 1 is perhaps an ancestral type from which all the other haplotypes have been generated recently. Haplotype 1 probably originated in Latin America and has recently colonized other regions of the world, probably via human migration [42].
In some studies, it has been observed that this subtype has been strongly related to rural populations [8, 43]. It is possible to hypothesize that the migration phenomenon [42] occurs mainly from the displacement of these rural communities infected with Blastocystis ST3 haplotype 1 spreading this parasite into the cities. Already in large cities, the transmission of Blastocystis ST3 haplotype 1 could happen from one person to another [43]. In addition, due to the opening of political borders between developed countries, this process of travel-associated infection is common, because travelers may act as carriers of this parasite [44].
Also, it is possible that the other haplotypes of Blastocystis ST3 could be colonizing different areas promoted by the human migratory phenomenon. This idea would explain the high representation of certain haplotypes throughout the American continent and others in European countries. For example, seven sequences of haplotype 9 correspond to South America and only one corresponds to Europe (Switzerland).
However, to test both hypotheses, it will be necessary to have a larger number of sequences and complete genomes from around the world. It is known that migration causes mobility of parasites and it is important to have information of other sociodemographic parameters of the host as the nationality or antecedents of previous travels to endemic areas [45]. In addition, the distribution of Blastocystis could be related to the lack of symptoms that occurs in many cases, because asymptomatic Blastocystis infections are not treated and therefore there are a large number of carriers of this parasite. It is known that these factors can influence the distribution of parasites [42, 46].
With respect to Morelos, all the sequences of Blastocystis ST3 were grouped into three haplotypes, which means that there is a low genetic diversity, a reduced rate of mutations, and little genetic differentiation; this suggests isolation and homogeneity in the population.
5. Conclusions
To our knowledge, this is the first study analyzing the haplotype diversity and distribution of Blastocystis ST3 subtypes in different human populations. In addition, our work facilitates the vision of a global distribution in Blastocystis ST3. We provide evidence of a recent expansion of this subtype that may be related to the migration of humans to other regions of the world. However, it is necessary to continue studying this parasite, in order to generate a more complete knowledge that allows us to know the course of Blastocystis infection, its epidemiology, and the causal factors that contribute to its dispersion dynamics and distribution.
Acknowledgments
The present work was supported by Grants IN218214 and IN-226511 from PAPIIT (DGAPA), Universidad Nacional Autónoma de México (UNAM); CONACyT 210-C01-140990 from the National Council for Science and Technology in Mexico (CONACyT); and FIS/IMSS/Prot/699 from the Mexican Institute of Social Security (IMSS). The authors would like to appreciate the collaboration of the Health Ministry of the State of Morelos, Mexico, for authorizing the establishments of the center of activity in the facilities and in the community of Xoxocotla. They also acknowledge the technical support provided by M.S. Martha Zaragoza, the Chemist Angeles Padilla, and Alejandro Flores. They also highly appreciate the informatics assistance of Angélica Serrano-Ahumada and Marco Gudiño. Leonardo D. Fernández is supported by CONICYT (FONDECYT Project no. 11170927) and by Universidad Bernardo O'Higgins (Project UBO/VRIP170201).
Contributor Information
Liliana Rojas-Velázquez, Email: lhily@yahoo.com.
Cecilia Ximénez, Email: cximenez@unam.mx.
Disclosure
Liliana Rojas-Velázquez is a doctoral student from Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM), and received Fellowship 348424/239901 from CONACYT. This paper constitutes a partial fulfillment of the Graduate Program.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
List of the sequences reported in the present study and those previously reported within the literature.
References
- 1.Yoshikawa H., Wu Z., Howe J., Hashimoto T., Geok-Choo N., Tan K. S. W. Ultrastructural and phylogenetic studies on Blastocystis isolates from cockroaches. Journal of Eukaryotic Microbiology. 2007;54(1):33–37. doi: 10.1111/j.1550-7408.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 2.Silberman J. D., Sogin M. L., Leipe D. D. Human parasite finds taxonomic home. Nature. 1996;380(6573):p. 398. doi: 10.1038/380398a0. [DOI] [PubMed] [Google Scholar]
- 3.Stechmann A., Hamblin K., Pérez-Brocal V., et al. Organelles in blastocystis that blur the distinction between mitochondria and hydrogenosomes. Current Biology. 2008;18(8):580–585. doi: 10.1016/j.cub.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark C. G., van der Giezen M., Alfellani M. A., Stensvold C. R. Recent Developments in Blastocystis Research. Amsterdam, Netherlands: Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 5.Alfellani M. A., Taner-Mulla D., Jacob A. S., et al. Genetic diversity of blastocystis in livestock and zoo animals. Protist. 2013;164(4):497–509. doi: 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Tan K. S. W. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clinical Microbiology Reviews. 2008;21(4):639–665. doi: 10.1128/cmr.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa C. V., Batista R. D. J., Igreja R. P., Levy C. M. D., Macedo H. W. D., Santos H. L. C. Distribution of Blastocystis subtypes isolated from humans from an urban community in Rio de Janeiro, Brazil. Parasites and Vectors. 2017;10(1):p. 518. doi: 10.1186/s13071-017-2458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Badry A. A., Abd ElWahab W. M., Hamdy D. A., Aboud A. Blastocystis subtypes isolated from irritable bowel syndrome patients and co-infection with Helicobacter pylori. Parasitology Research. 2017:1–11. doi: 10.1007/s00436-017-5679-4. [DOI] [PubMed] [Google Scholar]
- 9.Giacometti A., Cirioni O., Fiorentini A., Fortuna M., Scalise G. Irritable bowel syndrome in patients with Blastocystis hominis infection. European Journal of Clinical Microbiology & Infectious Diseases. 1999;18(6):436–439. doi: 10.1007/s100960050314. [DOI] [PubMed] [Google Scholar]
- 10.Yakoob J., Jafri W., Beg M. A., et al. Irritable bowel syndrome: is it associated with genotypes of blastocystis hominis. Parasitology Research. 2010;106(5):1033–1038. doi: 10.1007/s00436-010-1761-x. [DOI] [PubMed] [Google Scholar]
- 11.Jadallah K. A., Nimri L. F., Ghanem R. A. Protozoan parasites in irritable bowel syndrome: a case-control study. World Journal of Gastrointestinal Pharmacology and Therapeutics. 2017;8(4):201–207. doi: 10.4292/wjgpt.v8.i4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stensvold C. R., Suresh G. K., Tan K. S. W., et al. Terminology for Blastocystis subtypes - a consensus. Trends in Parasitology. 2007;23(3):93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Dogruman-Al F., Kustimur S., Yoshikawa H., et al. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Memórias do Instituto Oswaldo Cruz. 2009;104(5):724–727. doi: 10.1590/S0074-02762009000500011. [DOI] [PubMed] [Google Scholar]
- 14.Moosavi A., Haghighi A., Mojarad E. N., et al. Genetic variability of Blastocystis sp. Isolated from symptomatic and asymptomatic individuals in Iran. Parasitology Research. 2012;111(6):2311–2315. doi: 10.1007/s00436-012-3085-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa H., Wu Z., Kimata I., et al. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitology Research. 2004;92(1):22–29. doi: 10.1007/s00436-003-0995-2. [DOI] [PubMed] [Google Scholar]
- 16.Stensvold C. R., Alfellani M., Clark C. G. Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infection, Genetics and Evolution. 2012;12(2):263–273. doi: 10.1016/j.meegid.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Alfellani M. A., Stensvold C. R., Vidal-Lapiedra A., Onuoha E. S. U., Fagbenro-Beyioku A. F., Clark C. G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Tropica. 2013;126(1):11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Clark C. G. Extensive genetic diversity in Blastocystis hominis. Molecular and Biochemical Parasitology. 1997;87(1):79–83. doi: 10.1016/S0166-6851(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Padukone S., Mandal J., Parija S. Severe Blastocystis subtype 3 infection in a patient with colorectal cancer. Tropical Parasitology. 2017;7(2):122–124. doi: 10.4103/tp.TP-87-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojas L., Morán P., Valadez A., et al. Entamoeba histolytica and Entamoeba dispar infection in Mexican school children: Genotyping and phylogenetic relationship. BMC Infectious Diseases. 2016;16(1, article no. 485) doi: 10.1186/s12879-016-1812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meloni D., Poirier P., Mantini C., et al. Mixed human intra- and inter-subtype infections with the parasite Blastocystis sp. Parasitology International. 2012;61(4):719–722. doi: 10.1016/j.parint.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Ash L. R., Orihel T. C. Parasites: a guide to laboratory procedures and identification. American Society of Clinical Pathologists Press. 1987;4(2):p. 1988. [Google Scholar]
- 23.Scicluna S. M., Tawari B., Clark C. G. DNA barcoding of Blastocystis. Protist. 2006;157(1):77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Research. 1999;41:95–98. [Google Scholar]
- 25.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Librado P., Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 27.Clement M., Posada D., Crandall K. A. TCS: a computer program to estimate gene genealogies. Molecular Ecology. 2000;9(10):1657–1659. doi: 10.1046/j.1365-294X.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez-Miranda M. E., Jiménez-González D. E., Rodríguez-Campa M. E. Síndrome de intestino irritable: frecuencia y relación filogenética de Blastocystis sp. de pacientes mexicanos. Revista de Gastroenterología de México. 2011;76(4):309–315. [PubMed] [Google Scholar]
- 29.Rodriguez E., Mateos B., Gonzalez J. C. Transición parasitaria a Blastocystis hominis en niños de la zona centro del estado de Guerrero, México. Parasitología Latinoamericana. 2008 [Google Scholar]
- 30.Casero R. D., Mongi F., Sánchez A., Ramírez J. D. Blastocystis and urticaria: Examination of subtypes and morphotypes in an unusual clinical manifestation. Acta Tropica. 2015;148:156–161. doi: 10.1016/j.actatropica.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Malheiros A. F., Stensvold C. R., Clark C. G., Braga G. B., Shaw J. J. Short report: Molecular characterization of Blastocystis obtained from members of the indigenous tapirapé ethnic group from the Brazilian Amazon Region, Brazil. The American Journal of Tropical Medicine and Hygiene. 2011;85(6):1050–1053. doi: 10.4269/ajtmh.2011.11-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramírez J. D., Sánchez L. V., Bautista D. C., Corredor A. F., Flórez A. C., Stensvold C. R. Blastocystis subtypes detected in humans and animals from Colombia. Infection, Genetics and Evolution. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Tan T. C., Suresh K. G., Smith H. V. Phenotypic and genotypic characterisation of Blastocystis hominis isolates implicates subtype 3 as a subtype with pathogenic potential. Parasitology Research. 2008;104(1):85–93. doi: 10.1007/s00436-008-1163-5. [DOI] [PubMed] [Google Scholar]
- 34.Stenzel D. J., Boreham P. F. L. Blastocystis hominis revisited. Clinical Microbiology Reviews. 1996;9(4):563–584. doi: 10.1128/cmr.9.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AbuOdeh R., Ezzedine S., Samie A., Stensvold C. R., ElBakri A. Prevalence and subtype distribution of Blastocystis in healthy individuals in Sharjah, United Arab Emirates. Infection, Genetics and Evolution. 2016;37:158–162. doi: 10.1016/j.meegid.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 36.David É. B., Guimarães S., De Oliveira A. P., et al. Molecular characterization of intestinal protozoa in two poor communities in the State of São Paulo, Brazil. Parasites & Vectors. 2015;8(1, article no. 103) doi: 10.1186/s13071-015-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stensvold C. R., Clark C. G. Current status of Blastocystis: a personal view. Parasitology International. 2016;65(6):763–771. doi: 10.1016/j.parint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Vargas-Sanchez G.-B., Romero-Valdovinos M., Ramirez-Guerrero C., et al. Blastocystis isolates from patients with irritable bowel syndrome and from asymptomatic carriers exhibit similar parasitological loads, but significantly different generation times and genetic variability across multiple subtypes. PLoS ONE. 2015;10(4):1–13. doi: 10.1371/journal.pone.0124006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villalobos G., Orozco-Mosqueda G. E. R., Lopez-Perez M., et al. Suitability of internal transcribed spacers (ITS) as markers for the population genetic structure of Blastocystis spp. Parasites & Vectors. 2014;7:p. 461. doi: 10.1186/s13071-014-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramírez J. D., Sánchez A., Hernández C., et al. Geographic distribution of human Blastocystis subtypes in South America. Infection, Genetics and Evolution. 2016;41:32–35. doi: 10.1016/j.meegid.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Joy D. A. Early origin and recent expansion of Plasmodium falciparum. Science. 2003;300(5617):318–321. doi: 10.1126/science.1081449. [DOI] [PubMed] [Google Scholar]
- 42.Yomb J. C., Jonckheere S., Colin G., et al. Imported malaria in a tertiary hospital in Belgium: epidemiological and clinical analysis. Acta clinica Belgica. 2013;68(2):101–106. doi: 10.2143/ACB.2964. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira-Arbex A. P., David É. B., Guimarães S. Blastocystis genetic diversity among children of low-income daycare center in Southeastern Brazil. Infection, Genetics and Evolution. 2018;57:59–63. doi: 10.1016/j.meegid.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Hagmann S. H. F., Han P. V., Stauffer W. M., et al. Travel-associated disease among US residents visiting US GeoSentinel clinics after return from international travel. Journal of Family Practice. 2014;31(6):678–687. doi: 10.1093/fampra/cmu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bühler S., Rüegg R., Steffen R., Hatz C., Jaeger V. K. A profile of travelers - An analysis from a large Swiss travel clinic. Journal of Travel Medicine. 2014;21(5):324–331. doi: 10.1111/jtm.12139. [DOI] [PubMed] [Google Scholar]
- 46.Cheong H. S., Kwon K.-T., Rhee J.-Y., et al. Imported malaria in Korea: a 13-year experience in a single center. The Korean Journal of Parasitology. 2009;47(3):299–302. doi: 10.3347/kjp.2009.47.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the sequences reported in the present study and those previously reported within the literature.


