Abstract
Irisin is a myokine that leads to increased energy expenditure by stimulating the ‘browning’ of white adipose tissue. In the first description of this hormone, increased levels of circulating irisin, which is cleaved from its precursor fibronectin type III domain-containing protein 5, were associated with improved glucose homeostasis by reducing insulin resistance. Consequently, several studies attempted to characterize the role of irisin in glucose regulation, but contradictory results have been reported, and even the existence of this hormone has been questioned. In this Review, we present the current knowledge on the physiology of irisin and its role in glucose homeostasis. We describe the mechanisms involved in the synthesis, secretion, circulation and regulation of irisin, and the controversies regarding the measurement of irisin. We also discuss the direct effects of irisin on glucose regulatory mechanisms in different organs, the indirect effects and interactions with other hormones, and the important open questions with regard to irisin in those organs. Finally, we present the results from animal interventional studies and from human clinical studies investigating the association of irisin with obesity, insulin resistance, type 2 diabetes mellitus and the metabolic syndrome.
In humans, hormones can regulate glucose homeostasis directly, by modulating glucose uptake, storage and release, or indirectly, by interacting with other hormones that are important for glucose regulation, such as insulin and glucagon1. A chronic high-calorie diet combined with physical inactivity promotes obesity and a state of subclinical tissue inflammation, which results in insulin resistance and an imbalance in glucose metabolism that lead to the development of type 2 diabetes mellitus (T2DM)2,3.
Irisin is a myokine that is secreted after exercise and that is associated with increased energy expenditure because of its ability to stimulate the browning of white adipose tissue (WAT)4. When the hormone was first described, increased circulating levels of irisin, induced by adenoviral overexpression of its precursor, fibronectin type III domain-containing protein 5 (FNDC5), slightly reduced the weight of mice fed a high-fat diet (HFD) but substantially decreased levels of glucose and insulin, indicating an improvement in insulin resistance4. Subsequently, many investigators have tried to characterize the role of irisin in glucose regulation, reporting contradictory results and even questioning the very existence of the hormone. In this Review, we discuss the current knowledge about irisin in glucose homeostasis and T2DM development. We also review the discrepant results between different studies and propose future directions for further investigation.
Physiology of irisin
Synthesis and secretion
Irisin was first described in 2012 as a hormone that is secreted from the muscle cells of transgenic mice overexpressing Ppargc1a, which encodes the transcription cofactor peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α) that is involved in many pathways related to energy metabolism4. PGC1α stimulates the expression of FNDC5 and the synthesis of the transmembrane FNDC5 protein, which consists of 212 amino acids in humans and 209 amino acids in mice and rats5–7. The protein sequence includes a signal peptide, a fibronectin III domain, a hydrophobic transmembrane domain and a carboxy-terminal domain located in the cytoplasm. After proteolytic cleavage, glycosylation and probably dimerization of FNDC5, a new protein consisting of most of the fibronectin III domain is released. This protein, which consists of 112 amino acids, was named irisin; the amino acid sequence is identical in humans and mice4,8.
In humans, FNDC5 is highly expressed in skeletal muscle and in other organs that contain muscle, such as the heart, tongue and rectum9. Conversely, expression of FNDC5 is low in the pancreas and liver, which are key organs involved in glucose homeostasis9. Adipose tissue is also an important source of irisin. In rats, irisin is released from mature adipocytes of WAT, mainly from those in subcutaneous adipose tissue (SAT) and, to a lesser extent, from those in visceral adipose tissue10. However, brown adipose tissue (BAT) expresses almost no Fndc5 or irisin10. In mice, muscle-derived irisin represents ~72% of the total circulating levels of the protein, with the remaining 28% probably deriving from adipose tissue4,10. In humans, expression of FNDC5 in adipose tissue is 100–200 times lower than in skeletal muscle9,11,12, which suggests that adipose tissue is not the primary source of irisin. However, whether the increased expression levels of FNDC5 in muscle corresponds to increased synthesis of FNDC5 protein and, subsequently, to higher levels of released irisin is currently not known.
Circulation and detection
In addition to skeletal and cardiac muscle, irisin has also been detected in the brain (neurons and neuroglia), the skin (sebaceous glands) and in small amount in the liver, pancreas, spleen, stomach and testis of rats13. Circulating irisin is removed from the body mainly through the hepatobiliary system and the kidneys14. The reported circulating levels of irisin seem to differ greatly even in the same species, with concentrations being reported in human serum or plasma between 0.01 ng/ml and 2,000 ng/ml (REFS 15–19). These inconsistencies have raised doubts about the validity of the different assays and even about the actual existence of irisin in humans15–20.
The antibody that was used to detect irisin in the first paper to describe the hormone binds to the hydrophobic and C-terminal domains of FNDC5, which remain intracellular and are not secreted4. Consequently, the observed protein bands denoted as irisin might have been full-length secreted FNDC5 or nonspecific proteins that cross-reacted with the antibody21. However, some investigators reported that they have observed immunoreactivity with this antibody against recombinant irisin22. This antibody was later withdrawn from the market, and new antibodies targeting the fibronectin III domain of irisin were developed. These new antibodies have been used to detect circulating irisin in commercial ELISAs in clinical studies23–28 and gave protein bands in western blots at molecular sizes ranging from ~12 kDa, the expected molecular mass of irisin, to 35 kDa (REFS 24,25,29) (BOX 1).
Box 1. Irisin ELISAs.
EK-067-52 (Phoenix Pharmaceuticals)*
Published detection range: 0.328–204.9 ng/ml; minimum detection level: 4.15 ng/ml; linear range: 4.15–40.9 ng/ml
It measures above the expected values but has a spectrum of optical density values for standard concentrations that is wider than EK-067-29
Measured irisin levels fall consistently in the linear range130
Western blot analysis using the antibody of this ELISA kit detects a band at 25 kDa for recombinant irisin
It detects concentration spikes after the addition of recombinant irisin to the samples; limited availability
EK-067-52 is the best validated ELISA kit to date
EK-067-29 (Phoenix Pharmaceuticals)*
Published detection range: 0.1–1,000 ng/ml; minimum detection level: 1.29 ng/ml; linear detection range: 1.29–27.5 ng/ml
It measures irisin concentrations within the expected range according to tandem mass spectrometry
Western blot analysis using the antibody of this ELISA kit detects three bands at 25 kDa (glycosylated twice), 22 kDa (glycosylated once) and 15 kDa (unglycosylated)
The protein sequence detected in western blot analyses matches the sequence of irisin identified by matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry70
According to our observations, irisin concentrations measured with EK-067-29 often fall outside the linear range; the standard curve does not correlate well with the one of EK-067-52
Owing to the limited availability of EK-067-52, EK-067-29 is the best currently available irisin ELISA kit
AG-45A-0046EK-k101 (Adipogen)*
Published detection range: 1–5,000 ng/ml; sensitivity: 1 ng/ml
It seems to measure concentrations of irisin one order of magnitude higher than EK-067-52 or EK-067-29, and much higher than the values expected from mass spectrometry
CSB-EQ027943HU (Cusabio)
Published detection range: 3.12–200 ng/ml; sensitivity: 0.78 ng/ml; linear range: ~6.25–50 ng/ml (REFS 27,60)
It measures concentrations of irisin that are above the expected values and nonsignificantly higher than the concentrations obtained with EK-067-29 (REF. 27)
It has not been further evaluated
SEN576Hu (Cloud-Clone Corp.)
Published detection range: 0.015625–1.0 ng/ml
It has not been compared with other assays
The reported range for this antibody is far below the expected values in humans
To clarify these discrepancies, investigators found that a 32 kDa protein detected by one irisin antibody could be reduced to 24 kDa by deglycosylation, the expected molecular mass of an irisin dimer25. In another study, the investigators identified a protein of ~20 kDa, and they claimed that it was glycosylated irisin29. However, this band could not be detected using commercial assays, and the authors questioned the results from previous clinical studies29. Furthermore, another study identified that human irisin has a different start codon (ATA instead of ATG) in FNDC5 compared with mouse or rat irisin21. ATA start codons are viable but are generally associated with low eukaryotic mRNA translation efficiency30; a bioinformatic analysis of human FNDC5 mRNA predicted that a stem-loop structure or hairpin for human FNDC5, which could increase the translational efficiency despite the presence of the non-canonical start codon, could not form29. Moreover, in HEK293 cells, transfection of human FNDC5 containing an ATA start codon led to only 1% of the full-length protein being translated compared with transfection of FNDC5 derived from human or mouse vectors using ATG as the start codon21. In addition, a downstream in-frame ATG in the human FNDC5 sequence was translated into a truncated FNDC5 protein starting from Met76 and lacking the amino-terminal signal peptide; this led to cytoplasmatic accumulation of the shorter protein21. The investigators concluded that irisin is a transcribed pseudogene that cannot be effectively translated into the full-length FNDC5 protein in humans.
To address these criticisms, some investigators have used targeted mass spectrometry24. After removal of albumin and immunoglobulins and complete deglycosylation of the samples of plasma, a protein band was detected with a molecular mass of 12 kDa, the expected molecular mass of deglycosylated irisin. Moreover, these investigators confirmed that, in humans, irisin is mainly translated via the non-canonical ATA codon. This was demonstrated by targeted mass spectrometric analysis with the use of two unique peptides for the irisin sequence. The first peptide was downstream of the ATA codon and upstream of the first ATG codon in the FNDC5 mRNA sequence, whereas the second peptide was three amino acids downstream of the ATG codon. Irisin concentration was measured in human serum at an average of 3.6 ng/ml in four sedentary individuals and at an average of 4.3 ng/ml in six individuals undergoing aerobic interval training. The authors also stated that levels of irisin might even be underestimated, as a substantial amount of the protein was probably lost during sample preparation24. Although these findings have not yet been challenged, the studies supporting or questioning the existence of irisin have only been performed by a small number of research groups thus far. These findings need to be reproduced independently by other researchers.
The concerns about the quality and accuracy of results from clinical studies are similar to those raised in the past about several other hormones (such as luteinizing hormone, growth hormone (GH) and leptin)31–33. The presence of different protein fragments, the glycosylation of the protein, the presence of free or complexed protein forms in serum and plasma, and the cross reactivity of antibodies with other proteins can all alter the results of immunoassays34. We therefore need to develop assays with specific monoclonal antibodies to detect the non-glycosylated and glycosylated forms of irisin, standardize the preanalytical requirements (sample matrix, preparation and storage) and define reference values and the method of detection for irisin34. Of the available commercial ELISAs, five have been used in most irisin studies (BOX. 1). The development of assays that can reliably detect irisin in its physiological concentrations is crucial for valid and generalizable results in future studies.
Regulation of irisin
The role of exercise
In the first description of irisin, transgenic mice overexpressing the transcriptional co-activator PGC1α in muscle had increased expression of Fndc5 and irisin release4. PGC1α regulates mitochondrial biogenesis and function, as well as gene expression, in muscle cells35–37. As one of the important triggers of PPARGC1A expression is exercise, many studies have investigated the effect of exercise on irisin secretion and have reported contradictory results. For example, two studies did not find an association between levels of PGC1α, FNDC5, irisin and exercise21,38. In vitro exercise-mimicking treatment with forskolin and ionomycin in human primary muscle cell cultures stimulated expression of PGC1α but decreased expression of FNDC5 and irisin secretion38. Similarly, in vitro contraction of human skeletal muscle cells using electrical pulse stimulation increased mRNA levels of PPARGC1A but had no effects on FNDC5 mRNA levels21. Some in vivo studies using different physical exercise protocols have also failed to detect an association between levels of irisin or PGC1α and exercise. Fndc5 mRNA in the diaphragm muscles of obese Zucker rats (n= 16) and lean Zucker rats (n = 16) was unchanged after 9 weeks of aerobic training on a motorized treadmill39. In humans, mRNA levels of PPARGC1A and FNDC5 in skeletal muscle were significantly increased in 26 individuals after 12 weeks of training (FNDC5: 1.4-fold increase in healthy controls and twofold increase in people with pre-diabetes (P< 0.01); PPARGC1A: 6.1-fold increase in healthy controls and 4.9-fold increase in people with pre-diabetes (P < 0.01)), but circulating levels of irisin were paradoxically reduced from 160 ng/ml to 143 ng/ml (P< 0.01)40. Similarly, the expression of FNDC5 in human muscle (biopsy samples from vastus lateralis muscle; n = 9) was unchanged after an 8-week endurance training programme41. Finally, neither acute exercise (that is, low intensity aerobic exercise for 1 h) nor chronic exercise (that is, 21-week heavy-intensity endurance exercise with or without resistance exercise) changed the expression of PPARGC1A or FNDC5 in skeletal muscle (vastus lateralis) or serum levels of irisin in humans; the only exception was a single resistance exercise, which led to a twofold to fourfold increase in PPARGC1A mRNA levels accompanied by a 1.4-fold increase in serum levels of irisin42. However, many other animal and human studies have shown an increase in circulating levels of irisin after exercise23,26,27,43–50. For example, levels of Fndc5 mRNA were approximately threefold higher in muscle samples taken from exercising mice (n = 12) than in muscle of controls and twofold higher in muscle samples taken from human individuals (n = 8) after a controlled period of endurance exercise than in muscle of non-exercising individuals4. These investigators have also shown an increase from 3.6 ng/ml to 4.3 ng/ml in levels of irisin in the serum after 12 weeks of high-intensity aerobic training in humans (four sedentary individuals and six aerobically interval-trained individuals)24. In mice, the level of irisin detected with western blot was also twofold higher in skeletal muscle and 1.5-fold higher in serum after one bout of treadmill exercise, but without an accompanying change in Fndc5 mRNA levels (n = 28). Immunohistochemical analysis showed that irisin was located extracellularly between muscle fibres. Whether the increased levels of irisin after acute exercise are the result of increased physiological secretion from muscle cells or of release due to muscle damage remains unknown51.
In humans, the duration of exercise seems to be important for changes in circulating levels of irisin. For example, an acute bout of vibration exercise increased irisin levels by 9.5% in healthy untrained women (n= 14), and 6 weeks of training increased irisin levels by 18.1% in the same individuals. However, resting irisin levels remained unchained after the 6 weeks of training, indicating that acute, but not chronic, exercise triggers irisin release from muscle46. Other investigators have reported that levels of irisin peak after 3–60 min of exercise and return to baseline 6 h later49, but chronic exercise (ranging from 6 weeks to 1 year) did not alter circulating levels of irisin27,42–44,47,48. The type of acute exercise might also affect irisin, with some studies suggesting that aerobic exercise, as well as other resistance exercises or heavy strength training, stimulates the increase in circulating levels of irisin9,23,26,46,49,52. Finally, cold exposure increases irisin secretion in humans, probably through shivering-related muscle contraction. This shivering probably activates the same downstream pathways that are activated in exercise25.
In addition to PGC1α upregulation that stimulates FNDC5 expression4, the deprivation of intracellular muscle ATP after exercise might trigger synthesis of FNDC5 and release of irisin53. By contrast, the transforming growth factor β (TGFβ) effector protein SMAD3 might suppress the production of irisin54,55. Smad3−/− mice demonstrate increased energy expenditure due to browning of WAT56. After exercise, these mice had higher skeletal muscle levels of Fndc5 and Ppargc1a, as well as higher circulating levels of irisin, than wild-type mice, which shows that SMAD3 might negatively regulate irisin secretion. In cultured skeletal muscle cells, SMAD3 binds to the promoter regions of Fndc5 and Ppargc1a, and reduces their expression.
When interpreting the results of these exercise-based studies, one must remember that a high degree of heterogeneity exists between study designs, which makes reliable and generalizable conclusions difficult. For example, some studies that used chronic-exercise protocols were unable to detect changes in circulating levels of irisin, but these findings should not be interpreted as a lack of effect of exercise on irisin secretion. Moreover, studies that did not show that PGC1α was upregulated by exercise might have not used the appropriate experimental model to investigate the relationship between irisin and exercise. Furthermore, most human studies had few participants, and their results were based on commercially available antibody tests that have been questioned for their sensitivity (BOX 1).
According to the findings, in vitro exercise-mimicking protocols do not stimulate release of irisin from muscle, but variable in vivo results have been reported. However, most evidence from human studies suggests that levels of irisin are increased after acute exercise. Many important questions remain unanswered. As in vitro muscle contraction does not stimulate the release of irisin, the high serum levels of the hormone that are observed after acute exercise might be the result of muscle damage or of unidentified biochemical and molecular changes. Furthermore, failure of chronic exercise to upregulate circulating levels of irisin might indicate the existence of time-dependent changes in FNDC5 expression or irisin release by muscle tissue.
Other factors
Myostatin (which is encoded by MSTN) is a myokine that inhibits myogenesis, muscle differentiation and growth, and has been associated with suppression of irisin57,58. In Mstn-knockout mice, upregulation and phosphorylation of AMP-activated protein kinase (AMPK) activate PGC1α, thus leading to increased Fndc5 expression and release of irisin58. Similarly, HFD-fed mice treated with a myostatin antibody had a significant increase in Ppargc1a and Fndc5 expression, with a subsequent stimulation of browning of WAT.
Treatment of leptin-deficient ob/ob mice with leptin stimulates Fndc5 expression in myocytes in a nitric oxide-dependent manner, and leptin downregulates Fndc5 expression in subcutaneous adipocytes independently of nitric oxide59. Nitric oxide is involved in contractility and development of muscle, and its release is stimulated by leptin through upregulation of inducible nitric oxide synthase expression27,59,60. Consistent with these findings, leptin treatment of SAT explants from non-obese individuals can downregulate expression of FNDC5 (REF. 61). However, administration of leptin in humans at a physiological dose (0.01 mg per kg of body weight) or at a pharmacological dose (0.3 mg per kg of body weight) does not seem to alter circulating levels of irisin62.
Furthermore, treatment of adipocytes with α-lipoic acid can induce FNDC5 expression and secretion of irisin63. α-Lipoic acid is involved in mitochondrial bio-energetic function, and supplementation of it in humans shows beneficial effects in weight loss, glucose homeostasis and obesity64,65. Conversely, incubation of myotubes with one of the inflammatory cytokines IL-1β or tumour necrosis factor (TNF), or both, reduced FNDC5 protein synthesis by 37%, 47% and 57%, respectively63,66. This finding shows that, in inflammatory conditions, downregulation of FNDC5 expression might occur, probably in an attempt of the human body to preserve energy homeostasis by slowing the browning of adipocytes66.
In vitro exposure of human primary muscle cells derived from individuals who were lean or individuals with diabetes mellitus to palmitate or high glucose concentrations inhibited FNDC5 expression by ~40% and ~20%, respectively11. However, FNDC5 expression in muscle cells from individuals with T2DM is generally higher than in muscle cells from obese or lean people. This finding shows that additional body factors are involved in the lipid-mediated and glucose-mediated regulation of FNDC5 expression. Finally, some medications have been associated with changes in FNDC5 expression. In a study investigating whether chronic activation of peroxisome proliferator-activated receptor-α (PPARα) triggered by fenofibrate can increase beige cell depots, fenofibrate stimulated PPARα, which induced PPARGC1A and FNDC5 expression and subsequently stimulated browning through upregulation of UCP1, PR domain zinc finger protein 16 (PRDM16) and bone morphogenetic protein 8 (BMP8)67. Another study investigated whether metformin (a first-line treatment for T2DM that regulates glucose metabolism in the peripheral organs) or glibenclamide (a popular treatment for T2DM) affects irisin secretion. In diabetic db/db mice, metformin treatment increased expression of FNDC5 mRNA (approximately twofold) and protein (~1.3 fold) in muscle, as well as circulating levels of irisin (~20% increase); this finding was confirmed in vitro in C2C12 myotubes. By contrast, glibenclamide did not show similar effects68,69.
Effects on glucose homeostasis
When interpreting the findings of different studies, two limitations should be considered. First, many reports have not been reproduced independently. Second, many studies were performed before the quantification of irisin levels in humans using mass spectrometry and reported results that were obtained after exposure of tissues to concentrations of irisin well above the physiological normal levels.
Direct effects
Adipose tissue
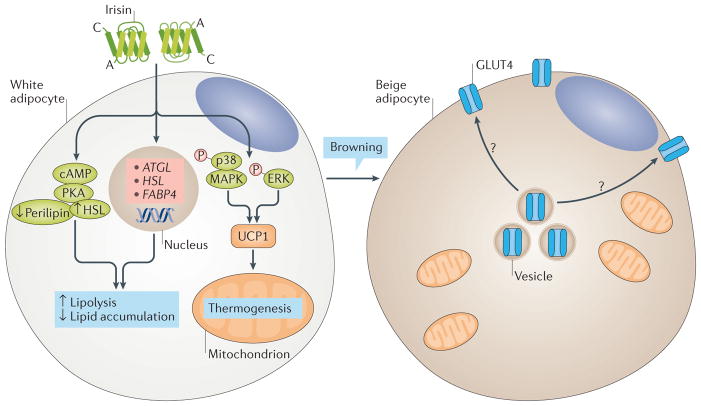
After secretion from muscle, irisin stimulates expression of UCP1 in adipocytes, leading to browning of WAT via p38 mitogen-activated protein kinase (MAPK) and extracellular-signal regulated kinase (ERK) pathways4,70 (FIG. 1). In humans, irisin seems to induce WAT browning in specific types of adipocytes and fat depots71. Human preadipocytes from SAT demonstrate a decrease in differentiation to mature adipocytes after irisin treatment, whereas the expression of genes and/or proteins related to browning (for example, UCP1, PPARγ and PRDM16) remains unaffected or is even decreased53,71,72. Furthermore, irisin does not affect browning-related gene expression in human perirenal adipose tissue, which has high expression of UCP1 and is therefore considered to be BAT72. By contrast, mature adipocytes from SAT demonstrate ex vivo an increase in expression of UCP1 protein (approximately twofold) and of genes related to browning (for example, UCP1 and PRDM16) after treatment with 50 nM irisin53,72. These effects are probably triggered by irisin-mediated activation of the ERK–p38 MAPK signalling pathway72. According to another study, irisin induces browning predominantly in human neck adipose depots, to a lesser extent in SAT25 and not at all in omental adipose tissue. In vivo studies in humans that might provide the conclusive results are currently lacking. In humans, BAT has ten times more insulin-mediated glucose uptake after cold exposure than WAT or visceral adipose tissue73. This increase in glucose uptake results from the upregulation of GLUT4 expression, without significant changes in the expression of GLUT1 or of genes encoding insulin receptors, such as IRS1, IRS2 or INSR73. Irisin seems to induce GLUT4 expression in human mature adipocytes53. However, whether this increase is also associated with increased translocation of GLUT4 to the cell membrane (similar to the one observed in muscle) and/or with increased glucose uptake in adipose tissue remains unknown. Finally, irisin increased the secretion of lactate from human mature adipocytes from SAT, which indicates a stimulation of glycolysis, probably by reducing oxidative respiration through uncoupling in the mitochondria53.
Figure 1. Candidate signalling pathways of irisin in adipocytes.
Irisin stimulates the browning of white adipose tissue (WAT) by inducing the expression of UCP1 gene and, consequently, of UCP1 protein via the p38 mitogen-activated protein kinase (MAPK) and extracellular-signal regulated kinase (ERK) pathway. Brown adipose tissue (BAT) has an increased number of mitochondria and increased energy expenditure due to elevated oxygen consumption, and accumulates smaller lipids than do white adipocytes. After cold exposure, BAT has higher glucose uptake and GLUT4 (also known as SLC2A4) expression than WAT. Irisin, which is also secreted from muscle after cold exposure, can stimulate browning; a possible involvement of irisin in glucose uptake and GLUT4 expression seems to be plausible but has not yet been investigated. Irisin also stimulates lipolysis via the cyclic AMP (cAMP)–protein kinase A (PKA)–perilipin–hormone-sensitive lipase (HSL) pathway and through the upregulation of the expression of PNPLA2 (also known as ATGL), HSL and FABP4.
Overexpression of Fndc5 in obese mice can reduce the size of adipocytes in SAT and stimulates lipolysis via the cyclic AMP–protein kinase A (PKA)–perilipin–hormone-sensitive lipase (HSL) pathway74. Adipocytes treated with irisin are smaller and accumulate fewer lipids than do control cells74. Irisin also stimulates basal and probably isoprenaline-induced lipolysis, inhibits lipid synthesis and stimulates intracellular lipid metabolism by regulating the expression of genes such as Pnpla2 (which encodes adipose triglyceride lipase) and Hsl and of proteins such as fatty acid-binding protein 4 (REFS 53,74,75). Consistent with these findings, HFD-fed mice treated with recombinant lentivirus expressing FNDC5 have a significant reduction in serum levels of cholesterol (~30%; P< 0,05), triglycerides (~50%; P < 0,05) and free fatty acids (~30%; P< 0,05), along with reduced perilipin levels (~30%) and adipocyte diameter in adipose tissues74. Ex vivo irisin and FNDC5 treatment can reduce the differentiation of human preadipocytes leading to decreased fat mass53.
Finally, in patients with obesity or with T2DM, FNDC5 expression, and consequently secretion of irisin from adipocytes, is lower (~25%) than in lean controls11,12. However, as body fat mass is substantially increased in obesity, the levels of adipose tissue-derived irisin might be similar, or even higher, in patients with obesity compared to lean individuals12.
Muscle
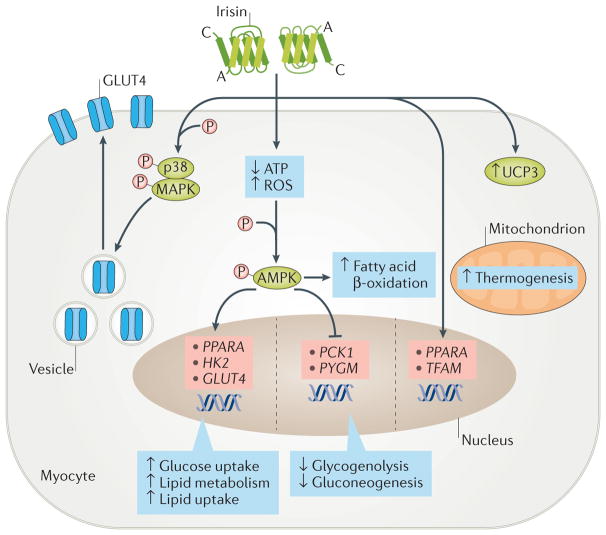
The treatment of primary human skeletal muscle cells with recombinant irisin (50 nM) for 1 h significantly increased uptake of glucose and fatty acid (~30–40%), which was similar to the uptake observed after exposure to insulin45. After 6 h of irisin treatment on these cells, the expression of genes that are involved in glucose transport and lipid metabolism in myocytes (such as GLUT4, HK2 and PPARA) was also upregulated (~30–80%), whereas the expression of genes that are involved in glycogenolysis (PYGM) or gluconeogenesis (PCK1) was suppressed (~20–40%). The changes in muscle metabolism were initiated by a decrease in levels of intracellular ATP, which led to the phosphorylation of AMPK and activation of its downstream pathway45 (FIG. 2). In another study, recombinant irisin (62 ng/ml) stimulated glucose uptake after activation of AMPK in differentiated L6 muscle cells76. The activation of AMPK was achieved through induction of reactive oxygen species (ROS) and led to activation of p38 MAPK, which subsequently induced the translocation of GLUT4 to the plasma membrane of these cells76 (FIG. 2). Intraperitoneal injection of irisin (0.5 μg per g of body weight) in obese and diabetic HFD-fed mice increased uptake and accumulation of glucose by stimulating GLUT4 translocation to skeletal muscle cell membranes. The same effects were seen in the murine C2C12 myoblast cell line treated with irisin (0.3–1.0 μg/ml) and cultured in high-glucose and increased-fatty-acid medium, and were completely attenuated after inactivation of the AMPK pathway with AMPKα2 siRNA77. Finally, irisin (200 nM) can attenuate palmitic acid-induced inhibition of insulin signalling by stimulating the phosphorylation of AKT and ERK in C2C12 cells in vitro78. Irisin, even in concentrations that are lower than those used in other studies (such as 5 nM), stimulates mitochondrial biogenesis by upregulating the gene expression of Tfam, Ppargc1a and Nrf1, as well as the gene and protein levels of UCP3 and GLUT4, in murine C2C12 cells79. Furthermore, irisin does not activate the nuclear factor-κB (NF-κB) pathway in C2C12 cells, which indicates that this myokine, in contrast to TNF, might not be involved in inflammatory responses in muscle79,80 (FIG. 2). Human myocytes from vastus lateralis demonstrate a dramatic increase in FNDC5 expression during differentiation. Treatment of these myocytes with irisin (10 nM and 50 nM) increased insulin-like growth factor 1 and decreased MSTN mRNA expression, both of which are considered important factors for muscle growth. These effects of irisin were mediated by the ERK pathway53.
Figure 2. Candidate signalling pathways of irisin in myocytes.
Irisin can activate the AMP-activated protein kinase (AMPK) pathway by reducing intracellular ATP levels, or by increasing reactive oxygen species (ROS) or intracellular calcium concentrations. Activation of the AMPK pathway stimulates the expression of GLUT4 (also known as SLC2A4), HK2 and PPARA genes and inhibits the expression of PYGM and PCK1 (also known as PEPCKC). The high expression of GLUT4 and HK2, combined with the increased translocation of GLUT4 protein from the cytoplasm to the membrane (mainly via the p38 mitogen-activated protein kinase (MAPK) pathway), induces glucose uptake by myocytes. Conversely, inhibition of PYGM and PCK1 expression reduces glycogenolysis and gluconeogenesis. In addition, the increased expression of PPARA stimulates lipid metabolism. The irisin–AMPK pathway also increases fatty acid β-oxidation. Finally, irisin stimulates biogenesis in mitochondria by regulating the expression of PPARA and TFAM genes and of UCP3 protein.
In humans, synthesis of FNDC5 and secretion of irisin are increased in the muscle of individuals with obesity but without T2DM, possibly in an attempt to maximize glucose uptake in the muscle and prevent hyperglycaemia9,11. Interestingly, after the onset of T2DM in treatment-naive patients, in vivo expression of FNDC5 in muscle is reduced by ~15%; however, in vitro myotubes isolated from these patients are more capable of expressing FNDC5, as its expression is higher in these cells than in myotubes taken from lean individuals38. These results indicate the existence, in the diabetic state, of an endogenous factor that activates expression of FNDC5 and secretion of irisin, such as glucose, insulin or fatty acids. As levels of irisin remain unchanged after a euglycaemic–hyperinsulinaemic clamp in patients with T2DM, insulin seems not to have an effect on irisin secretion regardless of the state of insulin resistance or obesity11. Conversely, treatment of cultured muscle cells with glucose or the saturated fatty acid palmitate can reduce FNDC5 expression by 20% and 40%, respectively11. This suppression of FNDC5 expression by palmitate in vitro is less prominent in myotubes from patients with T2DM than in those from lean controls, whereas the suppressive effect of glucose on FNDC5 expression is stronger in myotubes isolated from individuals with T2DM than in those from lean controls11. Therefore, glucose is probably an important regulator of irisin secretion from muscles in individuals with T2DM11,12.
Liver
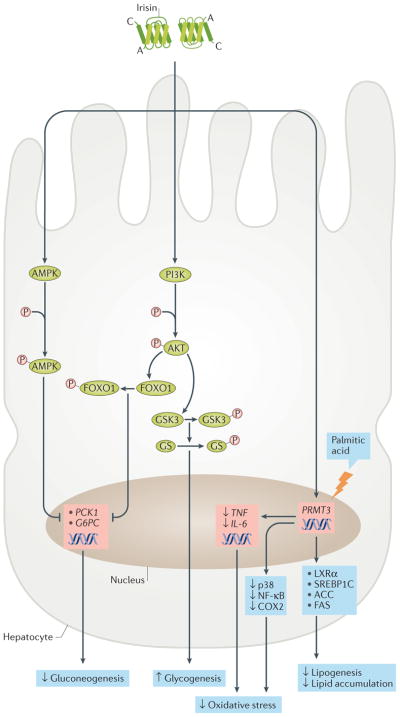
In hepatocytes with insulin resistance derived from human hepatocellular carcinoma cells or in mouse primary hepatocytes, treatment with irisin (20 nM) reduces gluconeogenesis through downregulation of PCK1 and G6PC via the phosphoinositide 3-kinase (PI3K)–AKT–FOXO1 pathway and stimulates glycogenesis through activation of glycogen synthase via the PI3K–AKT–glycogen synthase kinase 3 (GSK3) pathway81 (FIG. 3). Similarly, intraperitoneal injection of irisin (0.5 μg per g of body weight) in diabetic obese C57BL/6 mice decreased Pck1 and G6pc expression in the liver by activating the AMPK pathway77. Moreover, in primary hepatocytes from lean or obese mice, irisin reduced cholesterol content by inhibiting sterol regulatory element-binding protein 2 (SREBP2)82. These findings have been independently confirmed by investigators who also showed that constitutive androstane receptor (CAR) is involved in FNDC5 expression in the liver83. CAR seems to bind specifically to a direct repeat, separated by five nucleotides, of a nuclear receptor-response element of the FNDC5 promoter in HepG2 cells, stimulating FNDC5 expression. The secreted irisin from hepatocytes might act in an autocrine and/or paracrine manner.
Figure 3. Candidate signalling pathways of irisin in hepatocytes.
Irisin reduces gluconeogenesis by downregulating the expression of PCK1 (also known as PEPCKC) and G6PC via AMP-activated protein kinase (AMPK) and the phosphoinositide 3-kinase (PI3K)–AKT–FOXO1 signalling pathway. In addition, it stimulates glycogenesis via the PI3K–AKT–glycogen synthase kinase 3 (GSK3)–glycogen synthase (GS) pathway. Irisin inhibits palmitic acid-induced lipogenesis and lipid accumulation, as well as oxidative stress, by downregulating expression of PRMT3. This leads to reduced expression of several lipogenic markers (for example, liver x receptor-α (LXRα), sterol regulatory element-binding protein 1C (SREBP1C), acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS)) and inflammatory markers (such as the genes TNF and IL6, and the proteins cyclooxygenase 2 (COX2), nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MAPK)).
Moreover, irisin inhibits palmitic acid-induced lipogenesis by reducing the expression of lipogenic markers (Acaca, Fasn) and lipid accumulation in a mouse hepatic cell line (AML12) and in mouse hepatocytes ex vivo84. In addition, irisin prevents oxidative stress by downregulating inflammatory markers such as NF-κB, cyclooxygenase 2 (COX2), p38 MAPK, TNF and IL-6, as well as by slightly reducing the production of ROS, in hepatic cells. These effects are mediated by the inhibition of protein arginine N-methyltransferase 3 (REFS 84,85).
In humans, serum levels of irisin have been inversely associated with the presence of liver enzymes (alanine transaminase and aspartate transaminase) in serum and intrahepatic triglyceride content in a group of patients with obesity in China86; however, subsequent studies reported contradictory results. For example, higher levels of irisin in patients with nonalcoholic fatty liver disease (NAFLD) and increased circulating levels of irisin in patients with the metabolic syndrome and elevated levels of liver enzymes have both been reported87,88. Finally, levels of irisin in individuals with obesity, NAFLD or nonalcoholic steatohepatitis (NASH) are lower than in lean controls, but no difference exists between the three patient groups. Interestingly, in this study, irisin was also independently associated with portal inflammation28,89. Portal inflammation has been linked with progressive NAFLD or advanced disease28,89. Altogether, the role of irisin in NAFLD or NASH has not been adequately described and demands further investigation.
Pancreas
In the human pancreas, the levels of irisin that are detected in islets of Langerhans, serous acinar cells and intralobular duct cells are higher than in cells of muscle or liver, although these results should be interpreted cautiously, given the issues about the specificity of the commercially available irisin antibodies13. The direct effect of irisin on the endocrine pancreas in vitro and ex vivo has not yet been investigated. In humans, irisin is positively associated with circulating levels of insulin and HOMA of β-cell function (HOMA-β) in individuals with normal glucose tolerance (n= 254), indicating that irisin might be involved in the regulation of β-cell function90. However, in another study, irisin correlated neither with HOMA-β nor with fasting C-peptide or fasting insulin levels91.
Other organs
The direct effect of irisin on glucose metabolism in the kidney and nervous system in vitro and/or ex vivo has not been investigated (FIG. 4). Patients with chronic kidney disease have reduced circulating levels of irisin (58%), which is inversely correlated with levels of blood urea nitrogen and serum creatinine92,93. Patients with T2DM and renal insufficiency also have decreased circulating levels of irisin (~8%), which are positively associated with estimated glomerular filtration rate94. Furthermore, in a population of individuals with T2DM, macroalbuminuria was associated with circulating levels of irisin that were lower than those associated with microalbuminuria or normoalbuminuria95,96. Finally, in a large cross-sectional study including 1,115 community-living adults with obesity in China, high levels of irisin (highest quartile of the investigated population) were related to significantly reduced risk of chronic kidney disease (OR 0.572; P= 0.023) and marginally reduced risk of macroalbuminuria (OR 0.611; P= 0.050), suggesting that irisin might be associated with improved renal function97. Future studies should clarify whether the decrease in circulating levels of irisin that were seen in renal impairment are due to increased elimination and/or reduced reabsorption of irisin by the kidney or due to decreased secretion of irisin from muscle and adipose tissue.
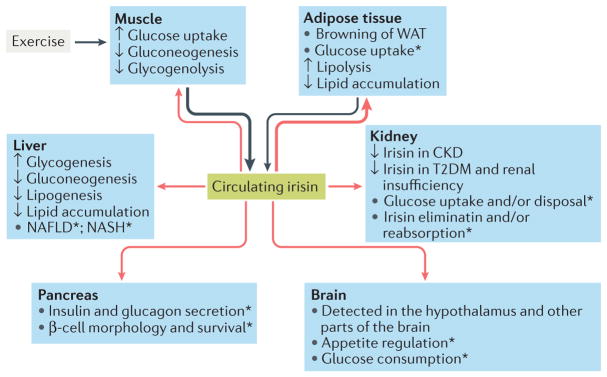
Figure 4. Effects of irisin on glucose homeostasis.
Irisin is primarily secreted by muscle during exercise and secondarily by adipose tissue (black arrows). Irisin reaches different organs via the blood (red arrows), leading to changes in their handling of glucose and lipid homeostasis. The most important target of irisin is adipose tissue, where it stimulates the ‘browning’ of white adipose tissue (WAT). The effects of irisin on muscle, adipose tissue and liver favour states of normoglycaemia and normolipidaemia.
*Although present in pancreas, muscle and brain, the role of irisin in these organs, as well as in kidney and liver (especially in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH)), has yet to be adequately investigated. CKD, chronic kidney disease; T2DM, type 2 diabetes mellitus.
In the nervous system, FNDC5 and irisin have been detected in Purkinje cells of the cerebellum, which send projections to the deep cerebellar nuclei and to the vestibular nuclei98. Moreover, in humans, irisin is localized in the paraventricular nucleus of the hypothalamus and in the cerebrospinal fluid98,99. The function of irisin in these areas remains unknown, although it might be involved in neuronal differentiation, in locomotor coordination during exercise and in metabolic homeostasis98. Knockdown of Fndc5 in neuronal precursors achieved via a doxycycline-inducible short hairpin RNA vector impaired the maturation of neurons and astrocytes in retinoic acid-treated mouse embryonic stem cells100. In addition, in retinoic-acid treated human embryonic stem cells, FNDC5 expression was increased during neural differentiation101. In mouse H19-7 HN cells (a fibroblast cell line that is derived from the hippocampus and that is used as a model of mitogenesis and differentiation), pharmacological concentrations (50–100 nmol/l) of irisin increased cellular proliferation via signal transducer and activator of transcription 3 (STAT3) without altering markers of hippocampal neurogenesis102. Furthermore, overexpression of Fndc5 in primary cortical neurons stimulated the expression of Bdnf, which increased the survival of neurons and supported new neuronal growth and differentiation103. Central administration of recombinant irisin into the third ventricle of rats (2.5 μg) activated neurons in the paraventricular nuclei of the hypothalamus leading to increased blood pressure and cardiac contractibility104. Moreover, in rats, central irisin treatment increased locomotion and oxygen consumption, as well as carbon dioxide and heat production105. Whether the increase in metabolic activity leads to improved glucose homeostasis by reducing insulin resistance and/or increasing glucose uptake in peripheral organs remains to be elucidated.
Despite being detected in the hypothalamus and other areas of the brain, the role of irisin in appetite regulation and glucose consumption in the brain has not been investigated yet. Only one study in individuals who did not have T2DM showed an association between higher pre-nocturnal energy intake and lower fasting levels of irisin the following morning106. However, levels of irisin during fasting did not predict subsequent energy intake, indicating that irisin probably does not regulate appetite in these individuals106.
Among the organs that are adversely affected by T2DM, irisin has been most extensively studied in endothelial cells. In both diet-induced obese mice and apolipoprotein E (Apoe)-knockout (KO) mice with streptozotocin-induced diabetes (Apoe-KO-streptozotocin mice), irisin treatment resulted in improved endothelial function attributable to the activation of the AMPK–AKT–endothelial nitric oxide synthase pathway107,108. In addition, irisin decreased atherosclerotic plaque area and inflammatory response in Apoe-KO-streptozotocin mice108. Also, in mice with streptozotocin-induced diabetes mellitus, intraperitoneal injection of irisin (0.5 mg per kg of body weight) improved bone marrow-derived endothelial progenitor cell function109. Furthermore, in in vitro studies using human endothelial cell lines, irisin inhibited glucose-induced apoptosis and upregulated proliferation and angiogenesis108,110,111. This effect was partly attributed to reduced oxidative stress (due to reduction of intracellular ROS levels and improvement of total antioxidant capacity)108,112.
Indirect effects of irisin
Several studies have reported synergistic effects of irisin and other hormones in the regulation of glucose homeostasis. Irisin treatment in obese mice has been associated with a significant increase (approximately threefold) in levels of betatrophin (which is also known as angiopoietin-like protein 8)70. Overexpression of betatrophin in the liver of mice stimulates β-cell proliferation, thus leading to a threefold increase in β-cell area and a twofold increase in pancreatic insulin content113. Based on these findings, a possible PGC1α–irisin–betatrophin axis has been proposed that regulates glucose homeostasis114. According to this theory, exercise induces the secretion of PGC1α from muscle tissue, which stimulates FNDC5 expression and consequently irisin release from muscle cells; irisin then induces expression of UCP1 in adipose tissue. Consequently, insulin resistance is reduced by the increase in energy expenditure triggered by the browning of WAT and by the increase in β-cell proliferation attributable to increased levels of betatrophin114. However, some studies have failed to reproduce these initial results and have therefore questioned the role of betatrophin in β-cell proliferation and even the existence of such an axis115–117. Betatrophin-deficient mice do not have any changes in β-cell expansion or in glucose metabolism in an insulin-resistant state induced by a HFD or after the administration of the insulin receptor antagonist S961 (REF. 117). Finally, no correlation between levels of betatrophin and irisin has been seen in patients with T2DM91. Taken together, the presence of a PGC1α–irisin–betatrophin axis is questionable and probably not important for glucose regulation. While this paper was in press the original paper on betatrophin was retracted118.
Leptin also participates in glucose homeostasis and might interact with irisin. In addition to the leptin-mediated stimulation in myotubes and to the downregulation of irisin secretion and FNDC5 expression in SAT61, leptin can also induce irisin-dependent myogenesis and inhibit irisin-mediated browning of adipocytes by downregulating UCP1 (REF. 59). Other studies have not found a similar association between levels of irisin and those of leptin or other adipokines27,119. Leptin administration (metreleptin) at a physiological dose (0.01 mg per kg of body weight) or at a high dose (0.1–0.3 mg per kg of body weight), in the short term (3 days) or the long term (16 weeks), in fasting or fed state, does not affect circulating levels of irisin in normal-weight individuals, in patients with obesity or in hypoleptinaemic young women with secondary amenorrhoea62.
Only one study has investigated the involvement of irisin in insulin function and signalling. In that report, in vitro murine myotubes (C2C12) treated with palmitic acid had increased insulin resistance due to inhibition of AKT and ERK phosphorylation78. In vitro, this inhibition was partially abolished by treatment with irisin (100 nM), indicating a positive effect of irisin on insulin function in muscle78. Moreover, several human studies reported a positive association between irisin and fasting levels of insulin but not between irisin and postprandial levels of insulin120–122 (see Supplementary information S1 (table)). Conversely, insulin administration in a euglycaemic–hyperinsulinaemic clamp failed to change irisin levels in patients with T2DM and obesity11. Future research should focus on the effect of irisin on insulin secretion and signalling, and vice versa, and on any possible interactions between the two pathways that might affect glucose homeostasis.
Finally, little information exists about the possible association between irisin and other hormones participating in glucose homeostasis (such as adrenaline, cortisol, GH and incretins). Serum levels of irisin follow a day–night rhythm, meaning that the likelihood of irisin regulating, or being regulated by, cortisol and GH cannot be excluded, as these two hormones follow a specific circadian circulating pattern131. Furthermore, levels of irisin in individuals with a wide range of BMIs, including patients with anorexia nervosa or with morbid obesity, do not correlate with levels of cortisol, TSH, C-reactive protein or ghrelin122. However, a possible relation of irisin with catecholamines, GH or incretins has not been investigated yet.
Irisin and metabolic diseases
Interventional animal studies
In the first description of irisin, wild-type BALB/c mice fed with a HFD for 20 weeks were injected intravenously with FNDC5-expressing adenoviral particles4. After 10 days, these mice had a similar weight compared to that of control mice, but significantly lower glucose levels after intraperitoneal glucose infusion (~20–30% after 60 min, and less after 90 min), as well as lower fasting levels of insulin (~50%), suggesting that irisin can reduce insulin resistance4. This finding was confirmed in a second study using C65BL/6 mice fed a HFD for 10 weeks, which showed lower glucose levels (up to 35% in 60 min) in an intraperitoneal glucose tolerance test, lower fasting levels of insulin (~50% lower) and a reduction in body weight (~5–10%) in mice treated for 14 days with recombinant human irisin compared with control mice70.
In a third report, diabetic HFD-fed mice treated with irisin had improved glucose tolerance and reduced epididymal fat mass (~20%), as well as reduced levels of total cholesterol and triglycerides in the serum, compared with control mice77. This improvement in glucose and lipid profile was achieved by increased glucose uptake combined with reduced gluconeogenesis in the liver. Furthermore, an increase in acetyl-CoA carboxylase-β phosphorylation in skeletal muscle, along with an increase in Ucp1 expression in their epididymal adipose tissue, was observed77. Similarly, in streptozotocin-induced and HFD-induced diabetic mice, subcutaneous perfusion of irisin could reduce fasting blood glucose and increase insulin sensitivity and glycogen content of the liver81. Furthermore, overexpression of Fndc5 in obese mice reduced hyperglycaemia, hyperinsulinism, hyperlipidaemia and blood pressure but also enhanced energy expenditure (measured by O2 consumption, CO2 heat production and total body activity) and lipolysis (measured by expression of perillipin and HSL, phosphorylation of HSL and adipocyte diameter in SAT and VAT)74. Finally, in mice with streptozotocin-induced diabetes, intraperitoneal and oral administration of irisin could reduce glucose levels (~30%) without affecting weight123. Taken together, these data indicate that, in mice, irisin can positively affect glucose homeostasis, lipid profile and other metabolic parameters related to obesity.
Human studies
Many clinical studies have described a positive association between circulating levels of irisin and weight or BMI in populations who did not have T2DM (see Supplementary information S1 (table)). This association has been seen in different subpopulations, such as patients with anorexia nervosa who had significantly lower (~15%) levels of irisin than normal-weight people or individuals with obesity122. Similarly, women with polycystic ovary syndrome who were overweight or with obesity had elevated levels of irisin (~15–20%) compared with individuals of normal weight60. Weight loss (−6.31 ± 0.195%) after dietary changes leads to a significant decrease in circulating irisin (15%), whereas weight regain returns irisin levels to baseline124. Most of these studies also showed a positive correlation between circulating levels of irisin and whole-body mass, fat mass and, occasionally, waist-to-hip ratio. In healthy individuals, most of the irisin in blood derives from muscle cells, but, in obesity, the amount of secreted irisin from adipose tissue is probably higher than in lean states owing to the increase in total fat mass15,124.
Another explanation for the association between irisin, BMI and fat mass might be the development of ‘irisin resistance’. Irisin secretion from muscles is increased in obesity, possibly to maximize energy usage and glucose homeostasis to achieve metabolic balance. The ‘hyperirisinaemia’ seen in obesity might, therefore, be a mechanism to compensate for the observed irisin resistance and to maximize the antiobesity and anti-hyperglycaemic effects of the hormone.
With the exception of reports in specific populations, such as children from Saudi Arabia43, the results of most studies agree that irisin is positively associated with BMI and markers of insulin resistance, such as HOMA and HOMA2 (REFS 43,121,125–128). This assertion was confirmed in a 2016 meta-analysis, in which the investigators showed that circulating levels of irisin are directly and positively, although weakly, associated with insulin resistance in adults who do not have T2DM129. Serum levels of irisin are also positively associated with fasting insulin and blood glucose levels in individuals who are healthy, in those with obesity but not T2DM, in children and in women with polycystic ovary syndrome120,128,130. However, food intake in children and young adults does not alter circulating levels of irisin131, despite stimulating insulin secretion and rising glucose levels. Similarly, in women with overweight or obesity, circulating levels of irisin are unaffected by the increase in glucose and insulin during an oral glucose tolerance test, which indicates that neither glucose nor insulin acutely stimulates irisin release63.
As irisin is associated with BMI and insulin resistance, one might expect that levels of irisin would be higher in populations with T2DM. However, most clinical studies, including a meta-analysis, have reported lower levels of irisin in patients with prediabetes or T2DM than in controls94–96,132–134. This finding might be explained by the reduction of FNDC5 synthesis and, consequently, irisin secretion seen in muscle tissue of patients with obesity and T2DM11. Moreover, we believe that an additional decrease in irisin secretion from adipose tissue, as a result of the inflammatory processes observed in obesity, cannot be excluded. The factor that is responsible for the switch from high muscle-specific secretion of irisin in obesity to low secretion in T2DM has not been identified yet, although ex vivo and in vitro studies suggest that chronic hyperglycaemia and hyperlipidaemia are possible triggers11,12. Consistent with the findings in patients with T2DM, in individuals with gestational diabetes mellitus, levels of irisin are also decreased (~30%; n= 632; meta-analysis)134–136. Levels of irisin in the blood might, therefore, be an important factor in the changes observed in metabolic health and disease. For example, in a fairly healthy population aged 40–70 years at baseline in South Korea, increased serum levels of irisin were independently associated with increased risk of T2DM (ORs for quartile 1 versus quartile 2 versus quartile 3 versus quartile 4 = 1 versus 0.80 versus 3.33 versus 4.10; P< 0.001)137.
Irisin levels are associated positively with the risk of the metabolic syndrome in white and black individuals (OR for highest quartile of irisin levels = 9.44) and associated negatively in individuals from China (OR 0.796; P = 0.027)120,130,138,139. Moreover, increased levels of irisin (highest quartile) are associated with major adverse cardiovascular effects in patients with established coronary artery disease after percutaneous coronary intervention, but the levels are lower in young patients (28–39 years old) who have had a myocardial infarction (~20%) than in young healthy controls140,141. Taken together, no consensus opinion exists regarding the association of irisin and the metabolic syndrome, and larger prospective studies are therefore needed to clarify the issue.
Conclusions
Detecting the factors and understanding the mechanisms that are involved in glucose homeostasis are crucial to develop early diagnostic and therapeutic strategies for T2DM. Irisin, which is involved in energy homeostasis, is a promising regulator of glucose metabolism. Through functions in muscle, liver and adipose tissue, irisin contributes to normoglycaemia (FIG. 4). This assertion is supported by the results from animal studies that show positive effects of treatment with irisin on glucose levels, weight and other metabolic parameters. However, the detailed contribution of irisin to many of the major mechanisms of glucose homeostasis has yet to be clarified. Future research should, therefore, focus on key aspects of irisin biology.
One crucial future step is to determine if a receptor for irisin exists and to characterize its mode of action. This finding might reveal additional functions of irisin and the signalling cascades that are involved in its effect. Moreover, the complete description of the mechanisms involved in irisin secretion by muscle tissue during exercise is also important. Such information might explain the differences between acute and chronic training on circulating levels of irisin and reveal additional stimulators of irisin secretion.
The effect of irisin on endocrine pancreas and especially on β-cell function, morphology and survival, as well as on insulin and glucagon secretion, has not yet been investigated. Similarly, the effects of irisin on glucose use in the brain and appetite regulation have not been reported, despite the presence of irisin in appetite centres of the hypothalamus. Furthermore, the involvement of irisin in glucose uptake or excretion from the kidney, or even its own renal elimination and/or reabsorption, remains unclear; however, clinical studies have indicated an association between circulating levels of irisin and renal function. Last, the interactions between irisin and insulin, glucagon, incretins or other hormones that are involved in glucose homeostasis have not been investigated in animals or humans and should be researched in the future.
Finally, inconsistencies in reported data highlight the necessity for more-accurate methods to measure irisin and for clinical studies better designed than current methods or reported investigations to reveal the role of irisin in T2DM and the metabolic syndrome. The potential use of irisin as a predictive marker for insulin resistance, T2DM or the metabolic syndrome should be addressed in well-designed prospective studies. Trials to assess the utility of irisin as a therapeutic agent in humans, either against obesity or T2DM, seem to be premature, on the basis of the current knowledge, but should be the main research goal for the near future.
Supplementary Material
Key points.
Irisin is secreted primarily by muscle and in small amounts by adipose tissue
Irisin improves glucose homeostasis, lipid profile and metabolic parameters in animals and acts on adipose tissue by inducing ‘browning’, as well as on muscle and liver
Inconsistencies in published data regarding the circulating levels of irisin highlight the need for accurate methods for irisin measurement and for improved study design
Levels of irisin are increased in states of obesity and decreased in patients with type 2 diabetes mellitus (T2DM)
Future studies should try to identify the irisin receptor and to investigate the effects of irisin on the endocrine pancreas and appetite centres in the brain; prospective clinical studies should investigate whether irisin is a predictive marker for insulin resistance, T2DM or the metabolic syndrome
Acknowledgments
C.S.M. is supported by US National Institutes of Health (NIH DK081913). J.M.F-R. is supported by Instituto Salud Carlos III (http://www.isciii.es); Centro de Investigación Biomédica en Red-Fisiopatología de la Obesidad y Nutrición (CIBERobn) (http://www.ciberobn.es); Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) (www.gencat.cat/agaur/) (2015FI B00570); and Fondo Europeo de Desarrollo Regional (FEDER).
Footnotes
These three ELISA kits have been used in more than 90% of the studies.
Author contributions
N.P. researched data for the article and wrote the manuscript. G.A.T. researched data for the article, wrote the manuscript and edited the article before submission. J.M.F-R., J.Y.H., K.H.P., J.S. and C.S.M. contributed to discussion of the content, wrote parts of the article and reviewed and/or edited the article before submission.
Competing interests statement
The authors declare no competing interests.
See online article: S1 (table)
References
- 1.Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–350. doi: 10.1046/j.1463-1326.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom P, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. This is the first study to describe the existence of irisin and its role in thermogenesis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCBI. Fibronectin type III domain-containing protein 5 precursor [Rattus norvegicus] NCBI; 2016. https://www.ncbi.nlm.nih.gov/protein/NP_001257910.1. [Google Scholar]
- 6.NCBI. Fibronectin type III domain-containing protein 5 preproprotein [Mus musculus] NCBI; 2016. https://www.ncbi.nlm.nih.gov/protein/NP_081678.1. [Google Scholar]
- 7.NCBI. Fibronectin type III domain-containing protein 5 isoform 2 preproprotein [Homo sapiens] NCBI; 2016. https://www.ncbi.nlm.nih.gov/protein/NP_715637.2. [Google Scholar]
- 8.Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J Biol Chem. 2013;288:33738–33744. doi: 10.1074/jbc.M113.516641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh JY, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roca-Rivada A, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurdiova T, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Navarrete JM, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 13.Aydin S, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–136. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Lv J, et al. Study on the distribution and elimination of the new hormone irisin in vivo: new discoveries regarding irisin. Horm Metab Res. 2015;47:591–595. doi: 10.1055/s-0035-1547261. [DOI] [PubMed] [Google Scholar]
- 15.Crujeiras AB, Pardo M, Casanueva FF. Irisin: ‘fat’ or artefact. Clin Endocrinol (Oxf) 2015;82:467–474. doi: 10.1111/cen.12627. [DOI] [PubMed] [Google Scholar]
- 16.Elbelt U, Hofmann T, Stengel A. Irisin: what promise does it hold? Curr Opin Clin Nutr Metab Care. 2013;16:541–547. doi: 10.1097/MCO.0b013e328363bc65. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann T, Elbelt U, Stengel A. Irisin as a muscle-derived hormone stimulating thermogenesis — a critical update. Peptides. 2014;54:89–100. doi: 10.1016/j.peptides.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Polyzos SA, Mantzoros CS. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism. 2015;64:937–942. doi: 10.1016/j.metabol.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Polyzos SA, Mathew H, Mantzoros CS. Irisin: A true, circulating hormone. Metabolism. 2015;64:1611–1618. doi: 10.1016/j.metabol.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Sanchis-Gomar F, Alis R, Pareja-Galeano H, Romagnoli M, Perez-Quilis C. Inconsistency in circulating irisin levels: what is really happening? Horm Metab Res. 2014;46:591–596. doi: 10.1055/s-0033-1363283. [DOI] [PubMed] [Google Scholar]
- 21.Raschke S, et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE. 2013;8:e73680. doi: 10.1371/journal.pone.0073680. This was the first study questioning the existence and importance of irisin in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bostrom PA, Fernandez-Real JM, Mantzoros C. Irisin in humans: recent advances and questions for future research. Metabolism. 2014;63:178–180. doi: 10.1016/j.metabol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab. 2015;100:E453–E457. doi: 10.1210/jc.2014-2416. [DOI] [PubMed] [Google Scholar]
- 24.Jedrychowski MP, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22:734–740. doi: 10.1016/j.cmet.2015.08.001. This study is the latest confirmation from the investigators that discovered irisin showing that irisin exists in circulating levels in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. This study further confirmed the existence of irisin in humans and its effects in browning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loffler D, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100:1289–1299. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]
- 27.Palacios-Gonzalez B, et al. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obesity (Silver Spring) 2015;23:729–732. doi: 10.1002/oby.21029. [DOI] [PubMed] [Google Scholar]
- 28.Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS. Irisin in patients with nonalcoholic fatty liver disease. Metabolism. 2014;63:207–217. doi: 10.1016/j.metabol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Albrecht E, et al. Irisin — a myth rather than an exercise-inducible myokine. Sci Rep. 2015;5:8889. doi: 10.1038/srep08889. This is the second study, after the study conducted by Raschke, S. et al. (reference 21), that questioned the existence of irisin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989;9:5073–5080. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidlingmaier M, Freda PU. Measurement of human growth hormone by immunoassays: current status, unsolved problems and clinical consequences. Growth Horm IGF Res. 2010;20:19–25. doi: 10.1016/j.ghir.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson CJ, Gaines Das R, Woollacott D. The first international standard for human leptin and the first international standard for mouse leptin: comparison of candidate preparations by in vitro bioassays and immunoassays. J Mol Endocrinol. 2001;27:69–76. doi: 10.1677/jme.0.0270069. [DOI] [PubMed] [Google Scholar]
- 33.Sturgeon CM, Ellis AR. Standardization of FSH, LH and hCG — current position and future prospects. Mol Cell Endocrinol. 2007;260–262:301–309. doi: 10.1016/j.mce.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Sanchis-Gomar F, Alis R, Lippi G. Circulating irisin detection: does it really work? Trends Endocrinol Metab. 2015;26:335–336. doi: 10.1016/j.tem.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegelman BM, Puigserver P, Wu Z. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):8–10. doi: 10.1038/sj.ijo.0801492. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 38.Kurdiova T, et al. Exercise-mimicking treatment fails to increase Fndc5 mRNA and irisin secretion in primary human myotubes. Peptides. 2014;56:1–7. doi: 10.1016/j.peptides.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Peterson JM, Mart R, Bond CE. Effect of obesity and exercise on the expression of the novel myokines, Myonectin and Fibronectin type III domain containing 5. PeerJ. 2014;2:e605. doi: 10.7717/peerj.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norheim F, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 41.Besse-Patin A, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (Lond) 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 42.Pekkala S, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Daghri NM, et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur J Clin Invest. 2015;45:775–781. doi: 10.1111/eci.12468. [DOI] [PubMed] [Google Scholar]
- 44.Ellefsen S, et al. Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur J Appl Physiol. 2014;114:1875–1888. doi: 10.1007/s00421-014-2922-x. [DOI] [PubMed] [Google Scholar]
- 45.Huh JY, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99:E2154–E2161. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 46.Huh JY, Mougios V, Skraparlis A, Kabasakalis A, Mantzoros CS. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism. 2014;63:918–921. doi: 10.1016/j.metabol.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Ijiri N, Kanazawa H, Asai K, Watanabe T, Hirata K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology. 2015;20:612–617. doi: 10.1111/resp.12513. [DOI] [PubMed] [Google Scholar]
- 48.Moraes C, et al. Resistance exercise training does not affect plasma irisin levels of hemodialysis patients. Horm Metab Res. 2013;45:900–904. doi: 10.1055/s-0033-1354402. [DOI] [PubMed] [Google Scholar]
- 49.Nygaard H, et al. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE. 2015;10:e0121367. doi: 10.1371/journal.pone.0121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 51.Brenmoehl J, et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci. 2014;10:338–349. doi: 10.7150/ijbs.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuchiya Y, Ando D, Takamatsu K, Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. 2015;64:1042–1050. doi: 10.1016/j.metabol.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014;38:1538–1544. doi: 10.1038/ijo.2014.42. This is the first study to describe the expression profile of FNDC5 (the precursor of irisin) and the factors predicting the circulating levels of irisin in humans. [DOI] [PubMed] [Google Scholar]
- 54.Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290:11431. doi: 10.1074/jbc.A114.617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290:7671–7684. doi: 10.1074/jbc.M114.617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong J, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (Lond) 2016;40:434–442. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez A, et al. Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in mice. Int J Obes (Lond) 2015;39:397–407. doi: 10.1038/ijo.2014.166. [DOI] [PubMed] [Google Scholar]
- 60.Li M, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:1485–1493. doi: 10.1210/jc.2014-2544. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez-Repiso C, et al. FNDC5 could be regulated by leptin in adipose tissue. Eur J Clin Invest. 2014;44:918–925. doi: 10.1111/eci.12324. [DOI] [PubMed] [Google Scholar]
- 62.Gavrieli A, Panagiotou G, Mantzoros CS. Leptin administration in physiological or pharmacological doses does not alter circulating irisin levels in humans. Int J Obes (Lond) 2016;40:1461–1463. doi: 10.1038/ijo.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huerta AE, et al. Circulating irisin and glucose metabolism in overweight/obese women: effects of α-lipoic acid and eicosapentaenoic acid. J Physiol Biochem. 2015;71:547–558. doi: 10.1007/s13105-015-0400-5. [DOI] [PubMed] [Google Scholar]
- 64.Koh EH, et al. Effects of α-lipoic acid on body weight in obese subjects. Am J Med. 2011;124:85.e1–85.e8. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Rochette L, Ghibu S, Muresan A, Vergely C. α-Lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93:1021–1027. doi: 10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- 66.Matsuo Y, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6:62–72. doi: 10.1002/jcsm.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rachid TL, et al. Fenofibrate (PPARα agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol Cell Endocrinol. 2015;402:86–94. doi: 10.1016/j.mce.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 68.Li DJ, et al. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol (Oxf) 2015;213:711–721. doi: 10.1111/apha.12421. [DOI] [PubMed] [Google Scholar]
- 69.Yang Z, Chen X, Chen Y, Zhao Q. PGC-1 mediates the regulation of metformin in muscle irisin expression and function. Am J Transl Res. 2015;7:1850–1859. [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–525. doi: 10.2337/db13-1106. This study confirmed the initial findings of Bostrom, P. et al. (reference 4) and also provided a functional mechanism for the observed browning effects of irisin on WAT. [DOI] [PubMed] [Google Scholar]
- 71.Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222:R25–R38. doi: 10.1530/JOE-14-0189. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530–E541. doi: 10.1152/ajpendo.00094.2016. [DOI] [PubMed] [Google Scholar]
- 73.Orava J, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 74.Xiong XQ, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852:1867–1875. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 75.Gao S, et al. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS ONE. 2016;11:e0147480. doi: 10.1371/journal.pone.0147480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee HJ, et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol. 2015;29:873–881. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xin C, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond) 2016;40:443–451. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 78.Yang Z, Chen X, Chen Y, Zhao Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol. 2015;8:6490–6497. [PMC free article] [PubMed] [Google Scholar]
- 79.Vaughan RA, et al. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab. 2014;16:711–718. doi: 10.1111/dom.12268. [DOI] [PubMed] [Google Scholar]
- 80.Vaughan RA, Gannon NP, Mermier CM, Conn CA. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J Physiol Biochem. 2015;71:679–689. doi: 10.1007/s13105-015-0433-9. [DOI] [PubMed] [Google Scholar]
- 81.Liu TY, et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond) 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 82.Tang H, et al. Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine. 2016;6:139–148. doi: 10.1016/j.ebiom.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mo L, et al. Irisin is regulated by CAR in liver and is a mediator of hepatic glucose and lipid metabolism. Mol Endocrinol. 2016;30:533–542. doi: 10.1210/me.2015-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park MJ, Kim DI, Choi JH, Heo YR, Park SH. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27:1831–1839. doi: 10.1016/j.cellsig.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Batirel S, Bozaykut P, Mutlu Altundag E, Kartal Ozer N, Mantzoros CS. The effect of irisin on antioxidant system in liver. Free Radic Biol Med. 2014;75(Suppl 1):S16. doi: 10.1016/j.freeradbiomed.2014.10.592. [DOI] [PubMed] [Google Scholar]
- 86.Zhang HJ, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–562. doi: 10.1016/j.jhep.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 87.Choi ES, et al. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PLoS ONE. 2014;9:e110680. doi: 10.1371/journal.pone.0110680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rizk FH, Elshweikh SA, Abd El-Naby AY. Irisin levels in relation to metabolic and liver functions in Egyptian patients with metabolic syndrome. Can J Physiol Pharmacol. 2016;94:359–362. doi: 10.1139/cjpp-2015-0371. [DOI] [PubMed] [Google Scholar]
- 89.Polyzos SA, Kountouras J, Anastasilakis AD, Margouta A, Mantzoros CS. Association between circulating irisin and homocysteine in patients with nonalcoholic fatty liver disease. Endocrine. 2015;49:560–562. doi: 10.1007/s12020-014-0473-x. [DOI] [PubMed] [Google Scholar]
- 90.Yang M, et al. Circulating levels of irisin in middle-aged first-degree relatives of type 2 diabetes mellitus — correlation with pancreatic β-cell function. Diabetol Metab Syndr. 2014;6:133. doi: 10.1186/1758-5996-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L, et al. Circulating levels of betatrophin and irisin are not associated with pancreatic β-cell function in previously diagnosed type 2 diabetes mellitus patients. J Diabetes Res. 2016;2016:2616539. doi: 10.1155/2016/2616539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wen MS, Wang CY, Lin SL, Hung KC. Decrease in irisin in patients with chronic kidney disease. PLoS ONE. 2013;8:e64025. doi: 10.1371/journal.pone.0064025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ebert T, et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur J Endocrinol. 2014;170:501–506. doi: 10.1530/EJE-13-1053. [DOI] [PubMed] [Google Scholar]
- 94.Liu JJ, et al. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J Diabetes Complicat. 2014;28:208–213. doi: 10.1016/j.jdiacomp.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 95.Hu W, Wang R, Li J, Zhang J, Wang W. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann Clin Biochem. 2016;53:67–74. doi: 10.1177/0004563215582072. [DOI] [PubMed] [Google Scholar]
- 96.Wang HH, Zhang XW, Chen WK, Huang QX, Chen QQ. Relationship between serum irisin levels and urinary albumin excretion in patients with type 2 diabetes. J Diabetes Complicat. 2015;29:384–389. doi: 10.1016/j.jdiacomp.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 97.Yang S, et al. Association of serum irisin and body composition with chronic kidney disease in obese Chinese adults: a cross-sectional study. BMC Nephrol. 2015;16:16. doi: 10.1186/s12882-015-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dun SL, et al. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Piya MK, et al. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab. 2014;306:E512–E518. doi: 10.1152/ajpendo.00308.2013. [DOI] [PubMed] [Google Scholar]
- 100.Hashemi MS, et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 101.Ghahrizjani FA, et al. Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene. 2015;557:123–129. doi: 10.1016/j.gene.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 102.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism. 2013;62:1131–1136. doi: 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wrann CD, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. This study links irisin and FNDC5 with neurocognitive function through a PGC1α–FNDC5–BDNF axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W, et al. Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther. 2015;29:121–127. doi: 10.1007/s10557-015-6580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang W, et al. Irisin: A myokine with locomotor activity. Neurosci Lett. 2015;595:7–11. doi: 10.1016/j.neulet.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlogl M, et al. Increased 24-hour ad libitum food intake is associated with lower plasma irisin concentrations the following morning in adult humans. Appetite. 2015;90:154–159. doi: 10.1016/j.appet.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501–H1508. doi: 10.1152/ajpheart.00443.2015. [DOI] [PubMed] [Google Scholar]
- 108.Lu J, et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-null diabetic mice. Atherosclerosis. 2015;243:438–448. doi: 10.1016/j.atherosclerosis.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 109.Zhu G, et al. Irisin increased the number and improved the function of endothelial progenitor cells in diabetes mellitus mice. J Cardiovasc Pharmacol. 2016;68:67–73. doi: 10.1097/FJC.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Song H, et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS ONE. 2014;9:e110273. doi: 10.1371/journal.pone.0110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu F, et al. Irisin induces angiogenesis in human umbilical vein endothelial cells in vitro and in zebrafish embryos in vivo via activation of the ERK signaling pathway. PLoS ONE. 2015;10:e0134662. doi: 10.1371/journal.pone.0134662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu D, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–147. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 113.Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Sanchis-Gomar F, Perez-Quilis C. The p38–PGC-1α–irisin–betatrophin axis: exploring new pathways in insulin resistance. Adipocyte. 2014;3:67–68. doi: 10.4161/adip.27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes. 2014;63:1283–1288. doi: 10.2337/db13-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cox AR, et al. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase β cell proliferation in mice. Diabetologia. 2015;58:1523–1531. doi: 10.1007/s00125-015-3590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gusarova V, et al. ANGPTL8/betatrophin does not control pancreatic β cell expansion. Cell. 2014;159:691–696. doi: 10.1016/j.cell.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yi P, Park J-S, Melton DA. Retraction notice to: betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2017;168:326. doi: 10.1016/j.cell.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bluher S, et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity (Silver Spring) 2014;22:1701–1708. doi: 10.1002/oby.20739. [DOI] [PubMed] [Google Scholar]
- 120.Moreno M, et al. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE. 2015;10:e0124100. doi: 10.1371/journal.pone.0124100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sesti G, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51:705–713. doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- 122.Stengel A, et al. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity — correlation with body mass index. Peptides. 2013;39:125–130. doi: 10.1016/j.peptides.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Duan H, et al. Anti-diabetic activity of recombinant irisin in STZ-induced insulin-deficient diabetic mice. Int J Biol Macromol. 2016;84:457–463. doi: 10.1016/j.ijbiomac.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 124.Crujeiras AB, et al. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism. 2014;63:520–531. doi: 10.1016/j.metabol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Chen JQ, et al. Serum irisin level is higher and related with insulin in acanthosis nigricans-related obesity. Exp Clin Endocrinol Diabetes. 2016;124:203–207. doi: 10.1055/s-0035-1565060. [DOI] [PubMed] [Google Scholar]
- 126.Crujeiras AB, et al. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J Hum Biol. 2014;26:198–207. doi: 10.1002/ajhb.22493. [DOI] [PubMed] [Google Scholar]
- 127.Huth C, et al. Irisin is more strongly predicted by muscle oxidative potential than adiposity in non-diabetic men. J Physiol Biochem. 2015;71:559–568. doi: 10.1007/s13105-015-0402-3. [DOI] [PubMed] [Google Scholar]
- 128.Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab. 2015;100:2123–2130. doi: 10.1210/jc.2015-1208. [DOI] [PubMed] [Google Scholar]
- 129.Qiu S, et al. Association between circulating irisin and insulin resistance in non-diabetic adults: a meta-analysis. Metabolism. 2016;65:825–834. doi: 10.1016/j.metabol.2016.02.006. This is a meta-analysis of several studies that shows that irisin is weakly, but positively, associated with insulin resistance. [DOI] [PubMed] [Google Scholar]
- 130.Park KH, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:4899–4907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anastasilakis AD, et al. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J Clin Endocrinol Metab. 2014;99:3247–3255. doi: 10.1210/jc.2014-1367. [DOI] [PubMed] [Google Scholar]
- 132.Choi YK, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Liu JJ, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27:365–369. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Zhang C, et al. Lower irisin level in patients with type 2 diabetes mellitus: a case-control study and meta-analysis. J Diabetes. 2016;8:56–62. doi: 10.1111/1753-0407.12256. [DOI] [PubMed] [Google Scholar]
- 135.Erol O, et al. Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study. J Matern Fetal Neonatal Med. 2016;29:3590–3595. doi: 10.3109/14767058.2016.1142967. [DOI] [PubMed] [Google Scholar]
- 136.Zhao L, et al. Circulating irisin is lower in gestational diabetes mellitus. Endocr J. 2015;62:921–926. doi: 10.1507/endocrj.EJ15-0230. [DOI] [PubMed] [Google Scholar]
- 137.Huh JH, Ahn SV, Choi JH, Koh SB, Chung CH. High serum irisin level as an independent predictor of diabetes mellitus: a longitudinal population-based study. Medicine (Baltimore) 2016;95:e3742. doi: 10.1097/MD.0000000000003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hwang YC, Jeon WS, Park CY, Youn BS. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc Diabetol. 2016;15:9. doi: 10.1186/s12933-015-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yan B, et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE. 2014;9:e94235. doi: 10.1371/journal.pone.0094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aronis KN, et al. Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes (Lond) 2015;39:156–161. doi: 10.1038/ijo.2014.101. [DOI] [PubMed] [Google Scholar]
- 141.Emanuele E, et al. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am J Med. 2014;127:888–890. doi: 10.1016/j.amjmed.2014.04.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.