Abstract
Coordinated electrical activity allows pancreatic β-cells to respond to secretagogues with calcium entry followed by insulin secretion. Metabolism of glucose affects multiple membrane proteins including ion channels, transporters and pumps that collaborate in a cascade of electrical activity resulting in insulin release. Glucose induces β-cell depolarization resulting in the firing of action potentials (APs), which are the primary electrical signal of the β-cell. They are shaped by orchestrated activation of ion channels. Here we give an overview of the voltage-gated potassium (Kv) channels of the β-cell, which are responsible in part for the falling phase of the AP, and how their regulation affects insulin secretion. β cells contain several Kv channels allowing dynamic integration of multiple signals on repolarization of glucose-stimulated APs. Recent studies on Kv channel regulation by cAMP and arachidonic acid and on the Kv2.1 null mouse have greatly increased our understanding of β-cell excitation–secretion coupling.
Keywords: delayed rectifier, electrical activity, hannatoxin, islet, Kv2.1, potassium channel, stromatoxin
Introduction
Glucose-induced electrical activity of the pancreatic β-cell is organized into slow depolarizing waves with a plateau from which action potentials (APs) rapidly fire. They are separated by quiescent periods at potentials below the AP threshold (figure 1). The β-cell AP is generated primarily through cation flux through ion channels modulated by glucose metabolism [1–4]. The produced ATP binds to the inward rectifier potassium channel complex (KATP) reducing its conductance of potassium as a reflection of the ATP/ADP ratio, thus causing an increase in the β-cell membrane potential [5,6]. The resulting depolarization activates voltage-gated channels that lead to the rising phase of the AP, including the L-type calcium channel and sodium channel(s). Continued depolarization also activates voltage-gated potassium channels (Kv), in particular Kv2.1, regulating membrane repolarization, with effects on AP width and frequency. Calcium entry additionally regulates calcium-activated potassium channels (Kca) that can also affect AP repolarization and the duration of the slow wave. The timing of AP firing is also regulated by the influence and extent of repolarizing vs. depolarizing ion flux around the activation threshold for voltage-gated channels. Although simple in shape, the AP is generated by a complex set of coordinated ion movements through several ion channels.
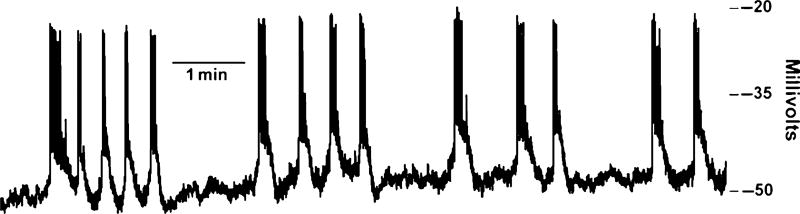
Fig. 1.
Electrical activity of a mouse islet treated with 14 mM glucose, exhibiting typical depolarizing waves topped with rapidly firing action potentials.
Initiation of the AP: KATP, Ca2+ and Na+ Channels
The resting membrane potential of the pancreatic β-cell is set by the activity of KATP [5,6]. KATP is an inward rectifier K+ channel (discussed in detail elsewhere in this volume) made of a hetero-octomer of the sulphonylurea receptor 1 (SUR1, ABCC8) and Kir6.2 (KCNJ11) membrane proteins [5,6]. This channel sets the membrane potential of the β-cell near −70 mV, determined by the equilibrium potential of K+ (EK). This is because of the activity of KATP allowing inward K+ flow when the cell is hyperpolarized and outward flow when the cell is slightly positive of EK. KATP is also regulated by polyamines and Mg2+ binding to the channel [7]. The KATP channel is sensitive to ATP and when the ratio of ATP/ADP increases, as following augmented glucose metabolism, flux through the channel is inhibited [5,6]. ATP inhibition of KATP allows the β-cell to reach the AP threshold by reducing the outward K+ flow and resulting in an intracellular accumulation of cations. Mutations in the genes encoding SUR1 (ABCC8) and Kir6.2 (KCNJ11) are rare but important causes of both persistent hyperinsulinaemia of infancy and neonatal diabetes, and polymorphisms in KCNJ11 have been associated with type 2 diabetes and gestational diabetes. While KATP channels are expressed in the brain, muscle and elsewhere, it seems that only in the β-cell do these channels function in just this way, with the possible exception of glucose-sensing neurons. More severe mutations do indeed have muscle and neurological features and this has been termed the developmental delay, epilepsy and neonatal diabetes (DEND) syndrome [8].
As KATP activity is reduced following an increase in the ATP/ADP ratio, the β-cell is slowly depolarized and reaches an activation threshold for voltage-dependent calcium channels (VDCC) [9]. Once activated, the VDCC of the β-cell, primarily the L-type calcium channels Cav1.2 and/or Cav1.3 (depending on species), allows inward Ca2+ flux down its electrochemical activity gradient [9–17]. Pharmacological inhibition of VDCC eliminates insulin secretion, so clearly they play an essential role in islet electrical activity and insulin secretion [9]. When Ca2+ entry is blocked with nifedipine or cobalt, the AP fails to fire even while the threshold for firing is met by glucose-induced KATP inhibition [18,19]. Following activation with glucose, Ca2+ influx shows an oscillatory pattern that is significantly slower than the duration of an individual AP with a time course that has been linked to the oscillatory pattern of islet insulin secretion [13–16]. Intracellular calcium [Ca2+]i changes within the β-cell do not alone account for the APs, which in some species is also clearly connected to Na+ influx. VDCC activity regulates Ca2+-activated K+ channels, which can influence AP repolarization and firing pattern [17].
The amount of Ca2+ entry also affects the duration of glucose-induced slow waves. When external Ca2+ is reduced to 1 mM, the islet slow waves are extended with more APs and reduced repolarization during quiescent periods [18]. However, when the extracellular Ca2+ is elevated to supra-physiological levels (5–10 mM), islets have shorter slow waves with fewer APs per wave and longer quiescent periods with greater repolarization between each slow wave [18,20]. These changes directly correlate with the amount of Ca2+ entry into the β-cell during glucose-induced depolarization, and thus the Ca2+ levels of the β-cell can determine many of the responses to glucose downstream of electrical activity [21–24].
Extracellular Na+ is also required for normal insulin secretion from both human and rodent islets [25–30]. Rodent β-cell APs fire in the absence of extracellular Ca2+ during glucose-induced depolarization; however, when Na+ is also removed with Ca2+ the APs fail to fire, implicating an important role for Na+ influx during the AP [30,31]. Human and canine β-cells on the other hand require external Na+ regardless of extracellular Ca2+ for a normal pattern of glucose-induced APs [29]. Two voltage-gated Na+ currents have been identified in rodent β-cells based on their differences in biophysical activation and inactivation [10,32]. Both are inhibited with a toxin specific for voltage-gated Na+ channels, tetrodotoxin (TTX, from Fugu rubripes). Interestingly, islet electrical activity is most affected by TTX under low glucose conditions (5–6 mM glucose), whereas TTX has minimal effect on islet electrical activity above 10 mM [32]. As normal blood glucose rarely climbs above 10 mM, activation of voltage-gated Na+ channels during glucose-induced depolarization will influence the upstroke of the resulting AP. Indeed, TTX treatment of β-cells undergoing glucose-induced (at 5 mM) APs causes a reduction in AP amplitude and broadens its width [32]. Although TTX-sensitive voltage-gated Na+ currents have been identified in the β-cell, other Na+ channels such as NaV1.7 and NaV1.3 may also combine to modulate glucose-induced sodium influx and resulting cellular excitability [10,33]. It remains uncertain which β-cell Na+ channels are functionally important, but it does seem that their contribution is species-specific and has greater importance in human than rodent β-cells.
Potassium Channels that Regulate Repolarization of the AP
Calcium-activated Potassium Channels
The three main groups of calcium-activated K+ (Kca) channels of the β-cell are large conductance (BK) channels, small and intermediate conductance (SK and IK) channels and a slowly activated K+ conductance termed Kslow [34–36]. The kinetics of the β-cell Kca currents allow for roles in both AP repolarization and slow wave duration. Although the molecular identity of Kslow is undefined, both SK and BK channels are expressed in β-cells. BK channel transcripts with multiple splice variants are highly expressed in the islet [37]. SK channels are also expressed in the rodent β-cell [36]. SK1, -2, -3 and -4/IK1 cDNAs have been identified in rodent islets and insulinoma cells by RT-PCR [36]. SK2 and SK3 protein expression is also found in mouse islets [36]. The activity of both SK and BK channels in various tissues modulate AP firing and shape and thus these channels may also affect glucose-induced APs of the β-cell.
The contribution of Kca channels to individual glucose-induced AP remains incompletely understood. Inhibitors of SK4/IK1 and BK channels including charybdotoxin, iberiotoxin and tetraethylammonium (TEA) have been used to decipher the roles of these two channels [38,39]. Glucose-induced APs are not affected by charybdotoxin or low concentrations of TEA [38–40]. However, when iberiotoxin is used to block BK channels when Kv2.1 is also blocked, there is a significant increase in AP amplitude [41]. The AP duration and the quiescent period between APs are also increased during combined blockade of Kv2.1 and BK channels during glucose stimulation [41]. This indicates that there may be multiple repolarizing K+ channels activated following the AP upstroke and depolarization including BK.
Small conductance Kca channels of the pancreatic β-cell may also influence islet insulin secretion. Apamin, a specific inhibitor of cloned SK1–3 channels, has no significant effect on glucose-induced electrical activity of rat islets [42]. However, glucose-induced Ca2+ fluctuations in mouse islets are increased in amplitude and frequency with apamin [36]. This effect was modulated in part through the SK3 channel. This was shown by knockdown of this subunit in a transgenic mouse model in which SK3 regulation with doxycycline was possible by introduction of the tet response element. Lowering SK3 expression reduced the Ca2+ changes induced by apamin [36]. Thus, SK3 channels play a detectable but modest role on islet Ca2+ fluctuations as long as other Kv channels are functional.
Calcium also regulates the duration of the slow wave of depolarization, from which APs fire, in part through its regulation of the Kca channel Kslow. Although the molecular identity of this channel remains elusive, K+ efflux from the cell has been extensively studied during the termination of the slow wave. A transient increase in K+ permeability has been shown to precede the termination of the slow wave [43,44]. The current responsible for the termination of the slow wave has been studied in intact islets [35]. The current induced, termed Kslow, is K+ permeable and inactivates over a time course similar to the quiescent period between glucose-activated slow waves [35]. The amount of Kslow current closely follows the Ca2+ levels and its inactivation follows a time course similar to the termination of the Ca2+ wave at the end of the slow wave [35]. Inhibition of Ca2+ influx eliminates the Kslow current and removal of extracellular Ca2+ induces a continued slow wave of depolarization [17,30,31,35,45].
The molecular identity of the Kslow current remains obscure. The current is inhibited up to 70% by high concentrations of the non-specific K+ channel inhibitor TEA [35]. Kslow from primary β-cells is insensitive to charybdotoxin and apamin; thus, the current is not composed of BK or SK subunits except that it is inhibited by UCL 1684, a non-peptide SK channel blocker. This indicates that the current could still be in the SK channel family [35,46]. UCL 1684 significantly inhibits SK channels as well as Kslow currents in β-cells, suggesting that the molecular identity of the Kslow current may be a heteromultimer.
Voltage-gated Potassium Channels
One of the primary functions of voltage-gated K+ channels is repolarization of the AP. They are encoded by the largest family of ion channel genes. Of the eleven families of Kv-related α-subunit genes so far reported, at least three families encode channel isoforms with currents superficially similar to those found in β-cells (table 1) [47,48]. These were originally named according to homology to three Drosophila genes: Shaker (Kv1.x), Shab (Kv2.x) and Shaw (Kv3.x). Members of Kv1, -2 and -3 give rise to delayed rectifier-type currents (figure 2). A fourth family, Shal (Kv4.x) comprises only rapidly activating and inactivating channels (similar to Kv1.4) and therefore Kv4.1–3 are thought unlikely to be expressed in β-cells (figure 2) [49]. K+ channel families five to eleven include some (Kv6–9) that do not express functional channels in mammalian cells on their own. Some subunits also form heteromultimers, generating additional current types with combinatorial properties [49,50]. The ability of isoforms to heteromultimerize is usually restricted and family-specific. For example, Kv1.x can combine with other Kv1.x but not with Kv2.x [49]. Some examples of ‘promiscuous coupling’ of Kv isoforms have been reported, such as between Kv5.x–Kv9.x (inactive as homomultimers) and Kv2.1 [51,52]. Kv5.x, Kv6.x, Kv8.x and Kv9.x may actually be subunits that are primarily involved in modulating Kv2.x expression or function. The heteromultimers produce channels that attenuate the amplitude of Kv2.x currents. Each modulatory subunit has its own specific properties of regulation of the functional Kv2 subunits, and they can lead to extensive inhibitions, to large changes in kinetics and/or to large shifts in the voltage dependencies of the inactivation process.
Table 1.
Expression of voltage-gated potassium (Kv) channels in pancreatic islets and expression of modulatory/silent Kv subunits in islets
| Gene | Alias | Expression | Detection | Reference |
|---|---|---|---|---|
| KCNA1 | Kv1.1 | None | PCR, IB | [59] |
| KCNA2 | Kv1.2 | None | PCR, IB | [59] |
| KCNA3 | Kv1.3 | None | PCR, IB | [59] |
| KCNA4 | Kv1.4 | β cell | PCR, IB | [59] |
| KCNA5 | Kv1.5 | β cell | PCR | [56,70] |
| KCNA6 | Kv1.6 | β cell | PCR, IB | [59] |
| KCNA7 | Kv1.7 | Islet | PCR | [48] |
| KCNA10 | Kv1.10 | ND | ||
| KCNB1 | Kv2.1 | β cell | PCR, IB, IHC | [48,59,62] |
| KCNB2 | Kv2.2 | Delta cell | PCR, IHC | [48] |
| KCNC1 | Kv3.1 | Alpha cell | PCR, ISH | [48] |
| KCNC2 | Kv3.2 | β cell | PCR, ISH, IHC | [48,58] |
| KCNC3 | Kv3.3 | None | PCR | [48] |
| KCNC4 | Kv3.4 | Delta cell | PCR, IHC | [48,71] |
| KCND1 | Kv4.1 | Pancreas | PCR | [48] |
| KCND2 | Kv4.2 | Islet | PCR, IB | [64] |
| KCND3 | Kv4.3 | Alpha cell | PCR, IHC | [71] |
| KCNH1 | Kv10.1 | Islet | PCR | [48] |
| KCNH2 | Kv11.1 | Islet | PCR | [48] |
| KCNF1 | Kv5.1 | None | PCR | [48] |
| KCNG1 | Kv6.1 | Alpha cell | PCR, ISH | [48] |
| KCNG2 | Kv6.2 | β cell | PCR, ISH | [48] |
| KCNG3 | Kv6.3 | None | PCR | [48] |
| KCNG4 | Kv6.4 | ND | ||
| KCNS1 | Kv9.1 | ND | ||
| KCNS2 | Kv9.2 | Islet | PCR, ISH | [48] |
| KCNS3 | Kv9.3 | β cell | PCR, ISH | [48] |
IB, immunoblot; IHC, immunohistochemistry; ISH, in situ hybridization; ND, not determined.
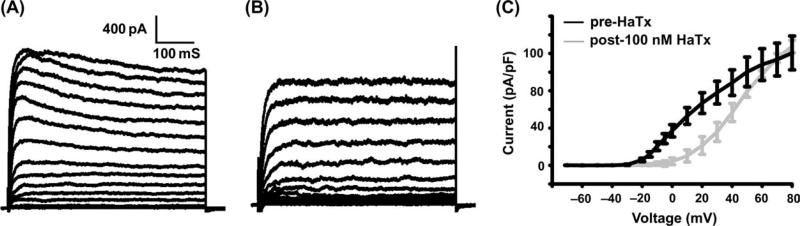
Fig. 2.
Hannatoxin significantly reduces β-cell voltage-gated potassium (Kv)-like currents. (A) Control β-cell Kv-like currents recorded in voltage clamp with voltage steps from −80 to +80 in 10 mV increments. (B) Control β-cell Kv currents 10 min following addition of 100 nM hannatoxin (HaTx). (C) Current vs. voltage plots for β-cells recorded as in A (black bar) and B (grey bar) ± s.e.m.’s (n > 7 for each condition).
The β-cell-delayed rectifier current activates slowly and inactivates slowly (figure 2). Rapidly inactivating Kv currents are not usually seen in primary β-cells (figure 2, upper panel). The Kv current is sensitive to TEA in the 5–7 mM range. Several investigators have made an effort to define the K+ channel gene or genes encoding this current. A summary of all the K+ channel genes known and their expression in β-cells is shown in table 1. Seven different isoforms of the Kv1.x gene family were initially identified in insulinoma cell lines and whole islets using the RT-PCR method [53]. These channels were identified by their sequence similarity to the rat brain K+ channels [54,55]. Kv1.5 (also termed hPCN1, KCNA5) was found to be highly expressed in human insulinomas [56]. Insulinoma cells overexpressing this channel had an ablated glucose-induced Ca2+ response, and transgenic mice overexpressing Kv1.5 specifically in β-cells exhibited reduced glucose tolerance [56]. Inhibiting Kv1 channels with a dominant-negative construct has little to no effect on β-cell repolarization [56]. Kv3.2 channel RNA and protein has also been identified in β-cells; however, their TEA-sensitive currents do not reflect the low sensitivity of the β-cell Kv currents to TEA. Kv2.1 channels are the primary β-cell delayed rectifier current [47,57,58], indicated by the voltage-gated kinetics of the current, as well as by RT-PCR, Western blot and immunohistochemical confirmation of its expression specifically in the endocrine β-cell [58–60]. Accordingly, most of the voltage-gated K+ current is eliminated in rat β-cells expressing a Kv2 dominant-negative construct [59].
Spider toxins that inhibit Kv2 channels also inhibit a majority of the Kv current of rodent and human β-cells [61–63]; these include the spider peptides stromatoxin, hannatoxin, guangxitoxin and SGTx1. These toxins are termed cystine knot toxins because of their disulphide bond-constrained structure; they bind to the S3–S4 extracellular loop of Kv2 channels, reducing channel activity by restricting the movement of the transmembrane domain S4 [61–63]. The toxins also inhibit Kv2.2 and Kv4 channels. As these channels are expressed in non-β-cells of the islet, their inhibition could also affect islet electrical activity and/or hormone secretion together with the inhibition of Kv2.1.
Inhibition of Kv channels with TEA or stromatoxin during trains of glucose-induced APs results in a significant increase in amplitude and duration of the AP with a slowing of the firing frequency (figures 3 and 4) [63]. Islets from a mouse model with an ablated Kv2.1 gene also show increased glucose-induced AP duration and reduced firing frequency compared with controls (unpublished observation). This change in electrical activity is reflected in the Ca2+ fluctuations of the islet, which usually show increased rates in response to inhibition of Kv2.1 during glucose stimulation [62,63]. Similarly, glucose-stimulated insulin secretion is also increased when Kv2.1 channels are inhibited [41,63]. Kv2.1 would thus be a therapeutic target if it could be specifically blocked in the islet [41,47,63], allowing increased glucose-induced insulin secretion in patients with type 2 diabetes. However, the Kv2.1 channel is expressed in many other tissues besides the pancreatic β-cell, including the brain, and thus blocking Kv2.1 specifically in the β-cell is a difficult task.
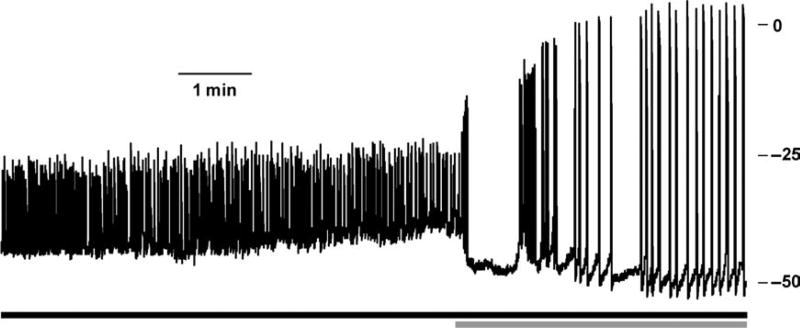
Fig. 3.
Islets respond to tetraethylammonium (TEA) with increased glucose-induced action potential (AP) amplitude and duration. C57 mouse islet glucose-induced (14 mM, black bar) APs treated with 15 mM TEA (grey bar).
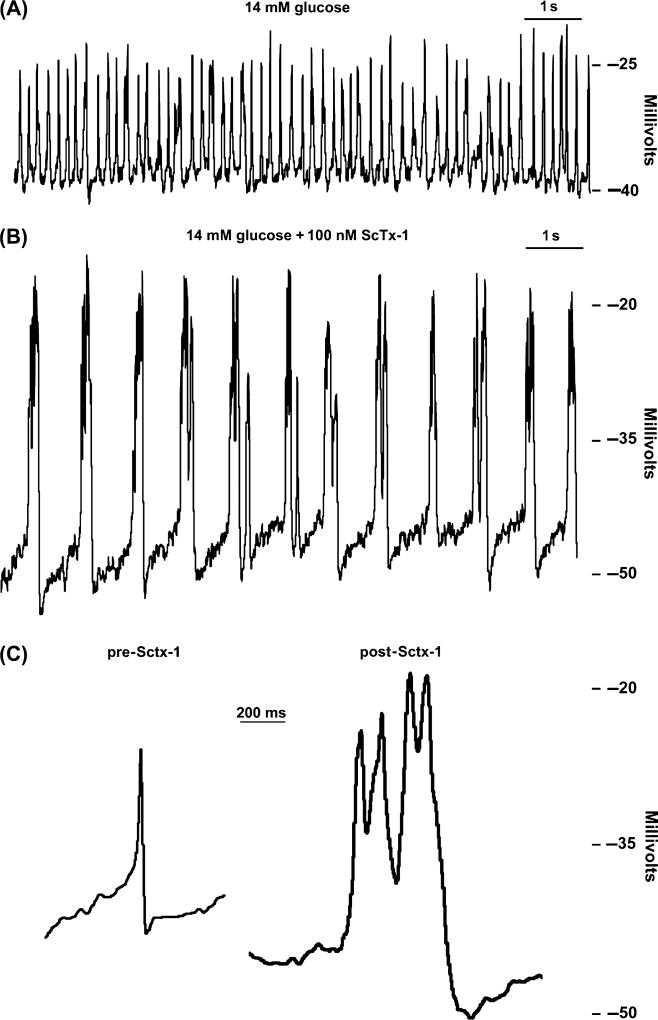
Fig. 4.
Stromatoxin (ScTx-1) significantly affects β-cell action potentials (APs). (A) Islet APs recorded in whole cell current clamp mode from an intact β-cell treated with 14 mM glucose. (B) APs from the same islet in panel (A) 2 min post-treatment with 100 nM ScTx-1. (C) Single APs recorded in 14 mM glucose alone (pre-ScTx-1) or with 14 mM glucose and 100 nM ScTx-1 (post-ScTx-1).
Interestingly, the incretin glucagon-like peptide 1 and related agonists have been shown to affect Kv2.1 activity. This peptide activates a G-protein-coupled receptor expressed on the β-cell, and the ensuing kinase cascade has been shown to reduce Kv2.1 currents [64]. Neuronal Kv2.1 channel activity in the brain is also dynamically modulated by its phosphorylation status in a Ca2+-dependent manner [65]. Thus, modulation of β-cell function through second messengers may also provide β-cell-specific inhibition of Kv2.1, allowing increased glucose-induced insulin secretion.
Kv2.1 activity is also modulated by the polyunsaturated fatty acids arachidonate and linoleic acid [66–68]. Non-esterified arachidonate and linoleic acid reduce β-cell Kv2.1-like currents and accelerate their inactivation [67,68]. Arachidonate also causes increased Ca2+ fluctuations from glucose-treated islets [68]. Hydrolysis of esterified linoleic acid and arachidonic acid amplifies glucose-induced insulin secretion [69]. Therefore, dynamic modulation of Kv2.1 channel function by fatty acids may play an important role in regulating the electrical responses of β-cells to glucose.
Conclusions
We have reviewed the important Ca2+, Na+ and K+ channels of the β-cell with a focus on new insights on the role of Kv channels. The growing intense focus on the role of the β-cell in all types of diabetes has increased the importance of understanding how electrical activity induced by glucose and other agonists is established and how it is altered in various pathological states. Pharmacologic intervention in hyperinsulinaemic, diabetic and hypoglycaemic states would also be aided by a better set of tools to specifically augment or inhibit β-cell electrical activity.
Acknowledgments
This work was supported by a NIH grant DK48494 to L. H. P. and also supported in part by a postdoctoral fellowship in β-cell research from Takeda Pharmaceuticals North America.
Footnotes
Conflicts of Interest:
All authors declare no conflicts of interest.
References
- 1.Dean PM, Matthews EK. Electrical activity on pancreatic islet cells. Nature. 1968;219:389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- 2.Dean PM, Matthews EK. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970;210:255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean PM, Matthews EK. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970;210:265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 5.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 6.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 7.Shyng S, Ferrigni T, Nichols CG. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. J Gen Physiol. 1997;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proks P, Girard C, Haider S, et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 10.Seino S, Chen L, Seino M, et al. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci U S A. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaney GC, Wheeler MB, Wei X, Perez-Reyes E, et al. Cloning of a novel alpha 1-subunit of the voltage-dependent calcium channel from the beta-cell. Mol Endocrinol. 1992;6:2143–2152. doi: 10.1210/mend.6.12.1337146. [DOI] [PubMed] [Google Scholar]
- 12.Vignali S, Leiss V, Karl R, Hofmann F, Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. J Physiol. 2006;572:691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo EA, Tornheim K, Deeney JT, et al. Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated rat pancreatic islets. J Biol Chem. 1991;266:9314–9319. [PubMed] [Google Scholar]
- 14.Barbosa RM, Silva AM, Tome AR, Stamford JA, Santos RM, Rosario LM. Control of pulsatile 5-HT/insulin secretion from single mouse pancreatic islets by intracellular calcium dynamics. J Physiol. 1998;510:135–143. doi: 10.1111/j.1469-7793.1998.135bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen MG, Bertram R, Sherman A. Intra- and inter-islet synchronization of metabolically driven insulin secretion. Biophys J. 2005;89:107–119. doi: 10.1529/biophysj.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunemaker CS, Zhang M, Wasserman DH, et al. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54:3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 17.Dukes ID, Roe MW, Worley JF, III, Philipson LH. Glucose-induced alterations in β-cell cytoplasmic calcium: coupling of intracellular calcium stores and plasma membrane ion channels. Curr Opin Endocrinol Diabetes. 1997;4:262–271. [Google Scholar]
- 18.Meissner HP, Schmeer W. The significance of calcium ions for the glucose-induced electrical activity of the pancreatic β-cells. In: Ohnishi S, Endo M, editors. The Mechanism of Gated Calcium Transport Across Biological Membranes. New York: Academic Press; 1981. pp. 157–165. [Google Scholar]
- 19.Lebrun P, Atwater I. Effects of the calcium channel agonist, BAY K 8644, on electrical activity in mouse pancreatic B-cells. Biophys J. 1985;48:919–930. doi: 10.1016/S0006-3495(85)83855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992;267:20713–20720. [PubMed] [Google Scholar]
- 21.Bokvist K, Eliasson L, Ammala C, Renstrom E, Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barg S, Eliasson L, Renstrom E, Rorsman P. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 2002;51:S74–S82. doi: 10.2337/diabetes.51.2007.s74. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell KJ, Pinton P, Varadi A, et al. Dense core secretory vesicles revealed as a dynamic Ca(2+) store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J Cell Biol. 2001;155:41–51. doi: 10.1083/jcb.200103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulla V, Renstrom E, Feil R, et al. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henquin JC, de Miguel R, Garrino MG, Hermans M, Nenquin M. Nutrient insulin secretagogues decrease 45Ca2+ efflux from islet cells by a mechanism other than the inhibition of the Na+–Ca2+ countertransport. FEBS Lett. 1985;187:177–181. doi: 10.1016/0014-5793(85)81237-0. [DOI] [PubMed] [Google Scholar]
- 26.Hellman B, Idahl LA, Lernmark A, Sehlin J, Taljedal IB. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974;138:33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donatsch P, Lowe DA, Richardson BP, Taylor P. The functional significance of sodium channels in pancreatic beta-cell membranes. J Physiol. 1977;267:357–376. doi: 10.1113/jphysiol.1977.sp011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Miguel R, Tamagawa T, Schmeer W, Nenquin M, Henquin JC. Effects of acute sodium omission on insulin release, ionic flux and membrane potential in mouse pancreatic B-cells. Biochim Biophys Acta. 1988;969:198–207. doi: 10.1016/0167-4889(88)90076-6. [DOI] [PubMed] [Google Scholar]
- 29.Pressel DM, Misler S. Sodium channels contribute to action potential generation in canine and human pancreatic islet B cells. J Membr Biol. 1990;116:273–280. doi: 10.1007/BF01868466. [DOI] [PubMed] [Google Scholar]
- 30.Hattori M, Kai R, Kitasato H. Effects of lowering external Na+ concentration on cytoplasmic pH and Ca2+ concentration in mouse pancreatic beta-cells: mechanism of periodicity of spike-bursts. Jpn J Physiol. 1994;44:283–293. doi: 10.2170/jjphysiol.44.283. [DOI] [PubMed] [Google Scholar]
- 31.Kitasato H, Kai R, Ding WG, Omatsu-Kanbe M. The intrinsic rhythmicity of spike-burst generation in pancreatic beta-cells and intercellular interaction within an islet. Jpn J Physiol. 1996;46:363–373. doi: 10.2170/jjphysiol.46.363. [DOI] [PubMed] [Google Scholar]
- 32.Barnett DW, Pressel DM, Misler S. Voltage-dependent Na+ and Ca2+ currents in human pancreatic islet beta-cells: evidence for roles in the generation of action potentials and insulin secretion. Pflugers Arch. 1995;431:272–282. doi: 10.1007/BF00410201. [DOI] [PubMed] [Google Scholar]
- 33.Philipson LH, Kusnetsov A, Larson T, Zeng Y, Westermark G. Human, rodent, and canine pancreatic beta-cells express a sodium channel alpha 1-subunit related to a fetal brain isoform. Diabetes. 1993;42:1372–1377. doi: 10.2337/diab.42.9.1372. [DOI] [PubMed] [Google Scholar]
- 34.Tabcharani JA, Misler S. Ca2+-activated K+ channel in rat pancreatic islet B cells: permeation, gating and blockade by cations. Biochim Biophys Acta. 1989;982:62–72. doi: 10.1016/0005-2736(89)90174-0. [DOI] [PubMed] [Google Scholar]
- 35.Gopel SO, Kanno T, Barg S, et al. Activation of Ca(2+)- dependent K(+) channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol. 1999;114:759–770. doi: 10.1085/jgp.114.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamarina NA, Wang Y, Mariotto L, et al. Small-conductance calcium-activated K+ channels are expressed in pancreatic islets and regulate glucose responses. Diabetes. 2003;52:2000–2006. doi: 10.2337/diabetes.52.8.2000. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer J, Wasson J, Salkoff L, Permutt MA. Cloning of human pancreatic islet large conductance Ca(2+)-activated K+ channel (hSlo) cDNAs: evidence for high levels of expression in pancreatic islets and identification of a flanking genetic marker. Diabetologia. 1996;39:891–898. doi: 10.1007/BF00403907. [DOI] [PubMed] [Google Scholar]
- 38.Atwater I, Ribalet B, Rojas E. Mouse pancreatic beta-cells: tetraethylammonium blockage of the potassium permeability increase induced by depolarization. J Physiol. 1979;288:561–574. [PMC free article] [PubMed] [Google Scholar]
- 39.Kukuljan M, Goncalves AA, Atwater I. Charybdotoxin-sensitive K(Ca) channel is not involved in glucose-induced electrical activity in pancreatic beta-cells. J Membr Biol. 1991;119:187–195. doi: 10.1007/BF01871418. [DOI] [PubMed] [Google Scholar]
- 40.Henquin JC. Role of voltage- and Ca2(+)-dependent K+ channels in the control of glucose-induced electrical activity in pancreatic B-cells. Pflugers Arch. 1990;416:568–572. doi: 10.1007/BF00382691. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald PE, Sewing S, Wang J, et al. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic beta-cells enhances glucose-dependent insulin secretion. J Biol Chem. 2002;277:44938–44945. doi: 10.1074/jbc.M205532200. [DOI] [PubMed] [Google Scholar]
- 42.Lebrun P, Atwater I, Claret M, Malaisse WJ, Herchuelz A. Resistance to apamin of the Ca2+-activated K+ permeability in pancreatic B-cells. FEBS Lett. 1983;161:41–44. doi: 10.1016/0014-5793(83)80726-1. [DOI] [PubMed] [Google Scholar]
- 43.Atwater I, Ribalet B, Rojas E. Cyclic changes in potential and resistance of the beta-cell membrane induced by glucose in islets of Langerhans from mouse. J Physiol. 1978;278:117–139. doi: 10.1113/jphysiol.1978.sp012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henquin JC, Meissner HP, Preissler M. 9-Aminoacridine- and tetraethylammonium-induced reduction of the potassium permeability in pancreatic B-cells. Effects on insulin release and electrical properties. Biochim Biophys Acta. 1979;587:579–592. doi: 10.1016/0304-4165(79)90010-2. [DOI] [PubMed] [Google Scholar]
- 45.Kozak JA, Misler S, Logothetis DE. Characterization of a Ca2+-activated K+ current in insulin-secreting murine betaTC-3 cells. J Physiol. 1998;509:355–370. doi: 10.1111/j.1469-7793.1998.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, Houamed K, Kupershmidt S, Roden D, Satin LS. Pharmacological properties and functional role of Kslow current in mouse pancreatic beta-cells: SK channels contribute to Kslow tail current and modulate insulin secretion. J Gen Physiol. 2005;126:353–363. doi: 10.1085/jgp.200509312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dukes ID, Philipson LH. K+ channels: generating excitement in pancreatic beta-cells. Diabetes. 1996;45:845–853. doi: 10.2337/diab.45.7.845. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Figueroa DJ, Austin CP, et al. Expression of voltage-gated potassium channels in human and rhesus pancreatic islets. Diabetes. 2004;53:597–607. doi: 10.2337/diabetes.53.3.597. [DOI] [PubMed] [Google Scholar]
- 49.Covarrubias M, Wei A, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- 50.Lee TE, Philipson LH, Kuznetsov A, Nelson DJ. Structural determinant for assembly of mammalian K+ channels. Biophys J. 1994;66:667–673. doi: 10.1016/s0006-3495(94)80840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hugnot JP, Salinas M, Lesage F, et al. Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels. EMBO J. 1996;15:3322–3331. [PMC free article] [PubMed] [Google Scholar]
- 52.Post MA, Kirsch GE, Brown AM. Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current. FEBS Lett. 1996;399:177–182. doi: 10.1016/s0014-5793(96)01316-6. [DOI] [PubMed] [Google Scholar]
- 53.Betsholtz C, Baumann A, Kenna S, et al. Expression of voltage-gated K+ channels in insulin-producing cells. FEBS Lett. 1990;263:121–126. doi: 10.1016/0014-5793(90)80719-y. [DOI] [PubMed] [Google Scholar]
- 54.Baumann A, Krah-Jentgers I, Muller R, Muller-Holtkamp F. Molecular organization of the maternal effect region of Drosophila: characterization of an IA channel with homology to vertebrate Na channel. EMBO J. 1987;6:3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and cDNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 56.Philipson L, Hice RE, Schaefer K, et al. Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel. Proc Natl Acad Sci U S A. 1991;88:53–57. doi: 10.1073/pnas.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Philipson LH. Beta-cell ion channels: keys to endodermal excitability. Horm Metab Res. 1999;31:455–461. doi: 10.1055/s-2007-978774. [DOI] [PubMed] [Google Scholar]
- 58.Roe MW, Worley JF, III, Mittal AA, et al. Expression and function of pancreatic β-cell delayed rectifier K+ channels: role in stimulus-secretion coupling. J Biol Chem. 1996;271:32241–32246. doi: 10.1074/jbc.271.50.32241. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald PE, Ha XF, Wang J, et al. Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion. Mol Endocrinol. 2001;15:1423–1435. doi: 10.1210/mend.15.8.0685. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald PE, Wang G, Tsuk S, et al. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K(+) channels in neuroendocrine islet beta-cells through an interaction with the channel N terminus. Mol Endocrinol. 2002;16:2452–2461. doi: 10.1210/me.2002-0058. [DOI] [PubMed] [Google Scholar]
- 61.Herrington J, Sanchez M, Wunderler D, et al. Biophysical and pharmacological properties of the voltage-gated potassium current of human pancreatic beta-cells. J Physiol. 2005;567:159–175. doi: 10.1113/jphysiol.2005.089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamarina NA, Kuznetsov A, Fridlyand LE, Philipson LH. Delayed-rectifier (KV2.1) regulation of pancreatic beta-cell calcium responses to glucose: inhibitor specificity and modeling. Am J Physiol Endocrinol Metab. 2005;289:578–585. doi: 10.1152/ajpendo.00054.2005. [DOI] [PubMed] [Google Scholar]
- 63.Herrington J, Zhou YP, Bugianesi RM, et al. Blockers of the delayed-rectifier potassium current in pancreatic beta-cells enhance glucose-dependent insulin secretion. Diabetes. 2006;55:1034–1042. doi: 10.2337/diabetes.55.04.06.db05-0788. [DOI] [PubMed] [Google Scholar]
- 64.MacDonald PE, Salapatek AM, Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K(+) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes. 2002;51:S443–S447. doi: 10.2337/diabetes.51.2007.s443. [DOI] [PubMed] [Google Scholar]
- 65.Misonou H, Mohapatra DP, Park EW, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- 66.McKay MC, Worley JF., III Linoleic acid both enhances activation and blocks Kv1.5 and Kv2.1 channels by two separate mechanisms. Am J Physiol Cell Physiol. 2001;281:1277–1284. doi: 10.1152/ajpcell.2001.281.4.C1277. [DOI] [PubMed] [Google Scholar]
- 67.Feng DD, Luo Z, Roh SG, et al. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology. 2006;147:674–682. doi: 10.1210/en.2005-0225. [DOI] [PubMed] [Google Scholar]
- 68.Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta cell delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem. 2007;282:7442–7449. doi: 10.1074/jbc.M607858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf BA, Pasquale SM, Turk J. Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry. 1991;30:6372–6379. doi: 10.1021/bi00240a004. [DOI] [PubMed] [Google Scholar]
- 70.Philipson LH, Rosenberg MP, Kuznetsov A, et al. Delayed rectifier K+ channel overexpression in transgenic islets and β-cells associated with impaired glucose responsiveness. J Biol Chem. 1994;269:27787–27790. [PubMed] [Google Scholar]
- 71.Gopel SO, Kanno T, Bargs S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting-cells in intact mouse pancreatic islets. J Physiol. 2000;528:497–507. doi: 10.1111/j.1469-7793.2000.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]