The 3rd International Lafora Workshop was held in Barcelona, Spain on September 1–3, 2017. The meeting was planned and hosted by Drs. Matthew Gentry, Director of the Lafora Epilepsy Cure Initiative and a Professor at the University of Kentucky; Joan Guinovart, Director of the Institute for Research in Biomedicine in Barcelona and Professor at the University of Barcelona; and Jose Serratosa, Chief of Neurology and Professor at the Autonoma University of Madrid. The workshop was sponsored by the National Institute of Neurological Disease and Stroke (NIH NINDS P01 NS097197), Chelsea’s Hope Lafora Children’s Research Fund, Valerion Therapeutics, and Ionis Pharmaceuticals. Approximately 50 students, postdocs, clinicians, academic and company scientists, and National Institutes of Health representation from the United States, Canada, and Spain as well as approximately 35 friends and family members of Lafora patients from the US, Spain, Italy, Poland, Bosnia and Herzegovina, Belgium, the Netherlands, Romania, and France.
The first Lafora workshop was held in June of 2014 and sponsored by Chelsea’s Hope, the North American Lafora advocacy group. Drs. Jim and Kim Rice, Chelsea’s Hope board members and whose oldest daughter Kristen died of Lafora disease (LD), initiated this event along with Dr. Jack Dixon, an award-winning biochemist from the University of California-San Diego whose lab worked on LD. In a curious turn of fate, the Rice and Dixon families became further connected when the Rice’s youngest daughter married Dixon’s son. With the help of Linda Gerber, another Lafora parent and the founder of Chelsea’s Hope, Dixon and the Rices organized the inaugural Lafora workshop, which brought together Lafora researchers and families from around the world for the first time.
This meeting allowed researchers to present and discuss their latest findings, agree upon a mechanism causing LD, and facilitated personal connections between scientists and families (Fig. 1). Additionally, it led ultimately to the successful funding of a P01 program project grant entitled “Lafora epilepsy: basic mechanisms to therapies.” This $9.1 million award established the Lafora Epilepsy Cure Initiative (LECI) (Fig. 2). At the second Lafora workshop, hosted again by Chelsea’s Hope and Dr. Dixon in San Diego in 2016, Lafora researchers reunited with families to announce their P01 funding success, present their newest discoveries, formulate a plan for identifying a cure, and offer hope to family members.
Figure 1.
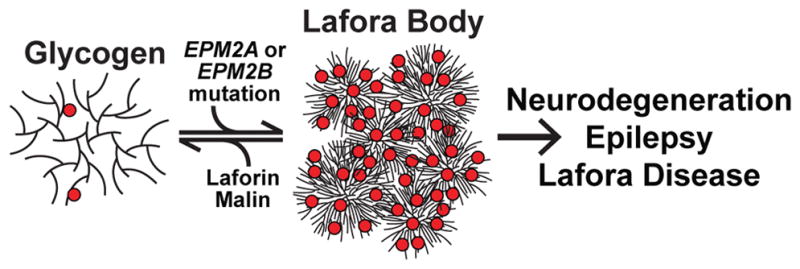
Glycogen is a soluble branched glucose polymer. The EPM2A gene encodes the glycogen phosphatase laforin and EPM2B encodes the E3 ubiquitin ligase malin. Mutations in EPM2A or EPM2B result in aberrant, hyperphosphorylated (red circles) glycogen inclusions. This abnormal, less soluble glycogen aggregates to form the Lafora bodies that cause neurodegeneration, epilepsy, and Lafora disease.
Figure 2.
The Lafora Epilepsy Cure Initiative (LECI) is funded by NIH grant NS097197. The primary investigator’s and projects for the grant are Drs. Gentry - Project #1: Personalized diagnosis - defining how glycogen metabolism and proteostasis impact LD; Minassian - Project #2: Genome editing, mRNA suppression and glycogen chain termination to inhibit glycogen storage as therapy for LD; Roach - Project #3: Suppressing glycogen storage with small molecule inhibitors as a therapeutic approach to LD; and Guinovart - Project #4: Defining the therapeutic window for the treatment of LD.
After Dr. Jose Serratosa’s proposal to the International League Against Epilepsy (ILAE) to host a session at the 32nd International Epilepsy Congress in Barcelona was accepted, it was decided that the third Lafora workshop would be held in conjunction with the International Congress. The Lafora workshop began on September 1 at the Palau de Congressos de Catalunya with a moving introduction from Dr. Frank Harris, a Lafora parent and now president of Chelsea’s Hope. Dr. Gentry followed with a history of LD research, an update on funding and the state of the LECI, and a discussion of future grant submissions as the basic science transitions to the translational and clinical phases.
Dr. Joan Guinovart gave the keynote address, describing his remarkable findings from a novel mouse model. Dr. Guinovart predicted that knocking out glycogenin, the enzyme that initiates glycogen synthesis, would have a similar effect as knocking out glycogen synthase, which his group had previously shown eliminates glycogen production and rescues LD. They were surprised to find that glycogenin knockout mice had increased glycogen accumulation in muscle and decreased exercise performance, discoveries that impact glycogen storage disorders and challenge our current understanding of glycogen metabolism.
The morning session, chaired by Drs. Serratosa and Gentry, continued with a stimulating talk from Dr. Felix Nitschke, a postdoctoral fellow in Dr. Berge Minassian’s lab at the University of Toronto. Dr. Nitschke’s elegant work described the transition of soluble glycogen to insoluble polyglucosan, the pathogenic cause of LD. Dr. Gentry then presented his lab’s work defining the effects of pathogenic mutations in laforin, the glycogen phosphatase that is mutated in over half of LD cases. Dr. Gentry’s group determined the crystal structure of this enzyme and their biochemical analyses establish personalized diagnoses for LD patients with laforin mutations. Next, Dr. Pascual Sanz from the Institute of Biomedicine of Valencia (Spanish Research Council, CSIC) presented fascinating data on metabolic dysfunction in cellular models of LD. Dr. Sanz discovered that LD fibroblasts display oxidative stress and defects in mitophagy, and he also discussed his progress on treating LD mice with 4-phenylbutyric acid and metformin, a drug that recently obtained orphan designation in Europe for the treatment of LD.
Drs. Gentzane Sánchez-Elexpuru and Marina Sánchez, from the lab of Dr. Serratosa, gave an excellent joint talk. Dr. Sánchez-Elexpuru, a Ph.D. student in the Serratosa lab, described her important findings regarding cardiac hypertrophy and systolic dysfunction in LD mice, suggesting LD has a metabolic cardiomyopathy component. Dr. Marina Sánchez presented intriguing in vivo data on LD mice using MRI and PET, concluding that cerebral glucose metabolism is altered in these mice. Dr. Peter Roach, Distinguished Professor of Biochemistry and Molecular Biology at Indiana University, concluded the morning session with a review of the current debate over the role of glycogen phosphorylation in the pathogenesis of the disease and an update on his progress identifying small molecule inhibitors of glycogen synthase, which would be used to therapeutically downregulate glycogen in LD.
After a break for lunch and lively discussions, Dr. Serratosa presented a clinical study on modifier effects in LD patients. Intriguingly, he found that although disease progression is often similar in siblings with LD, sometimes disease courses are very divergent, which could be attributed to genetic or epigenetic factors. Two joint presentations followed: Dr. Tracy McKnight, Director of Translational Research at Valerion Therapeutics and Katy Brewer, a Ph.D. student in the Gentry lab, described their work on a therapeutic enzyme that degrades Lafora bodies, the hallmark carbohydrate inclusions found in LD patients. Drs. Tamar Grossman, a Director of Antisense Drug Discovery at Ionis Pharmaceuticals, and Saija Ahonen, a postdoctoral fellow in the Minassian lab, presented on how antisense oligonucleotides (ASOs) can be used to downregulate glycogen metabolism to treat LD. Amazingly, they found that injection of ASOs targeting glycogen synthase vastly reduced Lafora body load in LD mice. Dr. Ahonen’s data were met with much excitement from the audience, as this is the first in vivo validation of a drug that could be used to treat LD. Further, investigators and audience members speculated that enzyme therapy and ASOs could be complementary: one would clear Lafora bodies, the other would prevent their formation.
Next, Dr. Anna DePaoli-Roach, Professor at Indiana University, presented her work to identify compounds inhibiting glycogen synthesis, specifically developing a cell-based assay for screening inhibitors of glycogen synthase. These are important studies that also test for toxicity and establish preliminary pharmacokinetics of lead compounds. Wrapping up the scientific portion of the afternoon, Dr. Jordi Duran from the Guinovart lab presented his compelling work on glycogen metabolism in the brain and the use of specific mouse models to understand cerebral Lafora body formation and metabolism. Dr. Antonio Delgado-Escueta, a leading Lafora neurologist and scientist at the University of California, Los Angeles, gave a final inspiring presentation entitled “How to Treat LD in 2017 and Beyond”.
Friday’s events concluded with a showing of a documentary about a Bosian family with Lafora disease. Snježana Gajic, a pediatrician living in Bosnia and Herzegovina, devoted her life to raising funds and spreading awareness of the disease when her two daughters Milana and Tatjana were diagnosed with Lafora. The film was produced and directed by Denis Bojic. After the film, scientists and families were invited to a traditional Catalan dinner at the historic Restaurant 7 Portes.
The second day of the workshop began with a P01 update and planning session at the Barcelona Institute of Science and Technology. LECI investigators met to discuss their progress toward each of the four central goals of the P01: defining patient-specific mechanisms and treatments targeting proteostasis; genetic approaches to inhibit glycogen storage; developing small molecule inhibitors; and defining the therapeutic window for LD treatment. After lunch, the investigators returned to the Congress Center to participate in the Lafora Symposium at the 2017 ILAE International Epilepsy Congress. Drs. Delgado-Escueta and Serratosa chaired the session where Drs. Gentry, Minassian, Roach, Guinovart, Delgado-Escueta, and Serratosa provided updates on their respective work. The session was very well attended and many world leaders and experts in epileptology expressed that they were impressed with the progress and quality of the session.
The last day of the workshop was an important day for the families of Lafora patients. Parents, siblings, cousins and friends came from around the world to meet with Lafora investigators, ask questions, and share stories about their affected family member or friend. Drs. Joan Tibau and Edmund Richer gave particularly touching accounts about their daughters living with LD. Philip Gattone, President and CEO of the North American Epilepsy Foundation, voiced his support to the Lafora cause. Drs. Deb Ramsdell, Hal Landy, and Dustin Armstrong, all of Valerion Therapeutics, and Drs. Tamar Grossman and Marc Gleichmann, both of Ionis Pharmaceuticals, affirmed their commitment to getting treatments to the clinic, announcing that the two companies were preparing to initiate a natural history study on a small cohort of patients.
Acknowledgments
Research reported in this publication is supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Numbers R01NS070899 and P01NS097197. We thank Cheylene Plummer and Mary Fern Waechter at the University of Kentucky College of Medicine for logistical support and planning the event, and we look forward to the 4th International Lafora Workshop, organized by Drs. Carolyn Worby and Kim Rice, which will be held in 2018 in San Diego.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose.