Abstract
Introduction
Despite high rates of venous thromboembolism (VTE) among patients with hematologic malignancies, few tools exist to assist providers in identifying those patients at highest risk for this potentially fatal complication. Laboratory biomarkers, such as d-dimer, have demonstrated utility in some clinical settings to distinguish patients at increased risk.
Materials and Methods
We performed a systematic review of the literature utilizing search terms including “biomarker”, “venous thromboembolism”, “hematologic malignancy”, “lymphoma”, “myeloma” and “leukemia” in the Medline database. A total of 25 studies investigating laboratory biomarkers of increased thrombotic risk in the setting of hematologic malignancy were identified and included in this review.
Results and Conclusions
The most studied biomarkers, d-dimer and fibrinogen, demonstrated some degree of efficacy in identifying high-risk patients at levels >4.0 mg/L or <1.0g/L respectively. Additional markers which demonstrated promise included thrombin generation, mean platelet volume, soluble VEGF, soluble P-selectin and extracellular vesicles. Other biomarkers reviewed, which did not consistently demonstrate significant associations with VTE included prothrombin fragments F1+2, factor VIII, protein C, protein S, von Willebrand antigen and activity, antithrombin, thrombin antithrombin complex, antiphospholopid antibody, plasminogen activator inhibitor, tissue factor pathway inhibitor and several variants associated with known hypercoagulable states (factor V Leiden, prothrombin gene variant, methylenetetrahydrofolate reductase variant). Data to support any of the biomarkers discussed here in routine clinical decision-making are currently lacking, but additional investigation in clinical studies, ideally in combination with clinical factors known to be associated with increased thrombotic risk, is warranted.
Keywords: Venous thromboembolism, Hematologic neoplasm, Hematologic malignancy, Biomarker, D-dimer, Fibrinogen
Introduction
Venous thromboembolism (VTE) is a common complication of the treatment of hematologic malignancy. Approximately 5% of adult patients with acute leukemia will experience VTE within 2 years of diagnosis.[1] A similar percentage of patients undergoing hematopoietic stem cell transplant (HSCT) will experience VTE within 180 days of transplant.[2] Despite these high rates, and the availability of potential interventions, such as low molecular weight heparin (LMWH) prophylaxis, we have few tools to predict which patients are at highest risk and might most benefit from such interventions. Furthermore, despite the associations between many therapies for hematologic malignancy (including asparaginase and immunomodulatory drugs (IMIDs) for myeloma) and VTE, tools to identify such risks in early stage trials are lacking. Clinical risk prediction models such as the Khorana score demonstrate utility in identifying patients in a general cancer population at elevated risk[3] but have not yet proven adequate to identify patients for whom there is a clear, beneficial intervention.[4, 5] Furthermore this score is highly dependent on factors, such as leukocyte, hematocrit and platelet counts, which are uniquely affected and variable in this population.
A 2013 review of the use of laboratory biomarkers to predict VTE among patients with malignancy concluded that d-dimer and soluble P-selectin both demonstrated association with risk of VTE among patients with various malignancies and therefore might be useful for risk prediction.[6] Additional biomarkers, such as prothrombin fragments F1+2, [7] thrombin generation[8] and tissue factor microparticles[9] have also shown promise but have significant limitations, including variable sensitivity and specificity depending on tumor subtype.[6] Relatively few patients suffering from hematologic rather than solid tumor malignancy were included in such studies however and treatments for and outcomes in these diseases remain fundamentally different than those of solid tumors.
Hematologic malignancy patients experience a number of unique risk factors for both VTE and hemorrhage, including prolonged periods of thrombocytopenia, extended use of central venous access devices and the use of previously mentioned, unique chemotherapeutic or targeted agents associated with high risk of VTE. Additionally, hematologic malignancies have higher rates of association with abnormal states of coagulation, such as disseminated intravascular coagulation (DIC), suggesting that biomarkers of coagulation may perform differently in these patients.[10] Here we report the results of a systematic review of the literature for studies evaluating the use of laboratory biomarkers for prediction of risk of VTE specifically in patients with hematologic malignancy.
Methods
We searched Medline (inception to September 2017) for relevant articles. See Supplemental Materials for the full search strategy which included “venous thromboembolism”, “biomarker”, “hematologic neoplasm”, “leukemia”, “lymphoma”, “multiple myeloma.” Inclusion criteria were (1) research studies including patients with hematologic malignancy, (2) inclusion of at least one laboratory-based biomarker used prospectively for prediction of VTE. Articles were excluded if outcomes specific to hematologic malignancy patients were not reported or otherwise distinguished from solid tumor or non-cancer patients. Additional exclusion criteria included (1) case reports or series including < 5 patients, (2) non-English language (3) not in humans (including in vitro or animal model studies).
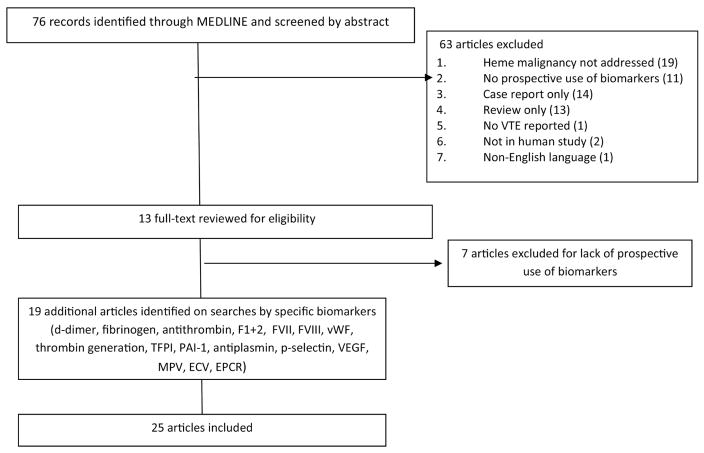
A total of 76 studies were identified with the initial search strategy. Sixty-three articles were excluded by abstract alone, 13 underwent full text review and six were included (Figure 1). Reasons for exclusion included: review/guidelines only (13), in vitro/animal or other not in human study (2), hematologic malignancy patients not included/addressed separately (19), no VTE reported (1), case report/series only (14), biomarker measurements not used prospectively (18), non-English language (1) study retraction (1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included and excluded studies
Following review of all included studies, separate searches of Medline were performed using the previous search criteria but replacing the term “biomarker” with specific terms for each biomarker identified on initial review. These terms included “d-dimer”, “fibrinogen”, “antithrombin”, “F1”, “Factor VII”, “Factor VIII”, “von Willebrand”, “thrombin generation”, “tissue factor plasminogen”, “plasminogen activator inhibitor”, “antiplasmin”, “p-selectin”, “VEGF”, “platelet volume” and “extracellular vesicles”. Nineteen additional studies which met criteria were identified by this method.
Results
We identified 18 potential groups of biomarkers addressed in 25 separate publications during literature review. The results of all 25 studies are included by study in Table 1. Here we discuss evidence by biomarker group.
Table 1.
Included Studies of Laboratory Biomarkers of Thrombosis for Use In Hematologic Malignancy
| Study | Subjects | VTE n (%) | Biomarker | Relationship to VTE vs no VTE |
|---|---|---|---|---|
| Aue 2011[20] | 32 adults with relapsed CLL | 5 (15%) | d-dimer | Median 0.5 ug/mL vs 0.42 ug/mL, p >0.05 |
| Protein S deficiency | 0/5 vs 5/27 | |||
| Low antithrombin | 0/5 vs 3/27 | |||
| FVL mutation | 0/5 vs 2/27 | |||
| Prothrombin gene mutation | 0/5 vs 2/23 | |||
| Auwerda 2007[22] | 135 adults with untreated myeloma | 14 (10%) | vWF (Ag, collagen-binding, Rco) | No relationship |
| FVIII | No relationship | |||
| Protein C activity | No relationship | |||
| Protein S activity | No relationship | |||
| Antiphospholipid antibody (LAC, ACA IgG & IgM) | No relationship | |||
| FV Leiden | No relationship | |||
| FII variant | No relationship | |||
| Ay 2008[26] | 91 adults with myeloma or lymphoma (596 solid tumor) | 4 (4.4%) | P-selectin | Median 45.9 [35.4–62.8] vs 42.1 [32.9–52.2] p = 0.025 HR for sP-selectin > 75th percentile: 2.3 (95% CI 1.2–4.5) |
| Ay 2009[12] | 94 adults with myeloma or lymphoma (727 solid tumor) | 7 (7.4%) | D-dimer | 0.96 (0.50–2.34) ug/mL vs 0.71 (0.34–1.33) ug/mL HR for 2-fold increase: 1.3 (95% CI 1.1–1.5) |
| F1+2 | 310 (213–416) pmol/L vs 249 (190–353) pmol/L p = 0.003 HR for 2-fold increase: 1.8 (95% CI: 1.3–2.6) |
|||
| Ay 2011[23] | 152 adults with myeloma or lymphoma (881 solid tumor) | 10 (6.6%) | Peak thrombin generation | 56 [431–677] nM vs 499 [360–603] p = 0.014 HR per 100nM increase: 1.15 (95% CI 1/02–1.30) |
| Azik 2013[32] | 92 adults 100 days post-HSCT | 5 (5.4%) | FV leiden | 0/5 vs 8/87 p >0.05 |
| MTHFR mutation | 0/5 vs 17/87 >0.05 | |||
| Prothrombin G20210A | 0/5 vs 2/87 >0.05 | |||
| Hyperhomocysteinemia | 1/5 vs 19/87 >0.05 | |||
| Elevated lipoprotein (a) | 1/5 vs 19/87 >0.05 | |||
| Protein C (mean) | 68±10% vs 76±21% >0.05 | |||
| Protein S (mean) | 76±12% vs 87±30% >0.05 | |||
| Antithrombin III (mean) | 102±11% vs 104±18% >0.05 | |||
| Beinart 2004[17] | 117 adults with ALL | 9 (7.7%) | Fibrinogen | < 50mg/dL: Specificity: 90%, LR: 6, RR: 10 (p = 0.0145) |
| Boersma 2016[21] | 168 adults with heme malignancy + CVC | 15 (8.9%) | FVIII (mean) | 203±62 % vs 166±59% p = 0.023 |
| PAI-1 (median) | 12.2 (3.5–22.7) vs 4.9(1.7–10.2) p = 0.062 | |||
| PAI-1 > 12.2 IU/mL | 8/15 vs 34/153 p = 0.008 | |||
| Protein S (mean) | 100±34 vs 93±29 p =0.417 | |||
| APC resistance (median) | 3.0 (2.63–3.79) vs 3.11 (1.76–4.80) p = 0.632 | |||
| Couturier 2015[18] | 708 adults with ALL/LL | 22 (3.1%)* | Fibrinogen (mean, at diagnosis) | 3.3 vs 4, p = 0.149 |
| Median AT nadir | 47.5 vs 51, p = 0.25 | |||
| Gheldof 2017[29] | 53 adults with acute leukemia | 4 (7.5%) | EVs (thrombin generation) | Elevated peak/lag ratio: 3/8 vs 0/47 |
| EVs (tissue factor activity) | >2pg/mL: 2/7 vs 1/48 | |||
| Kovacs 2014[16] | 153 adults post-auto HSCT for myeloma | 8 (5.2%) | d-dimer | 233.19 (47.00–715.00) ng/mL vs 200.11 (161.13–248.53) ng/mL |
| FVIII | 1.71 (1.13–2.91) IU vs 1.38 (1.27–1.49) IU | |||
| TAT | 3.43 (0.00–37.50) pg/mL vs 2.18 (1.65–2.89) ug/mL | |||
| Libourel 2016[11] | 404 adults with AML | 12 (4.7%) | d-dimer | d-dimer 0.5–4.0 mg/L: HR 2.79 (95% CI 0.84–9.28) d-dimer >4.0 mg/L: HR 12.03 (95% CI 3.39–42.64) |
| Alpha-2-antiplasmin | ≤ 0.8 IU/mL: HR 5.99 (95% CI 1.34–26.76) | |||
| Antithrombin | ≤ 0.8 IU/mL: HR 3.16 (95% CI 0.79–12.62) | |||
| Fibrinogen | <1: HR 12.38 (95% CI 1.54–99.18) | |||
| Mitrovic 2014[14] | 63 adults with APL | 9 (14.3%) | F II G20210A | OR 3.00 (95% CI 0.17–53.71) |
| FV Leiden | OR 3.00 (95% CI 0.17–53.71) | |||
| PAI-1 4G/5G | OR 0.10 (95% CI 0.01–0.91) | |||
| MTHFR C677T | OR 2.66 (95% CI 0.56–12.65) | |||
| Negaard 2008[13] | 93 adults with hematologic malignancy | 8 (8.6%) | Fibrinogen | No relationship |
| Antithrombin | No relationship | |||
| F1+2 | No relationship | |||
| D-dimer | No relationship | |||
| Tissue factor | No relationship | |||
| FVIIa-Antithrombin | No relationship | |||
| FVII | No relationship | |||
| FVIIa | No relationship | |||
| Free TFPI-1 | No relationship | |||
| Total TFPI-1 | No relationship | |||
| TFPI-1 activity | No relationship | |||
| Nowak-Gottl 2003[30] | 336 children with ALL | 30 (8.9%) | FV G1691A, FII G20210A, protein S, protein C, antithrombin deficiency, elevated Lp[a] | 46.5% of children with any one of these factors developed VTE |
| Nowak-Gottl 1999[31] | 289 children with ALL | 32 (11%) | MTHFR, P G20210A, FV Leiden, Lp(a), Protein C, Protein S, Antithrombin | VTE occurred in 27/58 subjects with a tested defect vs 5/231 patients without |
| Posch 2015[27] | 99 adults with lymphoma/myeloma | 5 (5%) | Soluble VEGF | HR (per 10pg/mL increase): 1/94 (95% CI 1.00–1.09) |
| Reidl 2013[28] | 273 adults with lymphoma/melanoma | 14 (5%) | Mean platelet volume | HR (per 1fL increase): 0.87 (95% CI 0.72–1.06) HR (MPV <75th percentile): 0.59 (95% CI 0.37–0.95) |
| Rozen 2016[24] | 56 children with ALL | 3 (5.4%) | Thrombin generation | Endogenous thrombin potential & peak values elevated in 2/3 with VTE |
| Rudd 2005[19] | 30 children with ALL | 4 (13%) | Antithrombin | Decreased level (compared to baseline) with all new thromboses (p = 0.012) |
| Protein C | No relationship | |||
| Protein S | No relationship | |||
| Streetly 2005[15] | 15 adults with myeloma | 3 (20%) | d-dimer | Elevated (>500 ug/L) in all cases |
| Undas 2015[25] | 48 adults with myeloma | 10 (21%) | Antiplasmin | 101.5 [86.3–111.8]% vs 104.1 [90.2–110.5]% p > 0.05 |
| Plasminogen | 97.5 [87–103.8] % vs 105.8 [97.4–113.1]% p = 0.03 | |||
| PAI-1 activity | 11 [9.9–12.8] vs 8.3 [6.4–10.5]% p = 0.004 | |||
| Factor VIII | 192.5 [183.5–266.5] % vs 188 [166.5–223]% p > 0.05 | |||
| Wermes 1999[33] | 73 children with ALL | 10 (27%) | FV Leiden, prothrombin mutation, MTHFR mutation, protein S, protein C, antithrombin deficiency | One of the above seen in 4/6 patients with VTE, 14/67 without |
CNS thrombosis only,
remaining 3 patients had DIC
vWF: von Willebrand Factor Ag: antigen, Rco: ristocetin cofactor, FVIII: factor VIII, LAC: lupus anticoagulant, ACA: anticardiolipin antibody, FV: factor V, FII: factor II, HR: hazard ratio, HSCT: hematopoietic stem cell transplant, LR: likelihood ratio, RR: risk ratio, CVC: central venous catheter, ALL: acute lymphocytic leukemia, LL: lymphoblastic lymphoma, AT: antithrombin, EV: extracellular vesicles, TFPI: tissue factor pathway inhibitor, VEGF: vascular endothelia growth factor, NHL: non-Hodgkin lymphoma, ()= range/95% CI, [] = IQR
D-dimer
Six identified studies reported VTE events in association with d-dimer levels. Four studies measured d-dimer at a baseline and evaluated for VTE at subsequent points during therapy. The largest study, performed by Libourel et al, included 404 patients with acute myeloid leukemia (AML). Hazard ratio (HR) for VTE among patients with d-dimer 0.5–4.0mg/L and >4.0mg/L vs ≤ 0.5mg/L were 5.58 (95% CI 0.62–49.97) and 32.05 (95% CI 3.58–286.83) (p = 0.002) respectively.[11] The second largest study utilized a subset of 111 patients from the Vienna Cancer and Thrombosis Study (CATS) with hematologic malignancies (lymphoma and multiple myeloma), including 8 patients who experienced VTE. Elevated d-dimer levels (>1.4 mg/L, the 75th percentile) were found to be positively associated with an increased risk of VTE (HR 1.8, IQR 1.0–3.2) among all patients enrolled in this study. While results were not reported separately for patients with hematologic vs other malignancies, the findings did not change on on multivariate analysis controlling for malignancy type (HR 1.8, IQR 1.0–3.2).[12]
A slightly smaller study, performed by Negaard et al, reported no association between d-dimer prior to treatment and VTE among a cohort of 93 patients with AML, chronic lymphocytic leukemia (CLL), multiple myeloma and non-Hodgkin lymphoma (NHL), including 8 patients with VTE (actual data not reported).[13] Similarly, Mitrovic et al examined d-dimer in 59 patients with acute promyelocytic leukemia (APL) and found no difference in mean levels between those who developed VTE (2.743 mg/L IQR 0.551–6.000) and those who did not (2.332 mg/L, IQR 0.024–11.340) (p = 0.879).[14]
Two studies evaluated change in d-dimer over the course of treatment. Streetly et al evaluated d-dimer levels in a cohort of 15 multiple myeloma patients undergoing treatment with a thalidomide analogue. While median d-dimer did not change over time as measured on day 0, day 21–28 and day 28 (282 mg/L, 98.4 mg/L and 220.9 mg/L, respectively), of four patients with baseline d-dimers >500 mg/mL three went on to develop VTE (d-dimers 505,000, 1506 and 795mg/L, normal range 4–52mg/L).[15] In a similar study Kovacs et al found a non-significant trend toward increasing d-dimer over time among 8 patients with multiple myeloma on thalidomide-prednisolone maintenance who developed VTE (233.19 mg/L, 95% CI 47.00–715.00 vs 403.92 mg/L, 95% CI 37.00 – 2900.00). This finding was not seen in the 72 patients who did not develop VTE (200.11 mg/L, 95% CI 161.13 – 248.53).[16]
Fibrinogen
Four studies examined the use of fibrinogen as a predictive biomarker for VTE. Two studies found a positive association, including Libourel et al, who found the HR for venous thrombosis among patients with fibrinogen < 1.0 g/L to be 12.38 (95% CI 1.54–99.18) in their cohort of AML patients.[11] A unique study by Beinart et al included 117 adult patients receiving L-aspariginase therapy for acute lymphocytic leukemia (ALL) reported a likelihood ratio (LR) of 6 and a relative risk (RR) of 10 (p = 0.0145) for VTE in patients with fibrinogen <50 mg/dL.[17]
The remaining two studies, by Negaard et al and Mitrovic et al, described above, found no significant association between fibrinogen and VTE.[13, 14] Mitrovic reported mean fibrinogen levels in those with VTE (3.9g/L, IQR 2.22–4.79) and those without (2.69 g/L, IQR 0.57–6.23)) (p = 0.105).[14] Negaard et al did not report levels separately.
Antithrombin
Five studies examined the use of antithrombin levels to predict risk of VTE, all but one of which found no association. Negative studies included Libourel et al, described previously, who reported a nonsignificant HR for VTE among patients with antithrombin ≤0.8 IU/mL at 3.16 (95% CI 0.79–12.62).[11] Negaard et al similarly found no difference in anthithrombin between patients with and without thrombosis.[13]
A unique study by Couturier et al evaluated antithrombin nadirs in patients with ALL or acute lymphoblastic lymphoma, receiving L-asparaginase therapy with antithrombin supplementation as necessary, looking specifically at central nervous system (CNS) thrombosis. No difference was noted between antithrombin nadir in those with (47.5%) vs without CNS-thrombosis (51%) (p = 0.25).[18]
The only clearly positive association was reported by Ruud et al who studied changes in antithrombin before and after treatment among 30 children undergoing ALL therapy, including one of two forms of asparaginase. Four episodes of acute thrombosis were identified and the authors correlated this with a decline in antithrombin (p = 0.012). However, mean antithrombin decreased in all patients during treatment (from 134% ± 18.8 to 75 ± 22.0 in one treatment arm, 125% ± 17.1 to 80 ± 18.0 in another).[19] Aue et al examined median levels of antithrombin in 32 adult patients receiving lenalidomide for chronic lymphocytic leukemia (CLL), including 5 who experienced DVT. Three of the 5 patients had low antithrombin levels (<57%)[20]
Thrombin Antithrombin
Only one study examined associations between thrombin antithrombin (TAT) changes over time and VTE. Kovacs et al found a trend toward decreasing (TAT) concentrations over time (at the beginning of therapy and after two months) among patients with multiple myeloma on thalidomide-prednisolone maintenance in patients who developed VTE (3.43 ug/mL, 95% CI 0.00–37.50 vs 2.90 ug/mL, 95% CI 1.00–7.50, p = 0.18). This difference was also seen in patients who did not develop VTE (2.18 ug/mL 95% CI 1.65–2.89 vs 2.04 ug/mL, 95% CI 1.0–2.59, p = 0.09).[16]
Prothrombin fragments F1+2
Two studies examined associations between prothrombin fragments F1+2 and VTE. Negaard et al, described previously, found no difference in F1+2 levels in hematologic malignancy patients with and without VTE (actual data not reported).[13]
Ay et al, however, reported F1+2 levels from patients enrolled in same Vienna CATS cohort of 111 patients with hematologic malignancies (lymphoma and multiple myeloma) found an association between elevated F1 + 2 levels at baseline and an increased risk of VTE (HR 2.0, 95% CI 1.2–3.6). Once again this was reported for all included patients (including those with solid malignancies) but the association persisted after multivariable analysis including tumor site and stage (HR 2.1, 95% CI 1.2–3.7).[12]
Factor VII
Only one study evaluated Factor VII (FVII) levels in hematologic malignancies. While median FVII was found to be decreased in patients with hematologic malignancies (89.1%, IQR 69.7–106.0) compared to healthy controls (97.2%, IQR 82.8–106.5) (p = 0.040), no such difference was found in factor VIIa (84mg/mL, IQR 48–114 vs 89 mg/mL, IQR 69–116, p = 0.173) and neither was associated with increased risk for VTE (actual data not reported).[13]
Factor VIII
Three studies included data on Factor VIII (FVIII) and VTE, only one of which identified a relationship. Boersma et al investigated 168 patients with hematologic malignancy and central venous catheters (CVC), including 15 patients with CVC-related thrombosis and 153 patients without CVC-related thrombosis. Patients who went on to develop thrombosis had higher factor VIII (FVIII) immediately following CVC placement (203 ± 62% vs 166 ± 59%, p = 0.023).[21]
Kovacs et al, described above, found a trend toward increasing FVIII over two months of thalidomide-prednisone maintenance, but identified no differences between those who developed VTE (1.71%, IQR 1.13–2.91 to 1.94%, IQR 1.33–3.06), and those who did not VTE (1.38%, 95% CI 1.27–1.49 vs 1.68%, 95% CI 1.52–1.85).[16]
Auerweda et al studied FVIII in 135 patients with untreated multiple myeloma and found no significant difference between the 14 patients with thrombosis and the 121 without (actual data not reported).[22]
Von Willebrand Antigen/Activity
Auwerda et al was also the only study to report data on von Willebrand factor (VWF) antigen (VWF Ag), and collagen-binding (VWF CB) and ristocetin-cofactor (VWF Rco) activities. Differences between the 14 patients experiencing VTE and the 121 not experiencing VTE did not reach statistical significance and were not reported formally.[22]
Thrombin Generation
A relationship between thrombin generation and VTE was reported in two separate studies. Ay et al once again utilized the Vienna CATS cohort, reporting data from 2008, at which time 152 patients with hematologic malignancy were enrolled. A total of 10 such patients with hematologic malignancyexperienced VTE. Median peak thrombin generation was found to be significantly higher among all patients with VTE (556 nM, IQR 432–677) vs those without (499 nM, IQR 360–603) (p = 0.014). Once again, while data were not reported separately for hematologic malignancy patients, multivariable analysis, including tumor site and stage, demonstrated consistent results, with peak thrombin time above the 75th percentile of the total study population (611 nM) associated with an increased risk of thrombosis (HR 1.9, 95% CI 1.2–3.0).[23]
Rozen et al also investigated the use of thrombin generation in 56 children with B lineage ALL. Three patients experienced thrombosis, two of whom consistently demonstrated endogenous thrombin potential (ETP) and peak values above the median (actual parameters not reported).[24]
Tissue Factor Pathway Inhibitor
Only one study by Negaard et al, previously described, addressed tissue factor pathway inhibitor (TFPI-I) and TFPI-I activity. Neither level was found to be associated with increased risk of thrombosis.[13]
Alpha-2-antiplasmin
Libourel et al, previously described, assessed alpha-2-antiplasmin, as a marker of DIC, in association with thrombosis. The HR for venous thrombosis among patients with alpha-2-antiplasmin ≤ 8IU/mL compared to >8IU/mL was 5.99 (95% CI 1.34–26.76).[11] Undas et al also examined antiplasmin in 10 myeloma patients with VTE and 38 patients without and found no association between VTE and median levels (101.5 (86.3–111.8)% vs 104.1 (90.2–110.5)%).[25]
P-selectin
Ay et al reported soluble (s) P-selectin levels from patients enrolled in the Vienna CATS study in 2008, at which time 91 patients with hematologic malignancies were enrolled, including 4 patients who experienced VTE. Median sP-selectin levels were found to be higher among patients with VTE (45.9 ng/mL IQR 35.4–62.8) compared to those without (42.1 ng/mL, IQR 32.9–52.2) (p = 0.025). A hazard ratio of 2.6 (95% CI 1.4–4.9) was demonstrated for risk of VTE among patients with sP-selectin ≥75 percentile vs not. While subgroup analyses by malignancy type (hematologic vs other) was not performed, median sP-selectin levels did not differ significantly between patients with lymphoma (40.6 ng/mL, IQR 31.2–53.9), melanoma (33.3 ng/mL, IQR 27.2–50.7) and those with solid tumors (range 40.9–45.2, p = 0.25).[26]
Soluble VEGF
Posch et al reported soluble vascular endothelial growth factor (sVEGF) levels in patients included the Vienna CATS study, at which time 99 patients with hematologic malignancies were enrolled including 5 patients who experienced VTE. Mean sVEGF levels were higher in patients who experienced VTE during follow-up (27.5 ± 74.5 pg/mL) vs those who did not (17.0 ± 34.6 pg/mL). Patients with sVEGF above the 75th percentile experienced increased rates of VTE (10.2%, 95% CI 6.4–14.9% vs 5.9%, 95% CI 4.2–7.9%, p = 0.03). Subgroup analysis was not performed, but cancer type (high vs very high risk tumor location), along with several other factors on multivariate analysis was found to explain only 3% of variation in sVEGF.[27]
Mean platelet volume
Riedl et al reported mean platelet volume (MPV) in patients enrolled in the Vienna CATS study, at which time 273 patients with hematologic malignancies were enrolled, including 14 patients who experienced VTE. An inverse association was noted between MPV and risk of thrombosis with a hazard ratio of 0.59 (95%CI 0.37–0.95) for development of VTE in patients with MPV above the 75th percentile. While subgroup analysis was not performed for patients with hematologic malignancies, the association between VTE and MPV persisted on multivariable competing risk analysis (HR 0.60, 95% CI 0.37–0.98) including tumor location.[28]
Extracellular vesicles
Gheldof et al studied 53 patients with acute leukemia, evaluating the procoagulant activity of extracellular vesicles isolated from the peripheral blood using an adapted thrombin generation assay. An elevated peak/lag ratio prior to treatment was found to be associated with thrombosis (3/8) and disseminated intravascular coagulation (DIC) (3/8). No thrombotic events occurred in 47 subjects without elevated peak/lag ratio (<1.7) on day 0.
The same study evaluated the tissue factor activity of extracellular vesicles using a commercially available kit (the Zymuphen MP-TF® kit). Elevated levels (>2pg/mL) were found to be associated with thrombosis (2/7) and significant hemorrhage (2/7). Two of the three without these complications experienced mild-moderate bleeding. Only one patient with a normal tissue factor activity developed DIC (on day 5 of treatment).[29]
Protein C and S
Three studies evaluated levels of protein S and/or protein C but only Boersma et al, who studied 168 adult patients with hematologic malignancy, reported numeric protein S levels in subjects with thrombosis. They reported mean protein S antigen levels of 100% ± 34 in patients with CVC-related thrombosis and 93% ± 29 (p = 0.417) in patients with vs without VTE.[21] Aue et al, previously described, examined median levels of protein S and found no overlap between 5 (of 32) patients with DVT and an additional 5 with low protein S (<55%) and no patients with low protein C (<59%).[20] Auwerda et al studied protein C and S levels in 135 adult patients with untreated multiple myeloma. No significant differences were found between the 14 patients who developed thrombosis and the 121 who did not.[22] Nowak-Gottl et al, [30, 31] Azik et al, [32] and Wermes et al, [33] described below, each evaluated for protein S and C deficiency as part of a larger panel of ‘hypercoagulable states’ but did not report specific associations of either with VTE.
PAI-1
Three studies evaluated for associations between plasminogen activator inhibitor (PAI-I) and VTE. Boersma et al, described above, did not find a significant difference in mean PAI-I levels between patients with CVC-associated thrombosis (12.2 IU/mL, IQR 3.5–22.7) and those without (4.9 IU/mL, 1.7–10.2) (p = 0.062) but did find a higher rate of PAI > 12.2 IU/mL among patients with thrombosis (8/15) vs without (34/153) (p = 0.08).[21] Mitrovic et al studied 59 patients with APL and found the PAI-1 4G/5G variant to be present in 11.1% of patients with VTE and 56% of patients without (p = 0.04), 55.6% and 20.0% of patients, respectively, were found to be homozygous for 4G/4G. PAI-1 levels were not reported. [14] Undas et al found higher PAI-1 activity among 10 patients with myeloma and VTE (median 11%, IQR 9.9–12.8) compared to 82 patients without (median 8.3%, IQR 6.4–10.5), (p = 0.004).[25]
Genetic Variants Associated with Hypercoagulability
Seven studies performed hypercoagulabilty evaluations including testing Factor V Leiden (or activated protein C resistance), the prothrombin gene G20210A variant, MTHFR polymorphisms, lipoprotein [a] and/or hyperhomocysteinemia.[14, 20, 22, 30–33] Four studies reported results in aggregate (association of any of the variants or other hypercoagulable states with VTE) rather than individual associations.
In two separate studies of children with ALL Nowak-Gottl reported an association between one of many hypercoagulable states and VTE. In one study of 289 patients 80% of patients with thrombosis had an inherited hypercoagulable state compared to 18.5% of all enrolled patients.[30] In another study of 336 patients a significant difference in thrombosis-free survival was reported between patients with vs without hypercoagulable states with thrombosis rates of 46.5% vs 2.2% (p < 0.001) respectively.[31] Azik et al screened 92 children for inherited hypercoagulable states and monitored them for 100 days post-hematopoietic stem cell transplant (HSCT). Three (60%) patients with VTE and 61 (70%) patients without VTE had one or more of these findings.[32] Wermes et al investigated inherited hypercoagulable states in 73 pediatric patients with ALL. Hypercoagulable states were found in 67% of patients with thrombosis but only 21% of patients without thrombosis.[33]
Auwerda et al, decribed previously, found no difference activated protein C ratio, anticardiolipin antibodies (IgG and IgM), FV Leiden and FII variants in 14 patients with thrombosis and 121 without. [22] Mitrovic et al, also described previously, upon comparing those with VTE to those without, did not find significant differences in rates of FII G20210A variant (11.1% vs 4.0%, p = 0.46), FV Leiden (11.1% vs 4.0% p = 0.46), MTHFR C677T (55.6% vs 32.0%, p = 0.22).[14] Aue et al, described above, observed no VTE in 3 patients with FV Leiden or prothrombin gene polymorphisms.[20] Boersma et al found similar mean APC resistance ratios in patients with vs without VTE of 3.0 (2.63–3.79) and 3.11 (1.76–4.80) (p = 0.632) respectively.[21]
Special subpopulations
Acute Promyelocytic Leukemia
Breccia et al studied 124 patients with acute promyelocytic leukemia (APL), including 11 patients with thrombosis and 113 without. Several biomarkers were compared between the group with thrombosis and the group without and significant differences were found in presenting white blood cell count (17×109/L vs 2.8×109/L, p = 0.002), bcr 3 PML/RARA transcript type vs bcr1-2 (9/11 vs 44/90, p = 0.01), FLT3-ITD mutation vs not (7/11 vs 26/77, p = 0.02), CD2+ expression (6/11 vs 17/87, p = 0.0001) and CD15+ expression (4/11 vs 8/95, p = 0.016).[34]
Mitrovic et al also studied associations between various markers and VTE in APL and found significant differences in bcr1-2/bcr3 (30%/70% in patients with VTE vs 61%/39% in patients without, p = 0.032) and FLT3-ITD expression (55.6% vs 22.2%, p = 0.028). CD2 and CD15 expression were also evaluated and were not found to be significantly different between subjects with VTE (55.6% and 11% respectively) and those without (21.4% and 34.9%) (p = 0.093 and 0.245.[14]
Thrombosis associated with Immunomodulatory Drugs in Myeloma
Aue et al demonstrated upregulation of tissue necrosis factor alpha, tissue factor and soluble vascular endothelial adhesion molecule during lenalidomide therapy in 32 patients with CLL. This upregulation was significantly higher in patients with lenalidomide-related DVTs vs no DVT or DVTs unrelated to lenalidomide (p = 0.003, 0.037 and 0.003), although actual differences were not reported. A similar trend was seen in upregulation of soluble thrombomodulin activity, but differences between patients with lenalidomide related DVT did not reach statistical significance (p = 0.082).[20]
Patients with monoclonal gammopathy
Spicka et al studied 49 patients with monoclonal gammopathies, evaluating for ‘defects in anticoagulation mechanisms’ including decreased antithrombin III (4%), protein C (2%) or S (6%), APC resistance (6%), increased PAI-1 (16%), increased t-PA (45%), decreased plasminogen (4%). Thrombotic events occurred in 46% of patients with such a defect and only 22% without. Subgroup analyses for individual defects were not performed.[35]
Conclusions
Here we have reviewed the results of 25 papers examining the use of various laboratory biomarkers to predict the risk of VTE among patients with hematologic malignancies. More than 18 potential biomarkers were examined, as well as several inherited hypercoagulable states/genetic variants. All studies were observational and the majority were retrospective and observed fewer than 20 incidents of thrombosis. Most studies focused on a single malignancy diagnosis (multiple myeloma, APL or ALL in children).
Among these studies, the d-dimer and fibrinogen stand out as the best studied, although findings were quite mixed. The largest studies reported associations between high levels of d-dimer (>4.0 mg/L or >1.4ug/mL) at baseline and VTE in patients with AML, myeloma and lymphoma, but elevation of d-dimer above the upper limit of normal was nearly universal with no consensus on a clear cut-off point at which it becomes predictive. Changes in d-dimer were less well studied, although an increase in d-dimer over the course of treatment may suggest increased risk of thrombosis in the post-HSCT population; the only study to address this was underpowered with only 8 events. Studies of fibrinogen suffered from similar limitations, with two studies identifying associations with VTE in patients with acute leukemia at different cut-offs (<1g/L and <0.5g/L). Interestingly the largest study, including 22 events in over 700 ALL patients, found no association. One potential difficulty in this and other studies of d-dimer and fibrinogen is the fact that abnormalities of coagulation resulting from DIC or therapies such as asparaginase likely play confounding roles. The use of these biomarkers for prediction of VTE in hematologic malignancy warrants further investigation in prospective trials, with special consideration for unique populations as mentioned above.
Evidence for association between antithrombin and VTE was quite weak, with the only positive associations between VTE and low levels identified in unique populations undergoing thrombogenic therapy (lenalidomide and asaparaginase based treatments). Results of studies on F1+2 were similarly mixed, although in this case the positive study was actually better powered, suggesting at least the potential for an association in patients with myeloma and lymphoma. Although further studies of F1+2 in different populations may be warranted, based on the current evidence neither of these factors should be used in clinical practice for the assessment of VTE risk.
Findings from the one positive study of FVIII were intriguing but limited only to patients with recent CVC placement for prediction of CVC-associated thrombosis and this marker warrants further study. At this time we find insufficient evidence to support use of FVIII, FVII, von Willebrand antigen/activity or TFPI657 for use in clinical decision making.
Several potential biomarkers, including thrombin generation, P-selectin, soluble VEGF and extracellular vesicles showed early promising initial results for use in this population. While these tests are not generally available and/or practical in clinical settings, consideration should be given to their use in research studies, first for validation and then potentially as a surrogate marker for thrombotic risk in clinical studies.
The MPV demonstrated similar promise, particularly intriguing in this population. We elected not to include standard hematologic parameters, such as leukocyte count, hemoglobin/hematocrit and platelet count in this review despite evidence for their utility in similar settings.[3] This was due to the fact that such parameters are linked closely to diagnosis, stage and type of treatment. MPV, which is easily available in any clinical setting with automated CBC technology, may serve as a useful surrogate for some of these parameters and warrants further investigation.
Results of testing for various hypercoagulable states, including low protein C and S levels, PAI-1 and various genetic variants were mixed but studies were generally of low quality and involved consideration of factors in aggregate. The significance of any one finding is not only difficult to interpret but has the potential to lead to harm if it leads the practitioner to introduce or continue anticoagulation in a setting in which it is not warranted. These assays are not appropriate for use in either the clinical or research setting.
In summary, the results of our review demonstrate promise for the potential use of biomarkers to increase precision in risk prediction for VTE in patients with hematologic malignancy, particularly d-dimer, fibrinogen and mean platelet volume. Additional tests, including thrombin generation, P-selectin, soluble VEGF and extracellular vesicles warrant further investigation for use in the research setting. At this time, however, no biomarker has demonstrated adequate sensitivity or specificity for use in clinical decision making or as an adequate surrogate marker in clinical studies and further studies are urgently needed. Until such studies are available risk assessments must continue to be based upon the individual patient’s history and clinical risk factors. In future studies careful consideration must be given to diagnosis and treatment, with the understanding that findings are likely to differ significantly between such populations.
Supplementary Material
Highlights.
Patients with hematologic malignancies are at high risk of thrombosis
Increased d-dimer is associated with an increased risk of VTE in patients with heme malignancy.
Mean platelet volume and thrombin generation may also be associated with risk of VTE.
Acknowledgments
The authors thank Douglas Shane for his invaluable assistance with the literature search.
This research was supported (in part) by the NHLBI under award number T32HL007093.
Abbreviations
- VTE
venous thromboembolism
- HSCT
hematopoietic stem cell transplant
- LMWH
low molecular weight heparin
- IMIDs
immunomodulatory drugs
- AML
acute myeloid leukemia
- IQR
interquartile range
- TAT
thrombin antithrombin
- FVII
factor VII
- FVIII
factor VIII
- CVC
central venous catheter
- TFPI
tissue factor pathway inhibitor
- VEGF
vascular endothelial growth factor
- MPV
mean platelet volume
- PAI
plasminogen activator inhibitor
- MTHFR
methyletetrahydrofolate reductase
- APL
acute promyelocytic leukemia
- ALL
acute lymphocytic leukemia
- CLL
chronic lymphocytic leukemia
- DVT
deep venous thrombosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ku GH, White RH, Chew HK, Harvey DJ, Zhou H, Wun T. Venous thromboembolism in patients with acute leukemia: incidence, risk factors, and effect on survival. Blood. 2009;113:3911–7. doi: 10.1182/blood-2008-08-175745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112:504–10. doi: 10.1182/blood-2007-10-117051. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Nisio M, Porreca E, Otten HM, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2014;8:CD008500. doi: 10.1002/14651858.CD008500.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. Journal of thrombosis and thrombolysis. 2016;41:81–91. doi: 10.1007/s11239-015-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–8. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 7.Rainer Ay CV, Dunkler Daniela, Simanek Ralph, Chiriac Alexandru-Laurentiu, Drach Johannes, Quehenberger Peter, Wagner Oswald, Zielinski Christoph, Pabinger Ingrid. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2009;27:4124-9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 8.Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. Journal Of Clinical Oncology: Official Journal Of The American Society Of Clinical Oncology. 2011;29:2099–103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 9.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, et al. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasugi K, Wada H, Hatada T, Okamoto K, Uchiyama T, Kushimoto S, et al. Prospective evaluation of hemostatic abnormalities in overt DIC due to various underlying diseases. Thromb Res. 2011;128:186–90. doi: 10.1016/j.thromres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Libourel EJ, Klerk CPW, van Norden Y, de Maat MPM, Kruip MJ, Sonneveld P, et al. Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood. 2016;128:1854–61. doi: 10.1182/blood-2016-02-701094. [DOI] [PubMed] [Google Scholar]
- 12.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:4124–9. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 13.Negaard HF, Iversen PO, Ostenstad B, Iversen N, Holme PA, Sandset PM. Hypercoagulability in patients with haematological neoplasia: no apparent initiation by tissue factor. Thromb Haemost. 2008;99:1040–8. doi: 10.1160/TH07-09-0541. [DOI] [PubMed] [Google Scholar]
- 14.Mitrovic M, Suvajdzic N, Elezovic I, Bogdanovic A, Djordjevic V, Miljic P, et al. Thrombotic events in acute promyelocytic leukemia. Thromb Res. 2015;135:588–93. doi: 10.1016/j.thromres.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Streetly M, Hunt BJ, Parmar K, Jones R, Zeldis J, Schey S. Markers of endothelial and haemostatic function in the treatment of relapsed myeloma with the immunomodulatory agent Actimid (CC-4047) and their relationship with venous thrombosis. Eur J Haematol. 2005;74:293–6. doi: 10.1111/j.1600-0609.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs MJ, Davies GA, Chapman JA, Bahlis N, Voralia M, Roy J, et al. Thalidomide-prednisone maintenance following autologous stem cell transplant for multiple myeloma: effect on thrombin generation and procoagulant markers in NCIC CTG MY.10. Br J Haematol. 2015;168:511–7. doi: 10.1111/bjh.13176. [DOI] [PubMed] [Google Scholar]
- 17.Beinart G, Damon L. Thrombosis associated with L-asparaginase therapy and low fibrinogen levels in adult acute lymphoblastic leukemia. Am J Hematol. 2004;77:331–5. doi: 10.1002/ajh.20230. [DOI] [PubMed] [Google Scholar]
- 18.Couturier MA, Huguet F, Chevallier P, Suarez F, Thomas X, Escoffre-Barbe M, et al. Cerebral venous thrombosis in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma during induction chemotherapy with l-asparaginase: The GRAALL experience. Am J Hematol. 2015;90:986–91. doi: 10.1002/ajh.24130. [DOI] [PubMed] [Google Scholar]
- 19.Ruud E, Holmstrom H, de Lange C, Natvig S, Albertsen BK, Wesenberg F. Thrombotic effects of asparaginase in two acute lymphoblastic leukemia protocols (NOPHO ALL-1992 versus NOPHO ALL-2000): a single-institution study. Pediatr Hematol Oncol. 2006;23:207–16. doi: 10.1080/08880010500506701. [DOI] [PubMed] [Google Scholar]
- 20.Aue G, Nelson Lozier J, Tian X, Cullinane AM, Soto S, Samsel L, et al. Inflammation, TNFalpha and endothelial dysfunction link lenalidomide to venous thrombosis in chronic lymphocytic leukemia. Am J Hematol. 2011;86:835–40. doi: 10.1002/ajh.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boersma RS, Hamulyak K, van Oerle R, Tuinenburg A, Ten Cate-Hoek AJ, Schouten HC. Biomarkers for Prediction of Central Venous Catheter Related-Thrombosis in Patients With Hematological Malignancies. Clin Appl Thromb Hemost. 2016;22:779–84. doi: 10.1177/1076029615579098. [DOI] [PubMed] [Google Scholar]
- 22.Auwerda JJ, Sonneveld P, de Maat MP, Leebeek FW. Prothrombotic coagulation abnormalities in patients with newly diagnosed multiple myeloma. Haematologica. 2007;92:279–80. doi: 10.3324/haematol.10454. [DOI] [PubMed] [Google Scholar]
- 23.Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:2099–103. doi: 10.1200/JCO.2010.32.8294. [DOI] [PubMed] [Google Scholar]
- 24.Rozen L, Noubouossie D, Dedeken L, Huybrechts S, Le PQ, Ferster A, et al. Different profile of thrombin generation in children with acute lymphoblastic leukaemia treated with native or pegylated asparaginase: A cohort study. Pediatric blood & cancer. 2017;64:294–301. doi: 10.1002/pbc.26228. [DOI] [PubMed] [Google Scholar]
- 25.Undas A, Zubkiewicz-Usnarska L, Helbig G, Woszczyk D, Kozinska J, Dmoszynska A, et al. Induction therapy alters plasma fibrin clot properties in multiple myeloma patients: association with thromboembolic complications. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2015;26:621–7. doi: 10.1097/MBC.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 26.Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008;112:2703–8. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 27.Posch F, Thaler J, Zlabinger GJ, Konigsbrugge O, Koder S, Zielinski C, et al. Soluble Vascular Endothelial Growth Factor (sVEGF) and the Risk of Venous Thromboembolism in Patients with Cancer: Results from the Vienna Cancer and Thrombosis Study (CATS) Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:200–6. doi: 10.1158/1078-0432.CCR-14-3358. [DOI] [PubMed] [Google Scholar]
- 28.Riedl J, Kaider A, Reitter EM, Marosi C, Jager U, Schwarzinger I, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS) Thromb Haemost. 2014;111:670–8. doi: 10.1160/TH13-07-0603. [DOI] [PubMed] [Google Scholar]
- 29.Gheldof D, Haguet H, Dogne JM, Bouvy C, Graux C, George F, et al. Procoagulant activity of extracellular vesicles as a potential biomarker for risk of thrombosis and DIC in patients with acute leukaemia. Journal of thrombosis and thrombolysis. 2017;43:224–32. doi: 10.1007/s11239-016-1471-z. [DOI] [PubMed] [Google Scholar]
- 30.Nowak-Gottl U, Ahlke E, Fleischhack G, Schwabe D, Schobess R, Schumann C, et al. Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101:2529–33. doi: 10.1182/blood-2002-06-1901. [DOI] [PubMed] [Google Scholar]
- 31.Nowak-Gottl U, Wermes C, Junker R, Koch HG, Schobess R, Fleischhack G, et al. Prospective evaluation of the thrombotic risk in children with acute lymphoblastic leukemia carrying the MTHFR TT 677 genotype, the prothrombin G20210A variant, and further prothrombotic risk factors. Blood. 1999;93:1595–9. [PubMed] [Google Scholar]
- 32.Azik F, Gokcebay DG, Tavil B, Isik P, Tunc B, Uckan D. Venous Thromboembolism after Allogeneic Pediatric Hematopoietic Stem Cell Transplantation: A Single-Center Study. Turkish journal of haematology: official journal of Turkish Society of Haematology. 2015;32:228–33. doi: 10.4274/tjh.2013.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wermes C, von Depka Prondzinski M, Lichtinghagen R, Barthels M, Welte K, Sykora KW. Clinical relevance of genetic risk factors for thrombosis in paediatric oncology patients with central venous catheters. European journal of pediatrics. 1999;158(Suppl 3):S143–6. doi: 10.1007/pl00014341. [DOI] [PubMed] [Google Scholar]
- 34.Breccia M, Avvisati G, Latagliata R, Carmosino I, Guarini A, De Propris MS, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21:79–83. doi: 10.1038/sj.leu.2404377. [DOI] [PubMed] [Google Scholar]
- 35.Spicka I, Rihova Z, Kvasnicka J, Cieslar P, Prochazka B, Klener P. Disturbances of anticoagulation and fibrinolytic systems in monoclonal gammopathies-another mechanism of M-protein interference with hemostasis. Thromb Res. 2003;112:297–300. doi: 10.1016/j.thromres.2003.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.