Abstract
Background
Stroke may be the initial manifestation of atrial fibrillation (AF). Limited studies, however, have evaluated racial differences in stroke prior to the diagnosis of AF.
Objective
We assessed racial differences in strokes that occurred prior to and after AF diagnosis in the Penn Atrial Fibrillation Free (PAFF) Study.
Methods
PAFF consists of 56,835 patients from the University of Pennsylvania Health System that were free of AF at the index visit. We developed an inception cohort of 3,507 patients with incident AF and without any remote history of stroke.
Results
Among the AF inception cohort, there were 538 ischemic strokes and 54 hemorrhagic ones. Nearly half (n=254) of the ischemic strokes occurred within a 6-month period prior to the diagnosis of AF. Of these, the majority of strokes occurred either on the day of (n=158) or within a 7 day period prior to (n=30) the diagnosis of incident AF. The remaining 284 ischemic strokes occurred a median 3.6 [IQR 1.9, 5.4] years after AF diagnosis. Compared to whites, blacks had an independently higher risk of having an ischemic stroke either prior to [adjusted OR 1.37, (95% CI, 1.03–1.81)] or after AF diagnosis [adjusted HR 1.67, 95% CI (1.30 – 2.14)].
Conclusions
In an incident AF population, nearly one-half of the ischemic strokes occurred prior to the diagnosis of AF. Compared to whites, blacks had a higher incidence of developing an ischemic stroke that persisted whether the stroke occurred in the period either preceding or following AF diagnosis.
Keywords: atrial fibrillation, race, ethnicity, risk factor, stroke, population
Introduction
Blacks have a greater incidence of stroke and stroke-associated disability than Whites.1–3 Multiple studies have demonstrated similar racial differences in the incidence of stroke among elderly patients with AF even after adjusting for anticoagulation use.4–7 These findings suggest that the etiology and consequences of AF including electromechanical changes in the heart and potential complications such as stroke and systemic thromboembolism may differ in blacks compared with whites. As such, clinical guidelines for the management of atrial fibrillation should emphasize not only the importance of population-based interventions to prevent stroke and thromboembolism, but also their potential for reducing racial disparities in the elderly with AF.
Stroke may be the initial manifestation of AF. Recent American Heart Association and American Stroke Association guidelines recommend extended cardiac rhythm monitoring over at least a 6-month period for the evaluation of AF in patients who have experienced a stroke without an apparent cause.8 In two recent studies, the 30-Day Event Monitor Belt for Recording Atrial Fibrillation after a Cerebral Ischemic Event Study (EMBRACE) and Cryptogenic Stroke and Underlying Atrial Fibrillation (CRYSTAL AF), investigators reported a significant increase in AF detection with longer-term monitoring that extended up to 36 months after the stroke event.9, 10 AF detection in these populations resulted in subsequent prescription of anticoagulation therapies.9 However, these studies enrolled a limited minority population, and only 2–4% of the study population was black. A better understanding of the burden of AF after an ischemic stroke is important as recurrent events may be prevented by earlier AF detection and the subsequent initiation of anticoagulation therapies. We created an inception cohort of adults with AF and evaluated racial differences in the risk of ischemic or hemorrhagic stroke that either preceded or followed the diagnosis of AF.
Methods
We assessed racial differences in ischemic and hemorrhagic strokes in the Penn Atrial Fibrillation Free (PAFF) study.11 PAFF is a large, multi-hospital cohort of patients from the University of Pennsylvania Health System that were free of AF or atrial flutter at the index visit. The cohort was developed in collaboration with the Penn Data Analytics Center, which aims to integrate the massive stores of clinical data across the health system for the purposes of research and quality improvement initiatives. For this project, we first developed an inception cohort of patients with incident AF and without any remote history of stroke. As a result, we had the unique ability to evaluate strokes that occurred during the time period preceding the diagnosis of AF. We also evaluated ischemic and hemorrhagic stroke risk after the diagnosis of AF.
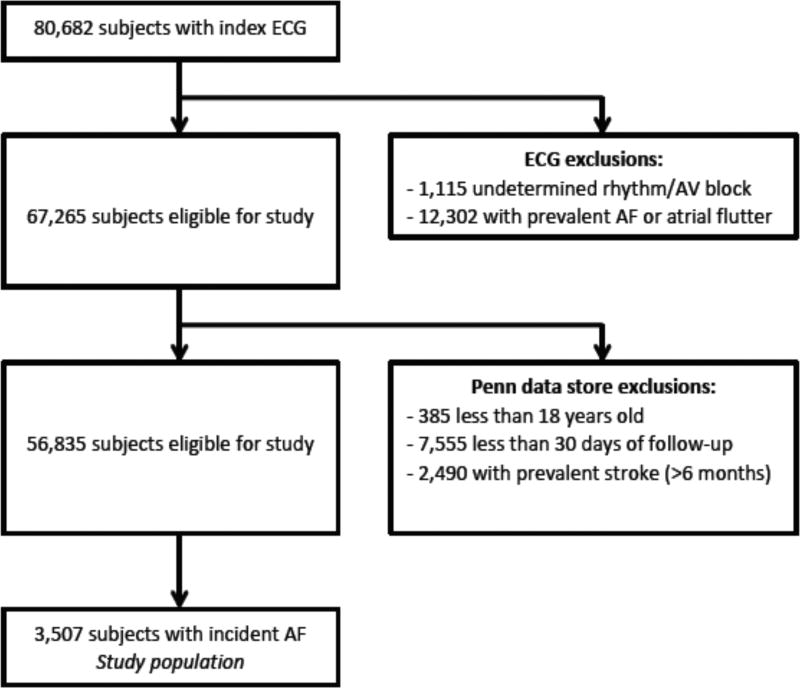
Figure 1 characterizes both the PAFF and AF inception cohorts. We included 80,682 patients who had an electrocardiogram (ECG) within the University of Pennsylvania Health System between June 1, 2004 and December 31, 2009.11 Individuals with an ECG demonstrating atrial fibrillation or atrial flutter (n=12,302), or an uninterpretable rhythm (n=1,115) were excluded, leaving 67,265 patients for additional analyses (Figure 1). We then linked all ECG records to administrative and electronic medical record data from the University of Pennsylvania Health System. In particular, emergency room, outpatient, and inpatient data including demographics, diagnoses, laboratory examinations, and medications were extracted from electronic medical records, which included all clinical encounters. Multiple sources including MUSE (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), Sunrise Clinical Manager (Eclipsys Corporation, Atlanta, GA), Epic (Verona, WI), Cerner (North Kansas City, MO), EmTrac (University of Pennsylvania, Philadelphia, PA), and Medview (University of Pennsylvania, Philadelphia, PA) were utilized. The MUSE database is an electronic depository of electrocardiographic information that spans the several hospitals within the health system. The Sunrise Clinical Manager was used to obtain inpatient data. Epic was queried for outpatient data; and Cerner was queried for both inpatient and outpatient laboratory data. EmTrac is an electronic medical record system exclusively for use by emergency room physicians. Medview is a database management system that coordinates transfer of data among the various other data repositories. Because the various clinical and laboratory settings within the University of Pennsylvania Health System had utilized their own medical recording system, the Penn Data Store (Penn Medicine’s Clinical Data Warehouse, Philadelphia, PA) created a comprehensive, combined database for the PAFF cohort.
Figure 1.
Design of the Penn Atrial Fibrillation Free Study
Patients with any clinical history of AF or atrial flutter, age less than 18 years or less than 30 days of clinical follow-up within the University of Pennsylvania Health System (n=7,940) were excluded from additional analyses. Finally, we excluded individuals with a prevalent diagnosis of stroke at the time of the initial ECG or more than 6 months prior to the diagnosis of AF (n=2,490). From this population of 56,835 patients without AF or a remote history of stroke, we identified 3,507 individuals with incident AF. This AF inception cohort comprised our primary study population. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Atrial Fibrillation and Covariates
We identified outpatients and inpatients with a new diagnosis of AF using either ECG coding from the MUSE database, data from electronic medical records, or ICD-9 codes (Supplemental Table 1). As part of a validation strategy, we selected the first 100 incident AF cases (i.e., oldest data available in the medical records) and confirmed arrhythmia manifestation in all these individuals using either ECG, telemetry recordings (inpatient or ambulatory), or cardiac implantable electronic device such as an implantable cardioverter defibrillator (ICD) or pacemaker.
Additional information related to demographics and comorbid conditions including age, sex, smoking status, hypertension, diabetes, obesity, heart failure, coronary heart disease, chronic kidney disease, and vascular disease, were obtained from either clinical encounters or ICD-9 codes (Supplemental Table 1). White and black races were recorded from the demographic self-identification form, which offered a choice of “White; Black or African American; Asian; Native American or Alaskan Native; Hawaiian Native or Pacific Islander; or Other.” Finally, our analysis evaluated medications including warfarin and antiplatelet agents such as aspirin, clopidogrel or dipyridamole. In particular, inpatient and outpatient records were queried for anticoagulants and anti-platelet drugs prescribed within 60 days of incident AF.
Stroke Ascertainment
Patients were followed over the course of clinical care from the date of index visit to December 31, 2013. Our primary outcomes were ischemic and hemorrhagic strokes that occurred within a 6-month period prior to or at any point after AF diagnosis. Strokes that occurred during the same hospitalization as incident AF were categorized as events that preceded the diagnosis of AF. All strokes included in these analyses were confirmed by both ICD-9 coding and a combination of either computed tomography (CT)/ magnetic resonance (MR) imaging and/or a neurologist’s evaluation. In particular, incident cases of ischemic or hemorrhagic stroke were first ascertained from ICD-9 codes obtained from all clinical encounters. Neuroradiology reports of computed tomography (CT) / magnetic resonance (MR) imaging and neurology evaluations were then reviewed through queries of the medical record to obtain confirmation of either ischemic or hemorrhagic strokes. Transient ischemic attacks were not included in these analyses.
Statistics
Baseline characteristics of participants were compared between blacks and whites using chi-squared or ANOVA tests. The association between race and either ischemic or hemorrhagic stroke was determined separately for strokes that preceded or occurred after the diagnosis of AF. We first used multivariable logistic regression to compare the odds of each stroke type in blacks and whites among the subgroup of patients that had a stroke within a 6-month period preceding the diagnosis of AF. In the subgroup where stroke occurred after the diagnosis of AF, we first calculated the unadjusted rates for incident stroke using Poisson regression and estimated risk differences comparing whites to blacks. Multivariable Cox proportional hazards regression models then compared the risk of either ischemic or hemorrhagic stroke in blacks to whites.
We then evaluated the risk of ischemic stroke across both races among those individuals that were either low risk for thromboembolic disease (CHA2DS2-VASc score = 0), intermediate risk (CHA2DS2-VASc score = 1), high risk (CHA2DS2-VASc score ≥2) and prescribed anticoagulant therapy and high risk and not prescribed anticoagulation. In all multivariable models, candidate variables for adjustment included those from the CHA2DS2-VASc score such as age, sex, hypertension, diabetes, heart failure, coronary heart disease, and other vascular disease. We also adjusted for smoking, obesity and CKD. In the analysis that evaluated racial differences in stroke risk after AF, we also adjusted for anticoagulation use. The proportional hazards assumption was not violated. Stata Statistical Software, release 11.0 (College Station, TX: StataCorp LP), and SPSS statistical software, release 19.0 (Armonk, NY:IBM Corp), were used for analyses. P<0.05 was considered statistically significant.
Results
Of the 56,835 patients eligible for inclusion into the PAFF study, 3,507 individuals developed AF and comprised our inception cohort. At the time of the first clinical encounter that documented AF, blacks were younger and more likely to be women than whites (Table 1). In addition, compared to whites, blacks were more likely to have a history of hypertension, diabetes, obesity, heart failure and chronic kidney disease. There were no racial differences in the prevalence of coronary heart disease, and blacks were less likely to have vascular disease. The CHA2DS2-VASc scores were higher for blacks compared with whites.
Table 1.
Baseline characteristics in White and Black Patients with Incident AF
|
|
|||
|---|---|---|---|
| White | Black | p-value | |
| Characteristics | (n=2,230) | (n=1277) | |
| Age (years)±SD | 64±13 | 63±16 | <0.001 |
| Men n(%) | 1462 (66) | 681 (53) | <0.001 |
| Current smoker n(%) | 1269 (57) | 696 (55) | 0.290 |
| Comorbidities | |||
| Hypertension n(%) | 1377 (62) | 1019 (80) | <0.001 |
| Diabetes n(%) | 305 (14) | 334 (26) | <0.001 |
| Obesity n(%) | 258 (12) | 305 (24) | <0.001 |
| Heart failure n(%) | 956 (43) | 705 (55) | <0.001 |
| Coronary heart disease n(%) | 1152 (52) | 696 (55) | 0.105 |
| Chronic kidney disease n(%) | 502 (23) | 504 (40) | <0.001 |
| Vascular disease n(%) | 661 (30) | 250 (20) | <0.001 |
| CHA2DS2-VASc ± SD | 2.1±1.5 | 2.5±1.5 | <0.001 |
| CHA2DS2-VASc | <0.001 | ||
| Low, score 0 n(%) | 289 (13) | 98 (8) | |
| Intermediate, score 1 n(%) | 549 (25) | 267 (21) | |
| High, score ≥2 n(%) | 1392 (62) | 912 (71) | |
| Medications | |||
| Warfarin n(%) | 870 (39) | 439 (34) | 0.006 |
| Aspirin n(%) | 1112 (50) | 661 (52) | 0.280 |
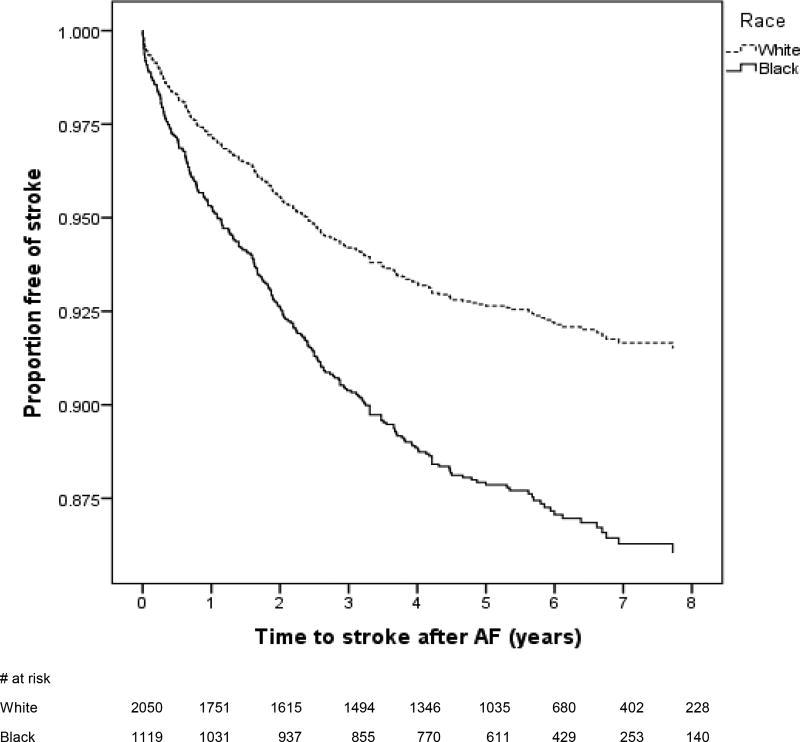
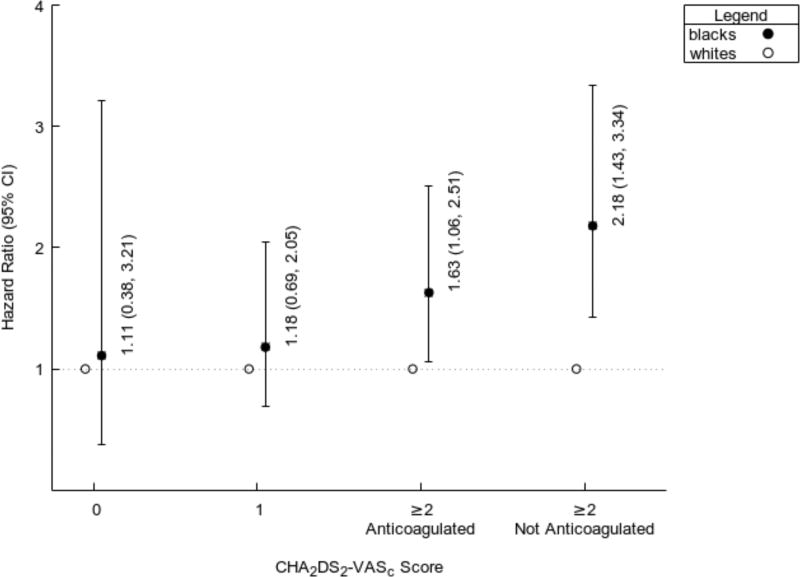
Among the 3,507 individuals with incident AF, there were 538 ischemic strokes and 54 hemorrhagic strokes. Nearly half (n=254) of the ischemic strokes occurred within a 6-month period prior to the diagnosis of AF. Of these, the majority of strokes occurred either on the day of (n=158) or within a 7 day period prior to (n=30) the diagnosis of incident AF. The remaining 284 ischemic strokes occurred a median 3.6 [IQR 1.9, 5.4] years after AF diagnosis. In strokes that occurred after an AF diagnosis, the annualized rate of ischemic stroke was 1.86%; and the rate of hemorrhagic stroke was 0.20% per year. Blacks with a new diagnosis of AF were 62% more likely to have had an ischemic stroke prior to AF diagnosis than whites who developed AF (Table 2). This higher risk persisted after multivariable adjustment for age, sex, smoking, hypertension, diabetes, obesity, heart failure, coronary heart disease, chronic kidney disease, and vascular disease. In strokes that occurred after the diagnosis of AF, blacks had 67% higher and independent risk of developing an ischemic stroke compared to whites (Figure 2 and Table 3). Finally, in the subgroup of patients who had a stroke after a diagnosis of AF, we did not observe any black-white differences in the risk of ischemic strokes among AF patients that were either low or intermediate risk for thromboembolism (Figure 3). The higher risk of ischemic stroke in blacks compared with whites was confined to those patients with a high risk of stroke defined as CHA2DS2-VASc ≥2. This elevated risk was observed in those with and without anticoagulation prescription.
Table 2.
Association of race and strokes that precede the diagnosis of AF
| Ischemic Strokes* | ||||
| Race | N | # ischemic stroke | Unadjusted OR (95% Cl) | Adjusted† OR (95% Cl) |
| White | 2050 | 137 (6.7%) | 1.00 (ref) | 1.00 (ref) |
| Black | 1119 | 117 (10.4%) | 1.62 (1.25, 2.10) | 1.37 (1.03, 1.81) |
| Hemorrhagic Strokes* | ||||
| Race | N | # hemorrhagic stroke | Unadjusted OR (95% CI) | Adjusted† OR (95% CI) |
| White | 1927 | 14 (0.73%) | 1.00 (ref) | 1.00 (ref) |
| Black | 1013 | 11 (1.1%) | 1.49 (0.68, 3.30) | 1.58 (0.68, 3.70) |
For these analyses, we used the total number of incident AF cases and excluded those who had a stroke after the diagnosis of AF. For the ischemic stroke analysis, we excluded those who had a hemorrhagic stroke. Similarly, for the hemorrhagic stroke analysis, we excluded those participants who had an ischemic stroke event.
Adjusted for age, sex, smoking, hypertension, diabetes, obesity, heart failure, coronary heart disease, chronic kidney disease, and vascular disease
Figure 2.
Multivariable adjusted incidence curves of ischemic stroke in blacks and whites. The Kaplan-Meier curves represent the incidence of ischemic stroke after adjustment for age, sex, smoking, hypertension, diabetes, obesity, heart failure, coronary heart disease, chronic kidney disease, vascular disease, aspirin and warfarin use.
Table 3.
Association of race and strokes that occur after the diagnosis of AF
| Ischemic Strokes* | ||||||
| Race | N | # ischemic stroke | Rate, %/yr (95% CI) | Rate difference (95% CI) | Unadjusted HR (95% Cl) | Adjusted† HR (95% Cl) |
| White | 2058 | 145 | 1.50 (1.29, 1.77) | 0.97 (0.49, 1.46); p<0.001 | 1.00 (ref) | 1.00 (ref) |
| Black | 1141 | 139 | 2.48 (2.10, 2.93) | 1.66 (1.31, 2.09) | 1.67 (1.30, 2.14) | |
| Hemorrhagic Strokes* | ||||||
| Race | N | # hemorrhagic stroke | Rate, %/yr (95% CI) | Rate difference (95% CI) | Unadjusted HR (95% CI) | Adjusted† HR (95% CI) |
| White | 1934 | 21 | 0.22 (0.15, 0.34) | −0.07 (−0.21, 0.07); p=0.13 | 1.00 (ref) | 1.00 (ref) |
| Black | 1010 | 8 | 0.15 (0.07, 0.30) | 0.69 (0.31, 1.55) | 0.72 (0.31, 1.70) | |
For these analyses, we used the total number of incident AF cases and excluded those who had a stroke event prior to the diagnosis of AF. For the ischemic stroke analysis, we further excluded those who had a hemorrhagic stroke. Similarly, for the hemorrhagic stroke analysis, we excluded those participants who had an ischemic stroke event.
Adjusted for age, sex, smoking, hypertension, diabetes, obesity, heart failure, coronary heart disease, chronic kidney disease, vascular disease, aspirin and warfarin use.
Figure 3.
Risk of Stroke by Subgroups of CHA2DS2-VASc and Anticoagulation Use
No interactions were detected between race and sex for the ischemic stroke subgroups that either preceded AF diagnosis (p-value for interaction = 0.87) or occurred after AF (p-value for interaction = 0.48). Finally, hemorrhagic strokes comprised nearly 10% of the stroke patients in our analysis, and we did not observe any significant racial differences (Tables 2 and 3).
Discussion
Nearly one-half of the ischemic strokes in patients with AF occurred in a 6-month window prior to the diagnosis of the arrhythmia. In particular, AF was diagnosed in the majority of these cases at the time of the stroke event, and our analysis is one of the first to demonstrate racial differences in ischemic stroke during this timeframe. The PAFF study design involved the creation of an inception cohort and provides an understanding of stroke risk within a several month period before and at any point after AF diagnosis. Our study also confirms recent reports of blacks with AF having a significantly higher risk of ischemic stroke compared with whites with AF.4–7 In our analysis, these racial differences were limited to individuals that had a CHA2DS2-VASc ≥2 regardless of anticoagulation use.
The PAFF study suggests a critical need for interventions that target the approximate 50% of ischemic strokes occurring prior to a diagnosis of atrial fibrillation. Effective strategies for reducing stroke risk will require a better understanding of the significance of subclinical AF and potentially novel cardiovascular risk markers such as left atrial structure and mechanics. Clinical studies should be designed to identify individuals in sinus rhythm that have subclinical AF and are at an increased risk for the development of AF and thromboembolic complications. Recent studies from large community-based cohorts have generated AF prediction algorithms that result in adequate discrimination in diverse populations of whites and blacks.12 Further, AF-SCREEN, an international collaboration of experts that are evaluating population-based screening strategies for AF, have recently published a White paper that acknowledges even brief periods of subclinical AF are a risk marker for adverse events.13 As such, they have advocated strongly for immediate, global efforts to pursue single-timepoint AF screening utilizing medical quality ECG tracings in populations at risk for AF and stroke. In addition, the 2016 European Society of Cardiology guidelines for AF management have recommended opportunistic screening in all patients contacting the health system ≥65 years of age.14 Ongoing clinical trials including the ARTESiA trial (Apixaban for the Reduction of ThromboEmbolism in Patients with Device-Detected Sub-Clinical Atrial Fibrillation, NCT01938248), the NOAH trial (Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High Rate Episodes, NCT02618577), and the Danish Loop study (Atrial Fibrillation Detected by Continuous ECG Monitoring, NCT02036450) are evaluating the benefit of anticoagulation to treat subclinical AF. Future research efforts will also need to clarify whether to incorporate race into a screening algorithm. Low-cost screening interventions in diverse populations have the potential to impact the nearly 50% burden of ischemic strokes that occur prior to the diagnosis of clinical AF.
Among the subgroup of individuals in whom the diagnosis of AF precedes stroke, our data highlight ongoing national trends in anticoagulation under-treatment.15, 16 In particular, despite a mean CHA2DS2-VASc score of 2.1 in whites and 2.5 in blacks, only 39% of whites and 34% of blacks were receiving anticoagulation. This burden of under-treatment is similar to the American College of Cardiology National Cardiovascular Data Registry of 429,417 outpatients with AF.16 Specifically, less than half of high-risk patients were receiving an oral anticoagulant prescription. Similarly, in a retrospective analysis from the Get With the Guidelines-Stroke program, of 94,474 patients with acute ischemic stroke and a history of AF, 84% were not receiving adequate anticoagulation at the time of the stroke.17 Our findings also extend prior observations of racial differences in post-AF ischemic stroke to include a younger population. Multiple studies utilizing Medicare registry data have demonstrated that blacks are at an increased stroke risk in the setting of AF even after adjusting for anticoagulation use.5, 18 Further, similar to the slightly higher prevalence of under-treatment seen in our population of blacks versus whites, prior studies have also reported racial differences in anticoagulation use.19, 20 Overall, our study highlights the importance of emphasizing oral anticoagulation in all AF populations, particularly blacks. Non-vitamin K antagonist oral anticoagulants offer a potential opportunity as they are easier to administer, more convenient and lack routine monitoring. However, Blacks comprised less than 2.0% of the more than 70,000 participants enrolled in the phase III clinical trials that resulted in the FDA approval of four oral anticoagulants for the prevention of thromboembolism.21–24 These novel therapies have the potential to reduce ischemic stroke burden and subsequent racial disparities.
Finally, our analysis provides unique insight into the risk of hemorrhagic stroke, which was confirmed by diagnostic codes, radiographic imaging, and a neurologist’s confirmation. The overall annualized rate of hemorrhagic stroke in our analysis was similar to that observed in prior AF populations taking anticoagulation.25 Specifically, investigators from Kaiser Permanente Southern California evaluated intracranial hemorrhage (ICH) rates in patients with AF. After assessing over 18,000 AF hospitalizations, they identified 173 ICH events that occurred at a rate of 0.27 ICH events per 100 person years.25 The hemorrhagic stroke events in our analysis resulted in limited statistical power to assess racial differences; however, our analysis is one of the first to demonstrate that hemorrhagic strokes comprise a significantly lower proportion of total strokes in AF patients compared with the general population.26–28 Hemorrhagic strokes comprised <10% of all strokes in the PAFF inception cohort. In population-based studies, hemorrhagic strokes comprised 20 to 30% of all strokes.26–28 The implications of these findings are important as patients with AF are often concerned about the risks of ICH and should recognize the disproportionately higher risk of ischemic versus hemorrhagic strokes in an AF population when considering long-term anticoagulation.
Several limitations of our study should be considered. The University of Pennsylvania Health System serves patients from a wide geographic distribution including Pennsylvania, New Jersey, Delaware, and Maryland; as a result, the study may not capture anticoagulant data prescribed outside of the health system. In addition, our data did not capture anticoagulation adherence as measured by the time in therapeutic range. Further, our study design utilized administrative data collection within a large healthcare network. As a result, we may not have collected information on all potential confounders including socioeconomic status measures such as income and education. Finally, our analysis excluded TIA because it is a subjective assessment of neurologic function. Its exclusion may decrease ascertainment of clinically significant neurologic disease in this population.
Conclusions
In an incident AF population, nearly one-half of the ischemic strokes occur prior to the diagnosis of AF. Compared to whites, blacks have a higher, independent risk of developing an ischemic stroke that either preceded or followed the diagnosis of AF. Future public health interventions should continue to highlight the importance of AF screening and anticoagulation therapies and evaluate whether such interventions can reduce the excess burden of ischemic strokes and subsequent racial differences.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health [K23DK089118; Dr. Deo]. This manuscript was also supported in part by the Pennsylvania Steel Company, Inc – EP Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
Disclosures: None
References
- 1.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–152. [PubMed] [Google Scholar]
- 2.Mathur R, Pollara E, Hull S, Schofield P, Ashworth M, Robson J. Ethnicity and stroke risk in patients with atrial fibrillation. Heart. 2013;99:1087–1092. doi: 10.1136/heartjnl-2013-303767. [DOI] [PubMed] [Google Scholar]
- 3.Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith-Hammond CA, Matchar DB, Oddone EZ. Racial variation in initial stroke severity. Stroke. 2000;31:563–567. doi: 10.1161/01.str.31.3.563. [DOI] [PubMed] [Google Scholar]
- 4.Kabra R, Girotra S, Vaughan Sarrazin M. Refining Stroke Prediction in Atrial Fibrillation Patients by Addition of African-American Ethnicity to CHA2DS2-VASc Score. J Am Coll Cardiol. 2016;68:461–470. doi: 10.1016/j.jacc.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shroff GR, Solid CA, Herzog CA. Atrial fibrillation, stroke, and anticoagulation in Medicare beneficiaries: trends by age, sex, and race, 1992–2010. J Am Heart Assoc. 2014;3:e000756. doi: 10.1161/JAHA.113.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37:1070–1074. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 7.Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/Ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008;39:2736–2743. doi: 10.1161/STROKEAHA.107.508580. [DOI] [PubMed] [Google Scholar]
- 8.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 9.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 10.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 11.Patel PJ, Borovskiy Y, Killian A, Verdino RJ, Epstein AE, Callans DJ, Marchlinski FE, Deo R. Optimal QT Correction Formula in Sinus Tachycardia for Identifying Cardiovascular and Mortality Risk: Findings from the Penn Atrial Fibrillation Free Study. Heart Rhythm. 2016;13:527–535. doi: 10.1016/j.hrthm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso A, Roetker NS, Soliman EZ, Chen LY, Greenland P, Heckbert SR. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman B, Camm J, Calkins H, et al. Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation. 2017;135:1851–1867. doi: 10.1161/CIRCULATIONAHA.116.026693. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu J, Maddox T, Kennedy K, Katz D, Marzec L, Lubitz S, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1:55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 17.Xian Y, O'Brien EC, Liang L, et al. Association of Preceding Antithrombotic Treatment With Acute Ischemic Stroke Severity and In-Hospital Outcomes Among Patients With Atrial Fibrillation. JAMA. 2017;317:1057–1067. doi: 10.1001/jama.2017.1371. [DOI] [PubMed] [Google Scholar]
- 18.Kabra R, Cram P, Girotra S, Vaughan Sarrazin M. Effect of race on outcomes (stroke and death) in patients >65 years with atrial fibrillation. Am J Cardiol. 2015;116:230–235. doi: 10.1016/j.amjcard.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas KL, Piccini JP, Liang L, Fonarow GC, Yancy CW, Peterson ED, Hernandez AF. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J Am Heart Assoc. 2013;2:e000200. doi: 10.1161/JAHA.113.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–587. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 23.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 25.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 26.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes: initial findings from the North East Melbourne stroke incidence study (NEMESIS) Stroke. 2001;32:1732–1738. doi: 10.1161/01.str.32.8.1732. [DOI] [PubMed] [Google Scholar]
- 27.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 28.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.