Abstract
Estrogen and progesterone, play essential roles in the development and progression of breast cancer. Over 70% of breast cancers express estrogen receptors (ER) and progesterone receptors (PR), emphasizing the need for better understanding of ER and PR signaling. ER and PR are traditionally viewed as transcription factors and directly bind DNA to regulate gene networks. In addition to nuclear signaling, ER and PR mediate hormone-induced, rapid extranuclear signaling at the cell membrane or in the cytoplasm which initiates downstream signaling to regulate either rapid or extended cellular responses. Specialized membrane and cytoplasmic proteins may also initiate hormone-induced extranuclear signaling. Rapid extranuclear signaling converges with its nuclear counterpart to amplify ER/PR transcription and specify gene regulatory networks. This review summarizes current understanding and updates on ER and PR extranuclear signaling. Further investigation of ER/PR extranuclear signaling may lead to development of novel targeted therapeutics for breast cancer management.
Keywords: Extranuclear signaling, nongenomic signaling, estrogen receptor, progesterone receptor, growth factor signaling, breast cancer
Introduction
1. Nature of extranuclear steroid hormone receptors
1.1. Classical estrogen receptor structure and activity
Early concepts of estrogen action proposed the involvement of both nuclear and extranuclear estrogen receptors (ER) driving ER activity, however most studies have focused on nuclear ER and its transcriptional activity [1–5]. There are two major types of estrogen receptors, ERα and ERβ, each produced from different genes with similar but not identical structure [2, 6]. On binding estradiol-17β (E2), ERα undergoes a change in conformation of the ligand-binding domain (LBD) to form a novel hydrophobic surface to modulate binding of coactivators and corepressors [2, 7, 8]. As a phosphoprotein, ER undergoes phosphorylation at serine and tyrosine residues after activation by E2 binding, and this contributes to receptor activity and DNA binding [9–11]. E2-bound ERs function as transcription factors by binding as homodimers to estrogen response elements (ERE) [7, 8] (Fig. 1).
Figure 1. Estrogen receptor signaling pathways in human breast tumors.
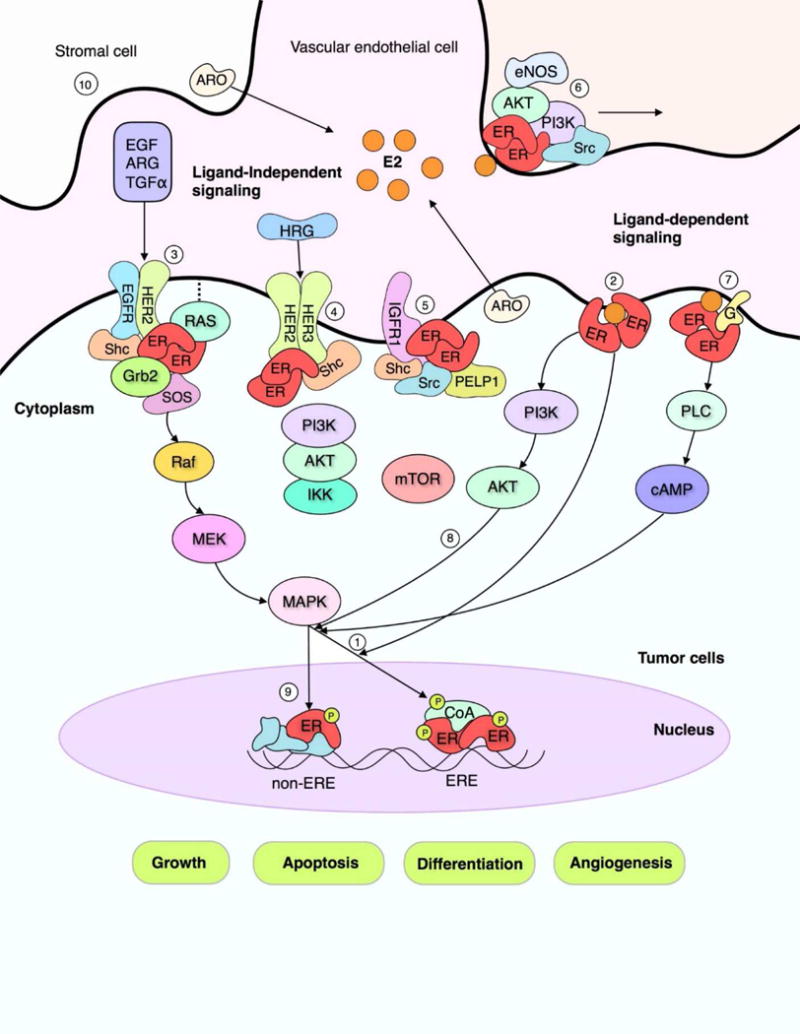
Proliferation and survival of BC cells is closely regulated by estradiol-17β (E2) and its receptors, ERα and ERβ, as well as growth factor receptors. In classic models of E2 action (1), E2 binds ER to promote dimerization and phosphorylation of ER. This allows direct binding of the E2-ER complex with steroid receptor coactivators and estrogen response elements (ERE) in DNA, leading to gene transcription to regulate growth, differentiation, apoptosis and angiogenesis. A subset of ERs occur in extranuclear sites, such as caveolae or lipid rafts in plasma membrane (2), and may interact with transmembrane growth factor receptors such as EGFR (3), HER2 (4), insulin-like growth factor receptor I (IGFR1) (5) and other signaling molecules, including components of Ras-MAPK and phosphatidylinositol 3-kinase (PI3K)/AKT pathways, Src kinases, Janus-activated kinase/signal transducer and activator of transcription signaling, nitric oxide synthase (NOS) (6), and G-proteins (7). Membrane-associated ER may undergo posttranslational modification, such as palmitoylation, and/or associate with adaptor proteins, such as Shc, PELP1, or lipid raft proteins. ERs and growth factor receptors may form a structured complex for signal transduction to MAPK and/or PI3K/AKT kinase that interacts, in turn, with nuclear ER and steroid receptor coactivators (8). Signaling for cell growth involves phosphorylation (P) of nuclear ER and coactivators that can occur in ligand-dependent as well as ligand-independent modes. ERE-dependent and alternate transcription sites may be activated (9). Further, E2 is produced locally in BC and in host supporting cells by the action of aromatase (ARO) (10) which is regulated by both nuclear and extranuclear ER. Pathways modified from Pietras and Marquez [52]; refer to text for more details.
Interestingly, about one third of E2-induced genes lack functional EREs, and estrogens indirectly regulate transcription of these genes by modulating activity of other transcription factors such as activator protein-1, Elk-1, serum response factor, cyclic AMP-responsive element binding protein, nuclear factor-kappaB (NFκB), and signal transducers and activators of transcription [7, 8, 12]. Blockade of ER signaling by interfering with E2 binding to ER is the basis for antiestrogen, tamoxifen, action. Tamoxifen is a partial agonist, termed a selective estrogen receptor modulator (SERM), that limits E2-stimulated proliferation in breast tumors [13]. Fulvestrant is an ER antagonist that downregulates cellular levels of ER and, unlike tamoxifen, has no agonist activity [14–18]. Further, inhibitors of aromatase (anastrazole, letrazole, exemestane) reduce E2 biosynthesis in target tissues and shows some advantages over tamoxifen as antitumor therapies [19–22]. Recent findings based on molecular analyses of ER in BC metastases show that ER mutations can emerge in patients treated with extended courses of endocrine therapy, in particular, endocrine resistance tumors occur mainly with mutations in critical residues in the LBD of ERα and β [23, 24]. The precise role of ERβ in ERα-positive and ERα-negative BC subtypes remains to be determined (Hamilton et al., 2015). ERβ is often reported to exert antiproliferative and pro-apoptotic effects in BC cells that co-express ERα [25, 26], while ERβ may promote proliferation and block apoptosis in BC cells that lack ERα expression [27–29].
1.2. Background on Extranuclear ER structure and activity
Estrogen and other steroid hormones are well known to accumulate and are retained in responsive cells [30] by specific interactions with cellular macromolecules, initially considered to be located outside the nucleus [31, 32]. Binding to the target-cell receptor was the proposed mechanism to initiate transfer of E2 to the nucleus where the ER/E2 complex promoted expression of phenotypic changes [31, 33, 34]. The genomic hypothesis of steroid hormone action has generally prevailed as the exclusive mechanism of hormone action due to the pioneering work by molecular and cellular biologists, whose studies extended details of these concepts to understand the late nuclear actions of steroid hormones in their target cells. Development of monoclonal antibodies for specific binding to nuclear ER consolidated this concept and has led to important clinical benefits for patients afflicted with ER-expressing BCs since these ER-positive tumors can be treated more effectively with targeted antiestrogens [1–3]. However, the emphasis on nuclear steroid actions overshadowed parallel observations on early extranuclear receptor-mediated signaling. More than 40 years ago, Pietras and Szego [35, 36] and Szego and Davis [37] first described rapid estradiol-induced actions, including changes in calcium fluxes and cyclic AMP generation that occurred in seconds to minutes after exposure of target cells to this sex steroid. These authors further provided evidence of estrogen binding with high affinity and specificity to a receptor molecule partially purified from target cell membranes [38, 39]. In the case of some hormone responses, rapid extranuclear effects may be sufficient to elicit a cascade of intracellular signals to alter specific cellular functions. One such example is the localized release of nitric oxide which is secondary to an instantaneous surge of calcium that occurs in endothelial cell response to E2 [40–42]. These events result in rapid vasodilation, thus bypassing the long (hours) transduction pathway response due to nuclear signaling. The local effects of E2 on the rapid electrophysiological activities of neurons are another examples of extranuclear receptor effects [43–45].
In evolutionary terms, steroid hormone recognition at the surface membrane appears to have been a primary response pathway in more primitive cells and organisms [46–48]. These early reports suggested that conserved functions of steroids from primitive cells to humans resulted from actions in part at the cell membrane. The concept of specific membrane-associated binding sites for steroid hormones is now supported by many independent laboratories [49–54]. Such membrane proteins appear to constitute a fraction of total receptor molecules (~5%) in target cells. Ultrastructural studies reveal extranuclear immuno-reactivity for ERα at membrane sites along dendritic spines and axon terminals of neurons [55]. Moreover, E2 may promote growth of mammary tumor cells, potentially in concert with ERE-dependent transcription, by stimulating downstream p42/p44 MAP kinase pathways [52]. Ligand-independent activation of steroid hormone receptors also occurs via peptide signaling systems. For example, ER can be activated in the absence of E2 through phosphorylation of tyrosine residues by EGF-stimulated MAP kinase [56–58]. Any comprehensive model of steroid hormone action must account for these important cellular interactions.
In vertebrates, the distribution of most sex steroid receptors favors nuclear localization, thereby allowing receptor binding to promoters and steroid receptor response elements in DNA of target cells to regulate specific gene expression. Although it is often assumed that steroid hormones enter target cells by passive diffusion, biophysical studies show that steroid hormones are largely lipophilic compounds that partition within the hydrocarbon core of lipid bilayer membranes. Steroid hormones appear to enter target cells by a saturable, temperature-dependent membrane-mediated process [59, 60]. Analysis of [3H]-estradiol-17β uptake in target cells was reported using analytical cell fractionation at progressive time periods, beginning within 10 seconds of exposure [61]. In these studies, E2 interacted specifically with membrane proteins in uterine target cells and underwent rapid internalization, resulting in intracellular delivery of a portion of E2 and its associated receptor protein. Concomitant with a decline in plasma membrane and endosomal subfractions, a significant amount of labeled E2 was shown to occur in Golgi and lysosome-mitochondrial compartments before later peaks shown in nuclear compartment. Similarly, membrane-associated binding sites for E2 may mediate rapid effects of E2 that contribute to proliferation of BCs [57]. After controlled homogenization and fractionation of human MCF-7 BC cells, the bulk of specific E2 binding was found in the nuclear fractions, but a significant portion of specific, high-affinity E2 binding-sites was also enriched in plasma membranes. These E2 binding-sites in BC cells co-purify with 5′-nucleotidase, a plasma membrane-marker enzyme and were free from major contamination by cytosol or nuclei. Electrophoresis of membrane fractions allowed detection of a primary 67-kD protein and a secondary 46-kD protein recognized by E2 and by a monoclonal antibody directed to the LBD of nuclear ER. Independent experiments also showed the presence of endogenous membrane and nuclear ERα in human BC cells that were identical by mass spectrometry analysis of subcellular proteins isolated from E2 affinity columns [62]. Related reports of steroid receptor localization in mitochondria requires further investigation, particularly concerning steroid regulation of metabolism in target tissues [51]. Using antibodies to nuclear ERα, Pappas et al. [63] identified a plasma membrane-associated protein by established immunohistochemistry (IHC) methods. Furthermore, E2 binding to a surface membrane protein was reduced significantly by prior treatment with antisense oligonucleotides to suppress ERα expression [64]. A number of independent reports confirmed ERα association with plasma membranes by use of controlled homogenization with quantitative subcellular fractionation [38]. Specific antibodies were directed to different domains of nuclear ERα in intact breast [56, 63, 65], NSCLC [66, 67], and pituitary tumor cells [68], as well as in nonmalignant vascular cells [40]. In addition, conformation of E2 binding of plasma membrane proteins was established through ERα knockout models in astrocytes [69]. Although ERs localize predominantly in tumor cell nuclei, a significant pool of ERs has been shown to localize in extranuclear sites in archival BC and NSCLC cells [41, 66, 70, 71]. Thus, important actions initiated by membrane-associated forms of ER may play a collaborative role with liganded-ERs in the nucleus to promote signaling for hormone-mediated proliferation and survival of BCs.
The ESR1 gene encodes for a major 66-kD transcript and a minor 46-kD isoform lacking portions of the NH2-terminal region of full-length ERα [22, 72]. The 46-kD ER also occurs in membranes of endothelial [22] and breast [73] cells, possibly forming part of a signaling complex. To assess the nature of membrane ER, nuclear ERα gene was transfected in ER-null Chinese hamster ovary cells, and this resulted in cellular expression of both membrane and nuclear ERs, and the transfected cells responded to E2 with rapid signal transduction [74]. Independent studies also showed that transfection of ESR1 and ESR2 genes resulted in expression of both nuclear and membrane-localized receptors, confirming that both forms originate from the same gene transcripts [52, 73, 74]. Similar studies were done with progesterone (PR) and androgen receptor (AR) demonstrating that non-nuclear forms of these proteins or splice variants originate from the same gene [75–78]. Studies based on knockdowns of ERα by small interfering RNA [68, 73] or knockouts of both ERα and ERβ in vivo [62] offer additional support for the hypothesis that membrane and nuclear ERs share a common origin. Further, membrane ERs do not occur in ER-negative MCF-7 BC subclones that lack nuclear ER [73]. These cells, unlike ER-positive MCF-7 cells, do not show rapid estrogen-induced phosphorylation of steroid receptor coactivator AIB1 [73]. Importantly, studies using mass spectrometry provide evidence that peptides derived from ERα occur in membrane fractions prepared from BC and vascular endothelial cells [79]. Together, these data indicate that membrane-associated ER derives predominantly from the same gene as nuclear ERα.
There is evidence that endogenous ERβ also localizes to plasma membranes in some tissues including BC [71]. ERβ was first reported in 1996 and is the second major receptor that mediates some actions of E2 in various organs [6, 80]. ERα and ERβ are encoded by different genes, yet ERβ has 96% homology with ERα in the DNA-binding domain and 60% homology in the LBD. However, ERβ is not identified in standard assays for ERα. Many studies also indicate that truncated forms of ERα or alternative steroid-binding proteins are expressed in a variety of organs. ERα isoforms, 46-kD [22] or 36-kD [24, 81, 82] in size, have been reported at the cell membrane, especially in BC cell lines. ERα isoforms of 46- and 36-kD are splice variants [22, 83, 84] but are not generally as abundant as ERα-66 kD in cells expressing both receptor forms. In comparison to the full length ERα-66 kD isoform, the ERα-36 kD isoform lacks the AF-1 and AF-2 transcription activation domains. Yet, the truncated ERα-36 kD isoform possesses an altered LBD and an intact DNA-binding domain, consistent with the report that ERα-36 kD lacks intrinsic transcriptional activity but can mediate extranuclear signaling [24]. ERα-36 kD is activated by both E2 and antiestrogens and can be detected in ERα-66 kD-positive and -negative BCs [85]. ERα-36 kD has been shown to mediate E2 and antiestrogen signaling via the MAPK/ERK pathway and stimulate cell proliferation [84]. ERα-36 kD is expressed in triple-negative breast cancer (TNBC) cell lines and associates with EGFR [24]. In addition to TNBCs, ERα-36 kD is overexpressed in apocrine and adenoid cystic carcinomas, tumors for which therapeutic treatment options are currently not available [24, 86]. Limited studies indicate ERα-36 kD overexpression associates with increased breast tumor growth, tamoxifen resistance and metastasis [87, 88]. However, efforts to evaluate the clinical utility of ERα-36 kD without the availability of specific and validated antibodies are daunting.
1.3. Alternative estrogen-responsive membrane proteins
It is reported that an orphan 7-transmembrane G-protein-coupled receptor, GPR30, serves as an estrogen membrane receptor and mediates E2 signaling from plasma membrane [89, 90] or endoplasmic reticulum [91, 92]. In some cell types, especially BC cells, there may be some coordination between GPR30 or other G-protein-coupled receptors and membrane ERα as part of a larger membrane signaling complex, thereby transmitting rapid-E2 downstream signals [93–95]. The function for GPR30 was first identified from studies showing MAPK activation by E2. Responses were detected in BC cells expressing GPR30 but not in cells lacking GPR30 [89]. Interestingly, ER-antagonists such as tamoxifen and fulvestrant also bind GPR30 [95], consistent with earlier studies showing that these agents are agonists for GPR30 [89]. Independent results indicate that tamoxifen activates PI3K through GPR30 but not ERα, suggesting a possible role of GPR30 in tamoxifen-resistant BCs and/or in the increased incidence of endometrial cancers in women treated with tamoxifen [96, 97]. Further, reduction of GPR30 expression has been reported to prevent growth stimulation of TNBC cells (that lack ERα) by E2 [98], conversely, other reports suggest GPR30/GPER expression correlates with better prognosis in ERα-positive BC patients [99, 100].
Despite these observations, a number of important questions remain about the physiological function of GPR30. It is not clear if GPR30 has redundant or overlapping functions with ERα, ERβ or other ER isoforms [101], or fully independent responses. From available reports, GPR30 is expressed in BC cells that do not express ERα including TNBC cells. It is not known whether drugs that selectively target GPR30 versus ERα and ERβ would be superior to drugs currently available for BC treatment. Although ERα and GPR30 may both occur in membranes of target cells, some investigators have not confirmed a functional linkage between membrane-localized ERα and GPR30 in BC and other cell types [5, 51]. Further, it remains to be confirmed whether estrogen binds with high affinity and specificity to GPR30, recently termed GPER1. Saturation binding by [H3]-E2 to the endogenous membrane GPR30 in mouse kidney and ERα-negative BC cell line SKBR3 showed relatively minute amounts of ligand specific binding [51, 95, 102]. In ERα-negative cells expressing GPR30/GPER1, studies have not confirmed specific binding by or signaling in response to E2 [62, 103]. Reasons for these experimental differences are unclear. Further, 2-methoxyestradiol, a final end product of estradiol metabolism with minimal to no binding affinity for either ERα or ERβ [104] appears to bind GPR30 and induce cellular responses primarily via GPR30 [105]. Notably, independent laboratories have created GPR30-knockout (KO) mice [106, 107] and report few phenotypic differences under basal conditions and no overlap with the phenotypes observed in ERα- or ERβ-knockout mice. At present, the endogenous primary ligand for GPR30 has yet to be identified. With recent reports of a very selective, non-steroidal GPR30 agonist [108] as well as a GPR30 antagonist [109], future studies of GPR30 function should be facilitated using these agents. Finally, another distinct membrane ER form under investigation termed Gq-mER has been shown to rapidly activate kinase pathways to stimulate downstream actions in central nervous system neurons [45].
1.4. Membrane association of estrogen receptors
Although more evidence is emerging on estrogen membrane receptors, how ER associates with membranes remains a challenging question. While ERα has many hydrophobic regions on Kyte-Doolittle plots, there appeared to be no readily apparent trans-membrane, glycosylphosphatidylinositol-anchor, or PDZ domains to foster membrane association [72]. However, it is reported that attachment of palmitic acid to an internal cysteine residue in ER promotes receptor trafficking to the plasma membrane [110]. Palmitoylation of ERα at cysteine-451 in the mouse and cysteine-447 in humans is required for about 5% of the total cellular ERα pool to localize to plasma membrane [66, 78, 83, 110]. A highly conserved nine-amino-acid motif in the LBDs of human ERα and ERβ seems necessary to mediate receptor palmitoylation and subsequent steroid signaling [78]. Several studies have clarified how subpopulations (~5% of total cellular receptors) of newly synthesized ER, as well as PR, are directed to plasma membrane via palmitoylation at specific cysteine residues. Further, membrane ER localization via palmitoylation is reported to be important for delaying ER degradation and for estradiol-induced ER occupancy at ERE sites, suggesting that ER membrane localization and extranuclear signaling may be a prerequisite for ER transcriptional activity [66, 78, 110, 111]. Palmitoylation at specific cysteine residues occurs from enzymatic actions of specific palmitoylacyltransferase proteins (PATs) DHHC-7 and -21, resulting in disulfide bond linkage of the palmitic fatty acid with ERα [112]. Importantly, all of these actions are highly conserved for membrane localization of both PR and androgen receptors (AR) [78, 112, 113]. Further, ERβ undergoes palmitoylation and subsequent membrane localization in some cell types thereby enabling rapid signaling in vitro and in vivo [114].
To better understand the impact of membrane-localized ER signaling for cell function, unique bioengineered mouse models have shown that membrane ER is required for normal organ development and functions. For example, in membrane ER-only mouse (MOER) model, ER-null mice were engineered to express the LBD of ERα targeted exclusively to the membrane of all cells. E2 treatment of cells derived from various organs of MOER mice triggered rapid activation of various kinase pathways and rapid generation of cyclic nucleotides, indicating that the membrane ER pool is sufficient in mediating acute signal transduction of E2 [115, 116]. In contrast, another mouse model was developed with selective loss only of membrane-localized ER but retaining abundant nuclear-localizedreceptors. Cells from these nuclear ER-only mice (NOER) failed to respond to E2 with the ERK and PI3K/AKT signaling as seen in wild-type ER mouse cells. Consequently, E2 stimulation of genes regulated by both rapid membrane signaling and nuclear ER transcription was significantly reduced. Of note, NOER mice lacking membrane ERα also exhibited several developmental abnormalities including infertility, abnormal pituitary hormone regulation, hypoplastic uteri, abnormal ovaries, stunted mammary gland ductal development and trabecular bone formation [115, 117]. These abnormalities were rescued in heterozygous NOER mice that were comparable to wild type mice in this model. Further work with such unique mouse models [118] and cell constructs will help to decipher the biological roles of membrane-associated and nuclear steroid hormone receptors [118].
To understand the nature of steroid receptor association with cell membranes, it is important to consider current concepts of supramolecular membrane organization (Fig. 2). The present view of the lateral organization of plasma membrane constituents was revised significantly from the original fluid-mosaic model wherein membrane proteins were considered to diffuse freely in a sea of lipids [119]. Rather, current data suggest the existence of membrane macro- and micro-domains that concentrate key signaling molecules for efficient coupling to effectors. The concept of a ‘signaling platform’ has been advanced to characterize a structure in which many different membrane-associated components are assembled in a coordinated fashion. Plasma membrane microdomains termed ‘lipid rafts’ arise from the phase behavior of lipid components. Raft association may concentrate receptors to interact with ligands and effectors on either side of the membrane, thus facilitating binding during signaling. Contrary to earlier notions, recent studies suggest that classical receptors for ER, PR and AR contain two transmembrane helices within their LBDs and harbor pore-lining regions in the plasma membrane that potentially regulate ion permeability [120, 121]. Na+, K+-ATPase, well-known to maintain an electrochemical gradient across the plasma membrane, similarly serves as a receptor for cardiotonic steroids [122]. In addition to the full-length ERα-66kD isoform, an N-terminus truncated ERα isoform (ERα-46kD) is reported to be a potential integral transmembrane protein that plays a key role in rapid endothelial responses to E2 [22].
Figure 2. Supramolecular organization of plasma membrane and occurrence of estrogen receptors (ER).
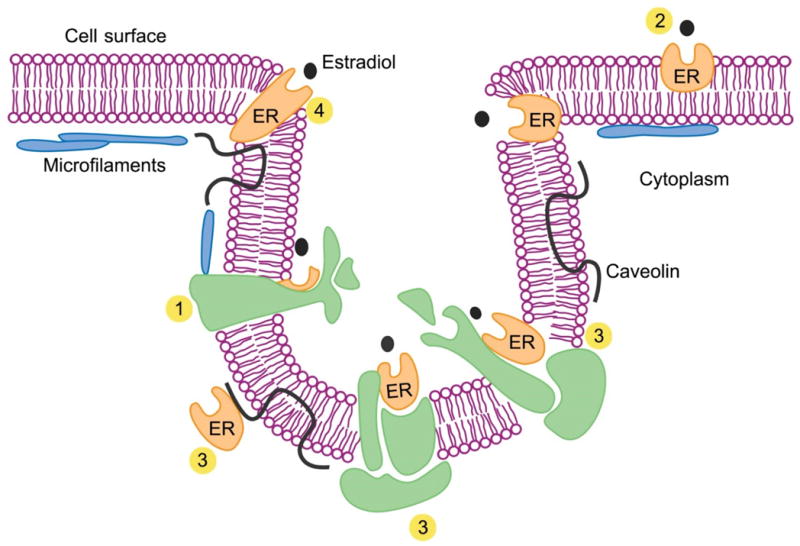
A model of the surface membrane from an estradiol (E2)-responsive cell in the region of a caveolar structure is shown. E2 may interact with one of several different forms of membrane-associated ERs. Full structural characterization of these ERs remain to be done. These molecules may be known membrane components, such as enzymes, G-proteins, ion channels, or receptors for nonsteroidal ligands, with unrecognized steroid binding sites (1); new isoforms of hormone receptors such as the truncated ERs that arise by alternative splicing (2); ‘classical’ receptors complexed with other membrane-associated proteins (3); or novel membrane proteins (4). Of note, splice variants of ER occur, and these give rise to proteins of different molecular size and possibly modified properties. Membrane insertion of receptors in primary transcript form would likely require one or more hydrophobic regions. ERα, for example, has several hydrophobic regions, but it is not known if these suffice for disposition as integral membrane proteins. Posttranslational modification of receptor protein leading to membrane targeting also occurs, including phosphorylation, glycosylation, nitrosylation and/or addition of lipid anchors or alterations such as palmitoylation or myristoylation. Evidence for palmitoylation of ER that fosters membrane association is documented in the text. Modified from Szego and Pietras [322].
While lipid rafts are moving cholesterol-rich domains in membranes that provide a matrix for signal transduction [73, 83, 123], caveolae and caveolae-like rafts are specialized forms of lipid rafts containing the structural proteins caveolin or flotillin, respectively. Caveolae are known to concentrate and assemble components of several signal transduction pathways (Fig. 2). As lipid rafts, caveolae are rich in cholesterol and sphingolipids, but, unlike rafts, they are lined intracellularly with clusters of caveolin protein, a cholesterol-binding molecule that contributes to membrane lipid organization. The list of caveolae-associated molecules includes receptor tyrosine kinases, G-protein-coupled receptors, protein kinase C, components of the MAPK pathway, and endothelial nitric oxide synthase (eNOS). In one such example, subpopulations of ERs are localized to caveolae in endothelial cells. In caveolae of plasma membrane isolated from endothelial cells, E2 directly stimulates ERs coupled with eNOS in a functional signaling complex to regulate local calcium levels and blood vessel contractility [124]. In another example, subpopulations of ERs are also enriched in caveolae in human BC cells and are coupled with downstream signaling pathways that promote tumor progression [73].
The precise nature of membrane-associated steroid receptors remains elusive, primarily because full structural characterization of receptors isolated from membranes is incomplete. Nonetheless, ER from target cell plasma membranes appears to be a protein species with high-affinity and saturable binding specific for E2. Further, current findings as detailed above suggest that membrane receptors for steroid hormones are, in certain instances, transcriptional copies or variants of nuclear receptors, and, in other instances, are products apparently unrelated to these [63]. Steroid receptors in membranes may also occur in multimeric complexes with other transmembrane molecules coupled to specific signaling cascades (Fig. 2). Apart from membrane receptor localization with caveolins and flotillins [73, 78], association with coactivator modulator of extranuclear actions of ER (MNAR)/proline-, glutamic acid-, and leucine-rich protein-1 (PELP1) [111] or transmembrane growth factor receptors as docking sites [125] are reported. It may well be that multiple forms of protein receptors for steroids coexist in plasma membranes, thus complicating efforts to isolate and characterize individual binding species in membranes. As suggested before, nature may have designed more than one way to open a given lock [46].
1.5 Classical nuclear progesterone receptor (PR)
The classical PR is a steroid hormone-activated receptor belonging to the nuclear receptor superfamily, type 1 [126]. PR is expressed as two major isoforms designated PR-A (94kDa) and PR-B (120kDa), both produced from the same gene on chromosome 11 band q13 [127]. Each isoform originates from two transcriptional (AUG) initiation sites from two distinct E2-inducible promoter regions [128, 129]. The difference between the two isoforms is the presence of the unique 164 amino acids upstream of the NH2-terminal region of PR-B, absent in PR-A. This unique-PR-B NH2 -terminal allows for different conformational, isoform-specific proteins that contribute to different interacting-binding partners and co-factors mediating distinct properties of the PR-B isoform [130, 131]. In common, the structures of PR-A and PR-B contain a C-terminal hormone-binding domain, a DNA binding domain, a hinge region, a nuclear localization sequence (NLS), and a shared NH2-terminal region. Two transcriptional activation domains TAF1 and TAF2 are present in the shared PR isoform sequence: TAF1 is located in the N-terminal, and TAF2 is located in the hormone-binding domain at the C-terminal [126, 132]. A third TAF (TAF3) is present in the unique-PR-B region [126, 133]. Another important feature of PR is the presence of a proline-rich SH3 motif in the N-terminal of both PR-A and PR-B, important for rapid, cytoplasmic (non-genomic) signaling, including binding of c-Src tyrosine kinases [134].
Traditionally, PR is well recognized for its role as a ligand-dependent transcription factor [126]. PR is part of a multi-protein chaperone complex and upon ligand binding, PR disassociates from this complex and forms homo- and hetero-dimers and interacts with co-activators, which then bind to specific progesterone response elements (PRE) found within the promoter regions of gene targets [132]. Upon progesterone binding, PR isoforms are phosphorylated with equal affinity and translocate to the nucleus [135]. Historically, PR-B was understood to function predominantly as an activator of transcription, while the PR-A isoform was mainly defined as an inhibitor of the transcriptional activity of PR-B and potentially other nuclear receptors (NRs) including ERα, glucocorticoid receptor, mineralocorticoid receptor and androgen receptor [136]. Albeit, when each isoform was independently expressed [137] or when the ratio of PR-A and PR-B was altered [138, 139], PR-A and PR-B demonstrated distinct and overlapping transcriptional responses, thus expanding the proposed functions of the PR-A isoform as a transcriptional activator.
Ligand-activated PR mediates major physiological roles in female reproduction [133]. Although expression of both isoforms is present in target cells, PR-B is essential for breast reproductive functions, and ablation of PR-A has no detrimental effect on breast function as described in mice; conversely PR-A is essential for normal ovarian functions [140]. PR is a well-recognized ERα-mediated target, but progesterone-bound/PR has also been shown to modulate ERα gene expression programming at the chromatin level [141]. In addition, a third PR isoform has been described and designated PR-C. PR-C isoform is truncated in the DNA-binding domain and is demonstrated to inhibit the PR-B isoform [142]. However, physiological roles of PR-C isoform remains to be further characterized [143].
In addition to the well-documented function of PR as a nuclear transcription factor, extranuclear function of PR have also been reported [76]. Progesterone binding to extranuclear PR results in rapid activation of the Src/MAPK signaling pathway [76]. This extranuclear activity occurs within seconds to minutes of progesterone binding, in the presence or absence of ERα [144]. These extranuclear actions of the PR are described in detail in section 2.2. Rapid PR extranuclear signaling mechanisms.
1.6 Membrane progesterone receptors (mPRs) and PGRMC1
Emerging in the progesterone signaling field is the increasing complexity of proposed progesterone receptors (PR) which extend beyond the classical intracellular PRs. Two types of putative membrane-specific progesterone receptors that are unrelated to classical PR-A and PR-B and with very different structures have been reported. These include the membrane progesterone receptor (mPR) with a molecular weight (MW) of ~40 kD and the progesterone receptor membrane component 1 (PGMRC1) with a MW of 26–28 kD [145].
1.6.1 Membrane progesterone receptors (mPRs)
Although mPRs have similar functional characteristics to the G-coupled receptor superfamily, they belong to the progesterone/progestin and adipoQ receptor (PAQR) class II family, which are a class of transmembrane proteins [146]. mPRs consist of 7-transmembrane domains with the N-terminal domain localized on the extracellular side of the plasma membrane [147]. There are five mPR subtypes identified, three in mammalian cells (mPRα, mPRβ, and mPRγ), and two characterized only in yeast (mPRδ and mPRε) [148].
Zhu and Thomas were the first to identify ligand-bound mPRs, distinct from classical PR, that activate rapid signaling of G-proteins and MAP kinase pathways [148, 149]. Direct interactions between G-proteins and mPRs were detected in co-immunoprecipitation studies, and disruption of G-protein-mPR interactions reduced progestin binding to mPRs and loss of ligand-dependent G-protein activation [147]. Given some disparities in the binding affinities of different progestins to nuclear PR versus mPR, mPR-specific agonists were identified, and these can now be exploited to study mPR-specific functions [150].
Thomas and colleagues described the first non-nuclear mPR on fish oocyte membranes [151]. Subsequently, mPR homologues with characteristics of fish mPR were identified in different species including humans [150–152]. In humans, mPR homologues were found in normal tissues, including breast, reproductive, neuroendocrine and immune cells, and were also detected in BC cell lines [151–153].
Three of the mPRs subtypes, mPRα, mPRβ and mPRγ, have been detected in breast and ovarian cancer cells and tissues with mPRα identified as the predominant subtype [154, 155]. To date, mPR is reported to occur in most BC cell lines and biopsies tested [149] and is associated with cell survival. Specific mPR-agonists or low doses of progesterone in PR-negative, mPR-positive BC cells have been shown to inhibit apoptosis, potentially via activation of MAPK p42/44 signaling pathways [155]. In aggressive TNBCs expressing the mPRα subtype, specific knockdown of mPRα expression is reported to block tumor growth and metastasis in vivo [156]. A recent study further showed that expression of mPRα is associated with pAkt, c-erbB2 and metallopeptidase 9 (MMP-9) expression and tumor size, suggesting a significant role for mPRα in BC progression (Wu, Sun, Wang et al., 2016). More studies are needed to determine the contribution of mPR and mPR-subtypes on BC development and their potential role in resistance to endocrine therapy.
1.6.2 Progesterone receptor membrane component 1 (PGRMC1)
A putative membrane-specific progesterone receptor, unrelated and distinct from the classical nuclear PR, has recently been identified. Human PGRMC1 is a member of the membrane-associated progesterone receptor (MAPR) family of cytochrome b5 (cytb5)-related heme/steroid-binding proteins, and is located on chromosome X (Xq22-q24) [157, 158]. PGRMC1 is a transmembrane protein with binding sites for Src homology 2 (SH2) and Src homology 3(SH3) domain-containing proteins [158]. PGRMC1 forms a stable dimer when bound to heme [159, 160], and this complex is necessary for downstream signaling initiated through multiple binding partners including multiple cyP450 proteins, plasminogen activator inhibitor 1 mRNA binding protein (PAIR-BP1), insulin induced gene 1 (Insig), and sterol regulatory element-binding protein (SREBP) [161]. Although the name implies that PGRMC1 binds progesterone, there is an ongoing debate whether PGRMC1 is a direct progesterone receptor or alternatively a modulator of the activities of other PR receptors or possibly both [162]. Hence, the function of PGRMC1 remains unclear, but this transmembrane protein may function as an adapter protein involved in protein interactions, intracellular signal transduction and/or membrane trafficking [158]. PGRMC1 is reported to be overexpressed in a number of cancers including lung, breast, ovarian, colon and thyroid [161, 163]. In lung and BC, PGRMC1 overexpression correlates with tumor growth, migration, anchorage-independent growth, and serum-independent proliferation [164, 165]. Specific inhibitors of PGRMC1 in lung and BCs elicit lower ERK activity and reduced cell viability by impeding cell cycle progression past the G1 checkpoint [164]. Dimerized PGRMC1 complexed with CYP450 has also been implicated in cancer cell chemoresistance, potentially by facilitating antitumor effects of chemotherapeutics such as doxorubicin via CYP450 [159, 160] Cahill and colleagues suggest that PGRMC1 is a regulatory-nexus protein, associated with cancer pathogenesis and a potential target for novel antitumor therapeutics [162].
2. Extranuclear sex steroid receptor: mechanism of action and signaling
2.1. Rapid ER extranuclear signaling mechanisms
Our ideas about sex steroid signaling have markedly expanded over the past 50 years. As ERα and ERβ are both ligand-activated transcription factors and not typical membrane receptors, trafficking of subsets of these receptors to cell membrane requires post-translational modifications. As highlighted above, two necessary modifications are palmitoylation and association with scaffolding proteins such as caveolins. It appears that it is through these complexes that ERs, which cannot by themselves activate G proteins or other signaling nodes, are able to initiate downstream intracellular signaling [166]. Palmitoylation results in interaction of ER with caveolin-1 that may serve as the transporter of ER to caveolae rafts in the membrane. ER and some isoforms are highly enriched in freshly isolated caveolae rafts [73, 167, 168] and probably initiate signal transduction from this site. On E2 stimulation, ERα dissociates from caveolin-1 allowing the activation of rapid signals critical to promote cell proliferation in BC cells [169]. Current data indicate that the process of palmitoylation occurs only on ER monomers. Hence, E2-induced ER dimerization limits the number of receptors that undergo this posttranslational modification and traffic to plasma membrane [112]. E2 binding to ERα results in receptor dimerization and activation of rapid signaling that involves, in part, interactions with discrete Gα and Gβγ proteins in target cells [74, 167]. Notably, ER have also been detected in early endosomes, which may play a role in trafficking of these receptors to the nucleus and/or in regulating receptor turnover [61]. Caveolin-1 also serves as a scaffold to bind/tether several critical signaling molecules to membrane caveolae raft sites, thereby enhancing interactions of ER with G-protein subunits and tyrosine kinases that promote acute downstream signals in seconds. Signaling to transactivation of growth factor receptors in cancer cells is reported to result in activation of MAPK and PI3K/AKT pathways that impact cell fate, proliferation, migration and other processes for malignant progression. Such ‘extranuclear’ regulation of cell functions may well act in concert with ‘nuclear’ effects of nuclear ER in BC cells [170].
2.2 Rapid PR extranuclear signaling mechanisms
In addition to its role as a transcription factor to direct nuclear action by binding to specific DNA sequences, accumulating evidence shows that PR can function outside the nucleus. The term ‘extranuclear’ activity of PR was suggested to specifically describe these PR functions outside the nucleus [171]. Human PR contains a polyproline (PPD) domain within the N-terminal domain (NTD) that directly interacts with and activates cell membrane/cytoplasmic signaling cascades through interaction with SH3 domains of c-Src, thereby leading to rapid activation of Src/Ras/Raf/MAPK and other downstream targets [134]. This rapid extranuclear activation of c-Src and MAPK is progesterone-dependent but independent of PR transcriptional activity. Increasing evidence, mainly in BC cell models, suggests that extranuclear actions of PR are an integral part of PR function [172]. Progesterone-bound PR shows dichotomous functions in promoting and inhibiting cell proliferation in most tissues depending on the stage of development, and PR extranuclear signaling could play a major role in this dichotomy. Most studies to date demonstrate that progestin-dependent activation of extranuclear signaling stimulates several key kinases and results in modifications of PR, co-activators or other transcription factors required for maximum PR activity [134, 144, 173, 174] (Fig 3). However, a recent study also indicates that extranuclear PR actions can interfere or block growth-promoting signaling pathways. Expression of PR-B in NSCLC cells [77], independent of progestins, was shown to inhibit cell proliferation via PR-PPD mediated inhibition of the EGFR-mediated MAPK signaling pathway [77].
Figure 3. Convergence of PR nuclear and extranuclear signaling pathways to regulate biological responses.
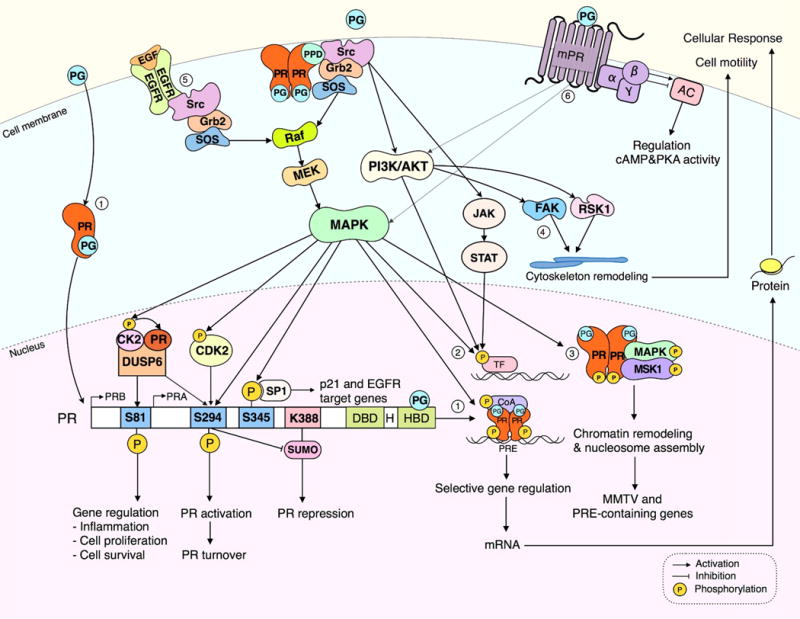
In the classic nuclear signaling pathway (1) progesterone activates progesterone receptor (PR) by binding and inducing conformational changes of the receptor causing in turn nuclear translocation, dimerization and binding to progesterone response elements (PRE) in the promoters or enhancer regions of PR target genes. Progestin treatment rapidly activates extranuclear signaling of a subpopulation of PR localized in the membrane or cytoplasm to transiently associate with c-Src through interaction with the PR polyproline domain (PPD) leading to activation of mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K/Akt), or Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathways. Activated MAPK further regulates PR transcriptional activity through phosphorylation of PR, co-activators (1) or other transcription factors (2). Rapid progestin activation of MAPK can directly phosphorylate PR or co-activators (1) or promote PR phosphorylations through CK2 and CDK2 (see text for details). Alternatively, activation of various cytoplasmic signaling pathways may phosphorylate and increase transcriptional activity of other transcription factors, independent of PREs (2). Rapid extranuclear activation of ER/PR complexes by progestins activates MAPK leading to a formation of phospho-PR/MAPK/Msk1 (Mitogen and stress-activated protein kinase 1), chromatin remodeling and enhanced MMTV and PRE-containing gene transcription (3). PR extranuclear activation of Src/PI3K/Akt may stimulate focal adhesion kinase (FAK) or ribosomal S6 kinase 1 (RSK1) triggering actin cytoskeleton remodeling and promotion of cell motility (4). Alternatively, PR extranuclear signaling may cross-communicate with growth factor signaling such as epidermal growth factor (EGF) leading to activation of MAPK and downstream events (5). Membrane localized progestin receptor (mPR) unrelated to the classical PR mediates progestin extranuclear signaling through GPCR-like membrane proteins via modulation of adenylate cyclase (AC), cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). mPR has been reported to activate MAPK and PI3K/Akt signaling pathways. More work is needed to define the physiological significance of extranuclear mPR activation of these several cytoplasmic signaling pathways in BC cells.
In addition to classical nuclear PR, recent data identify a novel truncated PR (PR-M) cloned from human adipose and aortic cDNA [175]. PR-M was predicted to contain a unique 16-amino acid sequence at the N-terminus lacking the A/B and the DNA-binding domain and was found to localize to mitochondria [176]. Progestin treatment induced a PR-M-mediated increase in mitochondrial membrane potential and oxygen consumption [176], but a detailed mechanism for PR-M mediated stimulation of cell respiration by progestins is unclear. More studies are clearly needed to determine the biological significance of PR-M in PR extranuclear signaling.
2.3. Cross-communication between extranuclear ER and growth factor receptors
Growth factor receptors such as EGFR and HER2 often concentrate in specific lipid rafts or caveolar domains of plasma membrane together with other signal transduction molecules. As noted above, extranuclear ERs also localize in these membrane domains, thereby promoting activation and transactivation of EGFR and HER2 receptors [73, 177] and interactions with other signaling molecules including insulin-like growth factor receptor (IGFR) I, the p85 regulatory subunit of PI3K, G-proteins, Src, and Shc, a protein that may couple ER with growth factor receptors [83, 178]. Coregulators, MNAR/PELP1 or metastasis-associated protein 1, also sequester ER in the extranuclear compartment to increase membrane action [169]. Activation of these pathways by E2 relays downstream proliferative and survival signals via MAPK and AKT that are important for BC survival, proliferation, migration and invasion. Further, MAPK stimulated by EGFR or/and HER2 signaling can, in turn, phosphorylate nuclear ER and receptor coactivators such as AIB1/SRC-3 [51, 73, 179, 180]. These events can be triggered by E2 (ligand-dependent signaling) or receptor kinases in the absence of E2 (ligand-independent ER activation), with the latter process underlying many forms of endocrine resistance in BC [181, 182].
2.4 Cross-communication between extranuclear-nuclear PR and growth factor signaling
About 70% of BCs are driven by ER activation [183]. In addition, several studies suggest that progestogen treatment significantly increases BC risk and promotes E2-induced cell proliferation [184, 185]. However, a recent report indicates that specific PR agonists inhibit E2-mediated cell proliferation, suggesting that PR functions as a molecular regulator of ER transcriptional activity via crosstalk between ER and PR in BC cells [141]. Of interest, two domains of PR-B were previously shown to be required for interaction with ER and for progestin activation of Src/MAPK signaling pathways [173]. Whether PR extranuclear signaling may play a role in modulating ER transcriptional activities remains to be explored further. Our current understanding of molecular mechanisms that underlie the convergence of extranuclear PR signaling with nuclear signaling is depicted in Figure 3.
Of 175 archival ER+/PR+ BC tissues investigated, about 55% exhibited ER/PR complexed with c-Src at the plasma membrane, and high levels of ER/PR/Src complexes were associated with poor BC prognosis [186]. BC cells expressing PR-B show enhanced responses to E2 and insulin-like growth factor (IGF-1), and this effect was independent of progestin and reported to be via PR-B/ER interaction with proline-, glutamate- and leucine-rich protein 1 (PELP-1) [187]. However, inhibition of PR-B expression or treatment with a PR-specific antagonist, onapristone, significantly inhibited cell growth and helped to partially restore tamoxifen sensitivity in resistant BC cells [187]. These studies suggest potential clinical benefit of ER/PR extranuclear signaling and indicate that ER/PR extranuclear signaling could play a crucial role in modulating ER/PR transcriptional activities. There is good evidence that progesterone treatment via PR-PPD rapidly activates c-Src and its downstream Ras/Raf/MAPK (ERK) signaling pathway and downstream transcription factor targets such as Elk-1 [134, 173]. Further, PR-B bearing mutations in the PR-PPD that abolish PR-SH3 interactions (PR-BΔSH3) fail to mediate rapid progestin-dependent activation of c-Src [134], and progestin fails to activate Cyclin-D1 (CCD1) gene transcription in BC cells expressing PR-BΔSH3 [144]. PR-PPD mediated activation of Src/MAPK signaling can result in PR phosphorylation at serine 345, allowing PR interaction with Sp1 through a tethering mechanism, to activate p21 and EGFR target genes [174] (Fig 3). MAPK phosphorylation of PR at serine 294 often associates with deSUMOylation on PR lysine-388 and hyperactivated PR [188]. Serine-294 phosphorylation and lysine-388 deSUMOylation of PR has been shown to regulate a unique set of genes found in BC with high ERBB2 expression and linked with decreased survival in tamoxifen-treated BC patients [189] (Fig 3).
Rapid extranuclear MAPK activation via interaction of PR and ER is essential for steps in the progestin activation of MMTV and other progesterone target genes. Activated phospho-MAPK translocates to the nucleus and forms a PR/MAPK and mitogen- and stress-activated protein kinase1 (Msk1) complex. This phospho-PR/MAPK/Msk1 complex is selectively recruited to the MMTV promoter by binding to BAF (BRG-1 associated factor) and p300/CRE-binding protein-associated factor (PCAF) and recruiting BAP and PCAF to MMTV hormone response element (HRE); thereby displacing a repressive histone complex containing HP1γ, that leads to activation of the MMTV promoter [190]. Selective inhibition of MAPK or its downstream signaling interferes with rapid progesterone-mediated chromatin remodeling and blocks transactivation of the MMTV promoter [191]. These results demonstrate the convergence of PR extranuclear and nuclear signaling pathways on progesterone-regulated gene transcription and indicate the significance of extranuclear signaling on overall gene regulation (Fig 3)
In addition to the Ras/Raf/MAPK signaling pathway, previous studies indicate that other mitogenic protein kinases, including cyclin-dependent kinase (Cdk2) and Casein Kinase II (CK2), phosphorylate PR and change PR transcriptional activities [192], suggesting that PR may serve as a mitogenic sensor in cells [193]. Cyclin A/Cdk2 is an important S-phase cell-cycle dependent kinase that phosphorylates large numbers of PR phosphorylation sites (8–14 sites) [194], such as Ser-162, Ser-190, Ser-400, and functions as a PR coactivator [195]. CyclinA/Cdk2 phosphorylation of PR regulates important PR activities including nuclear translocation, transactivation of PR-regulated genes, and hormone-induced degradation [194]. This intricate PR regulation by Cdk2 suggests significant roles of PR in the regulation of the cell cycle and proliferation.
CK2 is another important kinase that regulates PR functions. PR phosphorylation by CK2 on Ser-81 requires PR interaction with DUSP6, a negative regulator of the MAPK signaling pathway. This indicates that DUSP6 may serve as a scaffold to permit interaction between PR and the kinase that phosphorylates PR at Ser-81 [196]. Interestingly, PR phosphorylation at Ser-81 associates with a gene profile for mammary stem cell maintenance/renewal that is often altered in BC [196, 197]. PR with Ser-81 phosphorylation further regulates a set of genes associated with interferon/inflammation and STAT-signaling [197], thereby suggesting a potential link between PR regulation, inflammation and cancer [198]. Together, these data provide evidence that progesterone regulates gene expression by stepwise modifications ranging from the regulation of receptor activity and receptor degradation that require activation of several kinases and signaling pathways initiated by extranuclear functions of PR and ultimately chromatin organization, thereby indicating a natural crosstalk between nuclear and extranuclear PR (Fig 3).
3. Clinical implications of extranuclear steroid signaling in breast cancer
3.1. ER extranuclear signaling in breast cancer: ER assays to predict clinical outcome
At diagnosis, about 70% of BCs contain ERs and depend on E2 for growth. Since ER expression in BC is predictive of a clinical response to hormonal therapy, this has led to use of antiestrogens (tamoxifen, fulvestrant) and aromatase inhibitors in treating ERα-positive BC [16, 17, 20, 199, 200]. However, a substantial proportion of patients with localized BC, and essentially all patients with metastatic disease, become resistant to endocrine therapies. For more than 40 years, the IHC assay for nuclear ERα expression has been the predominant biomarker in BC, largely because of its important role in predicting potential benefit from endocrine therapy. Such observations led to current use of antiestrogens and AIs in treating ER-positive (ER+) BC. In addition, nuclear ER expression has important prognostic implications, with ER-negative tumors exhibiting a more aggressive phenotype. Earlier assays for ER depended on extensive homogenization of fresh-frozen tumor and preparation of cytosol extracts by centrifugation to perform a ligand-binding assay (LBA) [15, 200]. Of note, artifacts resulting from tumor homogenization in the LBA led to misinterpretations on the subcellular distribution of ER in cells. Unbound ER was found almost exclusively in cytosol, while ligand-bound ER was recovered in nuclear homogenate fractions. Later, it was recognized that these results were due to dissociation of unbound ER from the nucleus during homogenization of intact cells. This eventually led to the notion that ER was exclusively a nuclear-localized protein, a concept reinforced by localization of ER predominantly in tumor cell nuclei by use of monoclonal antibodies targeted to nuclear ERα [1, 4]. These antibodies were used to develop current assays for nuclear ERα. Further laboratory studies have revealed that the same experimental artifact that elicited dissociation of unbound ER from the nucleus during homogenization was also responsible for extracting another small pool of ERs from target cell membranes [36, 38, 39]. Investigations reviewed above confirm the presence of extranuclear membrane-associated ERα in BCs and further show that these ER help to stimulate BC gene transcripts and promote cancer progression [5, 41, 52, 83, 201]. Of note, activation of ER by a membrane-impermeant estrogen-dendrimer conjugate was shown to stimulate extranuclear responses in ER-expressing human BC cells, also contributing to regulation of BC cell number [53]. Of note, E2-induced growth of MCF-7 cells in vitro was blocked by treatment with the antibody to ERα and correlated closely with acute hormonal activation of MAPK and AKT kinase signaling [57]. In addition, E2-promoted growth of human BC xenografts in nude mice was significantly reduced by treatment in vivo with the ERα antibody. Thus, regulation of BC progression by E2 may be mediated by coordinated actions of ERs in nuclear and extranuclear compartments. These findings suggest that it may be important to measure both nuclear ERα and extranuclear membrane-associated ERα for greater diagnostic accuracy and to predict the response to BC treatment going forward.
Based on current IHC assays for nuclear ERα, only about half of advanced BCs with expression of ERα and/or PR respond to endocrine therapy, suggesting a need for improved assays designed to correlate with patient outcome [16]. The relatively poor specificity of nuclear ERα status in identifying tumors that will respond to hormone therapy has been documented in several studies and suggests that other factors are important [15, 16, 202, 203]. IHC results depend greatly on the type of antibody used, detection procedures, subcellular densities of ERs and problems due to long-term formalin fixation and antigen masking. Ongoing efforts to standardize IHC methods for detecting nuclear ERα, especially antibody validation and antigen retrieval methods, are helping to improve the reliability of this assay in archival BCs [204–208] [209]. These reports underscore the importance of fully elucidating the nature of the ER+ phenotype. To date, use of clinical IHC assays developed to detect nuclear ERα have shown relatively low levels of extranuclear ERα in standard formalin-fixed, paraffin-embedded BC specimens from the clinic [41, 210]. Although extranuclear ERα is detected in a number of cases, Welch et al. [210] suggest that the low incidence ranging from 0 to 3–4% using current assay methods is unlikely to be of routine clinical value. However, the criteria for positive extranuclear ER staining included immunoreactivity at least 25% or greater than that of nuclear ER staining. Using differing preanalytic fixation and processing methods and a different scoring system for intensity, Kim et al. [41] reported that extranuclear expression of either ER or PR was observed in 9.5% of clinical cases. Both studies used validated nuclear receptor antibodies. Given the abundance of data on extra-nuclear ER/PR expression in BC cell lines and preclinical models as detailed above, it will be important going forward to assess variables such as tissue fixation methods, antigen retrieval and processing protocols and tissue age that may impact extranuclear/membrane ER/PR versus nuclear receptor detection. Approaches to detect and quantify ER/PR forms present at very low levels or at a low density in subcellular compartments in human specimens are not yet available.
Recent work suggests that ER+ progenitor cells in the breast arise from primitive stem cells in fetal development [211]. ER+ progenitors proliferate in response to E2 and also produce paracrine factors that influence proliferation of adjacent ER-negative cells. Further, BC progression may result from transformation of these stem/progenitor cells. Findings indicate that only a minute subset of tumor cells (about 1%–10% of the primary BC mass) have tumorigenic potential [212, 213]. These tumor-initiating cells, termed cancer stem/progenitor cells (CSPC), are crucial in BC progression and are resistant to most chemotherapies. As such CSPC cells drive tumor formation, this model has important implications to understand BC progression, and to develop strategies for BC detection, prevention and treatment. Methods to detect in clinical specimens such minute subsets of CSPCs and potential extranuclear ER expression is another major challenge [214, 215].
The prognostic/predictive value of ERβ in BC is uncertain at this time, and conflicting reports are complicated by the identification of several variant forms of ERβ that are not detected by the current clinical IHC assays for ERα [15, 71, 216]. There is evidence that endogenous ERβ localizes to plasma membranes in clinical tissue specimens including BCs [71, 217]. Extranuclear ERβ2 expression, alone or combined with nuclear staining, predicted significantly worse overall survival among archival BC specimens [71]. Patients with only extranuclear ERβ2 had significantly worse outcome. In this series, nuclear and extranuclear ERβ expression differentially affected outcome, suggesting that measurement of these variables in BC could provide a more comprehensive picture of patient outcome to complement ERα assays. However, such data are limited by availability of specific, validated antibodies. Further, expression of ERβ1 is of particular interest because it is the only ERβ isoform that has an intact LBD, thereby serving as a potential drug target in clinic [218]. Of note, extranuclear signaling by ERβ has also been reported to be a significant factor in progression of medulloblastoma [219].
Besides full-length ERα-66 kD, the ERα-46 kD isoform is also expressed in a majority of archival BCs tested [220–222]. Binding affinities of both unliganded and fully-activated receptor forms toward coregulator peptides revealed that respective potencies of ERα-46 kD and ERα-66kD differed significantly, contributing to differential transcriptional activity of target genes to E2 stimulation. Expression of ERα-46 kD in BCs does not consistently correlate with expression of full-length ERα, but overall ERα-46 kD was expressed in ~70% of BC samples analyzed [220]. In BC cells in vitro with expression of both ERα-46 kD and ERα-66 kD, ERα-46 kD can impact cell proliferation depending on ratios of ERα-66 kD/ERα-46 kD [223, 224]. However, mice lacking the N-terminal A/B domain of ERα-66 kD were infertile due to lack of uterine epithelial responses to E2 normally mediated by full-length ERα [225]. Collectively, these findings indicate the importance of choice of antibodies used for BC diagnosis which may or may not detect ERα-46 kD or other ER forms, a factor that may have clinical relevance.
3.2 Metabolic effects of extranuclear ER signaling
Recent studies suggest that E2 regulates glucose metabolism in normal and malignant cells at least in part from membrane ER signaling [226]. A recent review details extensive effects of E2 in modulating normal organ metabolism [227]. Both ERα and ERβ play roles in normalizing insulin sensitivity and glucose homeostasis as revealed in ER-knockout models. Membrane ERα and ERβ are reported to have important roles in promoting insulin sensitivity and synthesis and secretion from pancreatic β-cells [228, 229]. Notably, high glucose and increased insulin signaling promote aggressive development of BC in mouse models [230], suggesting an important pathway by which ER stimulates BC development. Emerging reports further indicate that E2 signaling via AMPK modulates glycolytic pathways in BCs under reduced glucose conditions to promote the survival of BC cells in vivo [226].
Both ERα and ERβ are identified in the mitochondria of BC cells, primarily in the mitochondrial matrix [231, 232]. There is evidence in BCs that mitochondrial gene regulation occurs in part by means of E2 binding to ER in this intracellular organelle [233]. Additionally, mitochondrial ERβ is reported to mediate cytoprotection and survival of BC cells on E2 binding in mitochondria [62, 234].
3.3 ER extranuclear signaling and endocrine therapy in the clinic
Hormonal therapy was first used more than 120 years ago, marking the start of the current era of targeted antitumor treatment [235]. This approach is based on blocking activity of estrogens and their receptors. In the clinic, endocrine therapy is an important intervention for BCs that express ER and/or PR, and it has proven to be one of the most effective BC treatment strategies. Despite recent improvements in therapeutic options, development of endocrine resistance is one reason that BC is the 2nd most frequent cause of cancer death in women [199, 236, 237]. In most cases, ER is present in resistant tumors, and in many of these its activity continues to regulate BC growth [3]. Tamoxifen has been the most widely used hormone therapy for over 25 years, achieving a 39% reduction in BC recurrence and 31% reduction in mortality in nuclear ERα+ early BC [16, 20]. Although effective, tamoxifen has important drawbacks: a limited period of activity before resistance develops and undesirable side-effects in normal tissues such as uterus due to its activity as a partial agonist [18]. Of note, cooperative interactions between growth factor receptors and ER signaling pathways are identified in BC and NSCLC, and growth factor–mediated pathways, notably those of EGFR, HER2, and insulin-like growth factor receptor I, are critical in development of some types of antiestrogen resistance in BCs [17, 177, 238]. About 15–20% of BCs have overexpression of HER2 receptors, and increased HER2 expression correlates with poor clinical outcome and resistance to endocrine therapy [177, 238–242]. Such tamoxifen resistance is reported to associate with formation of HER2/ERα membrane-associated complexes that lead to non-genomic activation of both AKT and RPS6KA2, which in turn provides these BC cells with a survival advantage [243]. Similarly, overexpression of EGFR in about 50% of BCs correlates with endocrine resistance [244–247]. These data offered a rationale to target both ER and HER2 in ERα+/HER2-positive BCs in the clinic to overcome this type of endocrine resistance (Johnston et al., 2009).
As long as ER is present in tumors, growth may be stimulated by E2, partial agonists or ligand-independent action [17, 177, 238]. Introduction of aromatase inhibitors (AIs) for postmenopausal patients, either initially or after tamoxifen, has yielded better outcomes than the prior standard of 5 years tamoxifen [20]. However, in patients with advanced BC, only about 1/3 of nuclear ERα-positive BCs respond to AIs, and resistance can evolve due to ER activation by ER hypersensitivity, ligand-independent ER activation by activated growth factor receptor signaling [17, 177, 238] or emergence of receptor mutations. Unfortunately, in about 14% of metastatic ER+ BCs from patients with multiple prior endocrine therapies, there is evidence for acquisition of functionally-aberrant ESR1 with point mutations that often occur in the LBD of ERα [23, 248, 249]. Such mutated ESR1 variants continue to respond at least partially to ER antagonists such as fulvestrant which has a unique capability to downregulate and eliminate ER by induction of the ubiquitin-proteosome pathway[18, 250, 251] but higher drug doses are required to achieve wild-type ER levels of inhibition. These data underscore the need to find more potent ER antagonists and to implement use of hormonal treatments in combination with other synergistic agents such as cyclin-dependent kinase (CDK 4/6) inhibitors [252, 253]. There is currently no data on the status of extranuclear ER forms in metastatic tumors from the clinic with ESR1 mutations. However, there is evidence that hormonal antagonists such as fulvestrant, that block nuclear ER actions, also effectively block rapid E2-induced signaling in BC cells with wild-type ER [57, 73, 83], but therapies to specifically target membrane-associated ERs are yet to be developed.
While many studies on extranuclear steroid signaling have focused on estrogens, similar data confirm that extranuclear signaling by other sex steroids may be equally important in the clinic [50]. An experimental model whereby a biologically relevant androgen-mediated process is regulated completely independent of transcription was well-characterized in Xenopus laevis oocyte maturation [254]. Androgen-induced Xenopus oocyte maturation is mediated by classical androgen receptors (AR), as both the AR antagonist flutamide and AR knockdown by siRNA inhibit androgen-triggered maturation. Based on IHC and biochemical work, classical AR is expressed throughout the cell, with approximately 5% found in plasma membrane. These membrane-localized ARs regulate androgen-mediated maturation, as testosterone coupled to bovine serum albumin triggers oocyte maturation as well as free steroid. Of interest, prostate cancer is a well-characterized androgen-dependent tissue and is initially responsive to androgen deprivation (castration). However, over time, prostate cancer cells ultimately become castration-resistant due in part to AR amplification/mutation that allows responses to lower levels of androgens. In castration-resistant prostate cancer, the synthetic AR antagonist enzalutamide exhibits potent affinity to bind AR and blocks translocation of extranuclear AR to the nucleus to suppress binding of the ligand-bound receptor complex to DNA [255]. In the clinic, FDA-approved enzalutamide has had a major impact in improving the outcome of patients with castrate-resistant prostate cancer. Unexpectedly, enzalutamide is being assessed in clinical trials as a treatment for triple-negative breast cancers (TNBC) that lack expression of ERα, PR and HER2 [256].
TNBC occurs in 10–15% of patients, yet this disease subtype accounts for about half of all BC deaths. As TNBCs lack clinical expression of ERα, PR and HER2, they are not considered to be a target for hormonal therapy [257]. Although initially responsive to chemotherapy, TNBCs tend to relapse and metastasize early and have worse prognosis than other subtypes. Of note, several reports indicate that ERβ is expressed in TNBC cells [258, 259] and may play a role in TNBC progression [260–263]. ERβ forms occur in tumor cell nuclei, but also at extranuclear sites [71, 264]. ERβ can activate transcription by nuclear or indirectly by extranuclear pathways by interaction with coactivators/co-regulators such as PELP1 and SRAP [265] that in turn modulate signaling cascades to impact gene expression and TNBC progression [70, 71, 264, 266, 267]. As a consequence of such preclinical work, clinical studies to target ERβ are being planned in patients afflicted with metastatic TNBC [268].
3.4. ER extranuclear signaling in the tumor microenvironment and immunotherapy
Several steroid hormones regulate immune responses that contribute to significant changes in immune function during inflammatory and autoimmune diseases [269]. The regulation of immune responses by steroid hormones occurs at multiple levels including cell development, proliferation, cytokine or antibody production and apoptosis. Regulation of proliferation and apoptosis is especially critical in development of appropriate T- and B-cell repertoires. Of note, estrogen-binding proteins are reported to occur on plasma membranes of immune cells, and ERs are also reported to be expressed in myeloid-derived suppressor cells that act to block immune recognition of malignant cells [269, 270]. E2 binding to ERs and downstream activation of such immune suppressor cells are inhibited by fulvestrant, leading to promotion of antitumor activity in immune-competent mice. This work suggests that new approaches to management of BC such as repurposing of antiestrogens for combination therapy with immune checkpoint inhibitors may be forthcoming [271, 272]. Further studies are needed to detail membrane steroid receptor expression and signal transduction pathways in immune cells and in potential targets of the host immune system, particularly TNBCs [273].
3.5. ER and PR extranuclear signaling and resistance to endocrine therapy
Expression of PR and/or PR isoforms is a valuable marker for tumor aggressiveness and disease progression in breast, endometrium and lung cancers [274–276]. However, there are multiple hypotheses about how aberrations, loss and/or gain in PR expression is involved in cancer initiation, progression, metastasis and resistance to endocrine therapies. In normal mammary gland, PR+ cells are often found in non-proliferating cells [277]. However, the percentage of proliferating PR+/ER+ cells increases in hyperplasia and ductal carcinoma in situ (DCIS) states, and ER+/PR+ BC cells exhibit increased cell proliferation [278, 279]. Yet, loss of PR expression in BC is associated with endocrine resistance, BC aggressiveness and poor BC patient prognosis [280]. BC patients with PR-A rich tumors have worse disease-free survival as compared to those with PR-B rich tumors [281]. Selective loss of PR-B is also associated with poorly differentiated and progestin-resistant endometrium cancer [282]. Further, low PR expression predicts poor clinical outcome in NSCLC patients [276]; and treatment with PR antagonist (RU486) improves the survival of mice with spontaneous lung cancer [276]. Therefore, PR seems to mediate both proliferative and anti-proliferative functions depending on cellular and tissue contexts. How the presence of PR or PR isoforms affects cell growth and differentiation in these tissues and cancers remains poorly understood.
Accumulating evidence suggests that aberrant ERα/PR signaling in both the nucleus and the cytoplasm plays a critical role in altering endocrine pathways that lead to endocrine resistance in BC [283]. The presence or absence of ER and its ligand significantly affects the outcome of progesterone action in the nucleus [284], and ERα/PR extra-nuclear signaling pathways in the cytoplasm and membrane, are strongly implicated in hormone resistance [134, 144, 285–287]
There is considerable cross-communication between ERα and PR pathways, and both hormone pathways have been shown to co-regulate/cooperate in extranuclear signaling. E2 is reported to preferentially promote activation of the PR-B isoform [288]. In cells expressing ERα and the PR-B isoform, treatment with progestin, a synthetic progestogen, can attenuate antiestrogen-mediated growth arrest [144]. When ERα and PR are uncoupled, as in ERα-negative cells, rapid PR signaling can be exerted through PR-B interaction via the proline domain interacting with SH3 domains of the c-Src family of tyrosine kinases [76, 134, 144, 171, 289]. In addition, rapid extranuclear progestin activation has been postulated to play a role in gene regulation by mediating PR phosphorylation, thus acting as a feed-forward mechanism for PR nuclear transcriptional activity [290].
Experimental studies indicate that rapid, extranuclear PR signaling is mediated by the PR-B isoform which exerts rapid hormone signaling in the cytoplasm that interacts with several cellular signaling pathways. These pathways include those regulated by epithelial growth factor (EGF) [291], PI3K/AKT [292], IGF [293], Src receptor tyrosine kinases and RAS/p42/44 MAPK [76, 132–134, 144, 171, 289, 294]. Together, these pathways facilitate BC cell survival, cytoskeletal remodeling, proliferation and invasion. These acute signaling events are too rapid for classical transcriptional effects. As noted, extranuclear signaling of E2/ERα/Src occurs within minutes and is involved in transmitting signals that increase cell division or decrease apoptosis mediated by MAPK, and PI3K/AKT signaling pathways [295]. A functional Src interaction appears critical for extranuclear ERα function, and excessive extranuclear ERα/Src signaling associates with endocrine resistance [296]. Treatment with Src inhibitors is effective in blocking endocrine-resistant tumor progression [296], thus defining a potential role for pre-nuclear hormone receptor activity as a mechanism for endocrine-resistant BC phenotypes.
Additional reports suggest that a switch from ER/PR-mediated paracrine signaling for modulation of growth factor signaling pathways (e.g. EGF, HER2, Wnt, Src) to autocrine signaling contribute to BC progression [172] and potentially to the generation of endocrine-resistant tumors. How this information may be applied in endocrine therapy in the clinic remains unclear. However, treatments targeted to block rapid, hormone-dependent autocrine proliferation mechanisms may potentially have benefit in endocrine-resistant tumors.
3.6. Extranuclear PR signaling in NF-κB/p53 signaling
Epidemiological studies indicate that full-term pregnancy early in reproductive life provides a long-lasting and strong protection against BC [297], thus offering a clue that early, limited sex steroid stimulation may play a role in reducing later BC risk. This protective effect is reproduced by a 3-week exposure to low pregnancy-level doses of both estrogen and progesterone [298], but neither hormone alone is sufficient to induce the protective effect. Further, the absence of p53 tumor suppressor gene function was found to abrogate the protective effect of hormones against carcinogen-induced mammary carcinogenesis in mouse models. Limited data suggest that progesterone/PR and p53 may play a cooperative role in regulating chromosome stability [299]. Extranuclear PR signaling regulates p53 expression in human umbilical vein endothelial cells (HUVEC) [300], as progesterone treatment of HUVECs activates c-Src/Ras/Raf/MAPK/NFκB signaling to activate p53 gene transcription and control of cell cycle progression [300, 301]. Progesterone/PR also regulate p53 expression in BC, but it remains to be determined if similar mechanisms of extranuclear/nuclear PR activation of p53 occur in BCs. Thus, hormone stimulation at a critical period in mammary development appears to result in hormone-responsive cells with long-term changes in cellular regulatory loops governing proliferation and potentially responses to DNA damage.
3.7. Extranuclear ER/PR signaling - actin/cytoskeletal rearrangement, cell motility and metastasis
Cancer cell motility is essential in the process of BC invasion and metastasis. Cell migration is controlled by remodeling of the actin cytoskeleton by the formation of focal adhesion complexes and loss of stress fibers. This cytoskeletal reorganization associates with formation of specialized cell membrane structures such as membrane ruffles, filopodia and lamellipodia [302, 303]. Extranuclear ER/PR signaling is reportedly involved in actin remodeling and cell migration [301, 304]. E2 treatment of T47D ER+/PR+ BC cells induces rapid changes in the actin cytoskeleton with formation of membrane ruffles and pseudopodia [305]. These effects are achieved by acute interactions of ERα with G protein Gα13 resulting in recruitment of small GTPase-RhoA and activation of its downstream target Rho-associated kinase-2 (ROCK-2) and phosphorylation of moesin, an actin regulatory protein [306]. Similar signaling cascades mediated by ligand-bound ERα are also reported in endometrial cells [307]. These studies reveal that E2-activated ERα stimulates BC cell migration via activation of c-Src, paxillin and FAK, signaling molecules that play important roles in regulating focal adhesion dynamics to facilitate cell migration. Recent studies in MCF-7 BC cells similarly show that E2-mediated extranuclear signaling promotes cytoskeleton remodeling and cell migration via PELP1 scaffolding protein, c-Src, and integrin-linked kinase signaling pathways, with expression of PELP1 positively correlated with the invasiveness of BCs [308, 309].
Extranuclear PR signaling also plays a significant role in regulating cytoskeletal remodeling. Hence, PR-null MDA-MB-231 BC cells transfected with PR and exposed to progesterone exhibit increased expression of focal adhesion proteins, paxillin and FAK, increased numbers and diameters of stress fibers and increased cell migration and invasion [310]. Treatment with progesterone or synthetic progestin medroxyprogesterone acetate (MPA) also stimulates cell migration and invasion of HUVEC cells and T47D cells, a process preceded by rapid remodeling of the actin cytoskeleton and formation of membrane ruffles and pseudopodia [311]. These changes are mediated in part by phosphorylation of moesin through interaction between PR-A and G protein Gα13. MPA treatment also promotes PR interaction with c-Src resulting in activation of a PI3K/RhoA/ROCK-2 signaling cascade [311, 312]. Progesterone treatment further promotes cancer cell migration in 3D-matrices by activating formation of focal adhesion complexes as indicated by Tyr-397 phosphorylation of FAK via the c-Src/PI3k-Akt/RhoA/ROCK2 cascade [313]. Extranuclear PR signaling regulates BC cell migration by progesterone stimulation of the c-Src/AKT pathway, leading to activation of ribosomal S6 kinase 1 (RSK1) and increased phosphorylation of p27 at T198 with formation of a p27pT198/RhoA complex in cytosol. The p27pT198 RhoA complex prevents RhoA degradation and enhances cell migration; inhibition of RSK1 with RSK1 inhibitor BI-D1870 reversed these events. Together, these data suggest RSK1 activation plays a role in progesterone-stimulated BC cell migration [314], yet clinical-translation of these findings remains to be accomplished.
3.8. Extranuclear signaling by environmental estrogen and breast cancer risk
Increasing evidence suggests that exposure to environmental estrogens could be one of the major factors contributing to increased risks of BC [315]. Environmental estrogens such as bisphenol A (BPA), DES, organochloride pesticides and genistein have been shown to impact both nuclear and extranuclear ER signaling [316]. Rapid signaling from membrane ER in response to both E2 and xenoestrogen DES regulates the histone methyltransferase enhancer of Zeste homolog 2 (EZH2). Extranuclear ER signaling via PI3K/AKT kinase phosphorylates EZH2 at S21, reducing levels of trimethylation of lysine-27 on histone H3 in hormone-responsive cells, a process that can reprogram the profile of E2-responsive genes in target organs such as breast and uterus [317]. In addition, phytoestrogen genistein, but not BPA, induce extranuclear ER activation of PI3K/AKT kinase and phosphorylation of EZH2 leading to hormonal hypersensitivity and higher risk of uterine tumorigenesis in rats [318]. To date, these data indicate that rapid extranuclear ER signaling may provide a direct link between xenoestrogen-induced receptor signaling and modulation of epigenetic machinery during tissue development leading to increased hormone-related cancer risk. Development of improved screening methods to identify potentially harmful environmental agents and new interventions to manage such exposures are urgently needed [319]. One unfortunate clinical example is the long-term impact of fetal exposure to the synthetic estrogen diethylstilbestrol (DES). Maternal ingestion of DES was associated with later development of reproductive tract abnormalities including adenocarcinoma of the vagina in young female offspring [320, 321].
Concluding remarks
Numerous reports suggest that specialized proteins associated with target cell membranes are the first contact point for sex steroid hormones. For E2, progesterone and androgen, such extranuclear receptor molecules are often reported to share homology with nuclear forms. These primary interactions trigger a cascade of specific downstream cellular responses. Thus, rapid sex steroid hormone-dependent signaling is postulated as a prerequisite in part for later receptor-induced transcriptional activity, suggesting an integrated model of steroid receptor signaling where steroid hormone-dependent early extranuclear effects contribute to late receptor-dependent nuclear actions. Ongoing research continues to address whether extranuclear-initiated and nuclear transcriptional activities of sex steroid hormones are integrated to yield one overall effect or represent parallel pathways with distinct effects. More detailed understanding of molecular mechanisms, such as palmitoylation of ER and PR that target these receptors for membrane localization, remains to be elaborated in both normal and transformed cells.
While it is now well accepted that ER and PR can perform both nuclear and extranuclear functions and localize to cell membrane and nuclear pools, studies also suggest that alternative E2/progesterone-responsive membrane proteins such as GPR30, mPRs, or PGRMC1 may mediate extranuclear signaling. Physiological significance of these alternative molecules and their potential cross-communication with nuclear receptors clearly require further exploration.
Based on available evidence, nuclear and extranuclear ER/PR signaling appears to cooperate in mediating BC progression. Validation of extranuclear steroid hormone receptor structures and their specific biologic functions in health and disease may well lead to development of a new generation of targeted therapeutics to manage sex steroid hormone-responsive cancers in the clinic.
Highlights.
ER and PR play key roles in breast cancer development and progression.
Hormone-induced rapid extranuclear signaling affects signaling networks.
Extranuclear signaling modifies ER/PR transcription and gene networks.
History and updates on ER/PR extranuclear signaling are reviewed and discussed.
Acknowledgments
We thank our distinguished colleague and friend Dr. C.M. Szego, a pioneer in the field of extranuclear steroid hormone signaling, for thoughtful discussions and guidance.
Grant support: NIH/NCI U54 CA143930, California BCRP IDEA Award, U.S. Army DOD BCRP, Stiles Program in Integrative Oncology, Gateway Foundation, Hickey Foundation and Tower Cancer Research Foundation-Jessica M. Berman Breast Cancer Research Award to R.J.P. Grant for joint funding Rachadaphisek Sompoch Endowment fund and The Rachadaphisek Sompoch Endowment Fund (2016) Chulalongkorn University (CUHR59-003) to V.B.
Abbreviations
- AI
aromatase inhibitor
- BC
breast cancer
- DCIS
ductal carcinoma in situ
- E2
estradiol-17β
- ERα
estrogen receptor-alpha
- ERβ
estrogen receptor-beta
- ESR1
estrogen receptor gene
- LBD
ligand-binding domain
- NSCLC
non-small cell lung cancer
- PR-A
progesterone receptor-A
- PR-B
progesterone receptor-B
- TNBC
triple-negative breast cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 2.Green S, Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988;4:309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- 3.Jensen EV, Greene GL, Closs LE, DeSombre ER, Nadji M. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1–40. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- 4.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 5.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzenellenbogen BS. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod. 1996;54:287–293. doi: 10.1095/biolreprod54.2.287. [DOI] [PubMed] [Google Scholar]
- 8.O’Malley B GG, Closs L, et al. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1–40. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- 9.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 10.Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319(Pt 3):657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yudt MR, Vorojeikina D, Zhong L, Skafar DF, Sasson S, Gasiewicz TA, Notides AC. Function of estrogen receptor tyrosine 537 in hormone binding, DNA binding, and transactivation. Biochemistry. 1999;38:14146–14156. doi: 10.1021/bi9911132. [DOI] [PubMed] [Google Scholar]
- 12.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 13.McGuire WL, Horwitz KB, Pearson OH, Segaloff A. Current status of estrogen and progesterone receptors in breast cancer. Cancer. 1977;39:2934–2947. doi: 10.1002/1097-0142(197706)39:6<2934::aid-cncr2820390680>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Wakeling AE, O’Connor KM, Newboult E. Comparison of the biological effects of tamoxifen and a new antioestrogen (LY 117018) on the immature rat uterus. J Endocrinol. 1983;99:447–453. doi: 10.1677/joe.0.0990447. [DOI] [PubMed] [Google Scholar]
- 15.Dowsett M, Nicholson RI, Pietras RJ. Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast Cancer Res Treat. 2005;93(Suppl 1):S11–18. doi: 10.1007/s10549-005-9037-3. [DOI] [PubMed] [Google Scholar]
- 16.Elledge RM, Green S, Howes L, Clark GM, Berardo M, Allred DC, Pugh R, Ciocca D, Ravdin P, O’Sullivan J, Rivkin S, Martino S, Osborne CK. bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. J Clin Oncol. 1997;15:1916–1922. doi: 10.1200/JCO.1997.15.5.1916. [DOI] [PubMed] [Google Scholar]
- 17.Hurvitz SA, Pietras RJ. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113:2385–2397. doi: 10.1002/cncr.23875. [DOI] [PubMed] [Google Scholar]
- 18.Robertson JF, Lindemann J, Garnett S, Anderson E, Nicholson RI, Kuter I, Gee JM. A good drug made better: the fulvestrant dose-response story. Clin Breast Cancer. 2014;14:381–389. doi: 10.1016/j.clbc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C, S. Intergroup Exemestane A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 20.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A, G. Breast International Group 1–98 Collaborative A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 21.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, A.T. Group Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 22.Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DB, Miller WR. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 2003;63:6523–6531. [PubMed] [Google Scholar]
- 23.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–780. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu W, Katzenellenbogen BS. Estrogen Receptor-β Modulation of the ERα-p53 Loop Regulating Gene Expression, Proliferation, and Apoptosis in Breast Cancer. Hormones and Cancer. 2017:1–13. doi: 10.1007/s12672-017-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner M, Huang B, Gustafsson JA. Estrogen Receptor β as a pharmaceutical target. Trends in pharmacological sciences. 2017;38:92–99. doi: 10.1016/j.tips.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, Li X, Blanchard A, Bramwell V, Pritchard K, Tu D, Shepherd L, Myal Y, Penner C, Watson P. Expression of both Estrogen Receptor-beta 1 (ER-β1) and its co-regulator Steroid Receptor RNA Activator Protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with Estrogen Receptor-alpha (ER-α)-Negative Early Breast Cancer (EBC) Annals of Oncology. 2013;24:1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 28.Wisinski KB, Xu W, Tevaarwerk AJ, Saha S, Kim K, Traynor A, Dietrich L, Hegeman R, Patel D, Blank J. Targeting estrogen receptor beta in a phase 2 study of high-dose estradiol in metastatic triple-negative breast cancer: a Wisconsin Oncology Network Study. Clinical breast cancer. 2016;16:256–261. doi: 10.1016/j.clbc.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma R, Karthik GM, Lövrot J, Haglund F, Rosin G, Katchy A, Zhang X, Viberg L, Frisell J, Williams C. Estrogen Receptor β as a Therapeutic Target in Breast Cancer Stem Cells. Journal Of The National Cancer Institute. 2017;109:djw236. doi: 10.1093/jnci/djw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen EV JH. Basic guide to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414. [Google Scholar]
- 31.Gorski J, Toft D, Shyamala G, Smith D, Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- 32.Talwar GP, Segal SJ, Evans A, Davidson OW. The Binding of Estradiol in the Uterus: A Mechanism for Depression of Rna Synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1964;52:1059–1066. doi: 10.1073/pnas.52.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buller RE, O’Malley BW. The biology and mechanism of steroid hormone receptor interaction with the eukaryotic nucleus. Biochem Pharmacol. 1976;25:1–12. doi: 10.1016/0006-2952(76)90164-7. [DOI] [PubMed] [Google Scholar]
- 34.Schimke RT, McKnight GS, Shapiro DJ, Sullivan D, Palacios R. Hormonal regulation of ovalbumin synthesis in the chick oviduct. Recent Prog Horm Res. 1975;31:175–211. doi: 10.1016/b978-0-12-571131-9.50009-8. [DOI] [PubMed] [Google Scholar]
- 35.Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- 36.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 37.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proceedings of the National Academy of Sciences of the United States of America. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979;11:1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- 39.Pietras RJ, Szego CM. Metabolic and proliferative responses to estrogen by hepatocytes selected for plasma membrane binding-sites specific for estradiol-17beta. J Cell Physiol. 1979;98:145–159. doi: 10.1002/jcp.1040980116. [DOI] [PubMed] [Google Scholar]
- 40.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 41.Kim R, Kaneko M, Arihiro K, Emi M, Tanabe K, Murakami S, Osaki A, Inai K. Extranuclear expression of hormone receptors in primary breast cancer. Ann Oncol. 2006;17:1213–1220. doi: 10.1093/annonc/mdl118. [DOI] [PubMed] [Google Scholar]
- 42.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 43.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 44.Kelly MJ, Ronnekleiv OK. Minireview: neural signaling of estradiol in the hypothalamus. Mol Endocrinol. 2015;29:645–657. doi: 10.1210/me.2014-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushiro T, Nambara E, McCourt P. Hormone evolution: The key to signalling. Nature. 2003;422:122. doi: 10.1038/422122a. [DOI] [PubMed] [Google Scholar]
- 47.Pietras RJ, Nemere I, Szego CM. Steroid hormone receptors in target cell membranes. Endocrine. 2001;14:417–427. doi: 10.1385/ENDO:14:3:417. [DOI] [PubMed] [Google Scholar]
- 48.Kohidai L, Katona J, Csaba G. Effects of steroid hormones on five functional parameters of Tetrahymena: evolutionary conclusions. Cell Biochem Funct. 2003;21:19–26. doi: 10.1002/cbf.996. [DOI] [PubMed] [Google Scholar]
- 49.Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011;76:877–884. doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 51.Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66:271–280. doi: 10.1146/annurev-med-050913-021703. [DOI] [PubMed] [Google Scholar]
- 52.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 53.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz N, Verma A, Bivens CB, Schwartz Z, Boyan BD. Rapid steroid hormone actions via membrane receptors. Biochim Biophys Acta. 2016;1863:2289–2298. doi: 10.1016/j.bbamcr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 56.Marquez DC, Lee J, Lin T, Pietras RJ. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocrine. 2001;16:73–81. doi: 10.1385/ENDO:16:2:073. [DOI] [PubMed] [Google Scholar]
- 57.Marquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20:5420–5430. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- 58.Song RX, Chen Y, Zhang Z, Bao Y, Yue W, Wang JP, Fan P, Santen RJ. Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J Steroid Biochem Mol Biol. 2010;118:219–230. doi: 10.1016/j.jsbmb.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milgrom E, Atger M, Baulieu EE. Studies on estrogen entry into uterine cells and on estradiol-receptor complex attachment to the nucleus–is the entry of estrogen into uterine cells a protein-mediated process? Biochim Biophys Acta. 1973;320:267–283. doi: 10.1016/0304-4165(73)90307-3. [DOI] [PubMed] [Google Scholar]
- 60.Pietras CMSaRJ. Membrane recognition and effector sites in steroid hormone action. Academic Press; New Yorl: 1981. [Google Scholar]
- 61.Pietras RJ, Szego CM. Specific internalization of estrogen and binding to nuclear matrix in isolated uterine cells. Biochem Biophys Res Commun. 1984;123:84–91. doi: 10.1016/0006-291x(84)90383-8. [DOI] [PubMed] [Google Scholar]
- 62.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 63.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 64.Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 65.Nenci I, Marchetti E, Marzola A, Fabris G. Affinity cytochemistry visualizes specific estrogen binding sites on the plasma membrane of breast cancer cells. J Steroid Biochem. 1981;14:1139–1146. doi: 10.1016/0022-4731(81)90043-1. [DOI] [PubMed] [Google Scholar]
- 66.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 68.Norfleet AM, Clarke CH, Gametchu B, Watson CS. Antibodies to the estrogen receptor-alpha modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pawlak J, Karolczak M, Krust A, Chambon P, Beyer C. Estrogen receptor-alpha is associated with the plasma membrane of astrocytes and coupled to the MAP/Src-kinase pathway. Glia. 2005;50:270–275. doi: 10.1002/glia.20162. [DOI] [PubMed] [Google Scholar]
- 70.Hamilton N, Marquez-Garban D, Mah VH, Elshimali Y, Elashoff D, Garon EB, Vadgama J, Pietras R. Estrogen Receptor-beta and the Insulin-Like Growth Factor Axis as Potential Therapeutic Targets for Triple-Negative Breast Cancer. Critical reviews in oncogenesis. 2015;20:373–390. doi: 10.1615/CritRevOncog.v20.i5-6.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 72.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 73.Marquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 74.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 75.Acconcia F, Bocedi A, Ascenzi P, Marino M. Does palmitoylation target estrogen receptors to plasma membrane caveolae? IUBMB life. 2003;55:33–35. doi: 10.1080/1521654031000081256. [DOI] [PubMed] [Google Scholar]
- 76.Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Kawprasertsri S, Pietras RJ, Marquez-Garban DC, Boonyaratanakornkit V. Progesterone receptor (PR) polyproline domain (PPD) mediates inhibition of epidermal growth factor receptor (EGFR) signaling in non-small cell lung cancer cells. Cancer letters. 2016;374:279–291. doi: 10.1016/j.canlet.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. The Journal of biological chemistry. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 79.Xiang R, Shi Y, Dillon DA, Negin B, Horvath C, Wilkins JA. 2D LC/MS analysis of membrane proteins from breast cancer cell lines MCF7 and BT474. J Proteome Res. 2004;3:1278–1283. doi: 10.1021/pr049852e. [DOI] [PubMed] [Google Scholar]
- 80.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 81.Boyan BD, Sylvia VL, Frambach T, Lohmann CH, Dietl J, Dean DD, Schwartz Z. Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen. Endocrinology. 2003;144:1812–1824. doi: 10.1210/en.2002-221018. [DOI] [PubMed] [Google Scholar]
- 82.Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 83.Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nature reviews Molecular cell biology. 2016;17:783–797. doi: 10.1038/nrm.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- 86.Vranic S, Gatalica Z, Wang ZY. Update on the molecular profile of the MDA-MB-453 cell line as a model for apocrine breast carcinoma studies. Oncology letters. 2011;2:1131–1137. doi: 10.3892/ol.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li G, Zhang J, Jin K, He K, Zheng Y, Xu X, Wang H, Wang H, Li Z, Yu X, Teng X, Cao J, Teng L. Estrogen receptor-alpha36 is involved in development of acquired tamoxifen resistance via regulating the growth status switch in breast cancer cells. Mol Oncol. 2013;7:611–624. doi: 10.1016/j.molonc.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su X, Xu X, Li G, Lin B, Cao J, Teng L. ER-alpha36: a novel biomarker and potential therapeutic target in breast cancer. Onco Targets Ther. 2014;7:1525–1533. doi: 10.2147/OTT.S65345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Jr., Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 90.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265–266:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 93.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 94.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 95.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 96.Rieck GC, Freites ON, Williams S. Is tamoxifen associated with high-risk endometrial carcinomas? A retrospective case series of 196 women with endometrial cancer. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology. 2005;25:39–41. doi: 10.1080/01443610400024740. [DOI] [PubMed] [Google Scholar]
- 97.Senkus-Konefka E, Konefka T, Jassem J. The effects of tamoxifen on the female genital tract. Cancer treatment reviews. 2004;30:291–301. doi: 10.1016/j.ctrv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 98.Girgert R, Emons G, Grundker C. 17beta-estradiol-induced growth of triple-negative breast cancer cells is prevented by the reduction of GPER expression after treatment with gefitinib. Oncology reports. 2017;37:1212–1218. doi: 10.3892/or.2016.5306. [DOI] [PubMed] [Google Scholar]
- 99.Broselid S, Cheng B, Sjostrom M, Lovgren K, Santiago HL Klug-De, Belting M, Jirstrom K, Malmstrom P, Olde B, Bendahl PO, Hartman L, Ferno M, Leeb-Lundberg LM. G protein-coupled estrogen receptor is apoptotic and correlates with increased distant disease-free survival of estrogen receptor-positive breast cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:1681–1692. doi: 10.1158/1078-0432.CCR-12-2376. [DOI] [PubMed] [Google Scholar]
- 100.Sjostrom M, Hartman L, Grabau D, Fornander T, Malmstrom P, Nordenskjold B, Sgroi DC, Skoog L, Stal O, Leeb-Lundberg LM, Ferno M. Lack of G protein-coupled estrogen receptor (GPER) in the plasma membrane is associated with excellent long-term prognosis in breast cancer. Breast Cancer Res Treat. 2014;145:61–71. doi: 10.1007/s10549-014-2936-4. [DOI] [PubMed] [Google Scholar]
- 101.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, Filardo EJ. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol. 2014;382:950–959. doi: 10.1016/j.mce.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 103.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 104.LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res. 2002;62:3691–3697. [PubMed] [Google Scholar]
- 105.Thekkumkara T, Snyder R, Karamyan VT. Competitive Binding Assay for the G-Protein-Coupled Receptor 30 (GPR30) or G-Protein-Coupled Estrogen Receptor (GPER) Methods in molecular biology. 2016;1366:11–17. doi: 10.1007/978-1-4939-3127-9_2. [DOI] [PubMed] [Google Scholar]
- 106.Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 107.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 108.Sinicropi MS, Lappano R, Caruso A, Santolla MF, Pisano A, Rosano C, Capasso A, Panno A, Lancelot JC, Rault S, Saturnino C, Maggiolini M. (6-bromo-1,4-dimethyl-9H-carbazol-3-yl-methylene)-hydrazine (carbhydraz) acts as a GPER agonist in breast cancer cells. Current topics in medicinal chemistry. 2015;15:1035–1042. doi: 10.2174/1568026615666150317221549. [DOI] [PubMed] [Google Scholar]
- 109.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem Biophys Res Commun. 2004;316:878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 111.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23:188–199. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30:3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. The Journal of biological chemistry. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Dev Cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gustafsson KL, Farman H, Henning P, Lionikaite V, Moverare-Skrtic S, Wu J, Ryberg H, Koskela A, Gustafsson JA, Tuukkanen J, Levin ER, Ohlsson C, Lagerquist MK. The role of membrane ERalpha signaling in bone and other major estrogen responsive tissues. Sci Rep. 2016;6:29473. doi: 10.1038/srep29473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessieres E, Kim SH, Liere P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E283–290. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 120.Morrill GA, Kostellow AB, Gupta RK. Transmembrane helices in “classical” nuclear reproductive steroid receptors: a perspective. Nuclear receptor signaling. 2015;13:e003. doi: 10.1621/nrs.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim KH, Toomre D, Bender JR. Splice isoform estrogen receptors as integral transmembrane proteins. Mol Biol Cell. 2011;22:4415–4423. doi: 10.1091/mbc.E11-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weidemann H. Na/K-ATPase, endogenous digitalis like compounds and cancer development – a hypothesis. Front Biosci. 2005;10:2165–2176. doi: 10.2741/1688. [DOI] [PubMed] [Google Scholar]
- 123.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circulation research. 2000;87:e44–e52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 125.Song RX. Membrane-initiated steroid signaling action of estrogen and breast cancer. Semin Reprod Med. 2007;25:187–197. doi: 10.1055/s-2007-973431. [DOI] [PubMed] [Google Scholar]
- 126.McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 127.Law ML, Kao FT, Wei Q, Hartz JA, Greene GL, Zarucki-Schulz T, Conneely OM, Jones C, Puck TT, O’Malley BW, et al. The progesterone receptor gene maps to human chromosome band 11q13, the site of the mammary oncogene int-2. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2877–2881. doi: 10.1073/pnas.84.9.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 130.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–3115. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tora L, Gronemeyer H, Turcotte B, Gaub MP, Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988;333:185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- 132.Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–770. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 133.Edwards DP, Wardell SE, Boonyaratanakornkit V. Progesterone receptor interacting coregulatory proteins and cross talk with cell signaling pathways. J Steroid Biochem Mol Biol. 2002;83:173–186. doi: 10.1016/s0960-0760(02)00265-0. [DOI] [PubMed] [Google Scholar]
- 134.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 135.Christensen K, Estes PA, Onate SA, Beck CA, DeMarzo A, Altmann M, Lieberman BA, St John J, Nordeen SK, Edwards DP. Characterization and functional properties of the A and B forms of human progesterone receptors synthesized in a baculovirus system. Mol Endocrinol. 1991;5:1755–1770. doi: 10.1210/mend-5-11-1755. [DOI] [PubMed] [Google Scholar]
- 136.McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48:425–432. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 137.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. The Journal of biological chemistry. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 138.McGowan EM, Clarke CL. Effect of overexpression of progesterone receptor A on endogenous progestin-sensitive endpoints in breast cancer cells. Mol Endocrinol. 1999;13:1657–1671. doi: 10.1210/mend.13.10.0356. [DOI] [PubMed] [Google Scholar]
- 139.Graham JD, Clarke CL. Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res. 2002;4:187–190. doi: 10.1186/bcr450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- 141.Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A. Progesterone receptor modulates ER [agr] action in breast cancer. Nature. 2015;523:313–317. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62:287–297. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 143.Cork DM, Lennard TW, Tyson-Capper AJ. Alternative splicing and the progesterone receptor in breast cancer. Breast Cancer Res. 2008;10:207. doi: 10.1186/bcr2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McGowan EM, Russell AJ, Boonyaratanakornkit V, Saunders DN, Lehrbach GM, Sergio CM, Musgrove EA, Edwards DP, Sutherland RL. Progestins reinitiate cell cycle progression in antiestrogen-arrested breast cancer cells through the B-isoform of progesterone receptor. Cancer Res. 2007;67:8942–8951. doi: 10.1158/0008-5472.CAN-07-1255. [DOI] [PubMed] [Google Scholar]
- 145.Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 147.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 148.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression and characterization of a novel membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuo L, Li W, You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Research. 2010;12:R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids. 2010;75:314–322. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76:11–17. doi: 10.1016/j.steroids.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valadez-Cosmes P, Vazquez-Martinez ER, Cerbon M, Camacho-Arroyo I. Membrane progesterone receptors in reproduction and cancer. Mol Cell Endocrinol. 2016;434:166–175. doi: 10.1016/j.mce.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 154.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 155.Dressing GE, Alyea R, Pang Y, Thomas P. Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors. Horm Cancer. 2012;3:101–112. doi: 10.1007/s12672-012-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou L, Zhou W, Zhang H, Hu Y, Yu L, Zhang Y, Zhang Y, Wang S, Wang P, Xia W. Progesterone suppresses triple-negative breast cancer growth and metastasis to the brain via membrane progesterone receptor α. International Journal of Molecular Medicine. 2017;40:755–761. doi: 10.3892/ijmm.2017.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kimura I, Nakayama Y, Konishi M, Terasawa K, Ohta M, Itoh N, Fujimoto M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Current Protein and Peptide Science. 2012;13:687–696. doi: 10.2174/138920312804142110. [DOI] [PubMed] [Google Scholar]
- 158.Cahill MA. Progesterone receptor membrane component 1: an integrative review. The Journal of steroid biochemistry and molecular biology. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 159.Kabe Y, Nakane T, Koike I, Yamamoto T, Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nature communications. 2016;7 doi: 10.1038/ncomms11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kabe Y, Yamamoto T, Kajimura M, Sugiura Y, Koike I, Ohmura M, Nakamura T, Tokumoto Y, Tsugawa H, Handa H. Cystathionine β-synthase and PGRMC1 as CO sensors. Free Radical Biology and Medicine. 2016;99:333–344. doi: 10.1016/j.freeradbiomed.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 161.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacology & therapeutics. 2009;121:14–19. doi: 10.1016/j.pharmthera.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cahill MA, Jazayeri JA, Catalano SM, Toyokuni S, Kovacevic Z, Richardson DR. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer biology. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2016;1866:339–349. doi: 10.1016/j.bbcan.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Difilippantonio S, Chen Y, Pietas A, Schlüns K, Pacyna-Gengelbach M, Deutschmann N, Padilla-Nash H, Ried T, Petersen I. Gene expression profiles in human non-small and small-cell lung cancers. European journal of cancer. 2003;39:1936–1947. doi: 10.1016/s0959-8049(03)00419-2. [DOI] [PubMed] [Google Scholar]
- 164.Ahmed IS, Rohe HJ, Twist KE, Mattingly MN, Craven RJ. Progesterone receptor membrane component 1 (Pgrmc1): a heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. Journal of Pharmacology and Experimental Therapeutics. 2010;333:564–573. doi: 10.1124/jpet.109.164210. [DOI] [PubMed] [Google Scholar]
- 165.Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. Journal of Biological Chemistry. 2010;285:24775–24782. doi: 10.1074/jbc.M110.134585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Micevych PE, Wong AM, Mittelman-Smith MA. Estradiol Membrane-Initiated Signaling and Female Reproduction. Compr Physiol. 2015;5:1211–1222. doi: 10.1002/cphy.c140056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 168.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 169.Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 170.Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol. 2010;24:2114–2125. doi: 10.1210/me.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays Biochem. 2004;40:105–120. doi: 10.1042/bse0400105. [DOI] [PubMed] [Google Scholar]
- 172.Grimm SL, Hartig SM, Edwards DP. Progesterone receptor signaling mechanisms. Journal of Molecular Biology. 2016;428:3831–3849. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 173.Ballare C, Uhrig M, Betchtold T, Sancho E, Domenico MD, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone Receptor Rapid Signaling Mediates Ser345 Phosphorylation and Tethering to Sp1 Transcription Factors. Mol Endocrinol. 2008 doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saner KJ, Welter BH, Zhang F, Hansen E, Dupont B, Wei Y, Price TM. Cloning and expression of a novel, truncated, progesterone receptor. Molecular and cellular endocrinology. 2003;200:155–163. doi: 10.1016/s0303-7207(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 176.Dai Q, Shah AA, Garde RV, Yonish BA, Zhang L, Medvitz NA, Miller SE, Hansen EL, Dunn CN, Price TM. A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Molecular Endocrinology. 2013;27:741–753. doi: 10.1210/me.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 178.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 179.Mora J Font de, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 181.Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer. 2001;8:175–182. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- 182.Weinberg OK, Marquez-Garban DC, Pietras RJ. New approaches to reverse resistance to hormonal therapy in human breast cancer. Drug Resist Updat. 2005;8:219–233. doi: 10.1016/j.drup.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 183.Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tüchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Annals of surgery. 1993;218:13. doi: 10.1097/00000658-199307000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 185.Bines J, Dienstmann R, Obadia R, Branco L, Quintella D, Castro T, Camacho P, Soares F, Costa M. Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial. Annals of oncology. 2014;25:831–836. doi: 10.1093/annonc/mdu015. [DOI] [PubMed] [Google Scholar]
- 186.Poulard C, Treilleux I, Lavergne E, Bouchekioua-Bouzaghou K, Goddard-Léon S, Chabaud S, Trédan O, Corbo L, Le Romancer M. Activation of rapid oestrogen signalling in aggressive human breast cancers. EMBO molecular medicine. 2012;4:1200–1213. doi: 10.1002/emmm.201201615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D’Assoro AB, Ravindranathan P, Peng Y, Raj GV, Yee D, Lange CA. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1-and estrogen receptor-containing transcription complexes. Oncogene. 2015;34:506–515. doi: 10.1038/onc.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Molecular endocrinology. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 189.Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Research. 2012;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vicent GP, Nacht AS, Zaurín R, Ballaré C, Clausell J, Beato M. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Molecular Endocrinology. 2010;24:2088–2098. doi: 10.1210/me.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Molecular cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 192.Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Molecular and cellular biology. 2005;25:2885–2898. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dressing GE, Hagan CR, Knutson TP, Daniel AR, Lange CA. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocrine-related cancer. 2009;16:351–361. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Moore NL, Narayanan R, Weigel NL. Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity. Steroids. 2007;72:202–209. doi: 10.1016/j.steroids.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Molecular and cellular biology. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hagan CR, Knutson TP, Lange CA. A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic acids research. 2013;41:8926–8942. doi: 10.1093/nar/gkt706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Molecular and cellular biology. 2011;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC medicine. 2014;12:32. doi: 10.1186/1741-7015-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kaklamani VG, Gradishar WJ. Endocrine Therapy in the Current Management of Postmenopausal Estrogen Receptor-Positive Metastatic Breast Cancer. Oncologist. 2017 doi: 10.1634/theoncologist.2015-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McGuire WL, De La Garza M, Chamness GC. Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res. 1977;37:637–639. [PubMed] [Google Scholar]
- 201.Mintz PJ, Habib NA, Jones LJ, Giamas G, Lewis JS, Bowen RL, Coombes RC, Stebbing J. The phosphorylated membrane estrogen receptor and cytoplasmic signaling and apoptosis proteins in human breast cancer. Cancer. 2008;113:1489–1495. doi: 10.1002/cncr.23699. [DOI] [PubMed] [Google Scholar]
- 202.Neves JI, Begnami MD, Arias V, Santos GC. Antigen retrieval methods and estrogen receptor immunoexpression using 1D5 antibody: a comparative study. Int J Surg Pathol. 2005;13:353–357. doi: 10.1177/106689690501300407. [DOI] [PubMed] [Google Scholar]
- 203.Rhodes A, Jasani B, Barnes DM, Bobrow LG, Miller KD. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 205.Allred DC. Problems and solutions in the evaluation of hormone receptors in breast cancer. J Clin Oncol. 2008;26:2433–2435. doi: 10.1200/JCO.2007.15.7800. [DOI] [PubMed] [Google Scholar]
- 206.Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, Christensen K, Edwards DP. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids. 2002;67:799–813. doi: 10.1016/s0039-128x(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 207.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, Vijver M van de, Wheeler TM, Hayes DF, O. American Society of Clinical. P. College of American American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 208.Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, Grant M, Hasenburg A, Hille ET, Kay C, Kieback DG, Putter H, Markopoulos C, Kranenbarg EM, Mallon EA, Dirix L, Seynaeve C, Rea D. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29:1531–1538. doi: 10.1200/JCO.2010.30.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yaziji H, Taylor CR, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B, Floyd AD, Barry TS, Martin AW, Badve S, Baehner F, Cartun RW, Eisen RN, Swanson PE, Hewitt SM, Vyberg M, Hicks DG, C. Members of the Standardization Ad-Hoc Consensus Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16:513–520. doi: 10.1097/PAI.0b013e31818a9d3a. [DOI] [PubMed] [Google Scholar]
- 210.Welsh AW, Lannin DR, Young GS, Sherman ME, Figueroa JD, Henry NL, Ryden L, Kim C, Love RR, Schiff R, Rimm DL. Cytoplasmic estrogen receptor in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:118–126. doi: 10.1158/1078-0432.CCR-11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 212.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 214.Luo M, Clouthier SG, Deol Y, Liu S, Nagrath S, Azizi E, Wicha MS. Breast cancer stem cells: current advances and clinical implications. Methods in molecular biology. 2015;1293:1–49. doi: 10.1007/978-1-4939-2519-3_1. [DOI] [PubMed] [Google Scholar]
- 215.Sehl ME, Shimada M, Landeros A, Lange K, Wicha MS. Modeling of Cancer Stem Cell State Transitions Predicts Therapeutic Response. PLoS One. 2015;10:e0135797. doi: 10.1371/journal.pone.0135797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Balfe PJ, McCann AH, Welch HM, Kerin MJ. Estrogen receptor beta and breast cancer. Eur J Surg Oncol. 2004;30:1043–1050. doi: 10.1016/j.ejso.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 217.Speirs V, Green CA, Shaaban AM. Oestrogen receptor beta immunohistochemistry: time to get it right? J Clin Pathol. 2008;61:1150–1151. author reply 1151–1152. [PubMed] [Google Scholar]
- 218.Murphy LC, Leygue E. The role of estrogen receptor-beta in breast cancer. Semin Reprod Med. 2012;30:5–13. doi: 10.1055/s-0031-1299592. [DOI] [PubMed] [Google Scholar]
- 219.Belcher SM, Ma X, Le HH. Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocrinology. 2009;150:1112–1121. doi: 10.1210/en.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chantalat E, Boudou F, Laurell H, Palierne G, Houtman R, Melchers D, Rochaix P, Filleron T, Stella A, Burlet-Schiltz O, Brouchet A, Flouriot G, Metivier R, Arnal JF, Fontaine C, Lenfant F. The AF-1-deficient estrogen receptor ERalpha46 isoform is frequently expressed in human breast tumors. Breast Cancer Res. 2016;18:123. doi: 10.1186/s13058-016-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-alpha36 (ERalpha36) The Journal of biological chemistry. 2012;287:7169–7181. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jozan S, Julia AM, Carretie A, Eche N, Maisongrosse V, Fouet B, Marques B, David JF. 65 and 47 kDa forms of estrogen receptor in human breast cancer: relation with estrogen responsiveness. Breast Cancer Res Treat. 1991;19:103–109. doi: 10.1007/BF01980940. [DOI] [PubMed] [Google Scholar]
- 223.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Penot G, Le Peron C, Merot Y, Grimaud-Fanouillere E, Ferriere F, Boujrad N, Kah O, Saligaut C, Ducouret B, Metivier R, Flouriot G. The human estrogen receptor-alpha isoform hERalpha46 antagonizes the proliferative influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 225.Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, Lenfant F, Arnal JF. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–2233. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- 226.O’Mahony F, Razandi M, Pedram A, Harvey BJ, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol. 2012;26:2058–2070. doi: 10.1210/me.2012-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alonso-Magdalena P, Ropero AB, Garcia-Arevalo M, Soriano S, Quesada I, Muhammed SJ, Salehi A, Gustafsson JA, Nadal A. Antidiabetic actions of an estrogen receptor beta selective agonist. Diabetes. 2013;62:2015–2025. doi: 10.2337/db12-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fierz Y, Novosyadlyy R, Vijayakumar A, Yakar S, LeRoith D. Insulin-sensitizing therapy attenuates type 2 diabetes-mediated mammary tumor progression. Diabetes. 2010;59:686–693. doi: 10.2337/db09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol Metab. 2004;286:E1011–1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- 232.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 234.Razandi M, Pedram A, Jordan VC, Fuqua S, Levin ER. Tamoxifen regulates cell fate through mitochondrial estrogen receptor beta in breast cancer. Oncogene. 2013;32:3274–3285. doi: 10.1038/onc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Beatson G. On the treatment of inoperable cases of carcinoma of the mamma:suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;ii:162–165. [PMC free article] [PubMed] [Google Scholar]
- 236.Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 237.Pietras RJ. Biologic basis of sequential and combination therapies for hormone-responsive breast cancer. Oncologist. 2006;11:704–717. doi: 10.1634/theoncologist.11-7-704. [DOI] [PubMed] [Google Scholar]
- 238.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 239.Wright C, Nicholson S, Angus B, Sainsbury JR, Farndon J, Cairns J, Harris AL, Horne CH. Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992;65:118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 241.Laurentiis M De, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello G, Montagna E, Malorni L, Zinno L, Lauria R, Bianco AR, Placido S De. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 242.Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995;13:1129–1135. doi: 10.1200/JCO.1995.13.5.1129. [DOI] [PubMed] [Google Scholar]
- 243.Pancholi S, Lykkesfeldt AE, Hilmi C, Banerjee S, Leary A, Drury S, Johnston S, Dowsett M, Martin LA. ERBB2 influences the subcellular localization of the estrogen receptor in tamoxifen-resistant MCF-7 cells leading to the activation of AKT and RPS6KA2. Endocr Relat Cancer. 2008;15:985–1002. doi: 10.1677/ERC-07-0240. [DOI] [PubMed] [Google Scholar]
- 244.Nicholson RI, Gee JM, Knowlden J, McClelland R, Madden TA, Barrow D, Hutcheson I. The biology of antihormone failure in breast cancer. Breast Cancer Res Treat. 2003;80(Suppl 1):S29–34. doi: 10.1023/a:1025467500433. discussion S35. [DOI] [PubMed] [Google Scholar]
- 245.Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, Elledge RM. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 246.Jin K, Kong X, Shah T, Penet MF, Wildes F, Sgroi DC, Ma XJ, Huang Y, Kallioniemi A, Landberg G, Bieche I, Wu X, Lobie PE, Davidson NE, Bhujwalla ZM, Zhu T, Sukumar S. The HOXB7 protein renders breast cancer cells resistant to tamoxifen through activation of the EGFR pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2736–2741. doi: 10.1073/pnas.1018859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, Elledge RM. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–1242. doi: 10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang QX, Borg A, Wolf DM, Oesterreich S, Fuqua SA. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57:1244–1249. [PubMed] [Google Scholar]
- 249.Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, Li Z, Gala K, Fanning S, King TA, Hudis C, Chen D, Taran T, Hortobagyi G, Greene G, Berger M, Baselga J, Chandarlapaty S. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proceedings of the National Academy of Sciences. 1992;89:4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wijayaratne AL, McDonnell DP. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. Journal of Biological Chemistry. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 252.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 254.Sen A, Prizant H, Hammes SR. Understanding extranuclear (nongenomic) androgen signaling: what a frog oocyte can tell us about human biology. Steroids. 2011;76:822–828. doi: 10.1016/j.steroids.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 258.Novelli F, Milella M, Melucci E, Di Benedetto A, Sperduti I, Perrone-Donnorso R, Perracchio L, Venturo I, Nistico C, Fabi A, Buglioni S, Natali PG, Mottolese M. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 2008;10:R74. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen JQ, Russo J. ERalpha-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochim Biophys Acta. 2009;1796:162–175. doi: 10.1016/j.bbcan.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hartman J, Strom A, Gustafsson JA. Estrogen receptor beta in breast cancer–diagnostic and therapeutic implications. Steroids. 2009;74:635–641. doi: 10.1016/j.steroids.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 262.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 263.Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, Li CI. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103:470–477. doi: 10.1093/jnci/djr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tonetti DA, Rubenstein R, DeLeon M, Zhao H, Pappas SG, Bentrem DJ, Chen B, Constantinou A, Jordan V Craig. Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. J Steroid Biochem Mol Biol. 2003;87:47–55. doi: 10.1016/j.jsbmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 265.Yan Y, Li X, Blanchard A, Bramwell VH, Pritchard KI, Tu D, Shepherd L, Myal Y, Penner C, Watson PH, Leygue E, Murphy LC. Expression of both estrogen receptor-beta 1 (ER-beta1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-alpha)-negative early breast cancer (EBC) Ann Oncol. 2013;24:1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 266.Poola I, Fuqua SA, De Witty RL, Abraham J, Marshallack JJ, Liu A. Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:7579–7585. doi: 10.1158/1078-0432.CCR-05-0728. [DOI] [PubMed] [Google Scholar]
- 267.Hamilton N, Marquez-Garban D, Mah V, Fernando G, Elshimali Y, Garban H, Elashoff D, Vadgama J, Goodglick L, Pietras R. Biologic roles of estrogen receptor-beta and insulin-like growth factor-2 in triple-negative breast cancer. BioMed research international. 2015;2015:925703. doi: 10.1155/2015/925703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wisinski KB, Xu W, Tevaarwerk AJ, Saha S, Kim K, Traynor A, Dietrich L, Hegeman R, Patel D, Blank J, Harter J, Burkard ME. Targeting Estrogen Receptor Beta in a Phase 2 Study of High-Dose Estradiol in Metastatic Triple-Negative Breast Cancer: A Wisconsin Oncology Network Study. Clin Breast Cancer. 2016;16:256–261. doi: 10.1016/j.clbc.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, Nguyen JM, Curiel TJ, Cadungog MG, Singhal S, Eruslanov EB, Zhang P, Tchou J, Zhang R, Conejo-Garcia JR. Tumor Cell-Independent Estrogen Signaling Drives Disease Progression through Mobilization of Myeloid-Derived Suppressor Cells. Cancer Discov. 2017;7:72–85. doi: 10.1158/2159-8290.CD-16-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jaini R, Loya MG, Eng C. Immunotherapeutic target expression on breast tumors can be amplified by hormone receptor antagonism: a novel strategy for enhancing efficacy of targeted immunotherapy. Oncotarget. 2017;8:32536. doi: 10.18632/oncotarget.15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Welte T, Zhang XHF, Rosen JM. Repurposing Antiestrogens for Tumor Immunotherapy. Cancer discovery. 2017;7:17–19. doi: 10.1158/2159-8290.CD-16-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Disis ML, Stanton SE. Triple-negative breast cancer: immune modulation as the new treatment paradigm, Am Soc Clin Oncol Educ Book. 2015:e25–30. doi: 10.14694/EdBook_AM.2015.35.e25. [DOI] [PubMed] [Google Scholar]
- 274.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 275.Dai D, Wolf DM, Litman S, White MJ, Leslie KK. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone. Cancer Res. 2002;62:881–886. [PubMed] [Google Scholar]
- 276.Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H. Progesterone receptor in non-small cell lung cancer–a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65:6450–6458. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- 277.Clarke RB, Howell A, Potten CS, Anderson E. Dissoication between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 278.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4:197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Brisken C. Hormonal control of alveolar development and its implications for breast carcinogenesis. Journal of mammary gland biology and neoplasia. 2002;7:39–48. doi: 10.1023/a:1015718406329. [DOI] [PubMed] [Google Scholar]
- 280.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 281.Graham JD, Yeates C, Balleine RL, Harvey SS, Milliken JS, Bilous AM, Clarke CL. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Research. 1995;55:5063–5068. [PubMed] [Google Scholar]
- 282.Leslie KK, Kumar NS, Fox K. A novel mechanism underlying progestin resistance in endometrial cancer: loss of the nuclear localization of progesterone B receptor. Keystone Symposia: Nuclear receptor 2000Steamboat Springs, CO. 2000:105. [Google Scholar]
- 283.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Carroll JS, Hickey TE, Tarulli GA, Williams M, Tilley WD. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer. 2017;17:54–64. doi: 10.1038/nrc.2016.116. [DOI] [PubMed] [Google Scholar]
- 285.Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Edwards DP, Boonyaratanakornkit V. Rapid extranuclear signaling by the estrogen receptor (ER): MNAR couples ER and Src to the MAP kinase signaling pathway. Mol Interv. 2003;3:12–15. doi: 10.1124/mi.3.1.12. [DOI] [PubMed] [Google Scholar]
- 288.Graham JD, Roman SD, McGowan E, Sutherland RL, Clarke CL. Preferential stimulation of human progesterone receptor B expression by estrogen in T-47D human breast cancer cells. The Journal of biological chemistry. 1995;270:30693–30700. doi: 10.1074/jbc.270.51.30693. [DOI] [PubMed] [Google Scholar]
- 289.Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- 290.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 291.Dowsett M. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer. 2001;8:191–195. doi: 10.1677/erc.0.0080191. [DOI] [PubMed] [Google Scholar]
- 292.Klempner SJ, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov. 2013;3:1345–1354. doi: 10.1158/2159-8290.CD-13-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Osborne CK, Clemmons DR, Arteaga CL. Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol. 1990;37:805–809. doi: 10.1016/0960-0760(90)90423-i. [DOI] [PubMed] [Google Scholar]
- 294.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 295.Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha and Src. Trends Endocrinol Metab. 2005;16:347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 296.Vallabhaneni S, Nair BC, Cortez V, Challa R, Chakravarty D, Tekmal RR, Vadlamudi RK. Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Res Treat. 2011;130:377–385. doi: 10.1007/s10549-010-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sivaraman L, Conneely OM, Medina D, O’Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer research. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 299.Goepfert TM, McCarthy M, Kittrell FS, Stephens C, Ullrich RL, Brinkley BR, Medina D. Progesterone facilitates chromosome instability (aneuploidy) in p53 null normal mammary epithelial cells. Faseb J. 2000;14:2221–2229. doi: 10.1096/fj.00-0165com. [DOI] [PubMed] [Google Scholar]
- 300.Hsu SP, Lee WS. Progesterone receptor activation of extranuclear signaling pathways in regulating p53 expression in vascular endothelial cells. Molecular Endocrinology. 2011;25:421–432. doi: 10.1210/me.2010-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hsu SP, Yang HC, Kuo CT, Wen HC, Chen LC, Huo YN, Lee WS. Progesterone receptor-nfκb complex formation is required for progesterone-induced nfκb nuclear translocation and binding onto the p53 promoter. Endocrinology. 2014;156:291–300. doi: 10.1210/en.2014-1629. [DOI] [PubMed] [Google Scholar]
- 302.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et biophysica acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers – assembly, dynamics and biological roles. Journal of Cell Science. 2012;125:1855. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 304.Li Y, Wang JP, Santen RJ, Kim TH, Park H, Fan P, Yue W. Estrogen stimulation of cell migration involves multiple signaling pathway interactions. Endocrinology. 2010;151:5146–5156. doi: 10.1210/en.2009-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor I receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Giretti MS, Fu XD, De Rosa G, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu XD, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor alpha interacts with Galpha13 to drive actin remodeling and endothelial cell migration via the RhoA/Rho kinase/moesin pathway. Mol Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- 308.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic Potential of the Nuclear Receptor Coregulator Proline-, Glutamic Acid–, Leucine-Rich Protein 1/Modulator of the Nongenomic Actions of the Estrogen Receptor. Cancer research. 2007;67:5505–5512. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lin VC, Ng EH, Aw SE, Tan MG, Ng EH, Bay BH. Progesterone induces focal adhesion in breast cancer cells MDA-MB-231 transfected with progesterone receptor complementary DNA. Mol Endocrinol. 2000;14:348–358. doi: 10.1210/mend.14.3.0426. [DOI] [PubMed] [Google Scholar]
- 311.Fu XD, Flamini M, Sanchez AM, Goglia L, Giretti MS, Genazzani AR, Simoncini T. Progestogens regulate endothelial actin cytoskeleton and cell movement via the actin-binding protein moesin. Mol Hum Reprod. 2008;14:225–234. doi: 10.1093/molehr/gan010. [DOI] [PubMed] [Google Scholar]
- 312.Fu XD, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T. Extra-Nuclear Signaling of Progesterone Receptor to Breast Cancer Cell Movement and Invasion through the Actin Cytoskeleton. PLoS ONE. 2008;3:e2790. doi: 10.1371/journal.pone.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Fu XD, Goglia L, Sanchez AM, Flamini M, Giretti MS, Tosi V, Genazzani AR, Simoncini T. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Cancer. 2010;17:431–443. doi: 10.1677/ERC-09-0258. [DOI] [PubMed] [Google Scholar]
- 314.Wang HC, Lee WS. Molecular mechanisms underlying progesterone-enhanced breast cancer cell migration. Sci Rep. 2016;6:31509. doi: 10.1038/srep31509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Treviño LS, Wang Q, Walker CL. Hypothesis: Activation of rapid signaling by environmental estrogens and epigenetic reprogramming in breast cancer. Reproductive Toxicology. 2015;54:136–140. doi: 10.1016/j.reprotox.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chemical research in toxicology. 2011;24:6. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Molecular endocrinology. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho S-m, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Molecular Cancer Research. 2012;10:546–557. doi: 10.1158/1541-7786.MCR-11-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Watson CS, Hu G, Paulucci-Holthauzen AA. Rapid actions of xenoestrogens disrupt normal estrogenic signaling. Steroids. 2014;81:36–42. doi: 10.1016/j.steroids.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: association of maternal stilbestrol therapy with tumor appearance in young women. New England journal of medicine. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 321.Pietras RJ, Szego CM, Seeler BJ. Immunologic inhibition of estrogen binding and action in preputial-gland cells and their subcellular fractions. Journal of steroid biochemistry. 1981;14:679–691. doi: 10.1016/0022-4731(81)90002-9. [DOI] [PubMed] [Google Scholar]
- 322.Szego C, Pietras R. Membrane recognition and effector sites in steroid hormone action. Biochemical actions of hormones. 1981;8:307–463. [Google Scholar]


