Abstract
Background
Neutrophils are the most abundant white blood cells found in the amniotic cavity of women with intra-amniotic infection and/or inflammation. The current belief is that these neutrophils are of fetal origin. However, abundant neutrophils have been found in the amniotic fluid of women with a severe acute maternal inflammatory response but without a fetal inflammatory response in the placenta, suggesting that these innate immune cells can also be of maternal origin or a mixture of both fetal and maternal neutrophils.
Objectives
To investigate the origin of amniotic fluid neutrophils from women with intra-amniotic infection and/or inflammation, and to correlate these findings with acute histologic maternal and fetal inflammatory responses in the placenta.
Study Design
Amniotic fluid was collected from 15 women with suspected intra-amniotic infection and/or inflammation (positive microbiological cultures and/or interleukin (IL)-6 concentrations≥2.6 ng/mL). Amniotic fluid neutrophils were purified by fluorescence-activated cell sorting, DNA was extracted, and DNA fingerprinting was performed. DNA fingerprinting was also performed in the umbilical cord and maternal blood DNA. Fluorescence in situ hybridization (FISH) was assayed in women with male neonates. Blinded placental histopathological evaluations were conducted.
Results
1) DNA fingerprinting revealed that 43% (6/14) of the women who underwent a single amniocentesis had mostly fetal neutrophils in the amniotic fluid; 2) DNA fingerprinting showed that 36% (5/14) of the women who underwent a single amniocentesis had predominantly maternal neutrophils in the amniotic fluid; 3) DNA fingerprinting indicated that 21% (3/14) of the women who underwent a single amniocentesis had an evident mixture of fetal and maternal neutrophils in the amniotic fluid; 4) DNA fingerprinting revealed that a woman who underwent two amniocenteses (patient #15) had fetal neutrophils first, and as infection progressed, abundant maternal neutrophils invaded the amniotic cavity ; 5) FISH confirmed DNA fingerprinting results by showing that both fetal and maternal neutrophils are present in the amniotic fluid; 6) Most of the women who had predominantly amniotic fluid neutrophils of fetal origin at the time of collection delivered extremely preterm neonates [71% (5/7)]; 7) All of the women who had predominantly amniotic fluid neutrophils of maternal origin at the time of collection delivered term or late preterm neonates [100% (6/6)]; 8) Two of the women who had an evident mixture of fetal and maternal neutrophils in the amniotic fluid at the time of collection delivered extremely preterm neonates [67% (2/3)], and the third woman delivered a term neonate [33% (1/3)]; and 9) Most of the women included in this study presented acute maternal and fetal inflammatory responses in the placenta [87 % (13/15)].
Conclusion
Amniotic fluid neutrophils can be either predominantly of fetal or maternal origin, or a mixture of both fetal and maternal origin, in women with intra-amniotic infection and/or inflammation. The findings herein provide evidence that both fetal and maternal neutrophils can invade the amniotic cavity, suggesting that both the fetus and the mother participate in the host defense mechanisms against intra-amniotic infection.
Keywords: Acute chorioamnionitis, Clinical chorioamnionitis, Cytokine, Fetal inflammatory response, Fever, Funisitis, Human, Innate immune cells, Interleukin-6, Labor, Microbial invasion of the amniotic cavity, Parturition, Phagocytosis, Pregnancy, Preterm birth, Preterm labor, Term labor
Introduction
The current belief is that the amniotic fluid is sterile under normal circumstances; yet, it contains anti-microbial properties, a low number of white blood cells (i.e. leukocytes), and proteins implicated in fetal host defense mechanisms1-7. In women with intra-amniotic inflammation, however, leukocytes are abundant in the amniotic cavity8-13, and their presence is accompanied by increased concentrations of inflammatory mediators such as anti-microbial peptides14-18, cytokines19-21, and lipids22-33. This inflammatory process in the amniotic cavity can be initiated by microorganisms (i.e. intra-amniotic infection) or danger signals derived from necrosis or cellular stress (i.e. sterile intra-amniotic inflammation)34-39. In both conditions, the most abundant leukocytes in the amniotic cavity are the neutrophils8, 13.
Amniotic fluid neutrophils are a part of the innate immune host defense mechanisms that take place in the amniotic cavity of women with intra-amniotic infection40-42. This concept is supported by evidence demonstrating that amniotic fluid neutrophils 1) are a source of antimicrobial products17, 18, 43-45 and cytokines13, 2) can trap and kill bacteria invading the amniotic cavity by forming neutrophil extracellular traps (NETs)46, and 3) can phagocytize microorganisms commonly found in the lower genital tract, e.g., Streptococcus agalactiae (also known as Group B Streptococcus or GBS), Ureaplasma urealyticum, Gardnerella vaginalis, and Escherichia coli47.
Amniotic fluid neutrophils were initially thought to be of maternal origin48. Yet, experimental evidence showed that amniotic fluid neutrophils from women49 or rhesus monkeys50 undergoing preterm labor were of fetal origin. In such cases, these innate immune cells could originate from the fetal vessels of the chorionic plate51. However, abundant neutrophils have also been observed in the amniotic fluid of women with a severe maternal inflammatory response without a fetal inflammatory response in the placenta13, 46, indicating that further research is required to investigate whether, amniotic fluid neutrophils are of maternal origin or a mixture of both fetal and maternal neutrophils.
Fluorescence in situ hybridization (FISH) has been used to determine the origin of neutrophils infiltrating the amniotic cavity49 or chorioamniotic membranes.52 However, this technique is semi-quantitative and does not offer the complete assessment of chromosomal complement53. Short tandem repeat (STR) DNA profiling, also known as DNA fingerprinting, has emerged as a state-of-the-art method with high discriminating power and sensitivity for forensic DNA analysis and paternity testing54.
The aims of this study were to investigate the origin of amniotic fluid neutrophils from women with intra-amniotic infection and/or inflammation using DNA fingerprinting and to complement these findings with FISH, the conventional method. In addition, placental histopathological examinations were performed and correlated to the origin of amniotic fluid neutrophils.
Materials and Methods
Study population
This was a cross-sectional study of women who underwent transabdominal amniocentesis due to clinical indications or amniocentesis during cesarean delivery. Women were enrolled at Hutzel Women's Hospital of the Detroit Medical Center (April – November 2016). Amniotic fluid samples were acquired by an automatic cell counter (Cellometer Auto 2000, Nexcelom Bioscience, Lawrence, MA, USA) to obtain the viable cell numbers, most of which were white blood cells or leukocytes13. The inclusion criteria were as follows: 1) singleton pregnancy, 2) amniotic fluid samples without blood contamination, and 3) amniotic fluid samples with a large number of viable leukocytes (including mostly neutrophils and monocytes13) (>1 × 105 cells/mL) to perform fluorescence-activated cell sorting (FACS) of amniotic fluid neutrophils.
All of the women provided written informed consent to donate additional amniotic fluid for research purposes, according to protocols approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS).
Clinical definitions
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used when the estimation was inconsistent with menstrual dating. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by two or more of the following criteria: 1) uterine tenderness; 2) malodorous vaginal discharge; 3) fetal tachycardia (heart rate >160 beats/min); 4) maternal tachycardia (heart rate >100 beats/min); and 5) maternal leukocytosis (leukocyte count >15,000 cells/mm3)20, 55-58. Term delivery was defined as birth after 37 weeks of gestation, whereas preterm delivery was defined as birth between 20 and 36 6/7 weeks of gestation.
Intra-amniotic inflammation was diagnosed when the concentration of interleukin (IL)-6 in the amniotic fluid was ≥2.6 ng/mL59. Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture60-65. Intra-amniotic infection was defined as the presence of microbial invasion of the amniotic cavity with intra-amniotic inflammation36-39, 59, 66-76.
Sample collection
Amniotic fluid was retrieved by transabdominal amniocentesis under antiseptic conditions using a 22-gauge needle monitored by ultrasound. Amniotic fluid was also retrieved by amniocentesis during cesarean delivery under antiseptic conditions. Amniotic fluid samples were transported to the clinical laboratory in a capped sterile syringe and were cultured for aerobic and anaerobic bacteria, as well as for genital mycoplasmas8, 73, 77-80. Shortly after collection, a white blood cell (WBC) count was determined in each amniotic fluid sample using a hemocytometer chamber, according to methods previously described8. Glucose concentration was also determined81 and a Gram stain82 was performed in each amniotic fluid sample. Cultures, WBC count, glucose concentration, and Gram stain were not performed in all of the amniotic fluid samples collected during cesarean section since these samples were collected for research purposes only. However, both IL-6 concentration13 and the presence of bacteria (bacterial live/dead staining46, 83) were assessed in most of the amniotic fluid samples, as previously described.
Fluorescence-activated cell sorting (FACS) of amniotic fluid neutrophils
Amniotic fluid samples were passed through a sterile 15-μm filter (Cat# 43-50015-03; pluriSelect Life Science, Leipzig, Germany) to remove epithelial cells and centrifuged at 200 × g for 5 minutes at room temperature (n=16). The cell pellet (mostly leukocytes46) was washed with 1× phosphate-buffered saline (1× PBS; Life Technologies, Grand Island, NY), resuspended at 1×106 cells in 100μL of BD FACS stain buffer (Cat#554656; BD Biosciences, San Jose, CA) containing 20% human FcR blocking reagent (Miltenyi Biotec, San Diego, CA, USA), and incubated for 10 min at 4°C. Next, amniotic fluid cells were incubated with the following extracellular fluorochrome-conjugated anti-human antibodies (BD Biosciences) for 30 min at 4°C in the dark: CD15-FITC (fluorescein isothiocyanate) (clone W6D3, Cat#562370) and CD14-APC-Cy7™ (clone MφP9, Cat#557831). After a washing with 1× PBS, the cells were resuspended in pre-sort buffer (Cat# 563503, BD Biosciences) at a concentration of 5×106 cells/mL. Amniotic fluid neutrophils (CD15+CD14- cells) were purified using a BD FACSAria cell sorter (BD Biosciences) and BD FACSDiva 6.0 software (BD Biosciences). The purity of amniotic fluid neutrophils ranged from 92% to 99% (Figure 1). The purified neutrophils were then resuspended in RLT buffer (Qiagen, Germantown, MD) and stored at -80°C until use.
Figure 1. Fluorescence-activated cell sorting (FACS) of amniotic fluid neutrophils.
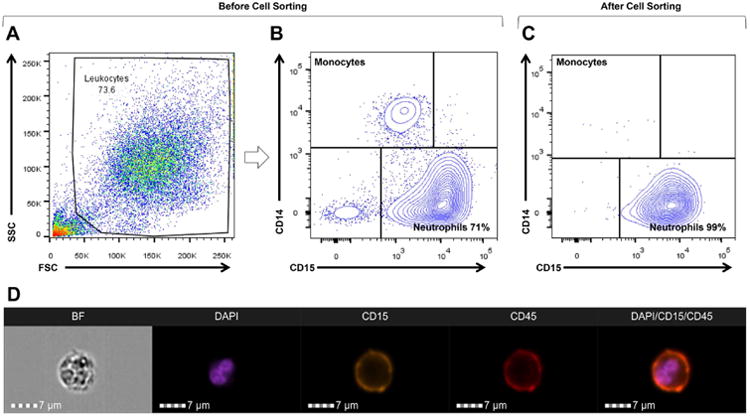
A) Representative image of the flow cytometry analysis of an amniotic fluid sample from a woman with intra-amniotic infection/inflammation containing different cell populations before cell sorting. B) Representative image of the flow cytometry analysis of an amniotic fluid sample from a woman with intra-amniotic infection/inflammation containing neutrophils (CD15+ cells) and monocytes (CD14+ cells) before cell sorting. C) Representative image of the flow cytometry analysis of purified amniotic fluid neutrophils (CD15+CD14- cells) from a woman with intra-amniotic infection/inflammation after cell sorting; purity, 99%. D) Flow cytometry imaging of a purified amniotic fluid neutrophil from a woman with intra-amniotic infection/inflammation. BF, bright field (gray scale); DAPI, nucleus/DNA (purple color); CD15, neutrophil marker (yellow color); and CD45, common leukocyte antigen/leukocyte marker (red color). A merged image showing the co-expression of CD15, CD45 and DAPI is also shown. Magnification bars are shown in all of the cases.
Imaging flow cytometry of amniotic fluid neutrophils
Amniotic fluid samples were passed through a sterile 15-μm filter to remove epithelial cells and centrifuged at 200 × g for 5 minutes at room temperature (n=6). The cell pellet (mostly leukocytes46) was washed with 1× PBS, resuspended in 80 μL of BD FACS stain buffer containing 20 μL of human FcR blocking reagent, and incubated for 10 min at 4°C. Next, amniotic fluid cells were stained with the following extracellular fluorochrome-conjugated anti-human antibodies (BD Biosciences) for 30 min at 4°C in the dark: CD45-APC (allophycocyanin) (Clone HI30, Cat#555485) and CD15-PE-CF594 (clone W6D3, Cat#562372). Following antibody staining, amniotic fluid leukocytes were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 20 min at room temperature. Amniotic fluid leukocytes were then rehydrated in 1× PBS and stained with 3 mmol/L of 4’,6-diamidino-2-phenylindole (DAPI) (Cat#D9542, Sigma-Aldrich Corporation, St. Louis, MO) in nuclear permeabilization buffer (Cat#00-8333-56, eBioscience, San Diego, CA) for 15 min at room temperature. Lastly, amniotic fluid leukocytes were resuspended in 50 μL of BD FACS stain buffer containing 1mg/mL of EDTA (Cat#15575-038, Life Technologies). Samples were acquired using an ImageStream®X Mk II imaging cytometer (Amnis, Seattle, WA) at the Microscopy, Imaging, and Cytometry Resources Core at the Wayne State University School of Medicine (Detroit, MI, USA) (http://micr.med.wayne.edu/). Images were obtained at a magnification of 60X using the low flow rate/high sensitivity INSPIRE software (Amnis) and the following lasers: 405nm, 488nm and 642nm. Acquired images were analyzed using the IDEALS 6.2 software (Amnis).
DNA fingerprinting
Genomic DNA was isolated from FACS-purified amniotic fluid neutrophils (n=16) using an AllPrep DNA/RNA Mini Kit (Qiagen), following the manufacturer's instructions. Fetal or maternal genomic DNA was isolated from frozen samples of either umbilical cord or maternal blood (buffy coat) using a DNeasy Blood & Tissue Kit (Qiagen), according to the manufacturer's instructions. DNA concentration and purity were assessed with the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). DNA samples were submitted for DNA fingerprinting to Genetica DNA Laboratories, a LabCorp brand (Burlington, NC, USA). Briefly, analytical procedures for PCR and capillary electrophoresis were performed on a 3130×l genetic analyzer (Applied Biosystems, Foster City, CA, USA). The 13 core CODIS STR loci plus PENTA E and PENTA D, and the gender-determining locus, amelogenin, were analyzed using the commercially available PowerPlex® 16HS amplification kit (Promega Corporation, Madison, WI, USA) and GeneMapper ID v3.2.1 software (Applied Biosystems). Appropriate positive and negative controls were concurrently used throughout the analysis. The interpretation of the DNA profile of each sample was performed by geneticists from LabCorp and returned as a written report. The reported sensitivity for this method was between 2% and 5%. A cut-off of 82% was used to establish the predominant origin of amniotic fluid neutrophils. This cut-off was based on the sensitivity of the FISH assay, which ranges from 82% to 99%49, 84-86. This allowed us to compare the DNA fingerprinting data herein with previously published FISH results49.
Fluorescence in situ hybridization (FISH) of amniotic fluid neutrophils
Amniotic fluid samples were passed through a sterile 15-μm filter to remove epithelial cells and centrifuged at 200 × g for 5 minutes at room temperature (n=4). The cell pellet (mostly leukocytes46) was washed with 1× PBS and resuspended in the same buffer at a concentration of 5×104 cells/150μL. Next, cytospin slides of amniotic fluid leukocytes were prepared onto Fisherbrand Superfrost microscope slides (Thermo Fisher Scientific) using a Shandon Cytospin 3 cytocentrifuge (Thermo Fisher Scientific) at 800 rpm for 5 min. Cytospin slides of amniotic fluid leukocytes were then fixed with 4% paraformaldehyde, washed with 1× PBS, dehydrated in graded ethanol at 30%, 60%, 80%, 95% and 100% (Sigma) for 2 min each, and air-dried. Lastly, the slides were stored at −80°C until use.
Fluorescent in situ hybridization (FISH) was performed using the MetaSystems TissueFISH Pretreatment Kit (Cat# D-0905-025-TF; Altlussheim, Germany), following the manufacturer's instructions. A DNA probe mix of repetitive sequences specific for the chromosome X centromeric region (green) and chromosome Y centromeric region (orange) was used (XCyting Centromere Enumeration Probe, Cat#D-0825-050-OG, MetaSystems). Briefly, the frozen slides were warmed to room temperature, washed with UltraPure™ DNase/RNase-Free Distilled Water (Thermo Fisher Scientific) for 1 min, and washed with 2XSSC solution (300 mmol/L sodium chloride and 30 mmol/L sodium citrate, pH 7.0) for 1 min. The slides were then pretreated with 1× pretreatment buffer at 96°C for 10 min, 2XSSC solution at room temperature for 5 min, and protease solution at room temperature for 5 min. The slides were rinsed with 2XSSC solution and dehydrated with 100% ethanol for 2 min. Next, 7 μL of the DNA probe mix were added directly to the slide and a 18×18 mm2 coverslip was placed on top of the slide, and sealed with rubber cement (Cat#11FIXO0125, MP Biomedicals, Santa Ana, CA, USA). The slides were then heated at 75°C for 5 min on a heat block to denature the probes/DNA and incubated at 37°C overnight in a HybEZ oven (Advanced Cell Diagnostics, Hayward, CA, USA). The next day, the slides were removed from the oven, the rubber cement was removed using forceps, and the coverslips were removed by rinsing with 2XSSC solution. The slides were washed with 0.4XSSC solution at 72°C for 2 min and with 2XSSC solution plus 0.05% Tween 20 (MP Biomedicals) at room temperature for 30 sec. Next, the slides were rinsed with distilled water, air-dried, and mounted in ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies). The DAPI and FISH probe signals were visualized on a Zeiss LSM 780 laser scanning confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) at the Microscopy, Imaging, and Cytometry Resources Core at the Wayne State University School of Medicine (http://micr.med.wayne.edu/). Confocal z-stacks were acquired using an EC Plan-Neofluar 40x/1.30 Oil DIC M27 lens. (Carl Zeiss Microscopy GmbH)
Placental histopathological examination
Sampling of the placentas was conducted according to established protocols by the Perinatology Research Branch. Five-μm-thick sections of formalin-fixed, paraffin-embedded tissue specimens were cut and mounted on SuperFrost™ Plus microscope slides (Erie Scientific LLC, Portsmouth, NH, USA). After deparaffinization, slides were rehydrated and stained with hematoxylin-eosin. A minimum of 5-full thickness sections of chorionic plate, 3 sections of umbilical cord, and 3 chorioamniotic membrane rolls from each case were examined by a placental pathologist (SMJ), who was blinded to clinical history and additional testing results. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading87-91. For more information about the staging and grading of the acute inflammatory lesions of the placenta, please refer to placental histology reviews87, 88, 91.
Results
Clinical characteristics of the study population
A total of 15 women who underwent a single amniocentesis or two amniocenteses before delivery and/or during cesarean delivery, were included in this study (n=16 amniotic fluid samples). Clinical characteristics of the study population, as well as the indication for amniocentesis, are shown in Table 1. The amniotic fluid samples had one or several of the following: 1) a positive microbiological culture, 2) elevated concentrations of IL-6 (≥2.6 ng/mL), 3) an increased WBC (>50 cells/mm3) or a viable cell (i.e. leukocytes; >100 cells/mm3) count, and 4) a positive bacterial live/dead staining (Table 1). Most of the amniotic fluid samples had a low glucose concentration (<14 mg/dL) (Table 1). Five of the women were diagnosed with clinical chorioamnionitis (Table 1). One woman underwent two amniocenteses: the first during pregnancy and the second during cesarean delivery (Table 1, samples 15-16). The most common microorganisms found in these amniotic fluid samples were Ureaplasma urealyticum and Mycoplasma hominis followed by Prevotella spp. (Table 1).
Table 1.
Clinical characteristics of amniotic fluid samples utilized for DNA fingerprinting assays.
| Sample | Clinical Chorioamnionitis |
Viable Cell Count* (cells/mm3) |
Gestational Age at Amniocentesis |
Indication for amniocentesis | IL-6 (ng/mL) |
Gram Stain |
Bacterial Live/Dead Staining |
Amniotic Fluid Culture |
WBC (cells/mm3) |
Glucose (mg/dL) |
Gestational Age at delivery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | 508 | 23.1 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 121 | Positive | Positive | Bacteroides ureolyticus, Garnerella vaginalis, Ureaplasma urealyticum | 750 | <1 | 23.6 |
| 2 | Yes | 786 | 22.1 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 123.7 | Negative | Negative | Negative | 390 | 7 | 23.9 |
| 3 | Yes | 638 | 36.9 | Research purposes (cesarean section) | 44.4 | NA | Positive | NA | NA | NA | 36.9 |
| 4 | No | 125 | 40.6 | Research purposes (cesarean section) | 39.9 | NA | Positive | NA | NA | NA | 40.6 |
| 5 | No | 9920 | 25.7 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 27 | Positive | Positive | Enterobacter aerogenes, Enterococcus faecalis, Mycoplasma hominis, Prevotella spp, Streptococcus viridans | 6938 | 4 | 25.7 |
| 6 | No | 100 | 18.9 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 121.3 | Positive | Positive | Bacteroides fragilis | 65 | 20 | 19.6 |
| 7 | No | 3660 | 21.3 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 118.7 | Negative | Positive | Staphylococcus hominis | 355 | <1 | 21.9 |
| 8 | Yes | 535 | 39.6 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 3.5 | Negative | Positive | Ureaplasma urealyticum, Mycoplasma hominis | 590 | <1 | 39.6 |
| 9 | No | 9600 | 38.1 | Research purposes (cesarean section) | 101.4 | Negative | Positive | Mycoplasma hominis, Ureaplasma urealyticum | NA | NA | 38.1 |
| 10 | No | 1160 | 22.3 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 125.5 | Positive | Positive | Mycoplasma hominis, Fusobacterium nucleatum | 700 | 10 | 22.7 |
| 11 | No | 3090 | 40.4 | Research purposes (cesarean section) | 54.6 | Negative | Positive | Ureaplasma urealyticum | NA | NA | 40.4 |
| 12 | No | 286 | 39.3 | Research purposes (cesarean section) | 0.5 | NA | Negative | Negative | NA | NA | 39.3 |
| 13 | No | 180 | 19 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 55.5 | Negative | Negative | Negative | 43 | 17 | 19.4 |
| 14 | Yes | 2200 | 35.6 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 70.6 | Positive | Positive | Mycoplasma hominis, Ureaplasma urealyticum, Prevotella spp, Streptococcus agalactiae, Streptococcus anginosus | 4000 | <1 | 35.6 |
| 15 | Yes | 860 | 39.9 | Diagnosis of intra-amniotic infection/inflammation (transabdominal) | 73.6 | Negative | Negative | Negative | 600 | <1 | 40 |
| 16 | Yes | 18800 | 40 | Research purposes (cesarean section) | 47.7 | Negative | Positive | Ureaplasma urealyticum | NA | NA |
Fluorescence-activated cell sorting (FACS) and imaging flow cytometry of amniotic fluid neutrophils
All of the amniotic fluid samples had a large population of neutrophils, which were identified by flow cytometry. Figures 1A-1C represent the gating strategy used to identify amniotic fluid leukocytes, including neutrophils and monocytes, before and after cell sorting. Before cell sorting, total leukocytes were identified using the forward-side scatter (FSC, cell size) and side scatter (SSC, cell granularity) parameters (Figure 1A). Neutrophils and monocytes were identified within the total leukocyte population by the expression of CD15 and CD14, respectively (Figure 1B). Amniotic fluid neutrophils were isolated by cell sorting and their purity ranged between 92% and 99% (Figure 1C). Hence, purified amniotic fluid neutrophil suspensions did not contain monocytes (CD14+ cells) or any other cells present in the amniotic fluid (Figure 1C).
Figure 1D represents the imaging flow cytometry of amniotic fluid neutrophils. Briefly, the structure of an amniotic fluid neutrophil is shown in bright field, the nucleus is evidenced by DAPI staining, and the identity of a neutrophil is demonstrated by the expression of CD15 and CD45 (Figure 1D). A merged image of DAPI, CD15, and CD45 is also shown in Figure 1D.
Amniotic fluid neutrophils in women with intra-amniotic infection and/or inflammation can be predominantly of fetal origin
DNA fingerprinting revealed that 43% (6/14) of the women who underwent a single amniocentesis had predominantly (83%-100%) fetal neutrophils in the amniotic fluid (Table 2 samples 1, 2, 5, 10, 12, and 13). In Figure 2, an example of the DNA fingerprinting of amniotic fluid neutrophils of fetal origin is shown (sample #12). The DNA fingerprinting of the amniotic fluid neutrophils is identical to the DNA fingerprinting of the fetus (Figure 2). By contrast, the DNA fingerprinting of amniotic fluid neutrophils differed from the one displayed by the mother (Figure 2).
Table 2. DNA fingerprinting of amniotic fluid neutrophils and placental pathological examination.
| Sample | Viable Cell Count* (cells/mm3) | Neutrophils of Maternal Origin (%) | Neutrophils of Fetal Origin (%) | Placental Pathology Results | |
|---|---|---|---|---|---|
| Acute Maternal Inflammatory Response | Acute Fetal Inflammatory Response | ||||
| 1 | 508 | 5 | 95 | Stage 3/Grade 2 | Stage 1/Grade 1 |
| 2 | 786 | 10 | 90 | Stage 3/Grade 2 | Stage 2/Grade 2 |
| 3 | 638 | 93 | 7 | Stage 2/Grade 1 | Stage 1/Grade 1 |
| 4 | 125 | 57 | 43 | Stage 2/Grade 1 | Stage 1/Grade 1 |
| 5 | 9920 | 17 | 83 | Stage 3/Grade 2 | Stage 1/Grade 1 |
| 6 | 100 | 68 | 32 | Stage 3/Grade 2 | Stage 2/Grade 1 |
| 7 | 3660 | 73 | 27 | Stage 3/Grade 2 | Stage 2/Grade 1 |
| 8 | 535 | 97 | 3 | Stage 1/Grade 1 | Stage 1/Grade 1 |
| 9 | 9600 | 98 | 2 | Stage 2/Grade 1 | Stage 2/Grade 1 |
| 10 | 1160 | 0 | 100 | Stage 2/Grade 1 | Stage 1/Grade 1 |
| 11 | 3090 | 100 | 0 | None | None |
| 12 | 286 | 0 | 100 | None | None |
| 13 | 180 | 10 | 90 | Stage 2/Grade 1 | Stage 1/Grade 1 |
| 14 | 2200 | 100 | 0 | Stage 3/Grade 2 | Stage 2/Grade 2 |
| 15 | 860 | 2 | 98 | Stage 2/Grade 1 | Stage 2/Grade 1 |
| 16 | 18800 | 97 | 3 | ||
Figure 2. DNA fingerprinting of amniotic fluid neutrophils that were mostly of fetal origin.

DNA fingerprinting of purified amniotic fluid neutrophils, the fetus (umbilical cord), and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amalogenin genes, vWA, D8S1179, TPOX, and FGA. Each electropherogram was separated into three sections: blue, green, and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye), and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to/above each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid neutrophils is identical to the DNA fingerprinting of the fetus.
Most of the amniotic fluid samples in which neutrophils were predominantly of fetal origin were collected from women diagnosed with intra-amniotic infection and/or inflammation (Table 1). Most of the women who had predominantly amniotic fluid neutrophils of fetal origin at the time of collection delivered extremely preterm neonates (<28 weeks of gestation, Table 1).
Amniotic fluid neutrophils in women with intra-amniotic infection/inflammation can be predominantly of maternal origin
DNA fingerprinting revealed that 36% (5/14) of the women who underwent a single amniocentesis had mostly (93%-100%) maternal neutrophils in the amniotic fluid (Table 1; samples 3, 8, 9, 11, and 14). In Figure 3, the DNA fingerprinting of amniotic fluid neutrophils of maternal origin is shown (sample #11). The DNA fingerprinting of the amniotic fluid neutrophils is identical to the DNA fingerprinting of the mother (Figure 3). By contrast, the DNA fingerprinting of amniotic fluid neutrophils differed from the one displayed by the fetus (Figure 3).
Figure 3. DNA fingerprinting of amniotic fluid neutrophils that were mostly of maternal origin.

DNA fingerprinting of purified amniotic fluid neutrophils, the fetus (umbilical cord), and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amalogenin genes, vWA, D8S1179, TPOX, and FGA. Each electropherogram was separated into three sections: blue, green, and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye), and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to/above each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid neutrophils is identical to the DNA fingerprinting of the mother.
All of the amniotic fluid samples in which neutrophils were predominantly of maternal origin were collected from women diagnosed with intra-amniotic infection/inflammation (Table 1). All of the women who had neutrophils predominantly of maternal origin in the amniotic fluid at the time of collection delivered term (≥37 weeks of gestation) or late preterm (34-36 completed weeks of gestation) neonates (Table 1).
Amniotic fluid neutrophils in women with intra-amniotic infection/inflammation can be a mixture of both fetal and maternal neutrophils
DNA fingerprinting revealed that 21% (3/14) of the women who underwent a single amniocentesis had an evident mixture of fetal and maternal neutrophils in the amniotic fluid (Table 2, samples 4, 6, and 7). In Figure 4, the DNA fingerprinting of amniotic fluid neutrophils that were an evident mixture of fetal and maternal neutrophils is shown (sample #4). The DNA fingerprinting of the amniotic fluid neutrophils included both fetal and maternal STR alleles; therefore, the DNA fingerprinting of amniotic fluid neutrophils resulted from the combination of the fetal and maternal DNAs (Figure 4).
Figure 4. DNA fingerprinting of amniotic fluid neutrophils that were of both fetal and maternal origin.

DNA fingerprinting of purified amniotic fluid neutrophils, the fetus (umbilical cord), and the mother (buffy coat from peripheral blood) is shown in electropherograms. Each electropherogram contains 16 genetic sites: D3S1358, TH01, D21S11, D18S51, Penta_E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta_D, X- and Y-specific amalogenin genes, vWA, D8S1179, TPOX, and FGA. Each electropherogram was separated into three sections: blue, green, and black. Each color indicates the dye used for the PCR multiplex: blue represents the genes amplified using fluorescein (FL dye), green represents the genes amplified using 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein (JOE dye), and black represents the genes amplified using tetramethylrhodamine (TMR dye). Each genetic site has STR alleles, which is represented by peaks. The number next to/above each STR allele (peak) is the number of repeats for each STR allele. The DNA fingerprinting of the amniotic fluid neutrophils included both fetal and maternal STR alleles; therefore, the DNA fingerprinting of amniotic fluid neutrophils resulted from the combination of the fetal and maternal DNAs.
All of the women who had an evident mixture of fetal and maternal neutrophils in the amniotic fluid at the time of collection were women diagnosed with intra-amniotic infection/inflammation (Table 1). Two of these women delivered extremely preterm neonates (<28 weeks of gestation), and the third woman delivered a term neonate (≥37 weeks of gestation) (Table 1).
One woman who underwent two amniocenteses had mostly fetal neutrophils in her first amniotic fluid sample (patient #15; sample#15: 860 cells/mm3, 98% of neutrophils of fetal origin, Table 1). In this sample, the concentration of IL-6 was 73.6 ng/mL but bacteria were not detected by conventional microbiological techniques or bacterial live/dead staining; therefore, this woman was diagnosed with intra-amniotic inflammation (Table 1). The next day, a second amniotic fluid sample was collected from the same woman (patient #15; sample 16), which had a larger number of leukocytes, and neutrophils were mostly of maternal origin (18800 cells/mm3, 97% of neutrophils of maternal origin, Table 1). In this second sample, the concentration of IL-6 was 47.7ng/mL and Ureaplasma urealyticum was detected by conventional microbiological techniques and bacterial live/dead staining; therefore, this woman was diagnosed as having intra-amniotic infection/inflammation (Table 1).
Identification of X and Y chromosomes by fluorescence in situ hybridization (FISH)
Next, we performed FISH in four of the amniotic fluid samples from women who delivered male neonates. Figure 5A is a confocal z-stack image of an amniotic fluid sample (sample #10) that includes neutrophils containing both X and Y chromosomes; therefore, these innate immune cells are of fetal origin. A 3D reconstruction of these amniotic fluid neutrophils shows that these cells are forming neutrophil extracellular traps (networks of extracellular fibers containing DNA) (Video 1), a mechanism for microbial killing46. Figure 5B is a confocal z-stack image of an amniotic fluid sample (sample #8) that includes neutrophils containing two X chromosomes; therefore, these innate immune cells are of maternal origin. A 3D reconstruction of these amniotic fluid neutrophils is shown in Video 2.
Figure 5. Fluorescence in situ hybridization of amniotic fluid neutrophils.
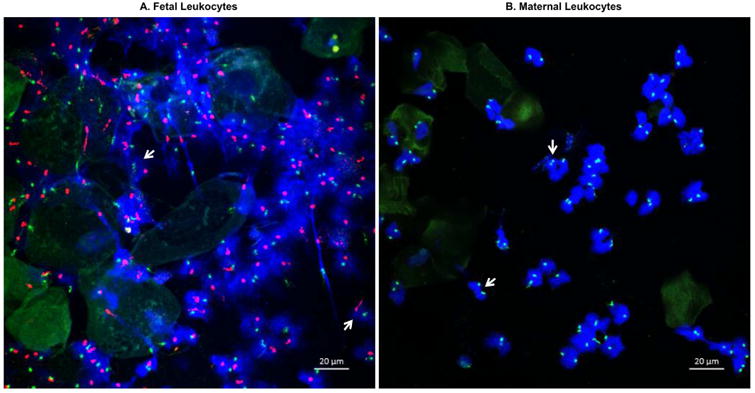
Amniotic fluid samples from women with intra-amniotic infection and/or inflammation who delivered male fetuses were obtained (n=4). Fluorescence in situ hybridization was performed to visualize X (green) and Y (red) chromosomes. A) Confocal z-stack image of an amniotic fluid sample including mostly fetal neutrophils containing both X and Y chromosomes (white arrows). B) Confocal z-stack image of an amniotic fluid sample including mostly maternal neutrophils containing two X chromosomes (white arrows). Magnification bars are shown in all of the cases. 400× magnification.
Is there an association between the origin of the amniotic fluid neutrophils and the placental histopathological examination?
Acute histologic fetal and maternal inflammatory responses were observed in most of the cases included in this study.
An example of the acute fetal and maternal histologic inflammatory responses observed in cases with amniotic fluid neutrophils predominantly of fetal origin is shown in Figures 6A-6D (Table 2, sample #2). Figure 6A shows the acute maternal inflammatory response observed in the chorioamniotic membranes (chorioamniotic membrane roll). This response includes neutrophilic migration into the amniotic connective tissue with degenerating neutrophils and amnion necrosis (i.e. necrotizing chorioamnionitis). Figure 6B shows neutrophilic infiltration into the membranes of the chorionic plate: such neutrophils can be either maternal (migrating from the intervillous space) or fetal (migrating from chorionic surface blood vessels). Figure 6C shows fetal neutrophils in the umbilical cord migrating from the umbilical artery into the Wharton's jelly and forming a band (acute umbilical arteritis). A magnification of this neutrophilic infiltration is shown in Figure 6D. One of the cases in which amniotic fluid neutrophils were mostly of fetal origin did not present either a maternal or fetal acute inflammatory response in the placental tissues (Table 2, sample #12).
Figure 6. Placental histopathological examination.
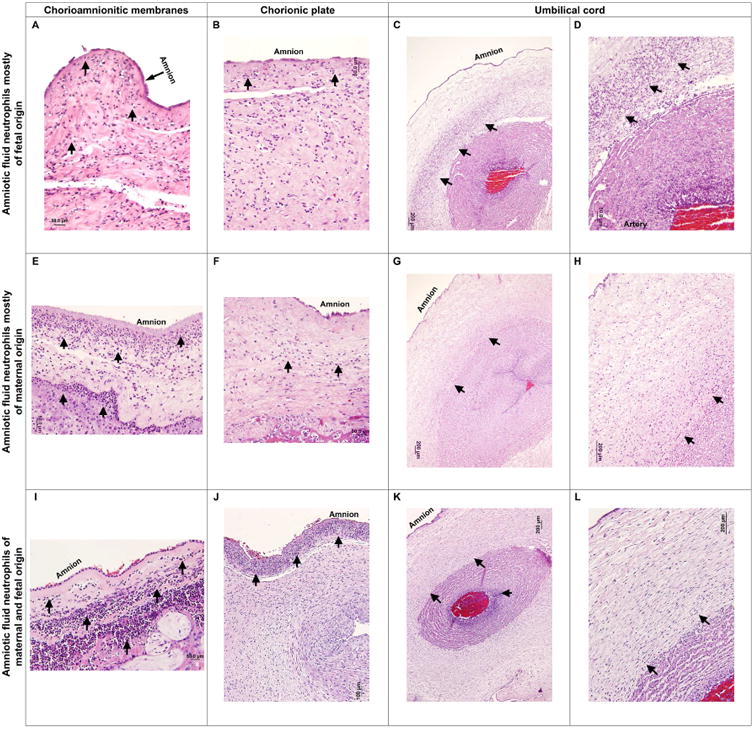
Histopathological examination of the chorioamnionitic membranes, chorionic plate membranes, and umbilical cord obtained from women who either had mostly fetal (A-D), maternal (E-H), or a mixture of fetal and maternal (I-L), neutrophils in the amniotic fluid. Acute maternal inflammatory responses observed in the chorioamniotic membranes (A, E, and I). Neutrophilic migration into the amniotic connective tissue (acute chorioamnionitis, black arrows). Epithelial necrosis of the amnion (A, necrotizing chorioamnionitis, black arrow). Acute inflammatory responses found in the chorionic plate membranes (B, F, and J). Neutrophilic infiltration of the chorionic plate membranes (black arrows). Acute fetal inflammatory responses in the umbilical cord (C, G, and K). Neutrophils seem to be migrating from the umbilical artery into the Wharton's jelly (umbilical arteritis, black arrows). A magnification of the neutrophilic infiltration in the Wharton's jelly (D, H, and L, black arrows) is also shown. Magnification bars are shown in all of the cases.
An example of the acute histologic maternal and fetal inflammatory responses observed in cases with amniotic fluid neutrophils predominantly of maternal origin is shown in Figures 6E-6H (Table 2, samples 16 and 17). Figure 6E shows an acute maternal inflammatory response in the chorioamniotic membranes (chorioamniotic membrane roll) with neutrophils extending to the amnion. Figure 6F shows acute inflammation in the membranes of the chorionic plate. Figure 6G shows fetal inflammation in the umbilical cord, characterized by the migration of neutrophils from the umbilical artery into the Wharton's jelly (acute umbilical arteritis). A magnification of this a neutrophilic infiltration is shown in Figure 6H (black arrows). One of the cases in which amniotic fluid neutrophils were mostly of maternal origin did not present either a maternal or fetal acute inflammatory response in the placental tissues (Table 2, sample #11).
In cases for which the amniotic fluid neutrophils were an evident mixture of maternal and fetal neutrophils (Table 2), the stage and grade of the maternal and fetal inflammatory responses were varied, but it is notable that in two cases the maternal inflammatory response was grade 2, while in all three cases the fetal inflammatory response was grade 1 (Table 2), suggesting that severe maternal inflammation may explain the presence of maternal neutrophils in the amniotic cavity. Figure 6I shows a severe acute maternal inflammatory response (chorioamniotic membrane roll) characterized by neutrophilic infiltration of the chorionic connective tissue and amnion of the chorioamniotic membranes. Figure 6J shows marked neutrophilic infiltration of the membranes of the chorionic plate. Figure 6K shows a fetal inflammatory response in the umbilical cord in the form of umbilical arteritis. This response is characterized by neutrophilic infiltration of the umbilical artery with some of these cells migrating into the Wharton's jelly (Figure 6K). A magnification of this a neutrophilic infiltration is shown in Figure 6L.
Discussion
Principal findings of the study
The principal findings of the study are as follows: 1) DNA fingerprinting revealed that 43% (6/14) of the women who underwent a single amniocentesis had predominantly fetal neutrophils in the amniotic fluid; 2) DNA fingerprinting showed that 36% (5/14) of the women who underwent a single amniocentesis had predominantly maternal neutrophils in the amniotic fluid; 3) DNA fingerprinting indicated that 21% (3/14) of the women who underwent a single amniocentesis had an evident mixture of fetal and maternal neutrophils in the amniotic fluid; 4) DNA fingerprinting revealed that one woman who underwent two amniocenteses (patient #15) had fetal neutrophils first, and as infection progressed, maternal neutrophils invaded the amniotic cavity; 5) FISH confirmed DNA fingerprinting results by showing that both fetal and maternal neutrophils are present in the amniotic fluid; 6) most of the women who had amniotic fluid neutrophils predominantly of fetal origin at the time of collection delivered extremely preterm neonates [71% (5/7)]; 7) all of the women who had amniotic fluid neutrophils predominantly of maternal origin at the time of collection delivered term or late preterm neonates [100% (6/6)]; 8) two of the women who had an evident mixture of fetal and maternal neutrophils in the amniotic fluid at the time of collection delivered extremely preterm neonates [67% (2/3)], and the third woman delivered a term neonate [33% (1/3)]; and 9) most of the women included in this study presented acute maternal and fetal inflammatory responses in the placenta [87% (13/15)]. Collectively, these data show that amniotic fluid neutrophils can either be predominantly of fetal or maternal origin, or a mixture of both fetal and maternal neutrophils, in women with intra-amniotic infection and/or inflammation. The findings herein provide evidence that both fetal and maternal neutrophils can invade the amniotic cavity, suggesting that both the fetus and the mother participate in the host defense mechanisms against intra-amniotic infection and/or inflammation.
Amniotic fluid neutrophils can be predominantly of fetal origin in women with intra-amniotic infection and/or inflammation
The fetal origin of amniotic fluid neutrophils was established when X and Y chromosomes were detected by FISH in the amniotic fluid leukocytes of four women with intra-amniotic infection who delivered premature male infants49. A subsequent study showed that amniotic fluid leukocytes of four pregnant macaques that received an intra-amniotic infusion of IL-1β, which causes preterm labor/birth92-96, were predominantly of fetal origin50. In the current study, DNA fingerprinting revealed that amniotic fluid neutrophils were predominantly of fetal origin when collected from mostly women with preterm gestations. Altogether, this evidence is consistent, indicating that a predominance of fetal neutrophils can be observed in the amniotic cavity of women with intra-amniotic infection and/or inflammation in preterm gestations.
In most of the cases for which amniotic fluid neutrophils were mostly of fetal origin, one or several bacteria of different genera were found in the amniotic cavity. In some of the cases, however, bacteria were not detectable by conventional microbiological techniques or live/dead staining, which is able to detect genital mycoplasmas46. These data suggest that fetal neutrophils invade the amniotic cavity in response to monomicrobial and polymicrobial infections as well as in the setting of sterile intra-amniotic inflammation, an inflammatory process induced by alarmins or danger signals36-39, 68, 97, 98. In either scenario, fetal neutrophils possibly invade the amniotic cavity by migrating from the chorionic plate or the fetus itself (e.g. lung)49 as none of the cases reported herein presented necrotizing funisitis. Indeed, a previous report implied that neutrophils migrate from the fetal vasculature of the chorionic plate (i.e. chorionic vasculitis) into the amniotic cavity51, particularly in those cases without acute umbilical phlebitis or arteritis.
One amniotic fluid sample containing predominantly fetal neutrophils did not show maternal or fetal inflammatory responses in the placental tissues. This sample was derived from a woman who had neither intra-amniotic infection (negative microbiological culture) nor inflammation (IL-6<2.6 ng/mL). Yet, the number of leukocytes was elevated in the amniotic fluid (286 viable cells/mm3), which suggests that a pro-inflammatory response took place in the amniotic cavity13. The fact that there was an elevated number of leukocytes, in the absence of a high concentration of IL-6, can be due to the possibility that the inflammatory process present in the amniotic cavity is mediated by an IL-6-independent pathway68. In this particular case, the inflammatory process in the amniotic cavity could be in its early stage since the fetus and the host (the mother) did not elicit severe immune responses in the umbilical cord and the chorioamniotic membranes, respectively.
Amniotic fluid neutrophils can be predominantly of maternal origin in women with intra-amniotic infection/inflammation
DNA fingerprinting revealed that amniotic fluid neutrophils are predominantly of maternal origin when collected from women in mostly late preterm (>35.6 weeks) and term (≥37 weeks) gestations. Interestingly, in all of the cases, Ureaplasma urealyticum was found in the amniotic cavity by conventional microbiological techniques or live/dead staining. This genital mycoplasma was either found to be alone or associated with Mycoplasma hominis, both of which are associated with spontaneous preterm labor and birth32, 62, 63, 78, 79, 99-104 and/or clinical chorioamnionitis73, 104, both regarded as major clinical pregnancy complications105-115. Genital mycoplasmas can induce severe inflammatory responses in the amniotic cavity and surrounding tissues116-120, which may be characterized by the migration of maternal neutrophils into this compartment, as demonstrated herein. In this scenario, neutrophils possibly invade the amniotic cavity by migrating from the maternal vasculature of the decidua or the intervillous space48. This is consistent with the findings reported herein showing that women included in this study had acute maternal inflammatory responses in both the chorioamniotic membranes and chorionic plate membranes. Previous studies indicated that infiltrating neutrophils in the chorioamniotic membranes of women with acute histologic chorioamnionitis, a manifestation of intra-amniotic infection/inflammation36, 87, 121-124, are of maternal origin, observed while using fluorescent in situ hybridization52, 125. Such infiltrating maternal neutrophils can participate in the mechanisms of host defense against intra-amniotic infection by forming neutrophil extracellular traps126. Taken together, these findings suggest that neutrophils migrate from the maternal vasculature into the amniotic fluid of women with intra-amniotic infection/inflammation to participate in the host defense mechanisms against pathogens invading the amniotic cavity.
A woman who was diagnosed with intra-amniotic infection had predominantly maternal neutrophils but did not present acute histologic maternal or fetal inflammatory responses in the placental tissues. Such an infection was caused by Ureaplasma urealyticum, detected by conventional microbiological techniques and bacterial live/dead staining. Women with intra-amniotic infection associated with this genital mycoplasma presented an infiltration of maternal neutrophils in the amniotic cavity and severe inflammatory responses in the chorioamniotic membranes and chorionic plate membranes. Therefore, it is likely that, in this particular case, acute inflammatory responses were not observed in the placenta because of the heterogeneity of the placental reaction patterns89.
Interestingly, amniotic fluid neutrophils of maternal origin were observed only during late preterm gestation (>35.6 weeks) and in the presence of bacteria. These findings suggest that the infiltration of maternal neutrophils is observed in the amniotic cavity when persistent infection occurs. Further studies are required to investigate whether the chemokine receptors and ligands expressed by amniotic fluid neutrophils of maternal origin are different from those of fetal origin.
Fetal and maternal neutrophils can be found in the amniotic fluid of women with intra-amniotic infection/inflammation
DNA fingerprinting, in some cases, showed that the amniotic fluid contains both fetal and maternal neutrophils in women diagnosed with intra-amniotic infection/inflammation. The majority of these cases presented grade 2 acute histologic maternal inflammatory responses in the placental tissues, providing further evidence that high-grade maternal inflammatory responses contribute to the abundance of maternal neutrophils in the amniotic cavity48.
One woman (patient #15) included in this study underwent two amniocenteses. The first amniotic fluid sample contained mostly fetal neutrophils but no bacteria, and the second amniotic fluid sample had mostly maternal neutrophils and was positive for Ureaplasma urealyticum. In this case, acute histologic maternal and fetal inflammatory responses (stage 2/grade 1) were observed in the placental tissues. This observation indicates that in some women infiltration of fetal neutrophils is associated with mild intra-amniotic inflammation, and if infection progresses, an invasion of maternal neutrophils into this compartment may be observed.
Strengths and Limitations
The current study represents the most extensive examination of the origin of amniotic fluid neutrophils in women having a term or a preterm delivery. This study was the first to utilize DNA fingerprinting, a technology that provides highly discriminating power and sensitivity,54 to study the origin of amniotic fluid neutrophils. The major finding of the study is that amniotic fluid neutrophils can also be of maternal origin or a mixture of both fetal and maternal neutrophils, a novel finding that extends the current belief that amniotic fluid neutrophils are solely of fetal origin49, 50.
Limitations of the current cross-sectional study include: 1) it does not allow the establishment of the timing of the fetal and/or maternal inflammatory responses in the amniotic cavity, and 2) it does not permit the characterization of such inflammatory responses based on the severity and progression of the infection.
Conclusion
Neutrophils are the most abundant WBC found in the amniotic cavity of women with intra-amniotic infection/inflammation8, 13. Indeed, their number is a useful marker for intra-amniotic inflammation8. In the current study, we provide DNA fingerprinting evidence indicating that, indeed, amniotic fluid neutrophils can be either predominantly of fetal or maternal origin, or a mixture of both fetal and maternal neutrophils, in women with intra-amniotic infection and/or inflammation. These results indicate that both the fetus and the mother can contribute to the mechanisms of host defense against intra-amniotic infection.
Supplementary Material
Video 1. A 3D reconstruction of a confocal z-stack image of an amniotic fluid sample including mostly fetal neutrophils containing both X and Y chromosomes. 400× magnification.
Video 2. A 3D reconstruction of a confocal z-stack image of an amniotic fluid sample including mostly maternal neutrophils containing two X chromosomes. 400× magnification.
Acknowledgments
We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. We also thank staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections. Finally, we thank Maureen McGerty and Tara N Mial for their critical readings of the manuscript.
Grant Support: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Footnotes
Disclosure statement: The authors report no conflict of interest.
Paper Presentation Information: Not applicable.
Disclaimer for authors employed by the U.S. Federal Government: Dr. Roberto Romero has contributed to this work as part of his official duties as an employee of the U.S. Federal Government
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970;106:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 2.Larsen B, Snyder IS, Galask RP. Bacterial growth inhibition by amniotic fluid. I. In vitro evidence for bacterial growth-inhibiting activity. Am J Obstet Gynecol. 1974;119:492–6. doi: 10.1016/0002-9378(74)90207-5. [DOI] [PubMed] [Google Scholar]
- 3.Schlievert P, Johnson W, Galask RP. Isolation of a low-molecular-weight antibacterial system from human amniotic fluid. Infect Immun. 1976;14:1156–66. doi: 10.1128/iai.14.5.1156-1166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlievert P, Johnson W, Galask RP. Amniotic fluid antibacterial mechanisms: newer concepts. Semin Perinatol. 1977;1:59–70. [PubMed] [Google Scholar]
- 5.Tafari N, Ross SM, Naeye RL, Galask RP, Zaar B. Failure of bacterial growth inhibition by amniotic fluid. Am J Obstet Gynecol. 1977;128:187–9. doi: 10.1016/0002-9378(77)90685-8. [DOI] [PubMed] [Google Scholar]
- 6.Niemela A, Kulomaa M, Vija P, Tuohimaa P, Saarikoski S. Lactoferrin in human amniotic fluid. Hum Reprod. 1989;4:99–101. doi: 10.1093/oxfordjournals.humrep.a136854. [DOI] [PubMed] [Google Scholar]
- 7.Pierce J, Jacobson P, Benedetti E, et al. Collection and characterization of amniotic fluid from scheduled C-section deliveries. Cell Tissue Bank. 2016;17:413–25. doi: 10.1007/s10561-016-9572-7. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Yoon BH, Mazor M, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200–10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, Romero R. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstet Gynecol. 1996;87:231–7. doi: 10.1016/0029-7844(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Varea A, Romero R, Xu Y, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2016 doi: 10.1515/jpm-2016-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller KA, Greig PC, Heine RP. Amniotic-fluid Lactoferrin: A Marker for Subclinical Intraamniotic Infection Prior to 32 Weeks Gestation. Infect Dis Obstet Gynecol. 1995;3:179–83. doi: 10.1155/S1064744995000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otsuki K, Yoda A, Saito H, et al. Amniotic fluid lactoferrin in intrauterine infection. Placenta. 1999;20:175–9. doi: 10.1053/plac.1998.0368. [DOI] [PubMed] [Google Scholar]
- 16.Pacora P, Maymon E, Gervasi MT, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904–10. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza J, Chaiworapongsa T, Romero R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 18.Soto E, Espinoza J, Nien JK, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836 e1–36 e18. doi: 10.1016/j.ajog.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kacerovsky M, Musilova I, Stepan M, Andrys C, Drahosova M, Jacobsson B. Detection of intraamniotic inflammation in fresh and processed amniotic fluid samples with the interleukin-6 point of care test. Am J Obstet Gynecol. 2015;213:435–6. doi: 10.1016/j.ajog.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet. 1986;1:1380. doi: 10.1016/s0140-6736(86)91685-5. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–7. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Wu YK, Mazor M, Hobbins JC, Mitchell MD. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988;34:141–5. doi: 10.1016/0952-3278(88)90137-8. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Wu YK, Mazor M, Oyarzun E, Hobbins JC, Mitchell MD. Amniotic fluid arachidonate lipoxygenase metabolites in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1989;36:69–75. doi: 10.1016/0952-3278(89)90020-3. [DOI] [PubMed] [Google Scholar]
- 26.Bry K, Hallman M. Prostaglandins, inflammation, and preterm labor. J Perinatol. 1989;9:60–5. [PubMed] [Google Scholar]
- 27.Mazor M, Wiznitzer A, Maymon E, Leiberman JR, Cohen A. Changes in amniotic fluid concentrations of prostaglandins E2 and F2 alpha in women with preterm labor. Isr J Med Sci. 1990;26:425–8. [PubMed] [Google Scholar]
- 28.Hsu CD, Meaddough E, Aversa K, et al. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am J Perinatol. 1998;15:683–7. doi: 10.1055/s-2007-999302. [DOI] [PubMed] [Google Scholar]
- 29.Lee SE, Park IS, Romero R, Yoon BH. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009;22:880–6. doi: 10.1080/14767050902994648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddipati KR, Romero R, Chaiworapongsa T, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014;28:4835–46. doi: 10.1096/fj.14-254383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JY, Ahn TG, Lee J, Yoon BH. Elevated prostaglandin F2a concentration in amniotic fluid is an independent risk factor for intra-amniotic inflammation and adverse pregnancy outcome in patients with preterm labor. Am J Obstet Gynecol. 2015;212:S360. [Google Scholar]
- 32.Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2016;29:2563–72. doi: 10.3109/14767058.2015.1094794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddipati KR, Romero R, Chaiworapongsa T, et al. Clinical chorioamnionitis at term: the amniotic fluid fatty acyl lipidome. J Lipid Res. 2016;57:1906–16. doi: 10.1194/jlr.P069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci. 2010;1205:118–22. doi: 10.1111/j.1749-6632.2010.05684.x. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. doi: 10.1111/aji.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- 41.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–8. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet Gynecol. 2007;30:793–8. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maymon E, Romero R, Chaiworapongsa T, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 44.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 45.Gravett MG, Novy MJ, Rosenfeld RG, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Lopez N, Romero R, Xu Y, et al. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci. 2016 doi: 10.1177/1933719116678690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017 doi: 10.1111/aji.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanc WA. Pathology of the placenta and cord in ascending and in haematogenous infection. Ciba Found Symp. 1979:17–38. doi: 10.1002/9780470720608.ch3. [DOI] [PubMed] [Google Scholar]
- 49.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol. 1997;176:77–81. doi: 10.1016/s0002-9378(97)80015-4. [DOI] [PubMed] [Google Scholar]
- 50.Macias AE, Wong SW, Sadowsky DW, et al. Maternal or fetal origin of rhesus monkey (Macaca mulatta) amniotic fluid leukocytes can be identified by polymerase chain reaction using the zinc finger Y gene. Am J Primatol. 2001;55:159–70. doi: 10.1002/ajp.1049. [DOI] [PubMed] [Google Scholar]
- 51.Lee SD, Kim MR, Hwang PG, Shim SS, Yoon BH, Kim CJ. Chorionic plate vessels as an origin of amniotic fluid neutrophils. Pathol Int. 2004;54:516–22. doi: 10.1111/j.1440-1827.2004.01659.x. [DOI] [PubMed] [Google Scholar]
- 52.Steel JH, O'Donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta. 2005;26:672–7. doi: 10.1016/j.placenta.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Reichard KK, Hall BK, Corn A, Foucar MK, Hozier J. Automated analysis of fluorescence in situ hybridization on fixed, paraffin-embedded whole tissue sections in B-cell lymphoma. Mod Pathol. 2006;19:1027–33. doi: 10.1038/modpathol.3800630. [DOI] [PubMed] [Google Scholar]
- 54.Jobling MA, Gill P. Encoded evidence: DNA in forensic analysis. Nat Rev Genet. 2004;5:739–51. doi: 10.1038/nrg1455. [DOI] [PubMed] [Google Scholar]
- 55.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–8. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Gilstrap LC, 3rd, Cox SM. Acute chorioamnionitis. Obstet Gynecol Clin North Am. 1989;16:373–9. [PubMed] [Google Scholar]
- 58.Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol. 1991;164:1317–26. doi: 10.1016/0002-9378(91)90707-x. [DOI] [PubMed] [Google Scholar]
- 59.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 61.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 63.Romero R, Shamma F, Avila C, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990;163:757–61. doi: 10.1016/0002-9378(90)91063-i. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769–78. doi: 10.1097/00003081-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–8. [PubMed] [Google Scholar]
- 66.Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275–9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruciani L, Romero R, Vaisbuch E, et al. Pentraxin 3 in amniotic fluid: a novel association with intra-amniotic infection and inflammation. J Perinat Med. 2010;38:161–71. doi: 10.1515/JPM.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329–43. doi: 10.1515/jpm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125 e1–25 e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 72.Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325 e1–25 e10. doi: 10.1016/j.ajog.2013.10.882. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2015;28:1510–9. doi: 10.3109/14767058.2014.961417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360–7. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29:349–59. doi: 10.3109/14767058.2015.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 78.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–24. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 79.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiGiulio DB, Gervasi MT, Romero R, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38:495–502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 82.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–9. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 83.Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–36. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamada H, Arinami T, Sohda S, Hamaguchi H, Kubo T. Mid-trimester fetal sex determination from maternal peripheral blood by fluorescence in situ hybridization without enrichment of fetal cells. Prenat Diagn. 1995;15:78–81. doi: 10.1002/pd.1970150117. [DOI] [PubMed] [Google Scholar]
- 85.Thilaganathan B, Sairam S, Ballard T, Peterson C, Meredith R. Effectiveness of prenatal chromosomal analysis using multicolor fluorescent in situ hybridisation. BJOG. 2000;107:262–6. doi: 10.1111/j.1471-0528.2000.tb11698.x. [DOI] [PubMed] [Google Scholar]
- 86.Gerami P, Mafee M, Lurtsbarapa T, Guitart J, Haghighat Z, Newman M. Sensitivity of fluorescence in situ hybridization for melanoma diagnosis using RREB1, MYB, Cep6, and 11q13 probes in melanoma subtypes. Arch Dermatol. 2010;146:273–8. doi: 10.1001/archdermatol.2009.386. [DOI] [PubMed] [Google Scholar]
- 87.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213:S21–8. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 89.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 90.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Khong TY, Mooney EE, Ariel I, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 92.Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol. 1994;171:1668–72. doi: 10.1016/0002-9378(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 93.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121–6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 94.Bethea CL, Gravett MG, Sadowsky DW, Haluska GJ, Axthelm MK, Novy MJ. Amniotic fluid prolactin is decreased by experimental intrauterine infection or interleukin-1beta infusion but not via prostaglandins in pregnant rhesus macaques. Biol Reprod. 1998;58:1385–93. doi: 10.1095/biolreprod58.6.1385. [DOI] [PubMed] [Google Scholar]
- 95.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, et al. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002;186:128–38. doi: 10.1067/mob.2002.118916. [DOI] [PubMed] [Google Scholar]
- 96.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 97.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016;75:3–7. doi: 10.1111/aji.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016;95:130. doi: 10.1095/biolreprod.116.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379–82. [PubMed] [Google Scholar]
- 100.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 101.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 102.Allen-Daniels MJ, Serrano MG, Pflugner LP, et al. Identification of a gene in Mycoplasma hominis associated with preterm birth and microbial burden in intraamniotic infection. Am J Obstet Gynecol. 2015;212:779 e1–79 e13. doi: 10.1016/j.ajog.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoneda N, Yoneda S, Niimi H, et al. Polymicrobial Amniotic Fluid Infection with Mycoplasma/Ureaplasma and Other Bacteria Induces Severe Intra-Amniotic Inflammation Associated with Poor Perinatal Prognosis in Preterm Labor. Am J Reprod Immunol. 2016;75:112–25. doi: 10.1111/aji.12456. [DOI] [PubMed] [Google Scholar]
- 104.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216:604 e1–-04 e11. doi: 10.1016/j.ajog.2017.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 106.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21:74–9. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J Perinatol. 2014;34:611–5. doi: 10.1038/jp.2014.81. [DOI] [PubMed] [Google Scholar]
- 110.Johnson C, Burd I, Northington F, Graham E. Chorioamnionitis and its effect on neonatal correction of acidemia in the very low birthweight infant. Am J Obstet Gynecol. 2015;212:S160. [Google Scholar]
- 111.Johnson CB, Jenkins DD, Bentzley JP, et al. Proton magnetic resonance spectroscopy and outcome in term neonates with chorioamnionitis. J Perinatol. 2015;35:1030–6. doi: 10.1038/jp.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mendez-Figueroa H, Abramovici A, O'Neil AE, Dahlke J, Pedroza C, Chauhan S. Chorioamnionitis without and with neonatal sepsis: newborn and infant outcomes. Am J Obstet Gynecol. 2015;2012:S318–S19. [Google Scholar]
- 113.Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016;29:1525–9. doi: 10.3109/14767058.2015.1053862. [DOI] [PubMed] [Google Scholar]
- 114.Haar EM, Gyamfi-Bannerman C, Randis TM. Abnormal brain imaging in infants exposed to chorioamnionitis and sepsis. Am J Obstet Gynecol. 2016;214:S11–S12. [Google Scholar]
- 115.Rushing N, Pippin L, Black L, Fortner K. Retrospective review of maternal and neonatal outcomes with discordant histologic and clinical chorioamnionitis in labor. Am J Obstet Gynecol. 2016;215:S823–S24. [Google Scholar]
- 116.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 117.Kacerovsky M, Pliskova L, Bolehovska R, et al. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol. 2012;206:342 e1–8. doi: 10.1016/j.ajog.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 118.Prince A, Ma J, Miller L, et al. Chorioamnionitis induced by intramniotic injection of IL1, LPS or Ureaplasma parvum is associated with an altered microbiome in a primate model of inflammatory preterm birth (PTB) Am J Obstet Gynecol. 2015;212:S153. [Google Scholar]
- 119.Senthamaraikannan P, Presicce P, Rueda CM, et al. Intra-amniotic Ureaplasma parvum-Induced Maternal and Fetal Inflammation and Immune Responses in Rhesus Macaques. J Infect Dis. 2016;214:1597–604. doi: 10.1093/infdis/jiw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Potts LC, Feng L, Jayes FL, Murtha AP. Novel ex vivo system to evaluate inflammation of gestational membranes in response to U. parvum. Am J Obstet Gynecol. 2016;214:S192–S93. [Google Scholar]
- 121.Horowitz J, Hussain N, Sanders M, Campbell W. Is maternal genital mycoplasma colonization associated with histologic chorioamnionitis? Am J Obstet Gynecol. 2015;212:S394. [Google Scholar]
- 122.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44:33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee J, Romero R, Lee KA, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol. 2016;214:366 e1–9. doi: 10.1016/j.ajog.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kunze M, Klar M, Morfeld CA, et al. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol. 2016;215:96 e1–8. doi: 10.1016/j.ajog.2016.01.181. [DOI] [PubMed] [Google Scholar]
- 125.McNamara MF, Wallis T, Qureshi F, Jacques SM, Gonik B. Determining the maternal and fetal cellular immunologic contributions in preterm deliveries with clinical or subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 1997;5:273–9. doi: 10.1155/S1064744997000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gomez-Lopez N, Romero R, Leng Y, et al. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol. 2017;77 doi: 10.1111/aji.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. A 3D reconstruction of a confocal z-stack image of an amniotic fluid sample including mostly fetal neutrophils containing both X and Y chromosomes. 400× magnification.
Video 2. A 3D reconstruction of a confocal z-stack image of an amniotic fluid sample including mostly maternal neutrophils containing two X chromosomes. 400× magnification.


