Abstract
Introduction
Continuous flow ventricular assist devices (cfVADs) continue to be limited by thrombotic complications associated with disruptive flow patterns and supraphysiologic shear stresses. Patients are prescribed complex antiplatelet therapies, which do not fully prevent recurrent thromboembolic events. This is partially due to limited data on antiplatelet efficacy under cfVAD-associated shear conditions.
Materials and Methods
We investigated the efficacy of antiplatelet drugs directly acting on three pathways: (1) cyclooxygenase (aspirin), (2) phosphodiesterase (dipyridamole, pentoxifylline, cilostazol), and (3) glycoprotein IIb–IIIa (eptifibatide). Gel-filtered platelets treated with these drugs were exposed for 10 min to either constant shear stresses (30 dyne/cm2 and 70 dyne/cm2) or dynamic shear stress profiles extracted from simulated platelet trajectories through a cfVAD (Micromed DeBakey). Platelet activation state (PAS) was measured using a modified prothrombinase-based assay, with drug efficacy quantified based on PAS reduction compared to untreated controls.
Results and Conclusions
Significant PAS reduction was observed for all drugs after exposure to 30 dyne/cm2 constant shear stress, and all drugs but dipyridamole after exposure to the 30th percentile shear stress waveform of the cfVAD. However, only cilostazol was significantly effective after 70 dyne/cm2 constant shear stress exposure, though no significant reduction was observed upon exposure to median shear stress conditions in the cfVAD. These results, coupled with the persistence of reported clinical thrombotic complication, suggest the need for the development of new classes of drugs that are especially designed to mitigate thrombosis in cfVAD patients, while reducing or eliminating the risk of bleeding.
Keywords: aspirin, phosphodiesterase inhibitors, glycoprotein IIb–IIIa inhibitors, thrombosis, shear stress, mechanical circulatory support
Introduction
Mechanical circulatory support (MCS), i.e. the use of exogenous or implanted pump systems to restore hemodynamics of the failing heart, has emerged as a mainstay of therapy for patients with advanced and end-stage heart failure [1]. MCS devices, while effective from a flow and blood pressure perspective, remain plagued by a range of complications, notably thrombosis [1]. Continuous flow ventricular assist devices (cfVADs), the primary MCS devices implanted today, are limited by pump-related thrombosis, occurring either within the pump, in pump inflow or outflow tracts, or secondarily via thrombus ingress from the upstream failing ventricle, leading to arterial and venous thromboembolic events, stroke, pump stop, and possible death [2–5]. The primary driver of pump-related thrombosis is non-physiologic flow associated with cfVADs, which repetitively and chronically subject circulating platelets to supra-physiologic shear stress (hypershear), resulting in shear-mediated platelet activation and thrombosis [6, 7]. To prevent thrombus formation, MCS-implanted patients are placed on life-long, antiplatelet and anti-coagulant therapy to mitigate risk. Sadly, despite the use of these anti-thrombotic regimens, thrombosis continues to occur, and is often accompanied unfortunately by concomitant bleeding as well, a recognized side effect of the medications employed [8, 9].
The primary anti-platelet agent utilized in current MCS management is aspirin, i.e. acetylsalicylic acid (ASA) [10, 11]. ASA inhibits platelet reactivity by acetylating cyclooxygenase (COX-1 and COX-2), thereby altering prostaglandin and thromboxane synthesis [12]. The majority of device implantation centers globally opt to use ASA as the sole anti-platelet agent, reported as being prescribed in 83.8% of HeartMate II (HMII, Thoratec Corporation, Pleasanton, CA) and 88.9% of HVAD (HeartWare, Framingham, MA) patients [13]. Device manufacturers may suggest dual anti-platelet therapy, consisting of ASA and an additional anti-platelet drug, such as dipyridamole (DP) [14]. DP is phosphodiesterase (PDE) type 3 and 5 inhibitor that increases intra-platelet cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), inhibiting platelet activation [15, 16]. Approximately 8.8% of HMII and 5.6% of HVAD patients are placed on ASA-DP dual antiplatelet therapy [13]. Pentoxifylline is a nonselective PDE inhibitor that inhibits platelet aggregation [15, 17], and is incorporated into some anti-thrombotic regimens as it is known to reduce whole blood viscosity and increase erythrocyte deformability [8]. In addition to COX and PDE inhibitors, direct acting antagonists for the GPIIb–IIIa receptor have been utilized to inhibit platelet aggregation. Eptifibatide, a potent inhibitor of fibrinogen binding to GPIIb–IIIa, is the most widely-used anti-platelet agent of its class, approved by the FDA for treatment of acute coronary syndrome and for use in percutaneous coronary interventions, due to its inhibition efficacy and modestly-prolonged bleeding times [18]. Eptifibatide has been used in a limited number of cases to mitigate HMII pump thrombosis, yielding mixed results with a concerning number of bleeding events [19–21]. Despite anti-platelet therapy, patients with MCS devices continue to have recurrent arterial and venous thromboembolic events in approximately 8% of HVAD patients [3] and pump thrombosis in 7% of HMII patients [5] annually. Thromboembolic events occur at a rate of 0.67 events per patient year across all cfVADs, as reported in INTERMACS [1]. These adverse events are partially a result of the limited understanding of antiplatelet drug efficacy under the supra-physiologic shear conditions encountered by platelets in cfVADs [24, 25]. The wall shear stresses in cfVADs are typically dynamic, approaching estimated shear stresses up to 2,000 dyne/cm2 in centrifugal devices [26] and up to 30,000 dyne/cm2 in axial devices, with peak fluid shear stresses approaching 4,000 to 6,000 dyne/cm2 in clinically implanted cfVADs [27, 29], as calculated with computational fluid dynamics modeling. Typical exposure times in VADs range on the order of 10 to 100 ms. These values are approximate as such proprietary parameters are not routinely released by device companies, giving an incomplete set of data, which may hinder a better understanding of platelet and blood trauma in devices and the design of less traumatic devices. Thus, establishing the efficacy of antiplatelet drugs under these hypershear conditions is crucial for optimizing pharmacotherapy of cfVAD patients.
In the present study, we expand on our initial report of the lack of efficacy of ASA as means of limiting hypershear-mediated platelet activation [24, 25], and herein examine the inhibitory efficacy of commonly prescribed direct-acting antiplatelet drugs, under similar constant and device-associated shear conditions. We evaluated the efficacy of three classes of platelet antagonists: (1) COX inhibitors, (2) PDE inhibitors, and (3) GPIIb–IIIa inhibitors. We utilized the COX-pathway inhibitor ASA as our starting point [24]. DP, pentoxifylline, and cilostazol were utilized as PDE inhibitor representatives. The latter reversibly inhibits agonist-induced aggregation by preventing PDE 3 from converting cAMP, leading to an increase in protein kinase A [15]. Cilostazol is commonly prescribed for intermittent claudication, a painful exercise-induced leg cramping in patients with peripheral artery disease, without prolonging bleeding time [30]. In this study, we did not examine other potentially powerful platelet activation antagonists which require metabolism, e.g. clopidogrel. We selected these agents for study as a common, but unsubstantiated clinical view is that they have greater potency for shear inhibition than ASA. We hypothesized that all three classes of direct-acting anti-platelet agents tested here have limited ability to inhibit or reduce shear-mediated platelet activation under device-associated shear profiles. Purified platelets treated with these three classes of drugs were exposed to constant shear stresses over an extended period of time, as well as dynamic shear stress waveforms extracted from simulated flight trajectories of platelets passing through a clinical cfVAD [27, 29]. Efficacy of the drugs was evaluated with the reduction in thrombin generation rates compared to untreated controls after shear exposure.
Materials and Methods
Platelet preparation
Informed consent was obtained from healthy adult volunteers of both sexes who had not taken aspirin or ibuprofen for two weeks, as per a University of Arizona IRB-approved protocol. Whole blood (30 ml) was drawn via antecubital venipuncture into 3 ml acid-citrate dextrose (ACD-A) and centrifuged at 500g for 15 min to obtain platelet-rich plasma (PRP), which was filtered through a column of Sepharose 2B beads (Sigma-Aldrich, St. Louis, MO, USA) to collect gel-filtered platelets (GFP) [31, 32]. GFP were diluted to a count of 20,000/µl in HEPES-buffered modified Tyrode’s solution, with 3 mM CaCl2 added 10 min prior to experiments [31, 33].
Treatment of gel-filtered platelets with anti-platelet drugs
Selection of the antiplatelet drugs was based on their common acceptance in clinical cardiovascular practice today, the frequency of their administration to device patients and the pathway of action. We selected drugs that inactivated cyclooxygenase (COX), and inhibited phosphodiesterase (PDE) and glycoprotein IIb/IIIa (GPIIb–IIIa).
Acetylsalicylic acid (ASA, aspirin) is a commonly administered COX pathway inhibitor in patients with cardiovascular disease due to its antiplatelet properties, and it is prescribed for almost all patients implanted with MCS devices [8]. Platelets in this study were treated with ASA (Sigma Aldrich, St. Louis, MO) dissolved in sodium bicarbonate solution (180 mg ASA, 270 mg citric acid, and 349 mg sodium hydrogen carbonate in 10 ml double-distilled H2O, ddH2O), obtaining a 0.1 M solution. This was further diluted to a 25 or 125 µM final concentration. These ASA concentrations correspond to clinical use dosages of 81 mg/day or 325 mg/day, respectively [8].
Several inhibitors of cyclic adenosine PDEs, which regulate platelet function by limiting the intracellular levels of cyclic nucleotides, are currently used in clinic as antiplatelet agents [15]. We investigated the effects of dipyridamole (DP), pentoxifylline, and cilostazol. DP was prepared from a stock solution (Persantine, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT) containing 5 mg/ml DP, 50 mg/ml polyethylene glycol (PEG) 6000, and 2 mg/ml tartaric acid dissolved in water. This was further diluted to a final concentration of 5, 10, or 25 µM. Pentoxifylline is a non-selective PDE inhibitor that also reduces whole blood viscosity and improves erythrocyte deformability. A 0.1 M solution was prepared by dissolving 280 mg pentoxifylline (Sigma Aldrich, St. Louis, MO) in 10 ml ddH2O. The pH was recalibrated to 7.4 by adding 0.1 M NaOH. Platelets were treated at a final concentration of 100 µM. Cilostazol is a selective PDE 3 inhibitor that increases cAMP, thereby increasing protein kinase A and directly inhibiting platelet aggregation. Cilostazol was prepared by pulverizing commercially available tablets (50 mg, Apotex Corporation, Weston, FL), then dissolving the powder in 10 ml dimethylformamide, obtaining a final concentration of 0.1 M. Platelets were treated at a final concentration of 50 µM.
GPIIb–IIIa inhibitors are some of the most potent antiplatelet agents, as they inhibit platelet aggregation, and are prescribed for some MCS patients. We obtained eptifibatide (Integrilin, Merck, Kenilworth, NJ), one such inhibitor, as a 100 ml infusion vial containing 0.75 mg/ml eptifibatide. Platelets were treated at a final concentration of 0.25 µg/ml.
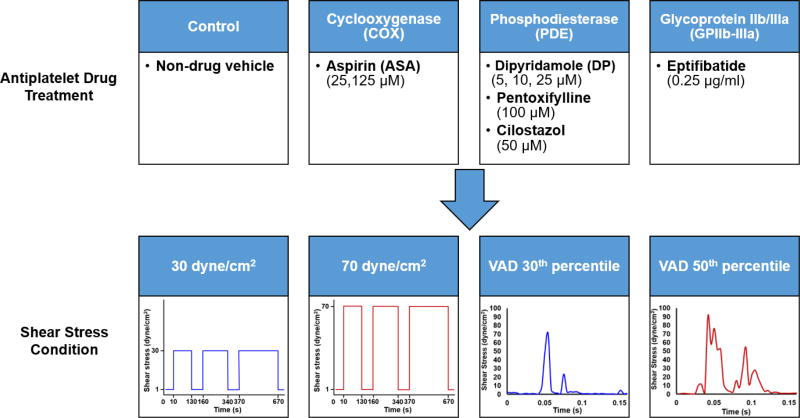
Platelets were incubated with the antiplatelet drug being tested at 37°C for 10 min prior to shear exposure. For each drug-treated platelet sample, a paired control experiment with platelets exposed to the solvent vehicle alone (control) was performed on the same day. The drug treatments are summarized in Figure 1.
Fig. 1. Exposure of drug-treated platelets to constant and dynamic device-related shear stress.
Gel-filtered platelets (GFP) were treated with a drug selected from one of three classes and exposed to either a constant or dynamic shear stress waveform, representative of a trajectory through a DeBakey cfVAD, in a Hemodynamic Shearing Device for 10 min.
Exposure of antiplatelet-treated platelets to constant and dynamic shear stress
Platelets were exposed to both constant and dynamic shear stress in the Hemodynamic Shearing Device, a computer-controlled cone-plate-Couette viscometer programmed with shear stress waveforms mimicking conditions found in blood recirculating devices [34, 35]. In the constant shear stress experiments, platelets were exposed to 30 dyne/cm2 or 70 dyne/cm. These shear stresses represent physiologic and pathologic magnitudes, respectively, encountered by platelets [36]. For the dynamic experiments, the waveforms were selected from the probability density function of the shear stress conditions extracted from platelet-like particle trajectories passing through a DeBakey cfVAD [27]. This function describes the distribution of the stress accumulations, or product of shear stress and exposure time, experienced by the particles during a single passage through the device. In particular, we selected waveforms corresponding to the 30th and 50th percentiles of the stress accumulation distribution, with an exposure frequency of 110 passages per min [24]. These percentiles were selected to mimic a “gentler” and median exposure, respectively, of platelets passing through a clinically implanted cfVAD. The magnitude of the waveforms was scaled by a factor of 52.5 as the maximum shear stress of the Hemodynamic Shearing Device cannot exceed 108 dyne/cm2 at 1 cP, but the waveforms retain their dynamic nature. Total experimental duration for each both constant and dynamic waveforms was 10 min, with samples taken at 0, 2, 5, and 10 min. At each time point, the Hemodynamic Shearing Device was slowed down to 1 dyne/cm2 for 30 s for sampling. The shear stress conditions are summarized in Figure 1. We note here that the emphasis is not necessarily on the shear stress magnitude alone, but also the continued exposure of platelets to these conditions, mimicking the stress accumulation these platelets encounter during repeated passages through cfVADs [34].
Platelet activation measurements
A modified prothrombinase-based chromogenic assay employing acetylated prothrombin was utilized to quantify the platelet activation state (PAS), a measure of thrombin generation [37]. PAS values were normalized against fully activated platelets obtained by sonication (10 W for 10 s, Branson Sonifier 150 with microprobe, Branson, MO) [32]. Normalized PAS values are reported as a fraction of the maximal thrombin-generating capacity, with a maximum value of 1.0.
Statistical Analysis
Prior to statistical analyses, normality of all drug-treated and control groups was checked using the Shapiro-Wilk test. For both constant and dynamic shear stress experiments, we calculated the differences in PAS between 0 and 10 min, ΔPAS, which was then compared between drug-treated and untreated (control) platelets. Depending on the normality of the data, either parametric or non-parametric (Kruskal-Wallis) one-way ANOVA was performed, with the Holm-Sidak or Dunn’s post hoc tests, respectively, to compare the ΔPAS for the different groups tested. For all cases, significance was achieved for p < 0.05. Results are presented as the mean ± standard error of the mean (S.E.M.), unless otherwise stated. All statistical analyses were performed using Sigmaplot 11.0 (Systat Software, San Jose, CA).
In addition, the shape of the PAS curve over a 10 min exposure time is described. A linear response shows that the PAS rate, or change in PAS over time, is steady over the exposure duration. A convex behavior shows that the PAS rate increases with respect to time during the exposure, whereas a concave behavior shows a slowdown in the PAS rate over the 10 min duration. In all experiments, there is no decrease in PAS from the start of the experiment, and thus the rate of PAS is always positive.
Results
Platelet Activation Due to Constant and Device-Related Shear Stress
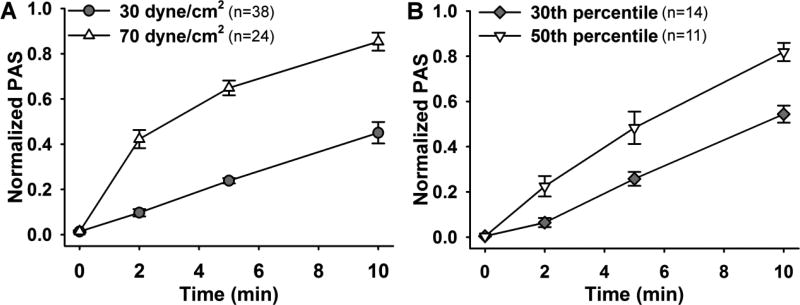
Prior to examining antiplatelet drug efficacy on shear-medicated platelet activation, we observed the response of platelets to constant and device-related dynamic shear stress waveforms. Exposure to 30 dyne/cm2 linearly increased the PAS over 10 min, to ΔPAS of 0.44 ± 0.03 (Fig. 2A, n = 38), whereas platelets exposed to 70 dyne/cm2 responded in a concave manner, resulting in ΔPAS of 0.83 ± 0.02 (Fig. 2A, n = 24). Similar behavior was observed for platelets exposed to dynamic shear stress waveforms extracted from DeBakey cfVAD simulations. A convex activation response was observed for platelets sheared with the 30th percentile waveform, yielding ΔPAS of 0.54 ± 0.04 (Fig. 2B, n = 14), whereas a concave behavior was observed for exposure to the 50th percentile waveform, with ΔPAS of 0.82 ± 0.04 (Fig. 2B, n = 11).
Fig. 2. Platelet activation due to constant and dynamic device-related shear stress.
Normalized PAS, a measure of platelet activation, was quantified for A) constant and B) cfVAD-related shear stress exposure in a Hemodynamic Shearing Device.
Effect of Aspirin on Sheared Platelets
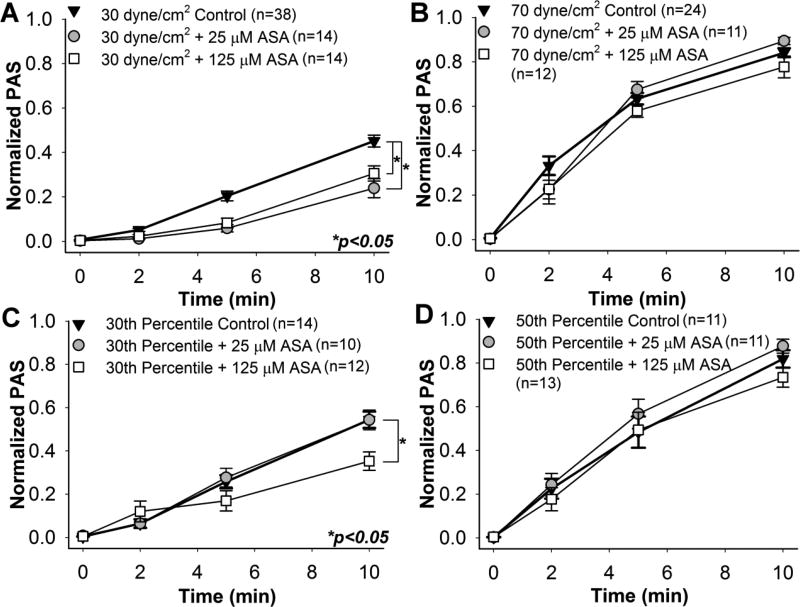
Confirming our previous observations [24], platelets pre-treated with 25 and 125 µM ASA, followed by constant shear exposure to 30 dyne/cm2 for 10 min, showed a significant reduction in the ΔPAS for both ASA concentrations when compared to control (Fig. 3A, n = 14, p < 0.05). This corresponds to a 46.7% and 32.1% reduction in ΔPAS after 25 and 125 µM ASA pre-treatment, respectively. However, no significant reduction was observed for the 70 dyne/cm2 condition (Fig. 3B, n = 11, p > 0.05). Similarly, platelets treated with 125 µM ASA and exposed to the 30th percentile DeBakey cfVAD waveform showed a 35.6% reduction in ΔPAS compared to untreated platelets (Fig. 3C, n = 12, p < 0.05), In contrast, no significant reduction was observed for 25 µM ASA-treated platelets after 30th percentile shear stress waveform exposure (Fig. 3C, n = 10, p > 0.05). Furthermore, ASA treatment had no effect on platelets exposed to the 50th percentile shear stress waveform (Fig. 3D, n = 12, p > 0.05). Platelets exposed to either the 30 dyne/cm2 constant shear stress condition or the 30th percentile dynamic cfVAD waveform showed a convex PAS behavior with respect to exposure time, whereas concave behavior was observed for the 70 dyne/cm2 constant shear condition and 50th percentile dynamic VAD waveform condition.
Fig. 3. Effect of aspirin (ASA) on sheared platelets.
GFP were pre-treated with 25 and 125 µM ASA, and their PAS measured during exposure to constant shear stresses of A) 30 dyne/cm2 and B) 70 dyne/cm2, as well as waveforms representing the C) 30th and D) 50thpercentiles of all shear stress trajectories passing through a DeBakey cfVAD. PAS for ASA-treated GFP was compared to non-drug control after 10 min.
Role of Phosphodiesterase Inhibitors on Sheared Platelets
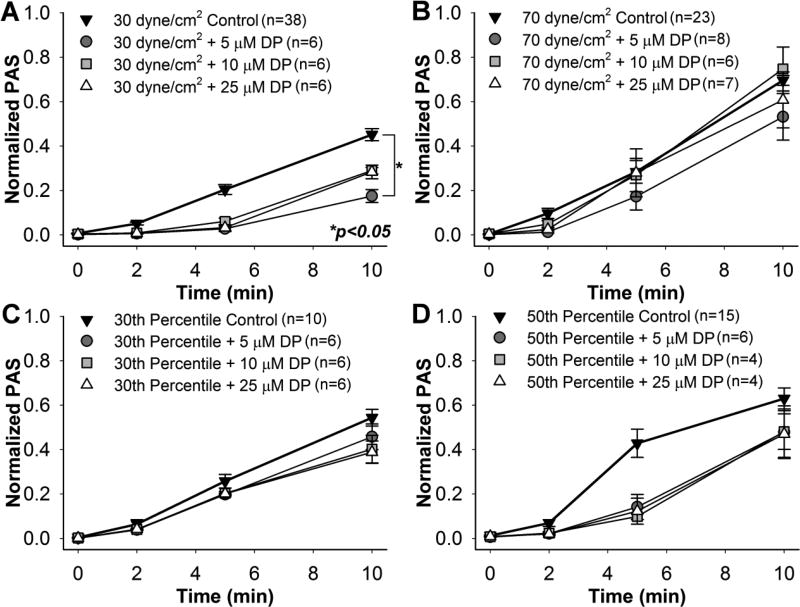
Pre-treatment with 5 µM DP yielded a 61.0% reduction in ΔPAS for platelets exposed to 30 dyne/cm2 for 10 min (Fig. 4A, n = 6, p < 0.05). Reductions observed for platelets pre-treated with 10 and 25 µM DP and exposed to 30 dyne/cm2 were non-significant (n = 6, p > 0.05). No differences were observed between the DP-treated platelets and control after 10 min exposure to the 70 dyne/cm2 (Fig. 4B, n = 7, p > 0.05), 30th percentile (Fig. 4C, n = 6, p > 0.05), or 50th percentile (Fig. 4D, n = 4, p > 0.05) shear stress waveforms, potentially due to a small sample size.
Fig. 4. Effect of dipyridamole (DP) on sheared platelets.
GFP were pre-treated with 5, 10, and 25 µM DP, and their PAS measured during exposure to constant shear stresses of A) 30 dyne/cm2 and B) 70 dyne/cm2, as well as waveforms representing the C) 30th and D) 50th percentiles of all shear stress trajectories passing through a DeBakey cfVAD. PAS for DP-treated GFP was compared to non-drug control after 10 min.
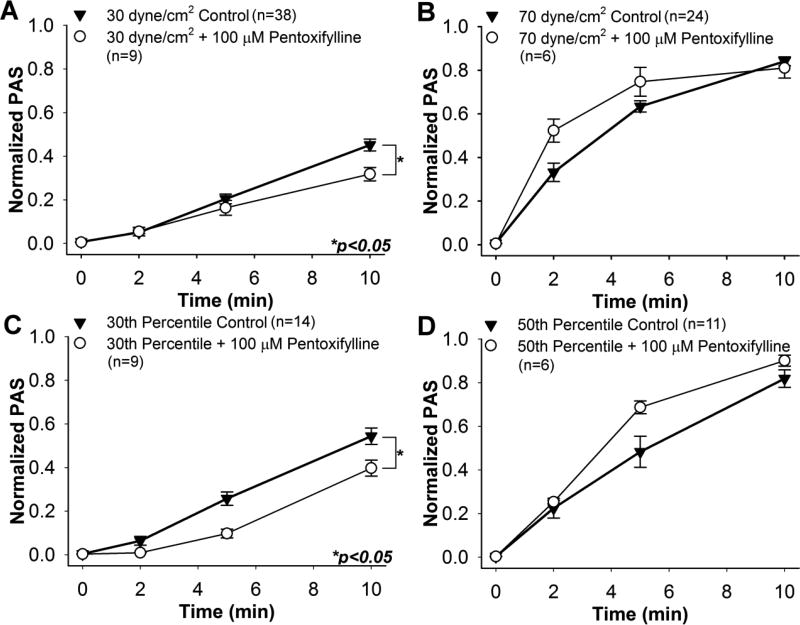
Pre-treatment with DP at all concentrations yielded a convex behavior in ΔPAS during shear exposure. Platelets treated with 100 µM pentoxifylline showed ΔPAS reductions of 29.6% and 27.0% after 10 min exposure to 30 dyne/cm2 (Fig. 5A, n = 9, p < 0.05) and the 30th percentile waveform (Fig. 5C, n = 9, p < 0.05), respectively. For both of these cases, ΔPAS increased in a convex manner during exposure. However, no significant difference was observed between the controls and pentoxifylline-treated platelets after 10 min exposure to the 70 dyne/cm2 (Fig. 5B, n = 6, p > 0.5) and 50th percentile (Fig. 5D, n = 6, p > 0.05) waveforms, with concave ΔPAS behavior observed for both. A slight, non-significant increase was observed in the PAS of the pentoxifylline-treated platelets compared to the control after 2 and 5 min of exposure to the 70 dyne/cm2 and 50th percentile waveforms.
Fig. 5. Effect of 100 µM pentoxifylline on sheared platelets.
PAS for pentoxifylline-treated GFP was measured during exposure to constant shear stresses of A) 30 dyne/cm2 and B) 70 dyne/cm2, as well as waveforms representing the C) 30th and D) 50thpercentiles of all shear stress trajectories passing through a DeBakey cfVAD. PAS for pentoxifylline-treated GFP was compared to non-drug control after 10 min.
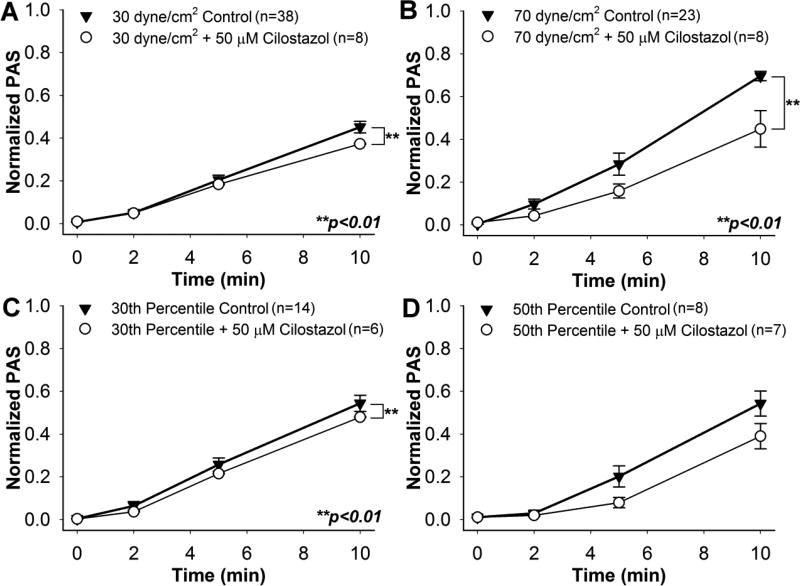
A clear difference emerged between control and 50 µM cilostazol-treated platelets for both constant shear stress conditions, as well as the 30th percentile waveform. Cilostazol-treated platelets exposed to 30 dyne/cm2 showed an 18.4% reduction in ΔPAS after 10 min (Fig. 6A, n = 8, p < 0.01), whereas those sheared at 70 dyne/cm2 yielded a 36.8% decrease (Fig. 6B, n = 7, p < 0.01). In addition, exposure of drug-treated platelets to the 30th percentile waveform resulted in an 11.8% ΔPAS reduction after 10 min (Fig. 6C, n = 6, p < 0.01). No significant change was noted for cilostazol-treated platelets as compared to the control after exposure to the 50th percentile waveform (Fig. 6D, n = 7, p > 0.05), potentially due to a small sample size, and a convex activating behavior was observed over the duration of the experiments for all shear exposures. It is important to note here that 50 µM cilostazol was the only drug that yielded a significant reduction in ΔPAS after exposure to 70 dyne/cm2 for 10 min, and that reduction in ΔPAS for drug-treated platelets as compared to the untreated controls was more apparent at higher constant and dynamic shear stress exposures.
Fig. 6. Effect of 50 µM cilostazol on sheared platelets.
PAS for cilostazol-treated GFP was measured during exposure to constant shear stresses of A) 30 dyne/cm2and B) 70 dyne/cm2, as well as waveforms representing the C) 30thand D) 50thpercentiles of all shear stress trajectories passing through a DeBakey cfVAD. PAS for cilostazol-treated GFP was compared to non-drug control after 10 min.
Effect of GPIIb–IIIa Inhibitor on Sheared Platelets
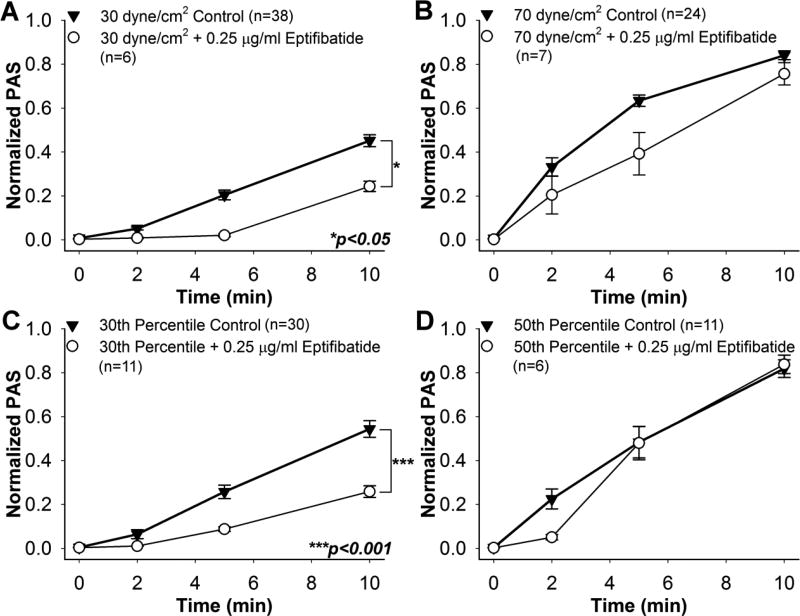
Treatment with 0.25 µg/ml eptifibatide yielded significant ΔPAS reductions of 45.8% and 52.7% after 10 min exposure to 30 dyne/cm2 (Fig. 7A, n = 6, p < 0.05) and the 30th percentile waveform (Fig. 7C, n = 11, p < 0.001), respectively, compared to the controls. However, eptifibatide treatment was ineffective after exposure to 70 dyne/cm2 (Fig. 7B, n = 7, p > 0.05) or the 50th percentile waveform (Fig. 7D, n = 6, p > 0.5). A convex ΔPAS response was observed during shear exposure for the 30 dyne/cm2 and 30th percentile experiments, whereas a concave response was observed for the 70 dyne/cm2 exposure. Eptifibatide-treated platelets exposed to the 50th percentile waveform yielded a convex activation response for the first 5 min, and was linear thereafter.
Fig. 7. Effect of 0.25 µg/ml eptifibatide on sheared platelets.
PAS for eptifibatide-treated GFP was measured during exposure to constant shear stresses of A) 30 dyne/cm2 and B) 70 dyne/cm2, as well as waveforms representing the C) 30th and D) 50th percentiles of all shear stress trajectories passing through a DeBakey cfVAD. PAS for eptifibatide-treated GFP was compared to non-drug control after 10 min.
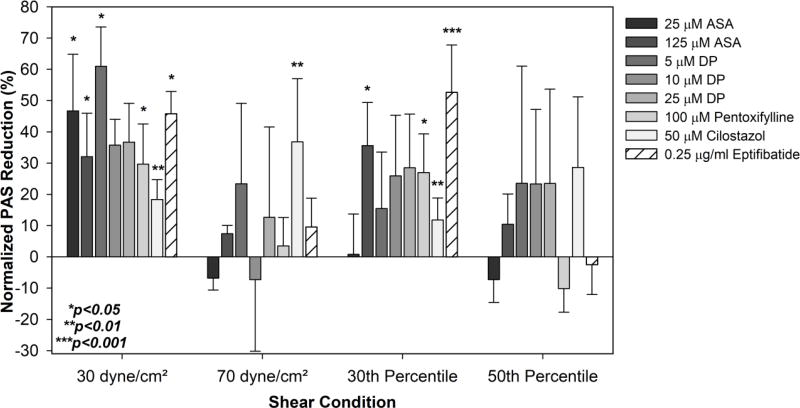
A summary of the percentage reductions in 10 min PAS is provided in Figure 8. All reductions were calculated with respect to the non-drug treated sheared vehicle control for the respective drug treatments.
Fig. 8. Summary of antiplatelet drug efficacy on sheared platelets.
The percentage reduction in drug-treated sheared platelet activation over the 10 min experimental duration, ΔPAS, was compared to untreated sheared controls.
Discussion
Antithrombotic therapy aimed at limiting VAD thrombosis in clinical use today has largely been developed empirically. As for most cardiovascular implants, e.g. prosthetic heart valves, clinical consideration and the approach to pharmacologic regimen formulation begins with and reflects thinking based upon traditional concepts such as Virchow’s triad [36, 38], which recognizes that some imbalance in blood inflammatory state or pro-thrombogenicity, coupled with an altered endothelial or foreign blood-contacting surface and altered flow leads to platelet and zymogen activation. This approach continues to generate clinical anti-thrombotic regimens employing well-accepted, though conventional, agents such as aspirin for anti-platelet effects and warfarin as an anti-coagulant. Unfortunately, utilization of this simple strategy for cfVADS has significantly fallen short, with notable and persistent thrombotic event rates continually being reported [1]. Further, a simple increase or modulation of current drug regimens conversely has led to higher rates of bleeding [39]. This is further exacerbated by the acquired von Willebrand syndrome imparted by cfVAD supra-physiological shear, resulting in loss of high molecular weight multimers, leading to further increased bleeding risk [40–42]. Attempting to balance this by mere reduction of anti-coagulant and anti-platelet dosing has led to increased clotting in cfVADs, as highlighted by recent findings of persistent and greater cfVAD thrombosis being reported [23, 43]. As such, conventional pharmacotherapy has focused on tackling the “symptoms” of device-associated thrombosis, rather than addressing a significant root cause – namely the paucity of understanding on anti-platelet and anti-coagulant drug activity under dynamic, high shear flow conditions, and conversely the relative unbalanced role of elements of Virchow’s triad in the case of MCS and cfVADs – i.e. the disproportionate and dominant role of hypershear as driving platelet activation in the free flow of blood passing through these devices [7]. In this study, we aimed to enhance our understanding of drug effectiveness in modulating shear-mediated platelet activation, through systematic exposure of anti-platelet agent-treated platelets to a variety of shear stress conditions and examining their response to this mechanical dosing. Herein we have found, similar to our initial reported observations with aspirin [24], that most conventional anti-platelet agents, i.e. the ones tested herein, have limited efficacy in modulating or limiting shear-mediated platelet activation.
Exposure to VAD-associated shear stresses
In this study we used both pre-defined constant shear stress waveforms, as well as those derived from numerical simulations of a clinical cfVAD (DeBakey), representing the dynamic nature of flow in such devices. It is important to note that such parametric studies on the efficacy of a variety of anti-platelet drugs under fluid shear stress conditions have not been previously published. ASA efficacy in reducing thrombin generation was first examined at the device level in a flow loop incorporating a DeBakey cfVAD [25]. Platelets treated with ASA and circulated for 30 min through the cfVAD showed 28% and 25% reduction in thrombin generation after direct treatment and 2 h after ingestion by healthy subjects, respectively. However, this approach required large blood donations and therefore a reduction in the number of parametric studies that could be performed. Parametric shear-based studies were first performed in a follow-up in vitro study by exposing platelets pre-treated with ASA to both constant and dynamic, device-related shear stresses, and measuring the time-varying thrombin generation [24]. These shear stress conditions have also been utilized in the present study. Constant shear stresses ranging from the physiological 1 dyne/cm2 to the pathological 70 dyne/cm2 were imposed for up to 10 min to examine activation due to both the magnitude of shear exposure, as well as the shear stress-exposure time dose. However, flow conditions in VADs are typically dynamic, disturbed, and pathologic, and therefore, platelets were exposed to representative shear stress waveforms extracted from numeric simulations of the DeBakey cfVAD [27]. These representative shear stress waveforms have their magnitudes scaled to the operating parameters of the Hemodynamic Shearing Device, but retain the dynamic shear stress rate profile of the original extracted waveforms. Thus, we are able to test efficacy of a variety of anti-platelet agents to device-specific shear stress histories.
Reduced efficacy of direct-acting antiplatelet drugs under shear
In this study we purposely examined conventional anti-platelet agents, as they are routinely employed and presently accepted for use by the cardiovascular therapeutic community, in the pharmacotherapy for cardiovascular disease patients with implanted therapeutics devices, including VADs. These included three classes of target or pathway inhibitors: COX (ASA), phosphodiesterase (DP, pentoxifylline, cilostazol), and GPIIb–IIIa (eptifibatide). The study was restricted to direct-acting conventional agents to avoid the need for metabolic activation of a prodrug. While the differing drugs tested had varying degrees of effectiveness in suppressing platelet activation under physiological constant shear stress (30 dyne/cm2) or mild stress accumulation after passing through a DeBakey cfVAD (30th percentile), only cilostazol showed a mildly effective reduction in platelet activation at supra-physiological shear stress (70 dyne/cm2). However, none of the drugs tested effectively reduced platelet activation after 10 min exposure to the median shear stress conditions encountered with each passage through the DeBakey cfVAD (50th percentile). This indicates that the direct-acting drugs tested are unlikely to individually inhibit platelet activation during repeated passages through VADs. There are more powerful antagonists (i.e. the P2Y12 antagonists clopidogrel and ticagrelor) with promising levels of effectiveness, but they either require metabolism or are beyond the scope of the conventional agents examined in this study and are rarely utilized clinically in the cfVAD patient population.
Efficacy of cilostazol under device-associated shear conditions
Cilostazol selectively inhibits the PDE 3 pathway by preventing the conversion of aggregation regulator cyclic adenosine monophosphate (cAMP) into 5’-AMP, thereby reducing thromboxane A2 production, leading to reversible aggregation inhibition induced by several biochemical agonists and shear stress [30]. A previous investigation of the effect of cilostazol on shear-induced platelet aggregation showed significantly reduced aggregation for 100 µM cilostazol-treated platelets exposed to a variable shear stress waveform (6–108 dyne/cm2 over a 5 min period) [44]. Furthermore, exposure of cilostazol-treated whole blood to collagen-treated surfaces at shear rates of 1500 s−1 generated thrombi with reduced heights without prolonging bleeding time [45]. Our observations of substantially reduced activation of cilostazol-treated platelets confirm that the PDE 3 inhibition is preserved under both constant and dynamic device-related conditions. However, the FDA has contraindicated the use of cilostazol for patients with any degree of heart failure [46, 47]. Thus, while our study shows a promising degree of platelet activation reduction in response to device-associated shear stress conditions, we do not recommend its use for antiplatelet therapy in advanced heart failure cfVAD recipients.
Mechanisms Operative in Shear-Mediated Platelet Activation, New targets and Future Perspectives
Our results suggest and are consistent with growing evidence that shear-mediated platelet activation involves additional mechanistic pathways, not primarily targeted by current pharmacologic agents [7]. Our results suggest that, at least for the level of inhibition imparted by the inhibitors tested, pathways and targets such as thromboxane and prostacyclin synthesis and COX [48, 49], cyclic nucleotide PDE and adenosine uptake [50], PDE 2, 3 and 5 [15, 50] and GPIIb–IIIa activation and ligand binding [50] may have a limited or partial mechanistic role in mediating and modulating the response to shear.
Several additional drugs have been proposed to address the shortcomings of the conventional antiplatelet drugs under VAD-associated conditions and warrant further study. The P2Y12 antagonists ticagrelor, cangrelor, and 2-MeSAMP have previously shown promise under low shear conditions [51]. Apyrase (CD39) has been considered as an inhibitor for ADP-induced activation, although this antagonist has shown limited efficacy under low shear conditions [51]. The phosphoinositide 3-kinase inhibitor TGX-221 has emerged as a potential alternative to GPIIb–IIIa inhibitors, demonstrating reduced aggregation and beta thromboglobulin release in extracorporeal circulation [52]. The efficacy of these agents has yet to be tested under the dynamic, hypershear milieu experienced in cfVADs and will be examined in future studies.
In contrast to pharmacologic targets, recent work has suggested that cell mechanobiological parameters such as cell stiffness [53], membrane fluidity [7, 54] and platelet membrane lipid composition [55] are vital in transduction of shear force signaling into the platelet, leading to activation. Further, recent work has shown that additive damage to the platelet membrane [56], most notably the high frequency components of shear [57] are key mechanistic components and potential targets, to limit mechano-destructive activity leading to platelet activation, the generation of exposed pro-thrombotic platelet membrane surfaces, all facilitating residence and activation of the X-IX-V complex driving thrombosis [58, 59].
The present study demonstrating limited efficacy of the drugs tested, coupled with the mechano-biological observations discussed, point out the need to direct efforts for agent development aimed at new and differing targets. Specifically, opportunity exists for development of agents that are shear-limiting, shear-modulating, or otherwise inhibitory of shear-imparted effects on the platelet. Several broad classes of target mechanisms for shear-imparted effects identified in recent studies include: (1) mechano-destruction, or repetitive additive damage exceeding a threshold, which may lead to an influx of activating mediators [34, 57, 60]; (2) mechano-activation, involving recruitment and activation of shear-sensitive channels and pores, allowing influx of specific activators [61]; and (3) mechano-transduction involving shear sensing and transduction via a range of biochemical-linkage pathways – involving transmembrane proteins beyond GPIb and GPIIb–IIIa pathways, the platelet membrane, sub-membrane assemblies, actin filaments and microtubules [62, 63].
Abrupt increases in cfVAD thrombosis were reported in 2014 [22, 23], with subclinical hemolysis suspected as the underlying cause. Recent studies have proposed mechanisms for this effect, including the platelet activating role [64] and protective effect [65] of erythrocyte-derived plasma-free hemoglobin on von Willebrand factor (vWF) degradation, which is counterintuitive to the observed vWF degradation and subsequent bleeding observed in cfVAD patients [66]. Thus, elucidating mechanisms protecting erythrocytes from mechanical destruction are another potential avenue for therapy.
Recent work from our group has demonstrated that modulation of platelet mechanical parameters holds promise in limiting shear-mediated platelet activation [7], leading to the advent of a new class of compounds termed “mechano-ceuticals,” i.e. agents which directly alter cellular mechanical properties, not acting as conventional pharmacologic agents or secondary messengers [67].
Limitations of the present study
In this study, we used gel-filtered platelets (GFP) at a diluted concentration (20,000 per µl) to observe the direct effects of shear stress and antiplatelet drug modulation on platelet activation. The use of purified platelets at a reduced concentration eliminated thrombin activity effects due to platelet-platelet collisions and cross-talk observed in prior studies [31]. Furthermore, our approach eliminated activation due to platelet diffusivity and margination through interactions with red blood cells (RBCs) [68], as well as platelet agonists, such as ADP and LDH, released during hemolysis [69]. Whole blood also contains plasma proteins, such as fibrinogen and von Willebrand factor, which contribute to platelet activation. Under shear stress, the proteins participate in platelet aggregation, making observation of bulk platelet activation difficult. In addition, gel filtration eliminates other proteins and ions, such as prothrombin, FXa and Ca2+, which participate in the common pathway of platelet activation. However, our modified prothrombinase-based assay protocol re-introduces these constituents, including an acetylated form of prothrombin, for proper generation of modified thrombin that can be quantified rapidly and is useful for time-course assays of shear-mediated platelet activation [32, 37]. We have chosen to use the prothrombinase-based PAS assay as it provides a sensitive, near real-time measurement of bulk thrombin measurement in response to a variety of agonists, including shear stress. However, this approach does not give indications of platelet-specific functional and molecular changes, such as surface receptor expression and fibrinogen binding, nor adhesion or aggregation behavior post-shear exposure. While past studies by our group have shown a strong correlation between PAS activity and P-selectin [70] or Annexin V [71] response under shear stress, the expression of these and other flow cytometric markers, as well adhesion and aggregation activity, under the specific conditions evaluated in this study warrant further attention.
The use of small sample sizes in several of the experiments may have yielded inadequate power to yield significance, with a possibility of type II statistical error. This may be observed particularly for the 10 min PAS results of the 50th percentile exposure of dipyridamole- and cilostazol-treated platelets (Figure 8). However, it is important to note that the average mean reductions in PAS at the higher constant and dynamic device-related shear stress exposures are lower than those at lower constant and dynamic shear stress exposures, suggesting lower efficacy at device-associated conditions. In addition, we did not analyze the 5 min PAS values, some of which may be significant with respect to the control, as our emphasis was on repeated exposure to both constant and dynamic shear stress conditions, and thus we focused on the endpoints of the experiments.
Conclusions
While conventional anti-platelet agents, such as those tested herein, are often prescribed as part of the pharmacotherapy of cfVAD recipients, when scrutinized under systematic and controlled constant and device-related shear stress conditions their effectiveness in preventing or modulating shear-mediated platelet activation appears limited. Some of these agents may be effective under low shear stresses or less damaging passages through cfVADs, and significant reductions in platelet activity may not have been achieved due to smaller sample sizes. However, of all the anti-platelet agents tested in this study, only the phosphodiesterase-3 inhibitor cilostaszol demonstrated partial effectiveness in limiting shear-mediated platelet activation under supra-physiologic, constant shear stress conditions, but ultimately failed when tested at median cfVAD-associated dynamic shear stress patterns. Furthermore, these drugs are associated with the propensity for bleeding in cfVAD patients. Our in vitro results, coupled with persistently reported cfVAD thrombosis rates and complications, suggest the need for the discovery and development of new classes of drugs that are especially designed to tackle shear-mediated thrombosis in cfVAD patients, while reducing or eliminating the risk of bleeding.
Highlights.
Direct-acting antiplatelet drugs targeting COX, PDE, and GPIIb–IIIa have limited efficacy in modulating shear-mediated platelet activation under both constant and dynamic levels of elevated shear stresses experienced in cfVADs
The PDE 3 inhibitor cilostazol significantly reduces platelet activation under elevated constant and mildly dynamic shear stresses, but is ineffective under median cfVAD-associated shear patterns
A clear need exists for the development of a new class of anti-platelet drugs able to modulate shear-mediated platelet activation at supraphysiologic shear stress levels associated with mechanical circulatory support devices such as cfVADs
Acknowledgments
Acknowledgment/Funding Sources
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (Quantum Grant Award No. 5U01EB012487-00) and by Fondazione Cariplo (Grant No. 2241-2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, John R, Caldeira C, Jeevanandam V, Boyle AJ, Sundareswaran KS, Sood P, Mehra MR. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.028303. [DOI] [PubMed] [Google Scholar]
- 3.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW H.B.t.T.A.T. Investigators. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, Adamson RM, Blood MS, Camacho MT, Idrissi KA, Petty M, Sobieski M, Wright S, Myers TJ, Farrar DJ I.I.C.I. HeartMate. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, Rogers J, Slaughter MS, Stevenson LW. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Heart Lung Transplant. 2015;34(12):1515–26. doi: 10.1016/j.healun.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Houel R, Mazoyer E, Boval B, Kirsch M, Vermes E, Drouet L, Loisance DY. Platelet activation and aggregation profile in prolonged external ventricular support. J Thorac Cardiovasc Surg. 2004;128(2):197–202. doi: 10.1016/j.jtcvs.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Slepian MJ, Sheriff J, Hutchinson M, Tran P, Bajaj N, Garcia JG, Scott Saavedra S, Bluestein D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J Biomech. 2017;50:20–25. doi: 10.1016/j.jbiomech.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ensor CR, Paciullo CA, Cahoon WD, Jr, Nolan PE., Jr Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann Pharmacother. 2011;45(1):60–77. doi: 10.1345/aph.1P459. [DOI] [PubMed] [Google Scholar]
- 9.Massicotte MP, Maul TM, Snyder TA, Baumann Kreuziger L. Mechanical Circulatory Support and Antithrombotic Therapy: Looking for the Holy Grail. ASAIO J. 2017;63(1):1–4. doi: 10.1097/MAT.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 10.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J, H.International Society for. Lung T. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Rossi M, Serraino GF, Jiritano F, Renzulli A. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg. 2012;15(4):733–40. doi: 10.1093/icvts/ivs297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24(11):1980–7. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- 13.Jennings DL, Horn ET, Lyster H, Panos AL, Teuteberg JJ, Lehmkuhl HB, Perez A, Shullo MA. Assessing Anticoagulation Practice Patterns in Patients on Durable Mechanical Circulatory Support Devices: An International Survey. ASAIO J. 2016;62(1):28–32. doi: 10.1097/MAT.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 14.Hohner E, Crow J, Moranville MP. Medication management for left ventricular assist device thrombosis. Am J Health Syst Pharm. 2015;72(13):1104–13. doi: 10.2146/ajhp140538. [DOI] [PubMed] [Google Scholar]
- 15.Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72(4):634–46. doi: 10.1111/j.1365-2125.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doligalski CT, Jennings DL. Device-Related Thrombosis in Continuous-Flow Left Ventricular Assist Device Support. J Pharm Pract. 2016;29(1):58–66. doi: 10.1177/0897190015615894. [DOI] [PubMed] [Google Scholar]
- 17.Nenci GG, Gresele P, Agnelli G, Ballatori E. Effect of pentoxifylline on platelet aggregation. Pharmatherapeutica. 1981;2(8):532–8. [PubMed] [Google Scholar]
- 18.Bledzka K, Smyth SS, Plow EF. Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circ Res. 2013;112(8):1189–200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Quthami AH, Jumean M, Kociol R, Pham DT, Kiernan M, DeNofrio D, Kapur NK. Eptifibatide for the treatment of HeartMate II left ventricular assist device thrombosis. Circ Heart Fail. 2012;5(4):e68–70. doi: 10.1161/CIRCHEARTFAILURE.112.966804. [DOI] [PubMed] [Google Scholar]
- 20.Bellumkonda L, Subrahmanyan L, Jacoby D, Bonde P. Left ventricular assist device pump thrombosis: is there a role for glycoprotein IIb/IIIa inhibitors? ASAIO J. 2014;60(1):134–6. doi: 10.1097/MAT.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 21.Tellor BR, Smith JR, Prasad SM, Joseph SM, Silvestry SC. The use of eptifibatide for suspected pump thrombus or thrombosis in patients with left ventricular assist devices. J Heart Lung Transplant. 2014;33(1):94–101. doi: 10.1016/j.healun.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant. 2014;33(1):102–4. doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 24.Valerio L, Tran PL, Sheriff J, Brengle W, Ghosh R, Chiu WC, Redaelli A, Fiore GB, Pappalardo F, Bluestein D, Slepian MJ. Aspirin has limited ability to modulate shear-mediated platelet activation associated with elevated shear stress of ventricular assist devices. Thromb Res. 2016;140:110–7. doi: 10.1016/j.thromres.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheriff J, Girdhar G, Chiu WC, Jesty J, Slepian MJ, Bluestein D. Comparative efficacy of in vitro and in vivo metabolized aspirin in the DeBakey ventricular assist device. J Thromb Thrombolysis. 2014;37(4):499–506. doi: 10.1007/s11239-013-0997-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selgrade BP, Truskey GA. Computational fluid dynamics analysis to determine shear stresses and rates in a centrifugal left ventricular assist device. Artif Organs. 2012;36(4):E89–96. doi: 10.1111/j.1525-1594.2011.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girdhar G, Xenos M, Alemu Y, Chiu WC, Lynch BE, Jesty J, Einav S, Slepian MJ, Bluestein D. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7(3):e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng. 2012;134(8):081002. doi: 10.1115/1.4007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu WC, Girdhar G, Xenos M, Alemu Y, Soares JS, Einav S, Slepian M, Bluestein D. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation- optimized HeartAssist 5 VAD. J Biomech Eng. 2014;136(2):021014. doi: 10.1115/1.4026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers KC, Oliphant CS, Finks SW. Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs. 2015;75(4):377–95. doi: 10.1007/s40265-015-0364-3. [DOI] [PubMed] [Google Scholar]
- 31.Sheriff J, Bluestein D, Girdhar G, Jesty J. High-shear stress sensitizes platelets to subsequent lowshear conditions. Ann Biomed Eng. 2010;38(4):1442–50. doi: 10.1007/s10439-010-9936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz-Heik K, Ramachandran J, Bluestein D, Jesty J. The extent of platelet activation under shear depends on platelet count: differential expression of anionic phospholipid and factor Va. Pathophysiol Haemost Thromb. 2005;34(6):255–62. doi: 10.1159/000093104. [DOI] [PubMed] [Google Scholar]
- 33.Neuenschwander P, Jesty J. A comparison of phospholipid and platelets in the activation of human factor VIII by thrombin and factor Xa, and in the activation of factor X. Blood. 1988;72(5):1761–70. [PubMed] [Google Scholar]
- 34.Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. Asaio J. 2008;54(1):64–72. doi: 10.1097/MAT.0b013e31815d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xenos M, Girdhar G, Alemu Y, Jesty J, Slepian M, Einav S, Bluestein D. Device Thrombogenicity Emulator (DTE) - Design optimization methodology for cardiovascular devices: A study in two bileaflet MHV designs. J Biomech. 2010;43(12):2400–9. doi: 10.1016/j.jbiomech.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–41. [PubMed] [Google Scholar]
- 37.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem. 1999;272(1):64–70. doi: 10.1006/abio.1999.4148. [DOI] [PubMed] [Google Scholar]
- 38.Lowe GD. Virchow's triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33(5–6):455–7. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 39.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038–47. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 40.Meyer AL, Malehsa D, Bara C, Budde U, Slaughter MS, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3(6):675–81. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 41.Bartoli CR, Restle DJ, Zhang DM, Acker MA, Atluri P. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: mechanical demolition and enzymatic cleavage. J Thorac Cardiovasc Surg. 2015;149(1):281–9. doi: 10.1016/j.jtcvs.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 42.Nascimbene A, Neelamegham S, Frazier OH, Moake JL, Dong JF. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127(25):3133–41. doi: 10.1182/blood-2015-10-636480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blitz A. Pump thrombosis-A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014;3(5):450–71. doi: 10.3978/j.issn.2225-319X.2014.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami N, Suzuki Y, Yamamoto M, Kihira H, Imai E, Wada H, Kimura Y, Ikeda Y, Shiku H, Nishikawa M. Inhibition of shear stress-induced platelet aggregation by cilostazol, a specific inhibitor of cGMP-inhibited phosphodiesterase, in vitro and ex vivo. Life Sci. 1997;61(25):PL 383–9. doi: 10.1016/s0024-3205(97)00986-7. [DOI] [PubMed] [Google Scholar]
- 45.Yagi H, Yamaguchi N, Shida Y, Hayakawa M, Matsumoto M, Sugimoto M, Wada H, Tsubaki K, Fujimura Y. Cilostazol down-regulates the height of mural platelet thrombi formed under a high-shear rate flow in the absence of ADAMTS13 activity. Eur J Pharmacol. 2012;691(1–3):151–5. doi: 10.1016/j.ejphar.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Chi YW, Lavie CJ, Milani RV, White CJ. Safety and efficacy of cilostazol in the management of intermittent claudication. Vasc Health Risk Manag. 2008;4(6):1197–203. doi: 10.2147/vhrm.s3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA. [accessed 8/9/2017];Pletal: Full Prescribing Information. 2015 < https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020863s023lbl.pdf>.
- 48.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 49.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345(25):1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 50.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9(2):154–69. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 51.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr Biol (Camb) 2010;2(4):183–92. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straub A, Wendel HP, Dietz K, Schiebold D, Peter K, Schoenwaelder SM, Ziemer G. Selective inhibition of the platelet phosphoinositide 3-kinase p110beta as promising new strategy for platelet protection during extracorporeal circulation. Thromb Haemost. 2008;99(3):609–15. doi: 10.1160/TH07-07-0452. [DOI] [PubMed] [Google Scholar]
- 53.Leung SL, Lu Y, Bluestein D, Slepian MJ. Dielectrophoresis-Mediated Electrodeformation as a Means of Determining Individual Platelet Stiffness. Ann Biomed Eng. 2016;44(4):903–13. doi: 10.1007/s10439-015-1383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi J, Tran P, Sen N, DeCook T, Slepian M. Desensitization of DMSO-treated platelets to common agonists via membrane modulation (598.5) FASEB J. 2014;28(1 Supplement) [Google Scholar]
- 55.Leung SL, Dimasi A, Heiser S, Dunn A, Bluestein D, Slepian M. Modulation of platelet membrane function via exogenous lipid moiety exposure alters platelet responsiveness to shear. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:266–9. doi: 10.1109/EMBC.2015.7318351. [DOI] [PubMed] [Google Scholar]
- 56.Sheriff J, Tran PL, Hutchinson M, DeCook T, Slepian MJ, Bluestein D, Jesty J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artif Organs. 2016;40(6):586–95. doi: 10.1111/aor.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Consolo F, Sheriff J, Gorla S, Magri N, Bluestein D, Pappalardo F, Slepian MJ, Fiore GB, Redaelli A. High Frequency Components of Hemodynamic Shear Stress Profiles are a Major Determinant of Shear-Mediated Platelet Activation in Therapeutic Blood Recirculating Devices. Sci Rep. 2017;7(1):4994. doi: 10.1038/s41598-017-05130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad SS, London FS, Walsh PN. The assembly of the factor X-activating complex on activated human platelets. J Thromb Haemost. 2003;1(1):48–59. doi: 10.1046/j.1538-7836.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 59.Lechtenberg BC, Murray-Rust TA, Johnson DJ, Adams TE, Krishnaswamy S, Camire RM, Huntington JA. Crystal structure of the prothrombinase complex from the venom of Pseudonaja textilis. Blood. 2013;122(16):2777–83. doi: 10.1182/blood-2013-06-511733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheriff J, Soares JS, Xenos M, Jesty J, Slepian MJ, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41(6):1279–96. doi: 10.1007/s10439-013-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP. Amplification of human platelet activation by surface pannexin-1 channels. J Thromb Haemost. 2014;12(6):987–98. doi: 10.1111/jth.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10(1):11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–12. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran PL, Pietropaolo MG, Valerio L, Brengle W, Wong RK, Kazui T, Khalpey ZI, Redaelli A, Sheriff J, Bluestein D, Slepian MJ. Hemolysate-mediated platelet aggregation: an additional risk mechanism contributing to thrombosis of continuous flow ventricular assist devices. Perfusion. 2016;31(5):401–8. doi: 10.1177/0267659115615206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartoli CR, Kang J, Zhang D, Howard J, Acker M, Atluri P, Motomura T. Left Ventricular Assist Device Design Reduces von Willebrand Factor Degradation: A Comparative Study Between the HeartMate II and the EVAHEART Left Ventricular Assist System. Ann Thorac Surg. 2017;103(4):1239–1244. doi: 10.1016/j.athoracsur.2016.06.112. [DOI] [PubMed] [Google Scholar]
- 66.Kang J, Zhang DM, Restle DJ, Kallel F, Acker MA, Atluri P, Bartoli CR. Reduced continuous-flow left ventricular assist device speed does not decrease von Willebrand factor degradation. J Thorac Cardiovasc Surg. 2016;151(6):1747–1754. e1. doi: 10.1016/j.jtcvs.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 67.NIBIB. [accessed August 9th.2017];Bioengineers work to head-off dangerous blood clots in patients with ventricular assist devices. 2015 < https://www.nibib.nih.gov/news-events/newsroom/bioengineers-work-head-dangerous-blood-clots-patients-ventricular-assist>.
- 68.Du VX, Huskens D, Maas C, Al Dieri R, de Groot PG, de Laat B. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost. 2014;40(1):72–80. doi: 10.1055/s-0033-1363470. [DOI] [PubMed] [Google Scholar]
- 69.Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, Lee JS, Christ G, Gladwin MT, Hantgan RR, Kim-Shapiro DB. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11(12):2148–54. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Claiborne TE, Girdhar G, Gallocher-Lowe S, Sheriff J, Kato YP, Pinchuk L, Schoephoerster RT, Jesty J, Bluestein D. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna Aortic Bioprosthetic Valves. ASAIO J. 2011;57(1):26–31. doi: 10.1097/MAT.0b013e3181fcbd86. [DOI] [PubMed] [Google Scholar]
- 71.Jesty J, Yin W, Perrotta P, Bluestein D. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 2003;14(3):143–9. doi: 10.1080/0953710031000092839. [DOI] [PubMed] [Google Scholar]