Abstract
Repeated exposure to childhood adversity (abuse, neglect and other traumas experienced before age 18) can have lifelong impacts on health. For HIV -infected adolescents and youth, such impacts may include onward transmission of HIV. To evaluate this possibility, the current study measured the burden of childhood adversity and its influence on risky health behaviors among perinatally-infected adolescents and youth. We surveyed 250 perinatally infected adolescents and youth (13–24 years) receiving care in Soweto, South Africa. Both male and female participants reported on childhood adversity (using the ACE-IQ), sexual behavior, and psychosocial state. Viral load was also abstracted from their charts. We used logistic regressions to test the association between cumulative adversity and behavioral outcomes. Half the sample reported eight or more adversities. Overall, 72% experienced emotional abuse, 59% experienced physical abuse, 34% experienced sexual abuse, 82% witnessed domestic violence, and 91% saw someone being attacked in their community. A clear gradient emerged between cumulative adversities and behavioral risk. Having experienced one additional childhood adversity raised the odds of risky sexual behavior by almost 30% (OR 1.27, 95% CI 1.09–1.48). Viral suppression was poor overall (31% had viral loads >400 copies/ml), but was not related to adversity. Adversity showed a robust relationship to depression and substance abuse. Childhood adversity is common, influences the current health of HIV-positive adolescents and youth, and puts their sexual partners at risk for HIV infection. Greater primary prevention of childhood adversity and increased access to support services (e.g., mental health) could reduce risk taking among HIV-positive adolescents and youth.
Keywords: HIV, adolescents, childhood adversity, adherence, sexual behavior
Introduction
The expansion of anti-retroviral therapy (ART) in the past decade has improved the life expectancy of HIV infected children leading to a rapidly emerging population of perinatally HIV-infected (PHIV) adolescents and young adults. Their numbers are expected to increase substantially over the next decade (Ferrand et al., 2009; UNAIDS, 2015). While mortality has improved dramatically, new clinical and psychosocial challenges are emerging. One major concern is that PHIV adolescents and youth have detectible viral loads and are engaging in high risk sex, meaning that they could pass the virus on to their partners and children. We know this age group has low ART adherence (Kim, Gerver, Fidler, & Ward, 2014; Nachega et al., 2009). Sexual risk taking has been studied less frequently in PHIV, but several Ugandan studies report low rates of consistent condom use (34–47%) (Birungi, Obare, Mugisha, Evelia, & Nyombi, 2009; Mbalinda, Kiwanuka, Eriksson, Wanyenze, & Kaye, 2015; Obare & Birungi, 2010). In a study of infected adolescents in South Africa, almost a third reported not using a condom at last intercourse (Toska et al., 2016).
Such behavioural risk taking may be exacerbated by high levels of childhood adversity among PHIV adolescents and youth. By definition, PHIV adolescents and youth have a mother who carried the virus, and thus are more likely to have experienced parental illness and related mental health problems (Petersen et al., 2010). Such adolescents and youth are more likely to have been orphaned, which heightens the risk of sexual, physical and emotional violence (L. Cluver, Orkin, Boyes, Gardner, & Meinck, 2011; Kidman & Palermo, 2016). Finally, growing up with a highly stigmatized chronic illness may predispose them to bullying by peers. In a US study, HIV-infected adolescents experienced an average of six childhood adversities (e.g., sexual abuse, abandonment), a notable increase relative to community samples (Radcliffe et al., 2007). However, the pattern of the HIV epidemic in the US is different to that of sub-Saharan Africa making it difficult to draw direct comparisons. It is possible that adverse childhood experiences (ACE) may be even more common in the South African context where endemic HIV and endemic violence coexist.
Importantly, the harmful impacts of ACEs reverberate across the life course (Jack P Shonkoff, Boyce, & McEwen, 2009). Studies have documented its impact on a spectrum of conditions ranging from depression to autoimmune disorders and cancers (Jack P Shonkoff et al., 2009). For PHIV adolescents and youth, adversity may also contribute to high risk sexual behavior and poor ART adherence. This would have long-term implications for both their own health (through poorly controlled HIV infection) and the public’s health (through onward transmission). Studies generally have shown a clear gradient between ACEs and sexually transmitted diseases, though all but one study has been in high income countries (Norman et al., 2012). The exception is a study in South Africa, which found ACEs were associated with HIV in youth (Jewkes, Dunkle, Nduna, Jama, & Puren, 2010).
One way that ACEs may be influencing later behaviors is through psychosocial sequelae. Chronic stress can alter brain physiology, resulting in social, cognitive and emotional impairments (Jack P Shonkoff et al., 2009; Jack P. Shonkoff et al., 2012). We know, for example, that ACEs (and associated chronic stress) increase alcohol abuse and depression (Brown, Riley, Butchart, & Kann, 2008; Jewkes et al., 2010). Both psychosocial sequelae contribute to and compound risky sexual behavior and poor adherence in behaviorally-infected (Murphy et al., 2001; Naar-King et al., 2006) and perinatally-infected adolescents and youth (Naar-King et al., 2006; Williams et al., 2006). Alternatively, adversity may disrupt normative transitions to adulthood – such as school drop-out – which are associated with increased sexual risk (Hargreaves et al., 2008).
Substantial literature exists demonstrating that ACEs have life-long consequences for health (Felitti Md et al., 1998), including in HIV-infected adults (Pence et al., 2012; Whetten et al., 2013). This research base has not been adequately described among adolescents and youth in low and middle-income countries. Given the challenges associated with growing up HIV-positive, an explicit focus on childhood adversity and its consequences is long overdue. This study aims to 1) examine the prevalence of ACEs among PHIV adolescents and youth in an endemic context, and 2) explore whether cumulative ACEs are associated with high risk sexual behaviors, psychosocial health and viral load suppression.
Methods
Study population
A convenience sample of HIV-positive patients was recruited from those attending the Paediatric Wellness Clinic, part of the Perinatal HIV Research Unit at Chris Hani Baragwanath Hospital in Soweto, South Africa. Patients were also recruited from those previously seen, but who had been subsequently referred to other clinics within the Soweto community following a government policy to decentralize HIV treatment. A smaller number was recruited from clinics within the Soweto community after obtaining approval from local health authorities. The inclusion criteria for participation was being aged 13–24 years; being aware of their HIV diagnosis; having documented HIV infection before age 10; and being literate in English. To check literacy, we had the adolescent read the appropriate signature pages in the assent/consent form; 11 adolescents could not read English and were excluded. Exclusion criteria included acute psychiatric illness and cognitive impairment. All minors provided assent with guardian consent. Participants aged 18–24 years provided consent to participate in the study. The protocol was granted approval by both Stony Brook University and the University of the Witwatersrand’s Human Research Ethics Committee.
Data collection
Surveys were in English and pre-programmed on Apple i-pads using the Qualtrics Survey application. Each participant received an individualized tutorial on how to use the touch screen and completed a practice module. Participants then completed the survey themselves in a private room at the clinic. After completion, all participants were given the opportunity to meet with a staff psychologist immediately or at later stage if they felt they needed additional support. For each participant, clinical data, HIV viral load and CD4 cell counts were also abstracted directly from medical records by the research coordinator.
Measures
The survey instrument was reviewed by an Adolescent Community Advisory Board and their feedback was incorporated. The survey captured adversity, sexual behavior, psychosocial health and relevant clinical and sociodemographic characteristics.
Adverse Childhood Experiences
ACEs were captured with the Adverse Childhood Experience - International Questionnaire (ACE-IQ). The World Health Organization notes that childhood adversities remain understudied in less developed countries (World Health Organization), and designed this questionnaire for international use in conjunction with South African researchers (World Health Organization). The ACE-IQ asks how often participants experienced 13 domains of adversity while they were growing up (i.e., during the first 18 years of life). For example, questions include “How often did your parents or caregivers not give you enough food even when they could easily have done so?”; “Did you see or hear a parent or household member in your home being slapped, kicked, punched or beaten up?”; and “Did your mother, father or caregiver die?” Each domain was scored using a binary option (i.e., any affirmative answer whether it was experienced once, a few or many times was coded as a yes). A cumulative score (0–13) was then calculated (World Health Organization). For the ACE scale, the Chronbach’s alpha was 0.72, indicating acceptable internal reliability.
Sexual Behavior
Respondents were asked about sexual debut (i.e., ever had sex) and current activity. Those who were sexually active were asked more detailed questions about their past year sexual behavior. A dichotomous variable representing high risk sexual behavior was coded one, if the participant was in any of the following in the past year: having concurrent partners (two or more in the same month), having three or more partners in the past year, or not using a condom with their most recent partner.
Adherence and Viral Load
To assess the respondent’s health and their potential to transmit the virus to their partners,. we reviewed their medical charts and recorded the most recent viral load and CD4 results. In 78 cases (31%), the laboratory testing and survey were conducted on the same day. In 43% of cases it was done in the prior year; and in 11% of cases it was done earlier. Viral suppression (reducing the number of copies of HIV in the blood to an undetectable level) was operationalized as having a viral load of less than 400 copies/ml on their most recent laboratory report.
Psychosocial health
Depression was measured using the Beck Depression Inventory (BDI). The BDI was analyzed using pre-established cut-points (i.e., 20+ points) to indicate moderate to severe depression (Beck, Steer, & Carbin, 1988). For the BDI, the Chronbach’s alpha was 0.91, indicating good internal reliability. We used the adolescent self-administered version of the CRAFFT Screening Questionnaire for alcohol and substance abuse (a score over 2 indicated a potential problem) (Knight et al., 1999).
Covariates
We collected information on age, gender, race, primary caregiver, and indicators of wealth. We include three wealth indicators: whether the respondent could get 100 Rand (about US$ 7) in a medical emergency (dichotomized into difficult/very difficult versus easy/very easy); whether people in their home go without food (later dichotomized into often/sometimes versus seldom/never), and whether their household owns a cell phone.
Analyses
Descriptive analyses are presented, overall and by gender. T-tests and chi-square tests are used to examine significant differences between genders. For ease of illustration, we categorized the continuous ACE score into lower (0–3), higher (4–7) and very high (8–13) adversity. However, tests of association make use of the continuous ACE score. Specifically, we used logistic regression models to test whether cumulative adversity scores are associated with the outcomes of interest (i.e., sexual risk behaviours, viral suppression and psychosocial health). Each outcome is modelled separately, without controlling for the other behavioral outcomes. All models adjust for age, gender, race, primary caregiver; we do not adjust for wealth due to missing data on 32 individuals. We ran a sensitivity analysis in which viral suppression was defined as less than 1000 copies/ml; results did not differ and are thus not presented. We used the same methods to test the association between individual adversities and the outcomes of interest. Each model includes only one adversity, adjusting for the above covariates. All analyses are conducted in Stata 13.
Results
Demographics
Table 1 summarizes the demographic make-up of the sample. There were slightly more girls than boys. Most respondents were adolescents and identified as Black African. About half reported that their primary caregiver was a parent; almost a quarter relied on a grandparent. This cohort was drawn from a disadvantaged community, their economic situation reflected such. They reported high rates of food insecurity (42%) and difficulty accessing monetary help in times of medical emergency (71%).
Table 1.
Characteristics of PHIV adolescents and youth, total and by gender
| Total (n=250) | Females (n=136) | Males (n=114) | Difference by Gender | |
|---|---|---|---|---|
|
| ||||
| mean (SD) | mean (SD) | mean (SD) | p-value | |
|
| ||||
| Demographics | ||||
| Age | 16.32 (2.67) | 16.29 (2.79) | 16.34 (2.54) | 0.888 |
| n (%) | n (%) | n (%) | p-value | |
|
|
||||
| Age group | 0.215 | |||
| Mid-adolescence (13–16) | 138 (55%) | 78 (57%) | 60 (53%) | |
| Late adolescence (17–19) | 77 (31%) | 36 (26%) | 41 (36%) | |
| Youth (20–24) | 35 (14%) | 22 (16%) | 13 (11%) | |
| Female | 136 (54%) | -- | -- | -- |
| Race | 0.134 | |||
| Black African | 221 (88%) | 124 (91%) | 97 (85%) | |
| Other | 29 (12%) | 12 (9%) | 17 (15%) | |
| Primary caregiver | 0.221 | |||
| Parent | 133 (53%) | 75 (55%) | 58 (51%) | |
| Grandparent | 55 (22%) | 25 (18%) | 30 (26%) | |
| Self | 11 (4%) | 5 (4%) | 6 (5%) | |
| Other | 51 (20%) | 31 (23%) | 20 (18%) | |
| Wealth Indicators | ||||
| Food Insecurity | 101 (42%) | 54 (42%) | 47 (44%) | 0.758 |
| Difficult to get 100R in a medical emergency | 166 (71%) | 89 (71%) | 77 (72%) | 0.939 |
| Cell phone in household | 220 (91%) | 119 (89%) | 101 (92%) | 0.534 |
| Sexual Risk | ||||
| Past year high risk sex | 49 (21%) | 22 (17%) | 27 (26%) | 0.042 |
| Viral Suppression | ||||
| Viral suppression | 173 (69%) | 85 (63%) | 88 (77%) | 0.012 |
| Psychosocial Health | ||||
| Depression | 78 (34%) | 49 (39%) | 29 (28%) | 0.087 |
| Alcohol and drug use problems | 45 (18%) | 19 (14%) | 26 (23%) | 0.070 |
measured among sexually active respondents only;
measured among those on ARVs only
Risk profile of perinatally-infected adolescents and youth
The risk for onward HIV transmission of HIV among perinatally HIV-infected adolescents and youth was high. Just over a third of respondents had sexually debuted (data not in tables). Within the cohort that was sexually active, most girls (59%) and almost all boys (82%) reported engaging in high risk sexual practices in the past year. Taken together, this meant that 17% of girls and 26% of boys in the total sample were engaged in high risk sex over the past year (Table 1). Maintaining viral suppression is important to halt disease progression; it also decreases the risk of HIV transmission. Yet in our sample, almost a third (31%) had detectable viral loads (VL>400c/ml).
When combined, 5% of girls and 10% of boys were both engaging in high risk sex and had detectible viral loads, meaning that they could pass the virus on to their partners. Risk increased with age: 20% of youth (age 20–24), 13% of those in late adolescence (age 17–19) and under 2% of those in mid-adolescence (age 13–16) were both engaging in high risk sex and had detectible viral loads. In the subset of respondents who had ever had sex, 23% were having risky sex with a detectible viral load.
Finally, this cohort was grappling with a high burden of psychosocial challenges. A substantial proportion were depressed (39% of girls and 28% of boys). The majority had used alcohol (52% and 61% respectively) or drugs to get high (23% and 35%) in the past year, and many were struggling with substance abuse problems (14% and 23% respectively).
Prevalence of adverse childhood experiences
Adversity was common. Over half HIV-positive adolescents and youth reported experiencing eight or more adversities during childhood; the average was seven out of the thirteen adversities queried (Table 2).
Table 2.
Prevalence of adverse childhood experiences, total and by gender
| Total | Females | Males | Difference by Gender | |
|---|---|---|---|---|
|
| ||||
| mean (SD) | mean (SD) | mean (SD) | p-value | |
|
| ||||
| ACE Score | 7.31 (2.78) | 7.21 (2.72) | 7.44 (2.84) | 0.527 |
| n (%) | n (%) | n (%) | p-value | |
|
|
||||
| Distribution of ACEs | 0.547 | |||
| Lower: 0–3 | 25 (10%) | 15 (11%) | 10 (9%) | |
| Higher: 4–7 | 94 (38%) | 47 (35%) | 47 (41%) | |
| Very high: 8–13 | 130 (52%) | 73 (54%) | 57 (50%) | |
| Individual ACE | ||||
| Physical abuse | 144 (59%) | 77 (57%) | 67 (60%) | 0.708 |
| Emotional abuse | 179 (72%) | 98 (73%) | 81 (72%) | 0.799 |
| Sexual abuse | 83 (34%) | 45 (34%) | 38 (34%) | 0.915 |
| Physical neglect | 120 (49%) | 65 (49%) | 55 (49%) | 0.925 |
| Emotional neglect | 204 (82%) | 110 (81%) | 94 (82%) | 0.749 |
| Substance abuser in household | 68 (28%) | 33 (26%) | 35 (29%) | 0.614 |
| Incarcerated household member | 76 (31%) | 44 (34%) | 32 (28%) | 0.375 |
| Someone with mental health issues in household | 38 (16%) | 20 (15%) | 18 (17%) | 0.768 |
| Witnessed domestic violence | 203 (82%) | 109 (81%) | 94 (82%) | 0.728 |
| Parents dead or divorced | 186 (75%) | 104 (78%) | 82 (73%) | 0.360 |
| Bullied | 152 (62%) | 75 (56%) | 77 (70%) | 0.024 |
| Community violence | 226 (91%) | 120 (90%) | 106 (93%) | 0.344 |
| Collective violence | 122 (49%) | 62 (46%) | 60 (53%) | 0.285 |
Violence was the most frequently reported adversity. Various forms of violence were directed against the individual, their family members, and in the general community: 82% witnessed a parent or other household member being treated violently in their home, 91% saw someone being attacked in their community (e.g., beaten up; stabbed; threatened with a gun), and 49% experienced collective violence (e.g., they or someone they knew well was beaten up by soldiers, police or gangs). In addition, 59% experienced physical abuse (e.g., spanking; kicking; whipping), 72% experienced emotional abuse (e.g., insults; humiliation; threats of or actual abandonment), and 34% experienced sexual abuse (e.g., being fondled or make to touch someone against their will; forced intercourse).
The wellbeing and healthy development of children also depends on the care available at home. Consistent with their mode of HIV infection, most respondents (75%) reported that a parent was absent due to death or divorce. Moreover, many were dealing with stressors within the family environment: a smaller proportion lived with a household member that had a substance abuse problem (28%), was depressed, mentally ill or suicidal (16%), or was ever incarcerated (31%).
Adverse childhood experiences were associated with risk of onward transmission of HIV
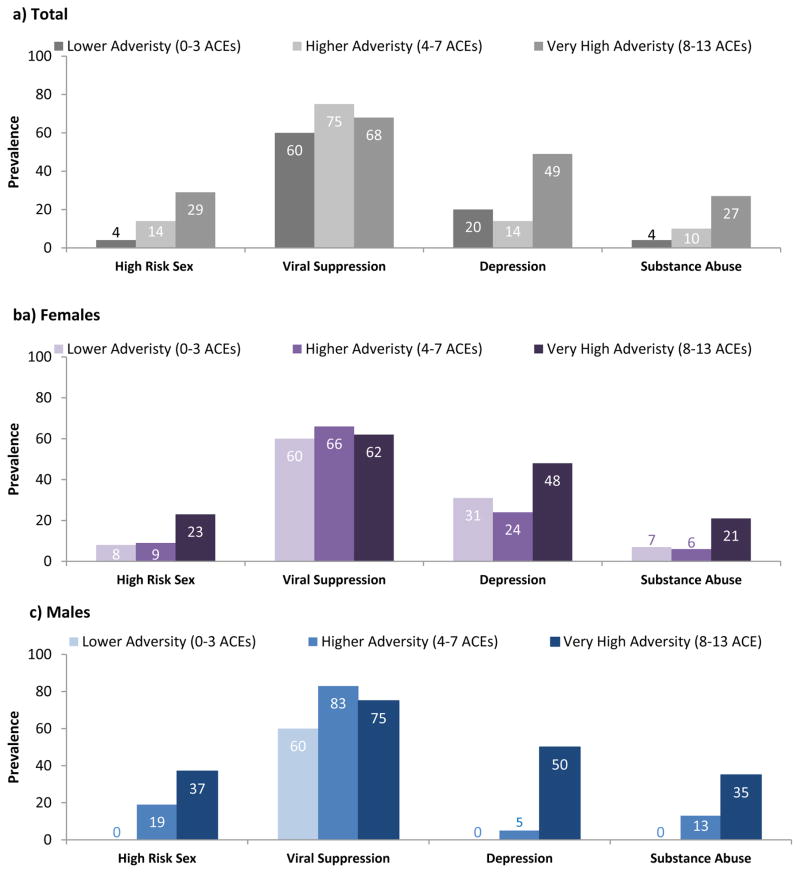
Cumulative ACEs were associated with risk behaviors that contribute to onward transmission. Figure 1 illustrates the clear gradient between adversity and sexual risk, with ACEs categorized for ease of visualization. Logistic regressions were used to formally test the association between the cumulative ACE score and each outcome; results are presented in Table 3. Having experienced one additional adversity in childhood raised the odds of risky sexual behavior (OR 1.27, 95% CI 1.09–1.48). This translates into roughly a three-fold increase in high risk sex for respondents reporting eight adversities as compared to those reporting only three. In addition, there were strong and consistent associations between individual adversities and HIV risk behavior, though the small sample size meant the confidence intervals were often wide. Experiencing emotional neglect was the strongest determinant of past-year high risk sex (OR 6.23; 95% CI 1.35–28.78), followed by physical and emotional abuse (Table 4). Viral load – which captures another aspect of onward transmission potential – was the only outcome that was not sensitive to cumulative adversity.
Figure 1.
Dose relationship between ACEs and health outcomes, by gender
Table 3.
Adjusted odds ratios predicting outcomes based on cumulative ACE score
| Total | Females | Males | |
|---|---|---|---|
|
| |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
|
| |||
| High risk sex | 1.27** | 1.27 | 1.27* |
| (1.09–1.48) | (0.99–1.63) | (1.04–1.55) | |
| Viral suppression | 1.00 | 0.97 | 1.04 |
| (0.91–1.11) | (0.74–1.11) | (0.88–1.22) | |
| Depression | 1.40*** | 1.28** | 1.67*** |
| (1.22–1.59) | (1.09–1.50) | (1.30–2.15) | |
| Substance use problem | 1.33*** | 1.27* | 1.38** |
| (1.14–1.55) | (1.01–1.58) | (1.12–1.70) | |
p<0.001,
p<0.01,
p<0.05; models adjust for age, race, primary caregiver, and gender (where appropriate)
Table 4.
Adjusted odds ratios predicting outcomes based on ACE modelled individually, total sample
| High Risk Sex | Viral Suppression | Depression | Substance Abuse | |
|---|---|---|---|---|
|
| ||||
| Individual ACE | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
|
|
||||
| Physical abuse | 2.77* | 1.08 | 1.28 | 1.34 |
| (1.23–6.25) | (0.61–1.91) | (0.70–2.33) | (0.66–2.70) | |
| Emotional abuse | 3.03* | 1.26 | 1.92 | 3.19* |
| (1.10–8.40) | (0.67–2.37) | (0.94–3.93) | (1.18–8.62) | |
| Sexual abuse | 1.40 | 0.94 | 3.11*** | 3.58*** |
| (0.68–2.87) | (0.52–1.69) | (1.70–5.69) | (1.79–7.16) | |
| Physical neglect | 1.59 | 1.27 | 4.29*** | 1.23 |
| (0.79–3.18) | (0.73–2.24) | (2.31–7.96) | (0.63–2.42) | |
| Emotional neglect | 6.23* | 2.11* | 3.22* | 2.34 |
| (1.35–28.78) | (1.05–4.25) | (1.31–7.90) | (0.78–7.02) | |
| Substance abuser in household | 1.98 | 0.79 | 1.21 | 1.75 |
| (0.94–4.14) | (0.43–1.46) | (0.64–2.27) | (0.86–3.57) | |
| Incarcerated household member | 1.34 | 0.79 | 1.73 | 3.73*** |
| (0.64–2.82) | (0.43–1.43) | (0.95–3.17) | (1.84–7.54) | |
| Someone with mental health issues in household | 1.50 | 1.52 | 4.71*** | 2.13 |
| (0.61–3.73) | (0.67–3.45) | (2.13–10.39) | (0.93–4.92) | |
| Witnessed domestic violence | 2.41 | 0.99 | 1.91 | 2.39 |
| (0.77–7.50) | (0.48–2.03) | (0.82–4.44) | (0.80–7.17) | |
| Parents dead or divorced | 0.93 | 0.56 | 1.40 | 5.46** |
| (0.40–2.20) | (0.27–1.17) | (0.67–2.92) | (1.58–18.88) | |
| Bullied | 1.96 | 0.86 | 3.72*** | 1.20 |
| (0.90–4.23) | (0.48–1.55) | (1.86–7.42) | (0.59–2.44) | |
| Community violence | -- | 2.35 | 9.63* | 4.50 |
| (0.93–5.96) | (1.20–77.03) | (0.58–35.07) | ||
| Collective violence | 1.97 | 0.77 | 2.81*** | 1.81 |
| (0.95–4.07) | (0.43–1.34) | (1.53–5.17) | (0.89–3.64) | |
p<0.001,
p<0.01,
p<0.05; models adjust for age, race, primary caregiver, and gender (where appropriate)
Adverse childhood experiences were strongly associated with poor psychosocial health
Cumulative ACEs showed a robust relationship with all three indicators of psychosocial health. Each additional adversity elevated the odds of depression (OR=1.408, 95% CI 1.22–1.59) and substance abuse (OR 1.33; 95% CI 1.14–1.55). Individually, sexual abuse demonstrated a strong association with depression (OR 3.11; 95% CI 1.70–5.69) and substance abuse (3.58; 95% CI 1.79–7.16)). In addition, community and collective violence significantly increased the odds of depression (OR=9.63; 95% CI 1.20–77.03 and OR=2.81; 1.53–5.17 respectively. Finally, parental death or divorce was the strongest individual predictor of substance abuse (OR 5.46; 95% CI 1.58–18.88).
Discussion
We found that HIV-positive adolescents and youth are embedded in violent contexts where adversity is common
This is one of the first studies of ACEs among PHIV adolescents and youth, a rapidly expanding population in South Africa. The majority (52%) of our study population experienced eight or more ACEs – far exceeding general population levels in high income countries (Anda et al., 2006; Bellis et al., 2014). For instance, in a study of eight European countries, only 5–14% experienced four or more adversities (Bellis et al., 2014). In US adults, 13% reported four or more ACEs (Anda et al., 2006). Levels of adversity also exceeded those reported among a general population sample of adolescents in South Africa, though stark differences in which adversities were measured1 make direct comparisons difficult (Lucie Cluver, Orkin, Boyes, & Sherr, 2015).
Violence was common in both private and public settings. At home, children experienced abuse directly as well as witnessed violence perpetrated against others. Almost 60% reported being physically abused, comparable to rates reported in other South African youth (Kaminer, Hardy, Heath, Mosdell, & Bawa, 2013; Meinck, Cluver, Boyes, & Loening-Voysey, 2016). In contrast, the rates of sexual and emotional abuse appear greatly elevated for PHIV adolescents and youth. For example, our sample reported sexual abuse three times more often than adolescents in a recent community-based sample from South Africa (Meinck et al., 2016). The gap in sexual abuse remains even in comparison to low-income, urban adolescents from Johannesburg (including Soweto) and Cape Town (Kaminer et al., 2013; Otwombe et al., 2015), and is consistent with evidence of higher sexual victimization among orphans across Africa (Kidman & Palermo, 2016). The heightened levels of abuse may be due in part to higher rates of orphaning among PHIV adolescents and youth, a risk factor for sexual abuse (Birdthistle et al., 2011; Kidman & Palermo, 2016). Finally, their own illness may also predispose them to violence: a meta-analysis found higher rates of sexual violence among children with disabilities (Jones et al., 2012).
Moreover, the vast majority (82%) of PHIV adolescents and youth reported witnessing violence in the home. This compares to only 32% of adolescents in the broader Johannesburg study (Otwombe et al., 2015). The stark difference may reflect the greater burden of domestic violence among adults living with HIV (Li et al., 2014), but could also reflect nuanced differences in study design. More definitive evidence will come from studies directly contrasting the experiences of PHIV adolescents and their non-infected peers.
Finally, the most commonly reported adversity was witnessing community violence (91%). This is similar to reports from other studies of urban youth in South Africa (Kaminer et al., 2013), and may reflect the larger explosion of violence in South Africa generally (Otwombe et al., 2015; Seedat, Van Niekerk, Jewkes, Suffla, & Ratele, 2009). A recent study suggests levels of violence in South Africa are several times higher than the global average: two thirds of adolescents had ever witnessed violence and almost a third had been the victim of violence outside the home (Otwombe et al., 2015).
Importantly, we found that the above childhood adversity influences the health of PHIV adolescents and youth, and may put their sexual partners at risk for HIV infection
PHIV are grappling with psychosocial health issues common to their age group: depression and alcohol abuse. In each case, we found that adversity amplifies these challenges. There is an evident gradient, which provides evidence for the cumulative stress theory (Anda, Butchart, Felitti, & Brown, 2010), and thus generalizability of this concept to a middle-income country.
The results for sexual health were consistent with previous literature on cumulative childhood adversity in the U.S., as well as with studies of individual adversities in Africa (Jewkes et al., 2010; Norman et al., 2012). However, ours is the first study to demonstrate these relationships among PHIV adolescents and youth. For those reporting eight adversities as compared to only three, there is a three-fold increase in high risk sex over the past year. Risk behaviors that begin during adolescence have life-long consequences for the individual’s health and for their partners’ health, and the peril is even greater when we are dealing with HIV-infected adolescents and youth. In our sample of sexually active HIV-positive respondents, we found almost a quarter had both a detectible viral load and were having high risk sex. Thus, a small but critical proportion could be passing HIV on to their partners. The flip side is, of course, that most HIV-positive youth were either abstaining from sex or using condoms. Future work could better our understanding of what leads to healthy trajectories, particularly for those facing high levels of adversity, and identify sources of resilience.
The evidence that adversity increases the risk for onward transmission was limited to sexual behavior; in our study, adversity did not have an impact on viral load. This is in contrast to previous literature from outside Africa. A U.S. study of children aged 8–15 found exposure to violence was associated with unsuppressed viral load (Kacanek et al., 2016), and another found recent stressful life events predicted non-adherence (Williams et al., 2006). If current adversity has a large influence on adherence, our study may have underestimated the association by using a lifetime adversity measure. Moreover, this study may have captured a relatively advantaged population: most of the respondents had the benefit of continuity of care from the same doctor over many years at a specialized research clinic. They did not receive additional study incentives, but were none-the-less able to make it to the research clinic for care and to complete the survey. The study missed those PHIV experiencing the greatest barriers to clinical care, including those who have disengaged from care altogether. Such sample limitations could bias the results towards the null.
There are other notable limitations to our exploratory study. The ACE-IQ was designed for retrospective recall among adults, and thus makes no distinction between past and current adversity for those still in childhood. For future studies, adapting the instrument to differentiate between past and current adversity would help elucidate which has a stronger influence on current behavioral outcomes. We included a wide age bracket (13–24), and acknowledge that younger adolescents and youth are at developmentally different stages. Moreover, there was no comparison group, and thus we are not able to definitively address whether PHIV adolescents and youth experience greater adversity than their non-infected or behaviorally-infected peers. Even without a comparison group, however, our study yields important findings: childhood adversity predicts high risk sexual behaviors for PHIV youth. Moreover, by examining the joint experience of sexual risk taking and viral load, we are able to show who is most likely to transmit HIV.
Greater primary prevention of childhood adversity will help reduce HIV onward transmission
A growing population of HIV-positive infants is surviving to adolescence, in large part due to expanded access to ART. Our study has shown that the prevalence of ACEs is very high in those growing up HIV-positive and is associated with HIV-related risk behaviors. Such results underscore the need for early intervention. Some of the strongest evidence to date on how to prevent violence within the home comes from parenting programs (Chen & Chan, 2016). Recent WHO reports on violence have cited parental education as a promising approach to reduce child maltreatment, while also bringing attention to structural factors (e.g., gender inequality) that must simultaneously be addressed to have a sustained impact (World Health Organization, 2016). As we strengthen primary prevention, it will be equally critical to extend care and support to those who have already experienced adversity. Routine care for pediatric HIV should include screening to identify patients who have been traumatized, and ensure access to age-appropriate services tailored to their lived experiences. The latter may include mental health services, interventions that build positive coping strategies and those that encourage healthy relationships.
Acknowledgments
Source of Funding: This research was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD083032 and supported by the South African Medical Research Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the South African Medical Research Council.
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD083032 and supported by the South African Medical Research Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the South African Medical Research Council. The authors thank the doctors, nurses, and patients of the Perinatal HIV Research Unit for their time, effort, and support of this project. In particular, we thank Wilma Pelser, Jacqueline Brown, Nkata Kekana and Mirriam Kunene for their dedication to the study participants and their support of this study.
Footnotes
Cluver et al measured orphanhood by AIDS; orphanhood by homicide; parental AIDS-illness; severe physical abuse; severe emotional abuse; sexual abuse or rape; domestic violence; community violence; and food insecurity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. American Journal of Preventive Medicine. 2010;39(1):93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, … Giles WH. The enduring effects of abuse and related adverse experiences in childhood. European archives of psychiatry and clinical neuroscience. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Bellis MA, Hughes K, Leckenby N, Jones L, Baban A, Kachaeva M, … Terzic N. Adverse childhood experiences and associations with health-harming behaviours in young adults: surveys in eight eastern European countries. Bulletin of the World Health Organization. 2014;92:641–655. doi: 10.2471/BLT.13.129247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdthistle I, Floyd S, Mwanasa S, Nyagadza A, Gwiza E, Glynn JR. Child sexual abuse and links to HIV and orphanhood in urban Zimbabwe. Journal of Epidemiology and Community Health. 2011;65(12):1075–1082. doi: 10.1136/jech.2009.094359. [DOI] [PubMed] [Google Scholar]
- Birungi H, Obare F, Mugisha JF, Evelia H, Nyombi J. Preventive service needs of young people perinatally infected with HIV in Uganda. AIDS Care. 2009;21(6):725–731. doi: 10.1080/09540120802511901. [DOI] [PubMed] [Google Scholar]
- Brown DW, Riley L, Butchart A, Kann L. Bullying among youth from eight African countries and associations with adverse health behaviors 2008 [Google Scholar]
- Chen M, Chan KL. Effects of Parenting Programs on Child Maltreatment Prevention: A Meta-Analysis. Trauma, Violence, & Abuse. 2016;17(1):88–104. doi: 10.1177/1524838014566718. [DOI] [PubMed] [Google Scholar]
- Cluver L, Orkin M, Boyes M, Gardner F, Meinck F. Transactional Sex Amongst AIDS-Orphaned and AIDS-Affected Adolescents Predicted by Abuse and Extreme Poverty. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;58(3):336–343. doi: 10.1097/QAI.0b013e31822f0d82. [DOI] [PubMed] [Google Scholar]
- Cluver L, Orkin M, Boyes ME, Sherr L. Child and adolescent suicide attempts, suicidal behavior, and adverse childhood experiences in South Africa: A prospective study. Journal of Adolescent Health. 2015;57(1):52–59. doi: 10.1016/j.jadohealth.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Felitti MdFVJ, Anda MdMSRF, Nordenberg MdD, Williamson MsPDF, Spitz MsMPHAM, Edwards Ba V, … Marks MdMPHJS. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. doi: http://dx.doi.org/10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferrand RA, Corbett EL, Wood R, Hargrove J, Ndhlovu CE, Cowan FM, … Williams BG. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS (London, England) 2009;23(15):2039. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves J, Morison L, Kim J, Bonell C, Porter J, Watts C, … Pronyk P. The association between school attendance, HIV infection and sexual behaviour among young people in rural South Africa. Journal of Epidemiology and Community Health. 2008;62(2):113–119. doi: 10.1136/jech.2006.053827. [DOI] [PubMed] [Google Scholar]
- Jewkes RK, Dunkle K, Nduna M, Jama PN, Puren A. Associations between childhood adversity and depression, substance abuse and HIV and HSV2 incident infections in rural South African youth. Child Abuse & Neglect. 2010;34(11):833–841. doi: 10.1016/j.chiabu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Bellis MA, Wood S, Hughes K, McCoy E, Eckley L, … Officer A. Prevalence and risk of violence against children with disabilities: a systematic review and meta-analysis of observational studies. The Lancet. 2012;380(9845):899–907. doi: 10.1016/S0140-6736(12)60692-8. [DOI] [PubMed] [Google Scholar]
- Kacanek D, Malee K, Mellins CA, Tassiopoulos K, Smith R, Grant M, … Puga A. Exposure to violence and virologic and immunological outcomes among youth with perinatal HIV in the Pediatric HIV/AIDS Cohort Study. Journal of Adolescent Health. 2016 doi: 10.1016/j.jadohealth.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer D, Hardy A, Heath K, Mosdell J, Bawa U. Gender patterns in the contribution of different types of violence to posttraumatic stress symptoms among South African urban youth. Child Abuse & Neglect. 2013;37(5):320–330. doi: 10.1016/j.chiabu.2012.12.011. doi: http://dx.doi.org/10.1016/j.chiabu.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Kidman R, Palermo T. The relationship between parental presence and child sexual violence: evidence from thirteen countries in sub-Saharan Africa. Child Abuse & Neglect. 2016;51:172–180. doi: 10.1016/j.chiabu.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–1956. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JR, Shrier LA, Bravender TD, Farrell M, Vander Bilt J, Shaffer HJ. A new brief screen for adolescent substance abuse. Archives of pediatrics & adolescent medicine. 1999;153(6):591–596. doi: 10.1001/archpedi.153.6.591. [DOI] [PubMed] [Google Scholar]
- Li Y, Marshall CM, Rees HC, Nunez A, Ezeanolue E, Ehiri J. Intimate partner violence and HIV infection among women: a systematic review and meta-analysis. Journal of the International AIDS Society. 2014;17(1) doi: 10.7448/IAS.17.1.18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbalinda SN, Kiwanuka N, Eriksson LE, Wanyenze RK, Kaye DK. Correlates of ever had sex among perinatally HIV-infected adolescents in Uganda. Reprod Health. 2015;12:96. doi: 10.1186/s12978-015-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck F, Cluver LD, Boyes ME, Loening-Voysey H. Physical, emotional and sexual adolescent abuse victimisation in South Africa: prevalence, incidence, perpetrators and locations. Journal of Epidemiology and Community Health. 2016 doi: 10.1136/jech-2015-205860. jech-2015-205860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Durako SJ, Moscicki AB, Vermund SH, Ma Y, Schwarz DF, Muenz LR. No change in health risk behaviors over time among HIV infected adolescents in care: role of psychological distress. Journal of Adolescent Health. 2001;29(3, Supplement 1):57–63. doi: 10.1016/s1054-139x(01)00287-7. doi: http://dx.doi.org/10.1016/S1054-139X(01)00287-7. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Templin T, Wright K, Frey M, Parsons JT, Lam P. Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care & STDs. 2006;20(1):44–47. doi: 10.1089/apc.2006.20.44. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, … Maartens G. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. Journal of acquired immune deficiency syndromes (1999) 2009;51(1):65. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obare F, Birungi H. The limited effect of knowing they are HIV-positive on the sexual and reproductive experiences and intentions of infected adolescents in Uganda. Population studies. 2010;64(1):97–104. doi: 10.1080/00324720903427575. [DOI] [PubMed] [Google Scholar]
- Otwombe KN, Dietrich J, Sikkema KJ, Coetzee J, Hopkins KL, Laher F, Gray GE. Exposure to and experiences of violence among adolescents in lower socio-economic groups in Johannesburg, South Africa. BMC Public health. 2015;15(1):1. doi: 10.1186/s12889-015-1780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, … Whetten K. Childhood trauma and health outcomes in HIV-infected patients: an exploration of causal pathways. Journal of acquired immune deficiency syndromes (1999) 2012;59(4):409. doi: 10.1097/QAI.0b013e31824150bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I, Bhana A, Myeza N, Alicea S, John S, Holst H, … Mellins C. Psychosocial challenges and protective influences for socio-emotional coping of HIV+ adolescents in South Africa: a qualitative investigation. AIDS Care. 2010;22(8):970–978. doi: 10.1080/09540121003623693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe J, Fleisher CL, Hawkins LA, Tanney M, Kassam-Adams N, Ambrose C, Rudy BJ. Posttraumatic stress and trauma history in adolescents and young adults with HIV. AIDS Patient Care And Stds. 2007;21(7):501–508. doi: 10.1089/apc.2006.0144. [DOI] [PubMed] [Google Scholar]
- Seedat M, Van Niekerk A, Jewkes R, Suffla S, Ratele K. Violence and injuries in South Africa: prioritising an agenda for prevention. The Lancet. 2009;374(9694):1011–1022. doi: 10.1016/S0140-6736(09)60948-X. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, Garner AS, … Wood DL. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Toska E, Cluver LD, Boyes ME, Isaacsohn M, Hodes R, Sherr L. School, supervision and adolescent-sensitive clinic care: combination social protection and reduced unprotected sex among HIV-positive adolescents in South Africa. AIDS and Behavior. 2016:1–14. doi: 10.1007/s10461-016-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS, U. AllIn#EndAdolescentAIDS. Geneva: 2015. http://allintoendadolescentaids.org/ [Google Scholar]
- Whetten K, Shirey K, Pence BW, Yao J, Thielman N, Whetten R, … O’Donnell K. Trauma History and Depression Predict Incomplete Adherence to Antiretroviral Therapies in a Low Income Country. PLoS ONE. 2013;8(10):e74771. doi: 10.1371/journal.pone.0074771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, Storm D, Montepiedra G, Nichols S, Kammerer B, Sirois PA … Team ftPC. Predictors of Adherence to Antiretroviral Medications in Children and Adolescents With HIV Infection. Pediatrics. 2006;118(6):e1745–e1757. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ) http://www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
- World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ): Guidance for Analysing ACE-IQ. Retrieved April 10, 2014, 2014, from http://www.healthinternetwork.com/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
- World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ): Rationale for ACE-IQ. Retrieved April 10, 2014, 2014, from http://www.healthinternetwork.com/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
- World Health Organization. INSPIRE: seven strategies for ending violence against children. Geneva: World Health Organization; 2016. [Google Scholar]