Abstract
Background
Altered knee kinematics after anterior cruciate ligament injury and reconstruction (ACLR) have been implicated in the development of posttraumatic osteoarthritis (PTOA), leading to poor long-term clinical outcomes.
Purpose
This study was conducted to determine (1) whether the average knee center of rotation (KCOR), a multidimensional metric of knee kinematics, of the ACL-reconstructed knee during walking differs from that of the uninjured contralateral knee; (2) whether KCOR changes between 2 and 4 years after surgery; and (3) whether early KCOR changes predict patient-reported outcomes 8 years after ACLR.
Study Design
Descriptive laboratory study.
Methods
Twenty-six human participants underwent gait analysis with calculation of bilateral KCOR during walking at 2 and 4 years after unilateral ACLR. Knee injury and Osteoarthritis Outcome Score (KOOS) and Lysholm score results were collected at 2, 4, and 8 years after ACLR in 13 of these participants.
Results
The ACL-reconstructed knee showed greater medial compartment motion because of pivoting about a more lateral KCOR (P = .03) than the contralateral knee at 2 years. KCOR became less lateral over time (P = .047), with values approaching those of the uninjured knee by 4 years (P = .55). KCOR was also more anterior in the ACL-reconstructed knee at 2 years (P = .02). Between 2 and 4 years, KCOR moved posteriorly in 16 (62%) and anteriorly in 10 (38%) participants. Increasing the anterior position of KCOR in the ACL-reconstructed knee from 2 to 4 years correlated with worsening clinical outcomes at 4 years (KOOS–Quality of Life, R2 = 0.172) and more strongly at 8 years (Lysholm score, R2 = 0.41; KOOS-Pain, R2 = 0.37; KOOS-Symptoms, R2 = 0.58; and KOOS–Quality of Life, R2 = 0.50).
Conclusion
The observed changes to KCOR during walking between 2 and 4 years after ACLR show progressive improvement toward kinematic symmetry over the 2-year follow-up. The correlation between increasingly abnormal kinematics and worsening clinical outcomes years later in a subset of participants provides a potential explanation for the incidence of PTOA after ACLR.
Keywords: ACL reconstruction, knee kinematics, center of rotation, patient-reported outcomes, osteoarthritis, gait analysis
Anterior cruciate ligament (ACL) tear, one of the most common knee injuries, greatly accelerates the development of posttraumatic osteoarthritis (PTOA).7,18,19,26 Although ACL reconstruction (ACLR) has been successful in allowing patients to return to high-level sports and physical labor, it has not been successful in preventing deteriorating function and PTOA. Several studies indicate that roughly half of ACL-reconstructed patients have progressed to symptomatic radiographic PTOA by 10 to 15 years after an injury.7,18,19,26 Given that ACL tears most frequently occur in teenagers and young adults,14 improved understanding of factors leading to worsening clinical outcomes and PTOA in this population is of crucial importance toward decreasing future morbidity from this injury.11
Altered kinematics during ambulation after ACLR have been implicated in subsequent PTOA development.4,23,24 The importance of the ACL in maintaining normal function has been identified in several studies that report common kinematic changes to the knee flexion angle and internal-external rotation angle of the knee during walking after ACLR.15,23,24 It has been suggested that these kinematic changes shift the location of repetitive joint contact loads during walking to regions of cartilage not conditioned for these loads because of regional variations in both cartilage structure and biology.3,8,9 If the new region of cartilage cannot adapt to the change in repetitive loading during ambulation, a degenerative pathway ensues.4,5,10,23,27
Given that the ACL can simultaneously influence multiple components of knee kinematics, and the potential importance of identifying kinematic changes that influence the deterioration of knee function and PTOA development, it is useful to determine whether a more comprehensive metric can be used to assess knee kinematics. The average knee center of rotation (KCOR) during the stance phase of walking may provide a novel mechanical marker that combines both translational and rotational motion in the transverse plane of the tibiofemoral joint, measured as the knee flexes during the weightbearing portion of walking,6 thus providing a more comprehensive assessment of knee kinematics during walking than a single metric alone. A strength of this metric is that it geometrically combines these variables as opposed to statistically combining them. Kinematic variables during walking are not completely independent of one another, and combining them statistically may yield insignificant results, despite contributions from several variables. Because kinematic changes after ACLR are both multifactorial and complex, combining variables this way may be useful in assessing PTOA risk.
Previous work has shown that KCOR is on the lateral side of the knee during the stance phase of walking in asymptomatic healthy knees, suggesting greater relative motion between the articular cartilage surfaces of the femur and tibia in the medial compartment compared with the lateral compartment.17 Given that about half of ACL-reconstructed patients develop symptomatic PTOA,18 and that changes in knee kinematics and joint moments occur after ACLR,15,23,24,28 it is also possible that some patients adopt changes in gait that alter the position of KCOR over time. Additionally, it is unknown whether these changes are associated with longer term changes in patient-reported outcomes (PROs) such as the Knee injury and Osteoarthritis Outcome Score (KOOS)22 and Lysholm score.20 Both of these PRO scores have been widely used to assess patient outcomes after ACLR18,19,26 and have recently been found to be associated with knee joint kinematics during dynamic pivoting activities29 and downhill running.16
The purpose of this study was to determine if KCOR is a potential clinically useful metric of PTOA after successful ACLR. It was hypothesized that (1) there are differences in KCOR between the ACL-reconstructed and contralateral knees, (2) KCOR of the ACL-reconstructed knee changes between 2 and 4 years after surgery, and (3) the changes seen in hypothesis 2 predict changes in PROs between 2 and 8 years after surgery that are not evident at 4 years.
METHODS
Twenty-six patients (mean age, 31.0 ± 6.4 years; mean body mass index [BMI], 24.8 ± 2.6 kg/m2; 2.6 ± 1.9 months between injury and surgery; 11 female; 15 right legs) who underwent unilateral ACLR and with no other history of serious injuries or surgery to either lower limb participated in this study after providing institutional review board–approved informed consent. Participants were tested at a nominal 2- (T1, 2.19 ± 0.31) and 4-year (T2, 4.34 ± 0.29) follow-up after ACLR. A subset of 13 participants (mean age, 30.5 ± 7.1 years; mean BMI, 24.5 ± 3.0 kg/m2; 2.3 ± 1.9 months between injury and surgery; 8 female; 7 right legs) returned for an 8-year (T3, 7.92 ± 0.57) follow-up evaluation. Eight-year data were not obtained on 13 participants for the following reasons: exclusion because of additional knee injuries or surgeries (n = 3), patient moved outside of a reasonable travel distance to participate (n = 1), no consent for recontact (n = 2), no interest (n = 1), and no response to attempted contact (n = 6).
All ACL-reconstructed participants had undergone single-bundle ACLR performed with a transtibial technique (24 Achilles tendon allografts, 2 patellar tendon autografts). Inclusion criteria required successful ACLR based on a clinical examination (KT-1000 arthrometer side-to-side difference <5 mm) and self-reported knee stability. Participants with resection of more than 25% of the meniscus in either compartment, clinical instability of the reconstructed knee, or a history of other serious injuries or surgery to either lower limb were excluded.
Participants completed 10-m gait trials at their preferred walking speed for both the ACL-reconstructed and contralateral legs. A 10-camera optoelectronic system (Qualisys) and a force plate (Bertec) embedded in the floor were used to measure participants’ motion at 120 Hz. A trial was successful when the entire foot of the test leg struck the force plate in the middle of the walkway. The software application BioMove (Stanford University) was used to calculate knee kinematics using the previously validated point cluster technique.2,12 The foot, shank, and thigh segments’ anatomic reference frames were determined as previously described13 using a standing reference pose collected before the walking trials.
KCOR was determined as previously described6,17 by first projecting lines coincident with the transepicondylar axis of the femur onto the transverse plane of the tibia using the coordinate transformation matrix, relating the femoral and tibial anatomic coordinate systems for every frame of motion capture during the stance phase of gait (Figure 1 and Video Supplement). The medial-lateral (ML) and anterior-posterior (AP) coordinates of KCOR were calculated over the entire stance phase by solving the least squares intersection of the projected lines. External joint moments were calculated using inverse dynamics and normalized to body weight and height (%BW×Ht). PROs were assessed at the 2-, 4-, and 8-year time points using questionnaires (KOOS, Lysholm) filled out the same day as the gait test. For both questionnaires, a lower score corresponds to worse clinical outcomes.
Figure 1.
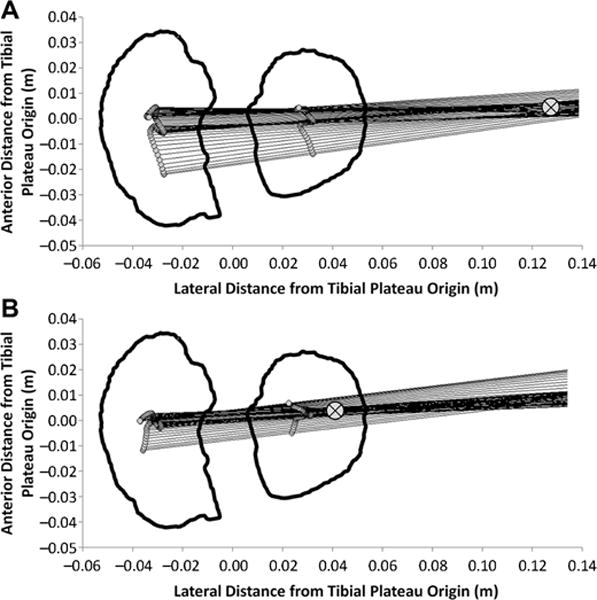
Medial-lateral axis of the femur projected onto the transverse plane of the tibial plateau is shown at each instant during the stance phase of walking for a characteristic participant with a highly lateral KCOR at (A) 2 years after ACLR and (B) the same participant at 4 years after ACLR with a more medial KCOR. ⊗ indicates the location of KCOR, which was calculated as the least squares intersection of the projected lines. Video versions of Figure 1A and 1B showing the time history throughout the stance phase of the medial-lateral axis of the femur can be found in the Video Supplement. ACLR, anterior cruciate ligament reconstruction; KCOR, knee center of rotation.
Cross-sectional differences between the ACL-reconstructed and contralateral knees and longitudinal changes in ML and AP coordinates of KCOR were compared using paired t tests and considered significant with P < .05. For this analysis, no correction for multiple comparisons was performed. Correlations between changes in KCOR between 2 and 4 years and changes in PROs between time points were tested using Pearson correlation coefficients and considered significant with P < .05. Matlab (version R2014b; The MathWorks) was used for all data processing and making the Video Supplement, and IBM SPSS Statistics (version 20; IBM Corp) was used for all statistical testing.
RESULTS
In both the ACL-reconstructed and contralateral knees, the average KCOR was on the lateral side of the knee for all time points (Figure 2), agreeing with previous work.17 There were significant differences in KCOR of the ACL-reconstructed knee both relative to the contralateral knee and over time. KCOR of the ACL-reconstructed knee was more lateral than that of the contralateral knee at 2 years after ACLR (P = .03) and became less lateral over time (P = .047), exhibiting no difference with the contralateral knee at 4 years (P = .55) (Figures 1 and 2 and Video Supplement). KCOR was also significantly more anterior in the ACL-reconstructed knee compared with the contralateral knee at both time points (P = .02 at 2 years, P = .015 at 4 years), and there was no significant change in the AP position of KCOR of the ACL-reconstructed knee between 2 and 4 years (P = .35) (Figure 2). Rather, there was a divergence in the direction of change between participants in this cohort, with KCOR in the ACL-reconstructed knee shifting anteriorly in 10 (38%) participants and shifting posteriorly in 16 (62%) participants (Figure 3). Given that the knee flexion moment is associated with net quadriceps muscle contraction, and that force generated by the quadriceps influences knee kinematics,25 a post hoc analysis was performed and showed that the changes in the AP position of KCOR were significantly correlated (P = .01, R2 = 0.23) with changes in the peak knee flexion moment over time (Figure 4). No significant changes in KCOR were observed in the contralateral knee between 2 and 4 years after ACLR.
Figure 2.
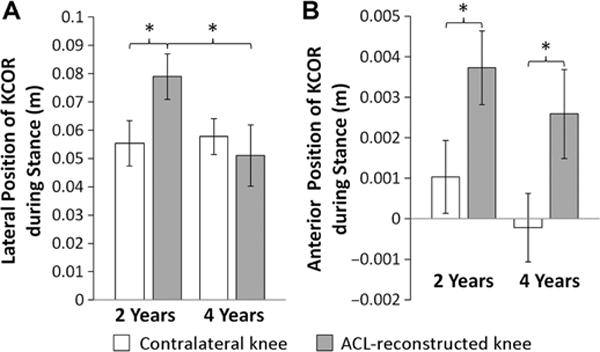
(A) Medial-lateral and (B) anterior-posterior position of KCOR of the contralateral and reconstructed knees at 2 years and 4 years after anterior cruciate ligament (ACL) reconstruction. Lateral and anterior directions are reported as positive values. *Statistically significant differences (P < .05) for cross-sectional and/or longitudinal comparisons. Data reported as mean ± SEM. KCOR, knee center of rotation.
Figure 3.
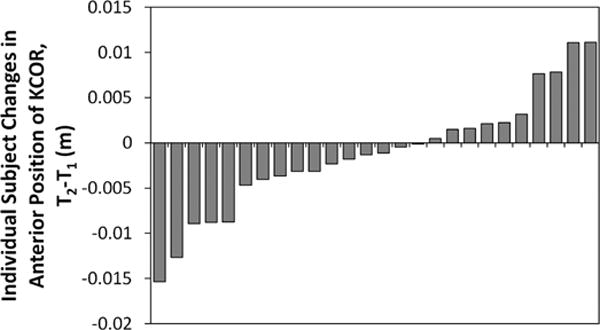
Distribution of individual participant changes in the anterior-posterior position of KCOR during the stance phase of walking from 2 years (T1) to 4 years (T2) after ACLR. Ten (38%) participants shifted anteriorly, and 16 (62%) shifted posteriorly. An anterior shift in KCOR over time is reported as a positive value. ACLR, anterior cruciate ligament reconstruction; KCOR, knee center of rotation.
Figure 4.
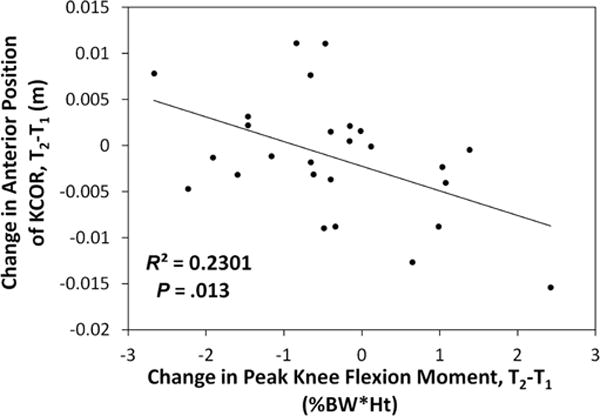
Correlation between changes in the anterior-posterior position of KCOR and changes in peak knee flexion moment from 2 years (T1) to 4 years (T2) after ACLR. An anterior shift in KCOR over time and an increased peak knee flexion moment correspond to positive changes for each. ACLR, anterior cruciate ligament reconstruction; KCOR, knee center of rotation.
Increasing anterior position of KCOR in the ACL-reconstructed knee from 2 to 4 years correlated with worsening clinical outcomes over time. Specifically, an increasingly anterior KCOR between 2 and 4 years after ACLR correlated with worsening clinical outcomes from 2 to 4 years as assessed by the KOOS subscale score for knee-related quality of life (KOOS-QOL; P = .035, R2 = 0.172) (Figure 5) in the full cohort of 26 patients who returned at 4 years. In addition, increasingly anterior KCOR between 2 and 4 years after ACLR correlated with worsening clinical outcomes as assessed by the Lysholm score (P = .018, R2 = 0.41) as well as the KOOS subscales for pain (KOOS-Pain; P = .035, R2 = 0.37), symptoms (KOOS-Symptoms; P = .004, R2 = 0.58) (Figure 6), and quality of life (KOOS-QOL; P = .01, R2 = 0.50). No significant correlations were observed between changes in anterior position from 2 to 4 years and changes in PROs from 2 to 4 years in the sub-cohort of 13.
Figure 5.
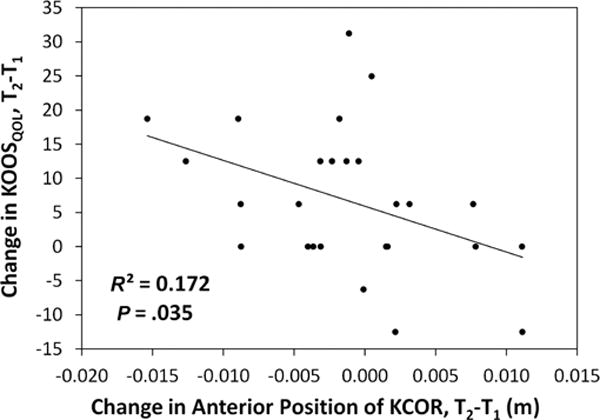
Correlation between changes in the KOOS quality of life subscore from 2 years (T1) to 4 years (T2) after ACLR and changes in the anterior-posterior position of KCOR from 2 years (T1) to 4 years (T2) after ACLR. An anterior shift in KCOR over time is shown as positive, and a worsening outcome in the KOOS quality of life subscore at 4 years is shown as negative. ACLR, anterior cruciate ligament reconstruction; KCOR, knee center of rotation; KOOS, Knee injury and Osteoarthritis Outcome Score.
Figure 6.
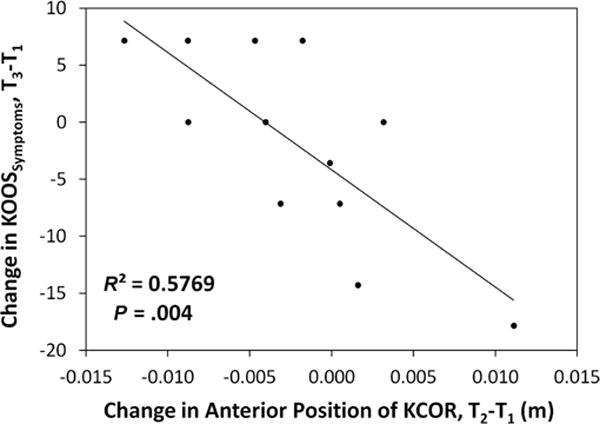
Correlation between changes in the KOOS symptoms subscore from 2 years (T1) to 8 years (T3) after ACLR and changes in the anterior-posterior position of KCOR from 2 years (T1) to 4 years (T2) after ACLR. An anterior shift in KCOR over time is shown as positive, and a worsening outcome in KOOS symptoms subscore at 8 years is shown as negative. ACLR, anterior cruciate ligament reconstruction; KCOR, knee center of rotation; KOOS, Knee injury and Osteoarthritis Outcome Score.
When considering the change in the ML position of KCOR from 2 to 4 years, an increasing lateral position in the ACL-reconstructed knee from 2 to 4 years correlated with worsening clinical outcomes from 2 to 4 years as assessed by the KOOS-QOL (P = .039, R2 = 0.166) in the cohort of 26 that returned at 4 years and the Lysholm score (P = .026, R2 = 0.375) in the subcohort of 13. No significant correlations were observed between ML changes in KCOR from 2 to 4 years and PROs at 8 years. Additionally, no significant correlations were observed between KCOR and KOOS subscale scores for function in sport and recreation or function in activities of daily living. Mean PRO scores for each time point are shown for the full cohort of 26 and the subcohort that returned at 8 years in Table 1.
TABLE 1.
Mean KOOS and Lysholm Patient-Reported Outcome Scores Over Timea
| KOOS Subscale
|
||||||
|---|---|---|---|---|---|---|
| Time Point | Pain | Symptoms | ADL | Sport/Rec | QOL | Lysholm |
| T1, 2 y (n = 26) | 96.1 ± 5.8 | 90.9 ± 10.5 | 98.0 ± 5.2 | 91.5 ± 10.5 | 79.3 ± 19.6 | 93.5 ± 5.7 |
| T2, 4 y (n = 26) | 95.9 ± 8.7 | 90.7 ± 14.9 | 98.6 ± 5.5 | 91.3 ± 19.3 | 86.1 ± 20.1 | 94.8 ± 6.0 |
| T3, 8 y (n = 13)b | 95.6 ± 5.7 | 91.4 ± 10.2 | 98.5 ± 3.5 | 93.8 ± 9.8 | 83.9 ± 14.5 | 93.2 ± 9.4 |
Data are reported as mean ± SD. ADL, activities of daily living; KOOS, Knee injury and Osteoarthritis Outcome Score; QOL, knee-related quality of life; Sport/Rec, sport and recreation.
n = 12 for KOOS at T3 (because of collection error, KOOS results were not collected for 1 of 13 participants at this time point).
DISCUSSION
The results of this study provide new insight into early kinematic changes over time after ACLR and how these changes may influence longer term clinical outcomes after surgery. The average KCOR was shown in this study to be sensitive to kinematic changes after ACLR that appear to predict subsequent PROs. The more lateral KCOR in the ACL-reconstructed knee at 2 years means that the reconstructed knee pivots about a more lateral center of rotation and experiences greater relative motion between the femur and tibia in the medial compartment relative to the lateral compartment than does the contralateral uninjured knee (Figures 1 and 2 and Video Supplement).6,17 Although it does not directly measure medial compartment motion between the femur and tibia, this finding does suggest that there are changes in the location and range of cartilage contact locations occurring during the stance phase of gait, especially in the medial compartment after ACLR. Such kinematic changes have been previously suggested to initiate degeneration in cartilage by shifting the location of the repetitive joint contact loads occurring during walking to cartilage not conditioned for these loads.3,4,23 When combined with an anterior shift in KCOR that was also observed at 2 years after surgery, this may help explain why PTOA typically initiates in the medial compartment of the knee after ACLR,7 irrespective of the damage commonly occurring to the lateral compartment at the time of ACL injury.21
When considering longitudinal changes in KCOR, the finding that it becomes less lateral over time between 2 and 4 years after ACLR to match values similar to the contralateral knee (Figures 1 and 2 and Video Supplement) suggests the normalization of motion across the medial compartment toward bilateral symmetry, which may reflect continued maturation of the ACL graft and/or neuromuscular recovery over this time period. The finding that this observed change toward a less lateral KCOR correlated with better clinical outcomes from 2 to 4 years as assessed by the KOOS-QOL in the cohort of 26 that returned at 4 years and by the Lysholm score in the subcohort of 13 that returned at 8 years suggests a positive effect of this return to symmetry. Indeed, this change in the ML position of KCOR between 2 and 4 years was not correlated with better clinical outcomes at 8 years, supporting the notion that kinematic improvement occurring during the earlier time frame improved knee function.
In contrast, the observed divergence (Figure 3) in the direction of change from 2 to 4 years in the AP position of KCOR and its correlation with the change in peak knee flexion moment from 2 to 4 years (Figure 4) suggests a potential for differential outcomes over time. A higher knee flexion moment corresponds to increased quadriceps contraction, pulling the tibia more anterior relative to the femur25 and shifting the calculated KCOR posteriorly and to a location more comparable with the contralateral uninjured knee. The observed changes to KCOR from 2 to 4 years in the ACL-reconstructed knee suggest that graft maturation and muscle adaptation toward improved kinematic symmetry continued over time in most but not all patients, providing a potential explanation as to why approximately half of patients develop PTOA after ACLR.18,19,26 It is also possible that persistent passive anterior subluxation of the tibia1 may influence changes in the AP position of KCOR over time. However, the correlation seen with peak knee flexion moment suggests that the AP position of KCOR is likely more influenced by changes in quadriceps function over time during nonpassive activities such as walking.
The divergence in the change to the AP position of KCOR within the cohort between 2 and 4 years after ACLR suggests that this may be a mechanical marker of change in knee function over time. The finding that increasing anterior position of KCOR between 2 and 4 years after ACLR correlated with worsening PROs at 4 years (Figure 5) and again more strongly and in more metrics for the subcohort at 8 years (Figure 6) further supports the hypothesis that early changes in KCOR predict longer term changes in clinical outcomes. While this analysis was strengthened by analyzing differences within participants both over time and relative to the contralateral knee, reducing variations that would exist with many of the variables considered in a between-participant analysis, this study evaluated a relatively small cohort of participants after ACLR, of which only half returned for the 8-year follow-up. While we found significant associations between gait metrics and long-term patient outcomes for this small subgroup, studies with larger cohorts that are powered to control for other covariates such as age, sex, and BMI are needed to determine the predictive value of this metric.
The earliest stages of joint degeneration and PTOA development are typically clinically silent.11 In our population of human participants with well-functioning ACL-reconstructed knees just 8 years after surgery, we observed that several participants exhibited changes greater than what is considered to be a minimal clinically important difference (MCID) of 8 points from 2 to 8 years for the KOOS22 (2/13 participants for KOOS-Symptoms [Figure 6], 2 for KOOS-Pain, and 4 for KOOS-QOL). The MCID is designed to determine if a change is clinically significant within a single patient. The observation that several participants in this select group of patients already show the MCID in several KOOS subscales just 6 years later further highlights the potential clinical utility of KCOR in providing an early warning of deteriorating clinical outcomes over time.
Average KCOR during walking, as defined in this study, is a unique metric that provides new information with high potential clinical relevance in the early assessment of patients sustaining ACL injuries. The data from this study show that continued improvement to knee kinematics did not occur in some participants between 2 and 4 years after ACLR, suggesting a need for a clinical follow-up longer than 1 year after surgery. The finding that worsening KCOR from 2 to 4 years correlated to poorer clinical outcomes at 8 years supports the hypotheses that KCOR, as well as the change in KCOR over time after ACLR, may be a useful metric for assessing knee kinematics and providing an early warning of deteriorating knee function. The results of this work support progressing to more comprehensive studies of larger ACL-reconstructed cohorts to determine whether KCOR and the change in KCOR over time predict not only PROs but also the PTOA risk as measured by advanced quantitative imaging and radiographs.
Supplementary Material
Acknowledgments
The authors thank Sean F. Scanlan and Michael E. Zabala for data collection at the 2- and 4-year time points.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding was received from the National Institutes of Health (grants R01 AR052784 to C.R.C. and AR039421 to T.P.A.) and the Department of Orthopaedic Surgery, Stanford University Medical Center.
Footnotes
Presented at the 42nd annual meeting of the AOSSM, Colorado Springs, Colorado, July 2016.
A Video Supplement for this article is available in the online version.
References
- 1.Almekinders LC, de Castro D. Fixed tibial subluxation after successful anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29(3):280–283. doi: 10.1177/03635465010290030301. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Alexander EJ, Toney MK, Dyrby C, Sum J. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. J Biomech Eng. 1998;120(6):743–749. doi: 10.1115/1.2834888. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Favre J, Erhart-Hledik JC, Chu CR. A systems view of risk factors for knee osteoarthritis reveals insights into the pathogenesis of the disease. Ann Biomed Eng. 2014;43(2):376–387. doi: 10.1007/s10439-014-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 6.Banks SA, Hodge WA. 2003 Hap Paul Award paper of the International Society for Technology in Arthroplasty: design and activity dependence of kinematics in fixed and mobile-bearing knee arthroplasties. J Arthroplasty. 2004;19(7):809–816. doi: 10.1016/j.arth.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42(5):1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 8.Bevill SL, Boyer KA, Andriacchi TP. The regional sensitivity of chondrocyte gene expression to coactive mechanical load and exogenous TNF-α stimuli. J Biomech Eng. 2014;136(9):091005. doi: 10.1115/1.4027937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevill SL, Briant PL, Levenston ME, Andriacchi TP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage. 2009;17(8):980–987. doi: 10.1016/j.joca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhari AMW, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 11.Chu CR, Beynnon BD, Buckwalter JA, et al. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39(7):1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non-weight bearing activities. J Orthop Res. 2004;22(4):794–800. doi: 10.1016/j.orthres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Favre J, Erhart-Hledik JC, Andriacchi TP. Age-related differences in sagittal-plane knee function at heel-strike of walking are increased in osteoarthritic patients. Osteoarthritis Cartilage. 2014;22(3):464–471. doi: 10.1016/j.joca.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–627. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50(10):597–612. doi: 10.1136/bjsports-2015-094797. [DOI] [PubMed] [Google Scholar]
- 16.Kalawadia J, Thorhauer E, Arilla FV, et al. Knee kinematics are related to patient-reported outcomes 6 months after anatomic ACL reconstruction. Orthop J Sport Med. 2015;3(2 Suppl) 2325967115S00031. [Google Scholar]
- 17.Koo S, Andriacchi TP. The knee joint center of rotation is predominantly on the lateral side during normal walking. J Biomech. 2008;41(6):1269–1273. doi: 10.1016/j.jbiomech.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 19.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 20.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 21.Mink JH, Deutsch AL. Occult cartilage and bone injuries of the knee: detection, classification, and assessment with MR imaging. Radiology. 1989;170(3 Pt 1):823–829. doi: 10.1148/radiology.170.3.2916038. [DOI] [PubMed] [Google Scholar]
- 22.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 23.Scanlan SF, Chaudhari AMW, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scanlan SF, Favre J, Andriacchi TP. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J Biomech. 2013;46(5):849–854. doi: 10.1016/j.jbiomech.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Shin CS, Chaudhari AM, Dyrby CO, Andriacchi TP. The patella ligament insertion angle influences quadriceps usage during walking of anterior cruciate ligament deficient patients. J Orthop Res. 2007;25(12):1643–1650. doi: 10.1002/jor.20463. [DOI] [PubMed] [Google Scholar]
- 26.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabala M, Favre J, Andriacchi TP. Prospective spatial patterns of tibial cartilage thickness changes following ACL reconstruction. Osteoarthritis Cartilage. 2013;21:S219. [Google Scholar]
- 28.Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013;46(3):515–520. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zampeli F, Pappas E, Giotis D, Hantes ME, Georgoulis AD. Kinematic predictors of subjective outcome after anterior cruciate ligament reconstruction: an in vivo motion analysis study. Knee Surg Sport Traumatol Arthrosc. 2012;20(4):785–792. doi: 10.1007/s00167-012-1902-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


