Abstract
The mechanisms of injured brain that establish poststroke seizures and epilepsy are not well understood, largely because animal modeling has had limited development. The main objective of this study was to determine whether an arterial occlusion model of cortical stroke in young adult and aged rats was capable of generating either focal or generalized epileptic seizures within 2 months of lesioning. Four- and 20-month-old male Fischer 344 (F344) sham-operated controls and those lesioned by transient (3 h) unilateral middle cerebral artery (MCA) and common carotid artery (CCA) occlusion (MCA/CCAo) were studied by video-EEG recordings up to 2 months post-procedure. The main findings were: 1) seizures (grade 3 and above) were recorded within 2 months in both young (4-month; 0.23/h) and aged (20-month; 1.93/h) MCA/CCAo rat groups; both MCA/CCAo rat groups had more seizures recorded than the respective control groups, i.e., no seizures in young controls and 0.52/h in old controls; 2) both age and infarction independently had effects on seizure frequency; however, there was no demonstrated interaction between the two factors; and 3) there was no difference in infarct volumes comparing 4- to 20-month-old MCA/CCAo animals. In addition, all lesioned and sham-operated animals demonstrated intermittent solitary myoclonic convulsions arising out of sleep. Morbidity and mortality of animals limited the extent to which the animals could be evaluated, especially 20-month-old animals. These results suggest that transient unilateral MCA/CCAo can result in poststroke epileptic seizures in both young adult and aged F344 rats within a relatively brief period of time following lesioning.
Keywords: Middle cerebral artery occlusion, Common carotid artery occlusion, Cortical infarction, Poststroke seizures, Poststroke epilepsy, Video-EEG
1. Introduction
The incidence and prevalence of unprovoked seizures and epilepsy increase in the elderly (Hauser et al., 1993; Scheuer & Cohen, 1993) and first occurrence rates for epilepsy are higher in the elderly than any other age group (Cockerell et al., 1995; Brodie & Kwan, 2005). Etiologies associated with this increase include cerebrovascular disease, brain tumors, toxic or metabolic disturbances, dementia, and trauma (Luhdorf et al., 1986; Loiseau et al., 1990; Ettinger & Shinnar, 1993; Thomas, 1997; Velez & Selwa, 2003); of these etiologies, ischemic cerebrovascular disease is the dominant cause of epilepsy in the elderly accounting for 25–50% of all cases. The reported long-term cumulative risk of PSE after a cerebrovascular event varies between 2%–15% (review, Zelano, 2016). The most important risk factor for developing epilepsy after an ischemic stroke is the involvement of the cerebral cortex (Heuts-van Raak et al., 1993). Lacunar, subcortical, and posterior circulation infarcts are infrequently associated with the development of epilepsy (Thomas, 1997; Bladin et al., 2000). In general, poststroke epileptic seizures are considered focal in onset, arising from areas in close proximity to the area of infarcted tissue (Loiseau, 1997). Given the importance of stroke as the primary cause of new-onset epilepsy in the elderly, in contrast, there is little understanding of the pathophysiological mechanisms of aged brain following stroke that cause or predispose to the development of epileptic seizures. The reason for this is clear - relatively few studies have focused on modeling poststroke epileptogenesis and PSE (reviews, Kelly, 2017; Reddy et al., 2017).
There are a limited number of studies that have examined arterial occlusion models of stroke and the subsequent development of acute seizures and/or epilepsy. Using young Sprague-Dawley rats and video-electroencephalogram (EEG) recordings, Hartings et al. (2003) showed that multiple ipsilateral or generalized electrographic seizures could be recorded within the first 2 h following transient intraluminal filament-induced middle cerebral artery occlusion (MCAo) without change in the animal’s behavior. Using young Sprague-Dawley rats and similar techniques, Karhunen et al. (2003) monitored animals at 3, 7, and 12 months post-lesioning but found no evidence of EEG or behavioral seizures. A subsequent study by Karhunen et al. (2006) monitored animals acutely and up to 12 months following endothelin-1-induced MCAo and found that early seizures did not predict the occurrence of epilepsy, the latter demonstrated in only one animal. Because MCAo in rats frequently involves the hippocampus, unlike MCAo in humans (Del Zoppo, 1998; Kelly 2002), our laboratory has used the technique of transient unilateral MCA and common carotid artery (CCA) occlusion (MCA/CCAo), which results in moderately large cortical infarcts of reproducible size and location, while sparing direct injury to the hippocampus (Aronowski et al., 1994, 1997). In a previous study conducted in our laboratory, MCA/CCAo was used to lesion 2.5-month-old Long-Evans rats, produced consistently-sized infarcts of the cerebral cortex, but failed to demonstrate the development of poststroke epilepsy in any animal as assessed by intermittent video-EEG recordings during the 6 months following lesioning (Kelly et al., 2006).
Because animal age and infarct size might be critical variables in the development of poststroke epileptogenesis, we performed transient unilateral MCA/CCAo in 4-month-old (young adult) and 20-month-old (aged) Fischer 344 (F344) rats followed by intermittent video-EEG recordings up to 2 months post-lesioning to determine whether epileptic seizures could occur within a relatively brief period of time following infarction while secondarily limiting the progression of aging-related morbidities in the aged cohorts. We hypothesized that MCA/CCAo lesioning would result in: 1) epileptic seizures in aged animals and to a less extent in young adult animals; and 2) larger infarcts in aged animals. Our objectives were to determine: 1) whether either the age or the infarction factor, or their potential interaction, affected the development of electrobehavioral evidence of poststroke epilepsy; and 2) the size and distribution of ischemic infarcts in the two age groups.
2. Material and Methods
2.1. Animal groups
The Institutional Animal Care and Use Committees (IACUCs) of the Allegheny-Singer Research Institute and the University of Texas Health Science Center at Houston approved all experimental procedures in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Twenty-four male F344 rats (4-months-of- age, n=12; 20-months-of-age, n=12) were randomly divided within each age group for sham-operation (n=6) or lesioning by transient (3 h) unilateral MCA/CCAo (n=6).
2.2. Production of ischemia
Animals were lesioned in the Department of Neurology, University of Texas Houston, using techniques identical to those used in our previous study (Kelly et al., 2006). Transient focal ischemia was induced by tandem occlusion of the left MCA and left CCA as described by Aronowski et al. (1997), with minor modifications. Animals were fasted overnight with free access to water and anesthetized with chloral hydrate (0.35–0.45 g/kg i.p.). The femoral artery was cannulated for measurement of blood pressure. Temperature was monitored from the right temporalis muscle and maintained at 37.0 ± 0.4°C during ischemia and the first hour of reperfusion by a feed-forward temperature controller (YSI Model 72, Yellow Springs, OH), heating lamp, and warming blanket. The CCA was isolated through a midline incision and tagged with a suture. An incision was made through the left temporalis muscle perpendicular to a line drawn between the external auditory canal and the lateral canthus of the left eye. Under direct visualization with the surgical microscope, a 1×3-mm rectangular burr hole was drilled 2–3 mm rostral to the fusion of the zygomatic arch and the squamosal bone to expose the MCA rostral to the rhinal fissure, and a 1-mm round burr hole was made 4 mm dorsal to the MCA exposure to allow measurement of cerebral perfusion (CP) using a laser Doppler flowmeter (LDF; model BPM2, Vasamedics Inc., St. Paul, MN). The beveled edge of a 23-gauge hypodermic needle was used to pierce and open the dura along the entire length of the rectangular burr hole. A 0.005-inch diameter stainless steel wire (Small Parts Inc., Miami, FL) was placed underneath the left MCA rostral to the rhinal fissure, proximal to the major bifurcation of the MCA, and distal to the lenticulostriate arteries. The artery was then lifted, and the wire rotated clockwise. The CCA was then occluded using atraumatic Heifetz aneurysm clips. After 3 h, reperfusion was established by removing the aneurysm clips from the CCA followed by rotating the wire counterclockwise and removing it from beneath the MCA. Interruption of flow through the MCA was inspected under the microscope and verified by CP measurements; only those animals that displayed reduction of CP following ischemia to less than 15% of the pre-ischemic value were included in the study. The same surgical procedure was performed on sham-operated animals except that the MCA and CCA were not occluded. Animals were returned to the vivarium for postoperative recovery and stabilization.
2.3. Behavioral testing
All lesioned animals underwent sensorimotor behavioral testing 24 h after surgery to investigate the correlation between behavioral score and infarct volume as described previously (Aronowski et al., 1996; Bland et al., 2000, 2001); behavioral testing was not performed in sham-operated animals because it was not relevant to the behavior/infarct volume correlation. The procedures are summarized below.
2.3.1. Measurement of forelimb placing
Animals were held by their torsos allowing their forelimbs to hang freely. Contralateral and ipsilateral forelimb placing was induced by gently brushing the respective vibrissae on the edge of a tabletop once per trial for 10 trials. A score of 1 was given each time the rat placed its forelimb on the edge of the tabletop in response to the vibrissal stimulation. The number of unsuccessful (missed) placements was counted. The difference between the number of unsuccessful placements with the affected and unaffected forelimb was used to estimate the degree of dysfunction.
2.3.2. Measurement of footfault asymmetry
Animals were placed on an elevated grid with square openings of 2.3 mm2 for 5 min. As the animals traversed the grid, a footfault was counted each time a paw slipped through an opening in the grid. The total number of footfaults with ipsilateral and contralateral forelimbs per a total of 50 steps was counted. The impairment level was defined as the difference between the number of contralateral and ipsilateral faults.
2.3.3. Measurement of asymmetry in the use of forelimbs for postural support
Animals were placed for 5 min into a Plexiglas cylinder (8” diameter and 12” high) placed in their home cage. Their behavior was observed for asymmetry in forelimb use during rearing, wall landing, and vertical movements along the wall of the Plexiglas cylinder. Each behavior was expressed as percent use of the contralateral (impaired) forelimb relative to the total number of ipsilateral plus contralateral limb-use observations.
2.3.4. Behavioral score summation
Scores from the individual behavioral tests were summated for each animal and the total score was used as an indicator of sensorimotor impairment with high scores indicating greater impairment than that associated with lower scores. Group mean scores for 4- and 20-month-old lesioned animals were calculated with standard error of the mean (SEM) and compared by Student’s unpaired t-test (p<0.05) using GraphPad statistical software.
2.4. Animal transport
In order to ensure adequate recovery from the surgical procedures, lesioned and sham-operated animals were supported and observed for approximately one week before being flown from Houston to Pittsburgh in a temperature-controlled cabin for subsequent video-EEG recordings.
2.5. Epidural skull screw electrodes
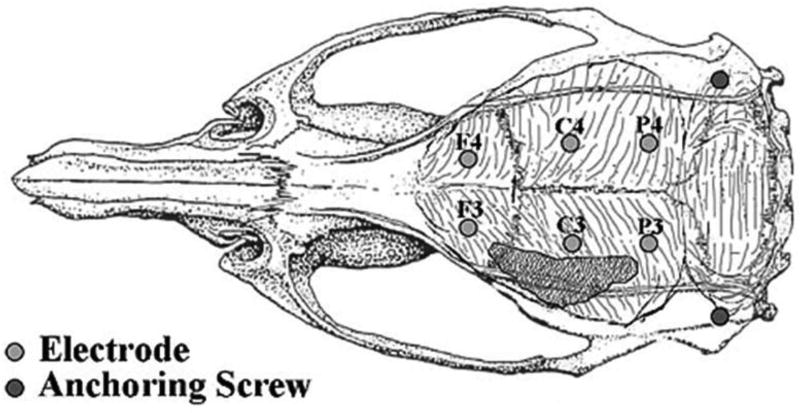
Screws were placed in the skulls of animals for EEG recordings 20.2 ± 1.2 days (mean ± SEM; range 17–26) after sham operation, and 27.8 ± 0.9 days (range 25–32) after MCA/CCAo. Animals were anesthetized with injections of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). After the loss of the tail pinch reflex, animals were positioned in a stereotaxic frame, petroleum jelly was applied over the eyes, and a midline incision was made along the scalp, which was reflected bilaterally. Burr holes were made in the skull for 6 recording and 2 anchoring stainless steel screws (MX-080-2; Small Parts Inc., Miami Lakes, FL). Three recording screws were placed parasagittally on each side of the skull to sample neocortical activities symmetrically. Odd numbered electrodes represented placements over the left (lesioned) hemisphere and even numbered electrodes represented placements over the right hemisphere; the rostrocaudal placement was designated as F=frontal, C=central, and P=parietal. Screw placements were: F3, F4 (+2.0 mm from bregma, 2.25 mm lateral from midline); C3, C4 (−3.0 mm from bregma, 2.75 mm lateral from midline); and P3, P4 (−7.0 mm from bregma, 2.75 mm lateral from midline). Two screws were placed laterally for anchoring the headset. Fig. 1 illustrates the position of the recording screws relative to the underlying brain and the superior aspect of the cortical infarction (see Results). An exposed end of an insulated copper wire was wrapped tightly around each recording screw while the other end of the wire was crimped into a six-conductor (RJ12) modular plug and secured to the skull surface with dental acrylic (Lang Dental Mfg., Wheeling, IL). The skin around the headset was sutured and the animals were allowed to recover from anesthesia. Bipolar EEG recordings were initiated approximately 7 days after placement of electrodes.
Figure 1.
Positions of screws in the rat skull used as electrodes or for anchoring the headset. Electrodes are designated to indicate relative positions over the cerebral cortex. F=frontal; C=frontoparietal; P=parietal; 3=left; 4=right. The shaded area represents the superior limit of the infarct, which occurs over the lateral convexity of the brain.
2.6. Digital video-EEG recordings
The digital video-EEG recording techniques used in this study were expanded from methods published previously (Kharlamov et al., 2003; Kelly et al., 2006). Four animal recording chambers, each equipped with a custom-designed swivel commutator (Dragonfly Inc., Ridgeley, WV), were used to monitor the animals and provided a low noise recording environment, full freedom of animal movement, and excellent visualization of animal behavior. Headset electrodes were connected to a 32-channel Stellate Systems video-EEG monitoring system that included a hardwired common reference arbitrarily linked to each animal’s F4 electrode. Individual animals were recorded with a standard montage of 8 EEG channels that sampled brain activity from both hemispheres using different bipolar electrode derivations. EEG signals were filtered at 1 and 70 Hz with a 60 Hz notch filter (Harmonie, Stellate Systems, Montreal). Closed-circuit television cameras connected to a video splitter unit (Advanced Technology Video, Inc., Redmond, WA) were used to monitor animal behavior. Digital video files were obtained for all animals (Diva, Stellate Systems) and recorded directly to high capacity (300 GB) hard disk drives using a removable hard drive bay. Animals were recorded during active wakefulness, passive wakefulness, and sleep during both daytime and nighttime hours. Based on our laboratory’s experience with aged F344 rats (Kelly et al., 2001a,b, 2003, 2011) and their increased morbidity and mortality compared to younger animals, video-EEG recordings were conducted up to 2 months after lesioning or sham operation, i.e., up to ~7-months-of age (4-month-old group) and ~23 months-of-age (20-month-age group), in order to maximize healthy survival of the 20-month-age group and to have equivalent monitoring times among the different cohorts before sacrifice of the animals for anatomical studies.
2.7. Video-EEG Data Analysis
Qualitative video-EEG analysis was performed off-line by a complete visual examination of the EEG recordings. Ictal behaviors were classified by a modification of the Racine (1972) scale: grade 3 – forelimb or hindlimb clonus; grade 4 – running and rearing; and grade 5 – jumping and falling; grade 1 and 2 behaviors were not scored because of the difficulty of discriminating normal vs. abnormal mouth, facial, and head nodding movements. Special attention was paid to intermittent focal and generalized EEG activities as determined by changes in waveform morphology, amplitude, and frequency. Available event detection software (Stellate SenSa) was not designed to be sufficiently discriminating for several EEG features of interest and therefore was not used. Standard descriptive terms for frequency ranges were used: delta (0.1–3.9 Hz); theta (4.0–7.9 Hz); alpha (8.0–12.9 Hz); and beta (13.0–30 Hz; Fisch, 1999). EEG interictal activities were distinguished from ictal activities based on waveform morphology and frequency and the associated absence or presence of behavioral change, respectively. Seizure frequency (number of grade 3, 4, and 5 seizures per hour) was used as the primary outcome measurement for investigating the “age” and “infarction” factor effects on the electrobehavioral evidence of poststroke epilepsy. A 2 × 2 factorial ANOVA test was applied to test the significance of each factor effect as well as the interaction effect between the two factors. A p-value < 0.05 was considered statistically significant.
2.8. Perfusion and preparation of tissue sections
At the end of the video-EEG monitoring period, animals were deeply anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with 0.9% saline followed by 4% ice-cold paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed, post-fixed overnight in the same fixative and cryoprotected in a buffered 30% sucrose solution for 3–4 days at 4°C. Gross examination of the brains was performed to confirm the presence of ischemic injury resulting from MCA/CCAo and reperfusion and to assess for other abnormalities such as tumor, hematoma, and compression by electrodes. Fixed brains were cut in 40 µm coronal sections, collected in a 1:50 series for the measurement of ischemic injury, placed in plastic tissue culture plates containing cryoprotectant solution (30% ethylene glycol/30% glycerol/40% 0.1 M PB containing 3.4 g NaCl), and stored at −20°C. Other tissue sections were used for immunohistochemical assessments with different cell-specific markers.
2.9. Immunohistochemistry
Primary antibodies used for the immunohistochemical staining were mouse anti-neuron specific nuclear protein (NeuN; 1:2000, Chemicon, Temecula, CA), rabbit anti-glial fibrillary acidic protein (GFAP; 1:500, Chemicon, Temecula, CA), and anti-vimentin (1:100, Chemicon, Temecula, CA). The secondary antibodies were biotinylated goat anti-mouse or anti-rabbit (1:250; Vector Lab., Burlingame, CA). Briefly, free-floating brain tissue sections were removed from the cryoprotectant solution and rinsed in 0.1 M PB (pH 7.4), followed by 30-min incubation in 0.3% hydrogen peroxide in PB. After several rinses in 0.1 M Tris-buffered saline (TBS, pH 7.4), tissue was incubated for 30 min in TBS containing 3% normal goat serum and 0.25% Triton X-100 (TX-100). Tissue sections were incubated overnight at 4°C with primary antibodies diluted with TBS containing 1% normal goat serum and 0.25% TX-100. After several rinses, the tissue was incubated for 2 h at room temperature with biotinylated secondary antibodies diluted in TBS containing 1% goat serum, followed by 1-h incubation in avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Lab., CA) and the treatment with imidazole acetate buffer (pH 9.6) containing 0.05% diaminobenzidine, 2.5% nickel ammonium sulfate, and 0.005% H2O2.
As a control for non-specific staining, rabbit pre-immune serum was substituted for the primary antibodies, and the tissue was processed using the aforementioned procedure. No positive immunoreactivity or recognizable background staining was observed under these conditions. Sections were mounted on gelatinized slides, dried, dehydrated in a series of graded ethanol solutions, immersed in xylene, and coverslipped with Permount (Fisher).
2.10. Morphometry
For the assessment of infarct volume, every 50th tissue section was immunolabeled with anti-NeuN antibodies were digitized by a Nikon slide scanner (model LS-2000, Nikon Corporation, Tokyo, Japan) and processed with a computer-assisted image analysis system (MCID, Imaging Research Inc., St. Catharines, Canada). For each tissue section, the areas of lost tissue were outlined manually on the computer screen as well as the outlines of the left and right hemispheres on each section. The volume of infarction was calculated as the integrated product of the cross-sectional area and the inter-section distance. Pearson’s correlation coefficient (r) was calculated to assess the relationship between behavioral scores and lesion volumes in 4- and 20-month-old animals. Values were calculated using GraphPad statistical software.
3. Results
3.1. Morbidity and mortality
Intermittent continuous digital video-EEG recordings were obtained from 17 of 24 (71%) animals up to the 2-month time point following sham operation or lesioning by MCA/CCAo (Table 1). Seven animals were not analyzed because: 1) one 20-month-old animal died during lesioning surgery; 2) three sham-operated 4-month-old animals died during transit from Houston to Pittsburgh; 3) two lesioned 4-month-old animals died due to anesthesia effects during our initial placements of screw electrodes; and 4) one lesioned 20-month-old animal with grade 5 seizures (Suppl. Fig. 1A) was excluded due to a large intracortical tumor (Suppl. Fig. 1B). Data were analyzed from three animals that died prematurely during the second month following lesioning: 1) one 20-month-old lesioned animal died 5 weeks after lesioning during class 5 seizure activity; 2) one 20-month-old lesioned animal died 7 weeks after lesioning due to failure to thrive; and 3) one 20-month-old sham-operated animal died 7 weeks after surgery due to abdominal surgical wound dehiscence.
Table 1.
Video EEG monitoring data
| Animals/ Totals |
Sham Operation Video+EEG |
MCA/CCAo Video+EEG |
|||||
|---|---|---|---|---|---|---|---|
| Age at surgery (mo) | 4 & 20 | 4 | 20 | 4 & 20 | 4 | 20 | 4 & 20 |
| Surviving animals (n) | 17 | 3 | 6 | 9 | 9 | 4 | 8 |
|
| |||||||
| Monitoring start day* | 29.0±0.0 | 22.7±0.8 | 24.8±1.2 | 31.0±1.7 | 31.0±1.6 | 31.0±1.0 | |
| Range (day) | 28–34 | 20–24 | 20–29 | 28–34 | 29–33 | 28–34 | |
|
| |||||||
| Recording duration: h | 3591 | 843 | 1289 | 2132 | 884 | 575 | 1459 |
| Light (h) | 1741 | 444 | 603 | 1047 | 419 | 275 | 694 |
| Dark (h) | 1850 | 399 | 686 | 1085 | 465 | 300 | 765 |
|
| |||||||
| Recording sessions (n) | 166 | 36 | 61 | 97 | 40 | 29 | 69 |
3.2. Behavioral testing
Composite scores of behavioral testing performed 24 h after lesioning were generated for 4-month-old (n=6) and 20-month-old (n=5) animals (Table 2). The mean score for 4-month-old animals was 7.9 (range 5.8 – 12.8), whereas the mean score for 20-month-old animals was 6.54 (range 4.4 – 8.4; p=0.18), suggesting no significant difference in behavioral impairment between the two animal groups shortly following lesioning.
Table 2.
Correlation analysis of infarct volume and behavioral score
| 4 mo lesioned animals (n = 6) | 20 mo lesioned animals (n = 5) | ||
|---|---|---|---|
| Behavioral Score | Lesion Volume (mm3) | Behavioral Score | Lesion Volume (mm3) |
| 6.7 | 35 | 8.4 | 70.7 |
| 5.8 | 14.22 | 5.8 | 101.47 |
| 7 | 25.65 | 7.7 | 60.95 |
| 5.8 | 6.13 | 6.4 | 7.6 |
| 9.3 | 84.9 | 4.4 | 11.46 |
| 12.8 | 37.51 | ||
| r = 0.5165 | r = 0.3998 | ||
| p = 0.2941 | p = 0.5049 | ||
| Overall: r = 0.3097; p = 0.3450 | |||
The p-value (two-tailed) represents the significance of the correlation coefficient, given the r value and degrees of freedom.
3.3. Video-EEG recordings
3.3.1. Continuous recordings
Video-EEG recordings were obtained during multiple individual recording sessions of lesioned and control animals (see Table 1). In general, active wakefulness of animals was maximal during night time recordings and was associated with EEG activity that was relatively suppressed with a predominance of theta to low beta-range activities of low to moderate amplitude. Passive wakefulness was common during daytime recordings characterized behaviorally by the animal at rest associated with EEG activities similar to those observed during active wakefulness but with increased delta activity. Sleep occurred during day and night recordings characterized by EEGs that demonstrated a nearly continuous admixture of polymorphic delta and theta activity of moderate to high amplitude and alpha and beta activity of low to moderate amplitude. Because recordings began, on average, 3–4 weeks after lesioning, and were terminated at or before the 2-month post-lesioning time point, overall recording times spanned a 4–5-week period for most animals.
3.3.2. Spike-wave discharges
All animals (17 of 17; 100%) demonstrated spontaneous generalized 7–9 Hz spike-wave discharges (SWDs) (Suppl. Fig. 2) typically associated with behavioral arrest, a common finding in laboratory rat strains (Kelly, 2004), and will not be described further.
3.3.3. Myoclonic convulsions
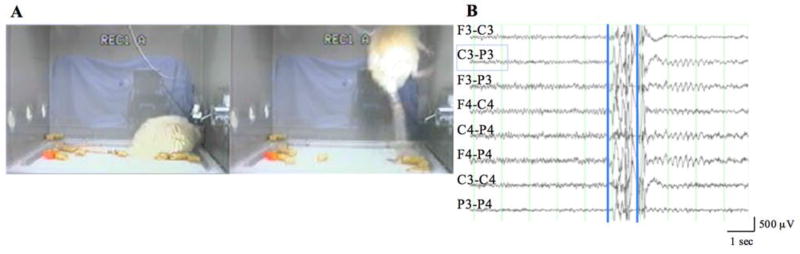
Surprisingly, all control as well as lesioned animals revealed evidence of a stereotypic myoclonic convulsion, suggestive of an age- and lesion-independent event. The event was an abrupt, brief, solitary myoclonic convulsion that occurred during sleep (Fig. 2A). These convulsions could be severe, at times resulting in the animal hitting the top of the recording chamber. Immediately after the convulsion, the animal would be awake and motionless for a brief period of time after which normal behavior resumed, albeit somewhat slowly. EEGs typically showed little to no evidence of significant change before the convulsion.
Figure 2.
Myoclonic convulsion occurring during sleep in a 20-month-old animal 1 month after lesioning. A. Left panel shows the animal asleep; right panel shows the animal during the myoclonic convulsion. B. Generalized low amplitude alpha (9 Hz) activity prior to movement artifact (1 sec) associated with the convulsion, followed by brief (2 sec) parietally dominant rhythmic theta (5.5 Hz) activity. First superimposed vertical cursor corresponds to A (left panel); second cursor to A (right panel). F=frontal; C=frontoparietal; P=parietal; 3=left; 4=right.
3.3.4. 4-month-old sham-operated animals
Aside from the SWDs and myoclonic convulsions described above, none (0/3) of these animals demonstrated any ictal behaviors.
3.3.5. 4-month-old MCA/CCAo animals
Three of the 4 (75%) 4-month-old MCA/CCAo animals had grade 3 seizures (n=6) lasting 8.5±1.2 (range 6.3–10.3) sec; in general, forelimb clonus occurred more frequently than hindlimb clonus. The EEG showed little apparent change during these events, and normal behavior resumed shortly after their occurrence. Two of the animals with grade 3 seizures also demonstrated single episodes (n=2) of grade 4 seizures lasting 34.6±0.5 (range 34.1–35.0) sec. Other than for a significant amount of movement artifact during the seizure, the EEG showed little apparent change during the event. One of the 4 (25%) animals had no seizures. None of the animals had grade 5 seizures. The mean seizure frequency in this group was 0.23/h.
3.3.6. 20-month-old sham-operated animals
All 6 (100%) 20-month-old sham-operated animals demonstrated grade 3 seizures (n=21) characterized by forelimb clonus lasting 11.3±2.5 (range 4.4–18.0) sec. Two animals had grade 4 seizures (n=4) lasting 54.2±33.3 (range 20.9–87.5) sec. The EEGs of these grade 3 and 4 seizures were similar to those described above. Two animals had grade 5 seizures (n=3) lasting 56.4±6.2 (range 50.2–68.9) sec. The EEGs of these grade 5 seizures were dominated by movement artifact. The mean seizure frequency in this group was 0.52/h.
3.3.7. 20-month-old MCA/CCAo animals
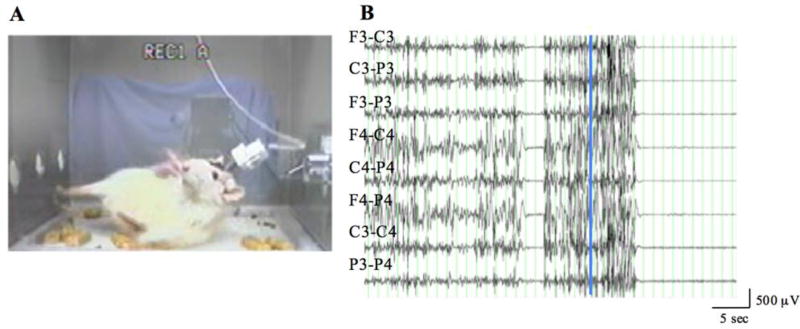
All 4 (100%) 20-month-old MCA/CCAo animals demonstrated grade 3 seizures (n=16) lasting 21.7±5.8 (range 14.4–39.1) sec, as well as grade 4 seizures (n=13) lasting 61.8±14.6 (range 32.6–72.9) sec. Two of the 4 (50%) animals had grade 5 seizures (n=4) lasting 89.1±9.0 (range 62.1–98.1) sec. The mean seizure frequency in this group was 1.93/h. Fig. 3 shows the EEG and animal behavior during a grade 5 seizure resulting in sudden death; the EEG was dominated by diffuse artifact throughout the recording, contrary to the onset and termination of the ictal discharge seen in Supplementary Fig. 1.
Figure 3.
Continuous grade 5 seizure activity and corresponding EEG recording of a 20-month-old animal 5 weeks after lesioning. A. Grade 5 convulsive seizure. B. Following the initial 3 seconds of recording, there is a 2-second period of isoelectricity during which the animal ceased convulsing, followed by a 10-second period of resumed grade 5 seizure activity before generalized isoelectricity and sudden death of the animal. Superimposed vertical cursor corresponds to A. F=frontal; C=frontoparietal; P=parietal; 3=left; 4=right. Infarct volume was 60.95 mm3. No specific abnormality was recognized on gross inspection of the brain other than the cortical infarct.
3.3.8. Statistical analyses on “age” and “infarction” factor effects
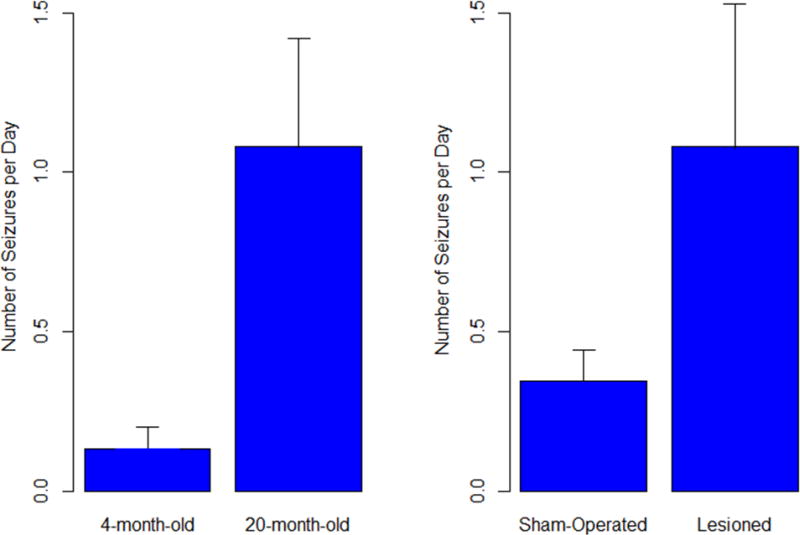
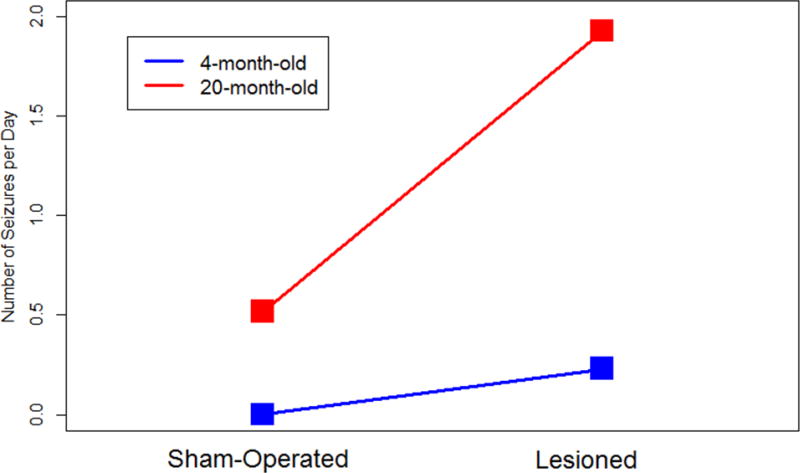
With respect to the seizure frequency in each of the animals, 2 × 2 factorial ANOVA revealed that both “age” and “infarction” factors had significant effects on seizure frequency (p = 0.012 and 0.014, respectively; Fig. 4); however, the interaction between the two factors was not significant (p = 0.097), although, as shown in Fig. 5, the effect on seizure frequency due to lesioning was apparently greater in 20-month-old rats than in 4-month-old animals.
Figure 4.
Seizure frequency analyzed by 2 × 2 factorial ANOVA revealed that both age and infarction factors had significant effects on seizure frequency (p = 0.012 and 0.014, respectively)
Figure 5.
Seizure frequency (seizures/day) in sham-operated and lesioned 4- and 20-month-old animals.
3.3.9 Statistical analyses on seizure duration
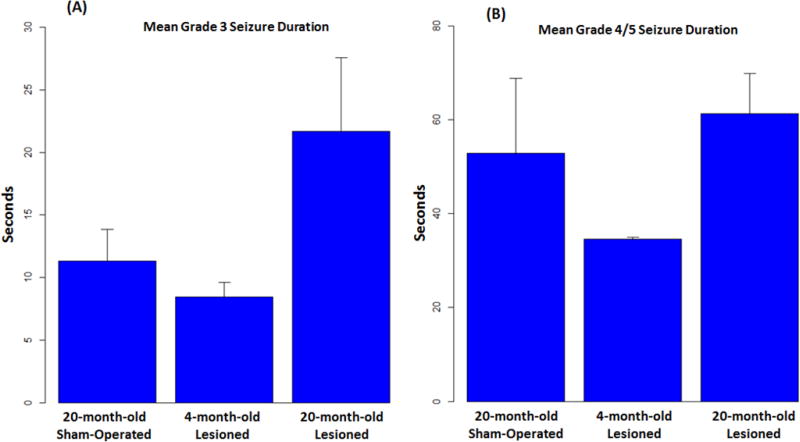
As a secondary investigation, for animal groups with seizures (grade 3 and above), mean seizure durations were compared using one-way ANOVA. Fig. 6 shows the mean seizure duration in each of the three compared groups (20-month-old sham-operated, 4-month-old lesioned, and 20-month-old lesioned) for grade 3 seizures and grade 4 and 5 seizures. It is clear that for grade 3 seizures, 20-month-old lesioned animals had much longer seizure durations than those in the other two animal groups. For grade 4 and 5 seizures, 4-month-old lesioned animals had much shorter seizure durations than those in the other two animal groups. However, statistical testing did not suggest a significant difference for the grade 3 seizure group (p=0.0923) or the grade 4/5 seizure group (p=0.365), likely due to the small sample size of the study.
Figure 6.
Convulsive seizure durations. A. Mean durations of grade 3 seizures in 20-month-old sham-operated and 4- and 20-month-old lesioned animals. B. Mean durations of grade 4 and 5 seizures in the same cohorts as in A.
3.4. Gross inspection of brain and dura
Gross examination of the brain and dura was performed following transcardial perfusion or premature death of the animal to identify potential anatomical abnormalities and to confirm the presence of cortical infarction in lesioned animals. MCA/CCAo resulted in a variably-sized infarct of the ipsilateral neocortex in both 4- and 20-month-old animal groups that typically appeared as a cystic cavity on the convexity of the left hemisphere within the distribution of the MCA. Headset removal from animals sham-operated at 4-months-of-age resulted in partial avulsion of the skull in each animal thereby precluding a careful examination of the dura. Assessment of the dura adherent to the inner table of the skull in all other animal groups revealed that no recording screw pierced the dura except for one small perforation at P3 in an animal sham-operated at 20 months of age; there was no evidence of hemorrhage in any brain. The dorsal cortex of sham-operated brains (n=8) revealed 2 to 6 small indentations, often symmetrically placed, caused by mild concave bowing of the skull in those areas underlying recording screws. Anchoring screws perforated the dura in 10 animals, either on one (n=8) or both (n=2) sides, resulting only in small indentations of the lateral cerebellar hemispheres. As described above (Suppl. Fig. 1B), an animal lesioned at 20-months-of-age had a large tumor of the right neocortex and was excluded from the study.
3.5. Immunohistochemical findings
3.5.1. NeuN immunoreactivity (IR)
NeuN IR gave no evidence of neuronal injury in the brain tissue of sham-operated controls at the time of analysis. In animals lesioned by MCA/CCAo, NeuN IR was absent in the necrotic core of the infarct, largely reflecting the anatomical distribution of the MCA. Infarct volume was quantified using NeuN IR at the same coronal levels in the 4- and 20-month-old groups at or before the 2-month time point following MCA/CCAo (Fig. 7). There was significant variability in the extent of the ischemic infarct in both age groups. The mean cortical infarct volume in 4-month-old animals was 35.8±10.8 mm3 (range 6.1 to 84.9 mm3, n=6), and in 20-month-old animals was 50.4±18.0 mm3 (range 7.6 to 101.5 mm3, n=5; t-test, p=0.49), indicating no significant difference in infarct volumes between the two age groups (Table 2).
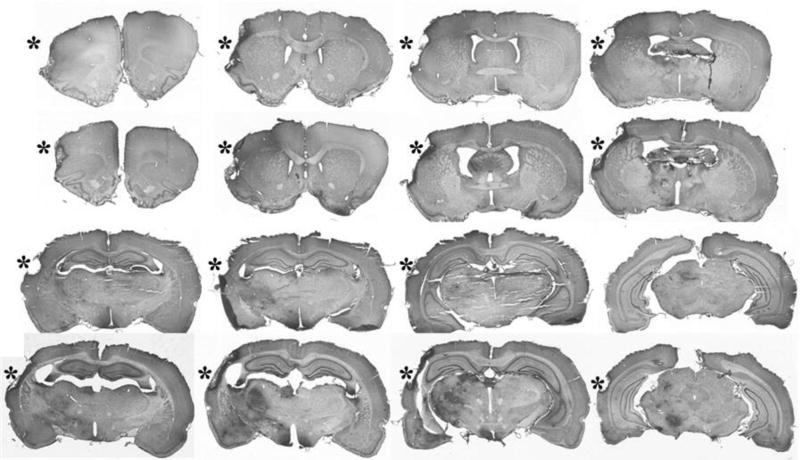
Figure 7.
NeuN-immunostained coronal brain sections demonstrate the infarct cavities (*) in the frontoparietal cortex of 4- and 20-month-old F344 animals 2 months after MCA/CCAo. Note the darkened areas of ischemic injury in the ipsilateral basal ganglia and thalamus, which appear more prominent in the 20-month-old animal.
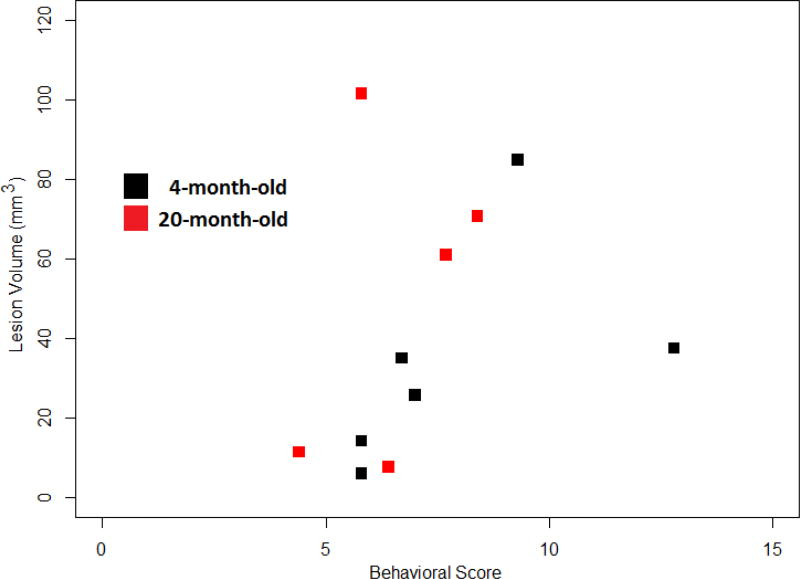
Figure 8 shows the behavioral score versus cortical infarct volume relation for all lesioned rats (six 4-month-old and five 20-month-old). Overall, it is clear that there exists a positive correlation between behavioral score and infarct volume (r = 0.31), with higher correlation in young animals (r = 0.52) than in older rats (r = 0.40). Statistical significance cannot be claimed here due to the small sample size; even with all rats, n = 11, there is only 37% power to claim significance when r = 0.5.
Figure 8.
Graphic representation of the relationship between behavioral score and cortical infarct volume. The plot separates young rats (black squares) from older rats (red squares). It is clear that, either in all rats or in each age group, there exists a positive correlation between behavioral score and cortical infarct volume.
Beyond the area of the infarct core, NeuN IR was decreased in the ipsilateral cerebral cortex, markedly so in the peri-infarct area (Fig. 9A) compared to the homotopic area (Fig. 9B), and decreased in the adjacent dorsolateral and ventrolateral striatum (data not shown).
Figure 9.
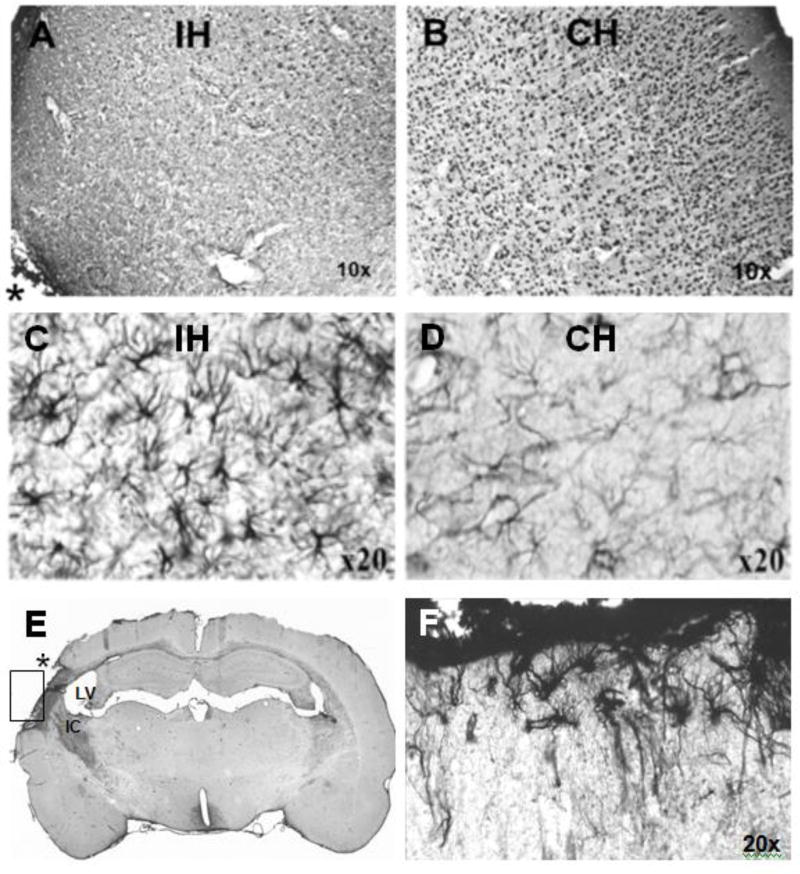
Immunoreactivity (IR) in 20-month-old lesioned animals. A. NeuN IR of the cerebral cortex of the hemisphere ipsilateral (IH) and proximal to the infarct (an asterisk [*] in the left lower corner of A marks the area of the infarct core) is markedly decreased compared to the homotopic area (B) of the contralateral hemisphere (CH). Note A and B are displayed as mirror images. C,D. GFAP IR was enhanced in the thalamus (C) compared to the homotopic area (D). E,F. Vimentin IR is increased in the ipsilateral hemisphere (E) in the scar boundary zone and the internal capsule (IC). An asterisk (*) marks the area of infarct; the lateral ventricle (LV) is enlarged. The scar boundary zone shown in (E) is rotated ~90 degrees clockwise and magnified in (F) demonstrating underlying reactive astrocytosis.
3.5.2. GFAP- and vimentin-positive astroglia
GFAP IR in sham-operated animals was present in astrocyte somata and their processes in both superficial and deep cortical layers, and throughout all brain regions; GFAP IR was similar in 4- and 20-month animals. Following MCA/CCAo, GFAP IR was robust in perilesional cortex gliosis (Fig. 9C) compared to the homotopic area (Fig. 9D). In addition, GFAP IR was enhanced in ipsilateral subcortical structures (data not shown).
Vimentin-expressing astrocytes in sham-operated animals were sparsely present in the corpus callosum, external and internal capsule, optic tract, fimbria of the hippocampus, and at the base of the lateral ventricles; in lesioned animals, the distribution pattern of vimentin IR was similar. Following MCA/CCAo, vimentin IR was markedly increased in the glial scar of the infarcted cortex (Fig. 9E,F). In general, vimentin IR was more sensitive than GFAP IR for demonstrating the distribution and astrocytic reactions following MCA/CCAo.
4. Discussion
4.1. Main findings
The main findings of this study were: 1) seizures (grade 3 and above) were recorded within 2 months in both young (4-month; 0.23/h) and aged (20-month; 1.93/h) MCA/CCAo rat groups; both MCA/CCAo rat groups had more seizures recorded than the respective control groups, i.e., no seizures in young controls, and 0.52/h in aged controls; 2) both age and infarction independently had effects on seizure frequency; however, there was no demonstrated interaction between the two factors; 3) there was no difference in infarct volumes comparing the two age groups. Additional findings included: 1) morbidity and mortality of animals limited the extent to which the animals could be evaluated, especially 20-month-old animals; 2) all lesioned and sham-operated animals demonstrated intermittent solitary myoclonic convulsions arising out of sleep; and 3) all animals demonstrated 7–9 Hz spike-wave discharges (SWDs), which were not analyzed.
4.2. Morbidity and mortality
Animal morbidity and mortality resulted in relatively small numbers of animals and limited monitoring time in both sham-operated and lesioned cohorts. The reasons for this were varied and included: 1) sudden death during lesioning, electrode implantation, and grade 5 seizure activity; 2) transport from Houston to Pittsburgh; and 3) tumor, abdominal wound dehiscence, and failure to thrive. In general, animals from the 20-month-old cohorts tended to be frail and experienced more complications than the 4-month-old cohorts. These issues operationally limited the overall monitoring duration (up to 2 months following sham operation or lesioning) in order to ensure relatively equivalent periods of recording times to assess and compare the potential development of epileptic seizures within the different age cohorts. These results suggest that use of young adult – or perhaps mid-aged animals – might be preferable to the use of aged animals in order to circumvent some of the morbidity and mortality issues experienced in this study.
4.3. Myoclonic convulsions
We cannot conclusively say why myoclonic convulsions were recorded in all animals but we believe that the convulsions may be manifestations of human sleep-related phenomena (hypnagogic or hypnopompic myoclonus), commonly known as hypnic jerks or nocturnal myoclonus, which are not regarded as epileptic seizures. This phenomenon may be related to the specific characteritics of the F344 strain as we have not observed similar convulsive activities in long term video EEG monitoring of both Sprague-Dawley (Kharlamove et al., 2003) and Long-Evans rats (Kelly et al., 2006).
4.4. Seizure occurrence and the effects of age and infarction
Because recordings did not begin until 3–4 weeks after lesioning, the study was not able to investigate the potential occurrence of provoked seizures within the first week after lesioning or sham operation. In addition, the limited periods of recordings prevented a comprehensive examination of how epileptic seizures might have evolved over an extended period of time. However, seizures were recorded in all animal groups except 4-month-old sham-operated animals. Grade 3 and 4 seizures were recorded in lesioned 4-month-old animals, and surprisingly, grade 3–5 seizures in sham-operated 20-month-old animals, as well as lesioned 20-month-old animals. The reasons for seizure occurrence in the sham-operated 20-month-old animals cannot be determined definitively, but may be related to a potential age-related propensity to seizures in the F344 strain that is enhanced by comorbidities.
Age and infarction factors had significant effects on seizure frequency, which was increased in 20-month-old animals compared with 4-month-old animals; however, there was no significant interaction between the two factors. These findings suggest that there was no clear advantage to use of aged animals, given the limitations stated above for this age group. With regard to seizure duration, 20-month-old lesioned animals had much longer grade 3 seizure durations than those in the 20-month-old sham-operated and 4-month-old lesioned groups; for grade 4/5 seizures, 4-month-old lesioned animals had much shorter seizure durations than those in the other two animal groups. However, statistical testing did not suggest a significant difference for either the grade 3 or grade 4/5 seizure groups, likely secondary to the small sample size of the study.
4.5. Infarct volume and associated anatomical changes
There was no significant difference between infarct volumes in the 4- and 20-month-old groups; a broad range of infarct volume was found in each group. Interestingly, the calculated infarct volume in the 4-month-old animals (35.8±10.8 mm3, n=6), sacrificed at ~7-months-of age, was similar to that of our previous study of Long-Evans rats (36.8±13.5 mm3, n=4), which were lesioned at 2.5-months-of age, studied in identical fashion, and sacrificed at 8.5-months-of-age (Kelly et al., 2006). In that study, no seizure activity was recorded from either lesioned (n=5) or sham-operated (n=4) Long-Evans animals.
When plotting behavioral score versus cortical infarct volume for all data collected, it is apparent that there exists a positive correlation between these two variables (Figure 8); the correlation is higher in young animals than that in the older animals. However, due to the low statistical power (<40% in 11 rats, six 4-month-old and five 20-month-old), the present study was not able claim a statistical significance of this relationship.
Immunohistochemical studies were performed to demonstrate the localization and extent of ischemic injury following transient unilateral MCA/CCAo, and to investigate morphological changes of the brain that might contribute to epileptogenesis. Multiple studies have shown increased vulnerability of aged brain to ischemic injury and subsequent neurological impairment. In the present study, evaluation of brain tissue beyond the limits of the infarct core revealed that ischemic injury in F344 animals was not restricted to the cortex within the distribution of the MCA, unlike the findings in Long-Evans animals in which the same procedure resulted in infarcts restricted to ipsilateral cortex when evaluated by triphenyltetrazolium (TTC)-stained tissue slices 24 hours after lesioning (Aronowski et al., 1997). In the present study, histopathological analysis two months after MCA/CCAo in F344 rats indicated that the localization and extent of ischemic injury in both animal age groups occurred primarily in ipsilateral cortex accompanied frequently by significant changes in the adjacent striatum and thalamus. The subcortical injury may be related specifically to the F344 rat strain and/or may demonstrate the features of secondary neuronal degeneration during long-term survival. Extensive apoptosis, delayed and selective neuronal loss, and reactive gliosis have been demonstrated in subcortical structures following MCAo in different animal models (Tamura et al., 1991; Soriano et al., 1996; Watanabe et al., 1997, 1998; Dihne et al., 2002; Hobohm et al., 2005). Secondary degeneration in the thalamus has been observed clinically in patients with cerebral infarction in the territory supplied by the MCA (Herve et al., 2005), and should not be mistaken for further cerebral infarction (Ogawa et al., 1997). Mechanisms of secondary brain injury may depend on the region of brain affected initially, and may be due to retrograde degeneration of thalamocortical projections (Nordborg & Johansson, 1996) and neurotransmitter imbalances because of a loss of GABAergic inhibition (Dihne et al., 2002).
The variability of ischemic injury in cortical and subcortical structures likely influenced the neurological impairment and recovery following MCA/CCAo, and may have predisposed to or caused the development of epileptic seizures. The variability in location and extent of ischemic injury in both 4- and 20-month-old F344 animals suggested that the level of ischemia or the response to ischemia was not uniform across groups and possibly due to different factors. Ischemic injury in the aged animals did not result in larger infarcts than those induced in younger animals, a finding that stands in contrast to studies that reported a larger volume of ischemic tissue in older animals compared to younger ones (Futrell et al., 1991; Davis et al., 1995; Wang et al., 2003) or when assessed at day 7 or 24 h after the induction of the stroke (Kharlamov et al., 2000; Rosen et al., 2005), respectively. Interestingly, however, is that a study with young and aged mice in the MCAo model that measured the volume of atrophy at a later time point, i.e., 30 d after ischemia, showed that the brain tissue loss was less in aged than in young animals (Manwani et al., 2011).
Our findings provided evidence of increased activation of astrocytes, which is commonly found in normal brain aging and CNS lesions. For example, increased expression of vimentin and GFAP has been observed following ischemia (Petito et al., 1990, Schmidt-Kastner et al., 1990, 2005 Lin et al., 1993; Jorgensen et al., 1993; Petito and Halaby, 1993) and traumatic brain injury (Baldwin & Scheff, 1996; Hill et al., 1996). In temporal lobe epilepsy, a glial scar is frequently associated with the epileptogenic focus, and is similar to that found after brain injury (Hoeppner and Morrell, 1986). In the present study, signs of glial activation in the thalamus, which typically precede secondary degeneration, were seen in most 20-month-old animals, but not in all 4-month old animals, and were related to severity of ischemic injury. Age-related deficits in the brain recovery might be associated with a stronger reactive astrocytic response in aged animals (Goss and Morgan, 1995; Gordon et al., 1997), and prolonged and increased activation of astrocytes, especially in the hippocampus and thalamus, might contribute to poststroke epilepsy. Conversely, spontaneous epileptic seizures may also cause reactive gliosis (Drage et al., 2002). Large cortical infarcts and the associated morphological changes of neurons and astrocytes by occlusion/reperfusion and secondary degeneration/injury in subcortical structures may also contribute to poststroke epileptogenesis (Tamura et al., 1991; Burn et al., 1997; Berg, 2003, Lamy et al., 2003, Camilo and Goldstein, 2004).
4.6. Study limitations
There were several limitations to this study. Foremost among them were the effects of morbidity and mortality that limited the number of animals in the different cohorts and restricted the times of recordings to a very brief duration compared to standard practices using continuous video-EEG monitoring following experimentally-induced brain injury. Specifically, the small sample sizes of the different cohorts resulted in low power to detect significant differences among them. In addition, the decision to limit the recording time for the 4-month-old cohorts to that felt necessary for the survival of the 20-month-old cohorts (up to 2 months post-lesioning) was at the expense of potentially additional ictal data that may have been obtained with extended monitoring of the 4-month-old cohorts. It remains uncertain as to why 20-month-old sham-operated animals demonstrated grade 3 and 5 seizures in this study but raises the possibility of an age-related propensity to seizure activity in the F344 strain, perhaps enhanced by underlying morbidities. Although recording screws caused mild concave bowing of the skull sufficient to secondarily cause mild indentations of the brain in some animals (Kadam et al., 2017), the screws did not perforate the brain; anchoring screws did perforate the dura in several animals but only in the lateral cerebellar hemispheres, which are not likely to cause or contribute to ictogenesis in the different animals. Eliminating these issues in a larger study would clarify and delineate the age-related propensity of poststroke epileptogenesis following severe ischemia induced by arterial occlusion.
5. Conclusions
The findings of this study indicated that when using Fisher 344 rats, spontaneous seizures could be recorded within 2 months following transient unilateral occlusion of the MCA and CCA, which resulted in moderately-sized ipsilateral infarcts of both cortical and subcortical structures. Although there was no demonstrated interaction between age and infarction, both factors had significant effects on seizure frequency; there was no difference in infarct volumes between the two age groups. The findings suggest that the potential development of poststroke epilepsy may be enhanced by using young adult or mid-aged animals rather than juvenile animals, and that potentially confounding comorbidities are best avoided by not using aged animals.
Supplementary Material
HIGHLIGHTS.
Seizures occurred within 2 months in 4- and 20-month lesioned and 20-month sham rats
Both age and infarction independently had effects on seizure frequency
There was no interaction between age and infarction on seizure frequency
There was no difference in infarct volumes between 4- and 20-month lesioned animals
All animals had intermittent solitary myoclonic convulsions arising out of sleep
Acknowledgments
Supported by Health Research Formula Fund RFA 01-07-26 from the Pennsylvania Department of Health (Tobacco Settlement Fund), the Targeted Research Initiative for Seniors of the Epilepsy Foundation funded by a grant from the Centers for Disease Control and Prevention, the NIA Animal Allocation Program for Pilot Study Research Support, and NINDS R21NS052722 (KMK). Funding sources had no role in the conduct of the research or the preparation of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronowski J, Ostrow P, Samways E, et al. A graded bioassay for determination of brain rescue from experimental acute ischemia. Stroke. 1994;25:2235–2240. doi: 10.1161/01.str.25.11.2235. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Samways E, Strong R, et al. An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficit. J. Cereb. Blood Flow Metab. 1996;16:705–713. doi: 10.1097/00004647-199607000-00022. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Grotta J. Reperfusion injury: demonstration of brain damage produced by reperfusion following transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Scheff SW. Intermediate filament change in astrocytes following mild cortical contusion. Glia. 1996;16:266–275. doi: 10.1002/(SICI)1098-1136(199603)16:3<266::AID-GLIA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Berg AT. Seizures and epilepsy after ischemic stroke. Epilepsy Curr. 2003;3:129–130. doi: 10.1046/j.1535-7597.2003.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch. Neurol. 2000;57:1617–1622. doi: 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schallert T, Strong R, et al. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke. 2000;31:1144–1152. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- Bland ST, Pillai RN, Aronowski J, et al. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav. Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Kwan P. Epilepsy in elderly people. BMJ. 2005;331:1317–1322. doi: 10.1136/bmj.331.7528.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Dennis M, Bamford J, et al. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ. 1997;315:1582–1587. doi: 10.1136/bmj.315.7122.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilo O, Goldstein LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004;35:1769–1775. doi: 10.1161/01.STR.0000130989.17100.96. [DOI] [PubMed] [Google Scholar]
- Cockerell OC, Eckle I, Goodridge DM, et al. Epilepsy in a population of 6000 re-examined: secular trends in first attendance rates, prevalence, and prognosis. J. Neurol. Neurosurg. Psychiat. 1995;58:570–576. doi: 10.1136/jnnp.58.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, et al. Experimental stroke and neuroprotection in the aging brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ. tPA: A neuron buster, too? Nat. Med. 1998;4:148–150. doi: 10.1038/nm0298-148. [DOI] [PubMed] [Google Scholar]
- Dihne M, Grommes C, Lutzenburg M, et al. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke. 2002;33:3006–3011. doi: 10.1161/01.str.0000039406.64644.cb. [DOI] [PubMed] [Google Scholar]
- Drage MG, Holmes GL, Seyfried TN. Hippocampal neurons and glia in epileptic EL mice. J. Neurocytol. 2002;31:681–692. doi: 10.1023/a:1025747813463. [DOI] [PubMed] [Google Scholar]
- Ettinger AB, Shinnar S. New-onset seizures in an elderly hospitalized population. Neurology. 1993;43:489–492. doi: 10.1212/wnl.43.3_part_1.489. [DOI] [PubMed] [Google Scholar]
- Fisch BJ. Fisch & Spehlmann’s EEG Primer: Basic Principles of Digital and Analog EEG. Elsevier; Amsterdam: 1999. [Google Scholar]
- Futrell N, Garcia JH, Peterson E, et al. Embolic stroke in aged rats. Stroke. 1991;22:1582–1591. doi: 10.1161/01.str.22.12.1582. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Schreier WA, Ou X, et al. Exaggerated astrocyte reactivity after nigrostriatal deafferentation in the aged rat. J. Comp. Neurol. 1997;388:106–119. [PubMed] [Google Scholar]
- Goss JR, Morgan DG. Enhanced glial fibrillary acidic protein RNA response to fornix transection in aged mice. J. Neurochem. 1995;64:1351–1360. doi: 10.1046/j.1471-4159.1995.64031351.x. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Williams AJ, Tortella FC. Occurrence of nonconvulsive seizures, periodic epileptiform discharges, and intermittent rhythmic delta activity in rat focal ischemia. Exp. Neurol. 2003;179:139–149. doi: 10.1016/s0014-4886(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hervé D, Molko N, Pappata S, et al. Longitudinal thalamic diffusion changes after middle cerebral artery infarcts. J. Neurol. Neurosurg. Psychiatry. 2005;76:200–205. doi: 10.1136/jnnp.2004.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuts-van Raak EP, Boellaard A, De Krom MC. Supratentorial brain infarcts in adult-onset seizures, the Maastricht epilepsy case register. Seizure. 1993;2:221–227. doi: 10.1016/s1059-1311(05)80131-1. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Barbarese E, McIntosh TK. Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J. Neuropathol. Exp. Neurol. 1996;55:1221–1229. doi: 10.1097/00005072-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Hobohm C, Gunther A, Grosche J, et al. Decomposition and long-lasting downregulation of extracellular matrix in perineuronal nets induced by focal cerebral ischemia in rats. J. Neurosci. Res. 2005;80:539–548. doi: 10.1002/jnr.20459. [DOI] [PubMed] [Google Scholar]
- Hoeppner TJ, Morrell F. Control of scar formation in experimentally induced epilepsy. Exp. Neurol. 1986;94:519–536. doi: 10.1016/0014-4886(86)90235-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen MB, Finsen BR, Jensen MB, et al. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp. Neurol. 1993;120:70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Kadam SD, D'Ambrosio R, Duveau V, et al. Methodological standards and interpretation of video-EEG in adult control rodents. Epilepsia. 2017;48(Suppl.4):10–27. doi: 10.1111/epi.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhunen H, Pitkanen A, Virtanen T, et al. Long-term functional consequences of transient occlusion of the middle cerebral artery in rats: a 1-year follow-up of the development of epileptogenesis and memory impairment in relation to sensorimotor deficits. Epilepsy Res. 2003;54:1–10. doi: 10.1016/s0920-1211(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Nissinen J, Sivenius J, et al. A long-term video-EEG and behavioral follow-up after endothelin-1 induced middle cerebral artery occlusion in rats. Epilepsy Res. 2006;72:25–38. doi: 10.1016/j.eplepsyres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Kharlamov A, Hentosz TM, et al. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001a;47:189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Kume A, Albin RL, et al. Autoradiography of L-type and N-type calcium channels in aged rat hippocampus, entorhinal cortex, and neocortex. Neurobiol. Aging. 2001b;22:17–23. doi: 10.1016/s0197-4580(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Poststroke seizures and epilepsy: clinical studies and animal models. Epilepsy Curr. 2002;2:173–177. doi: 10.1046/j.1535-7597.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Ikonomovic MD, Abrahamson EE, et al. Alterations in hippocampal voltage-gated calcium channel α1 subunit expression patterns after kainate-induced status epilepticus in aging rats. Epilepsy Res. 2003;57:13–30. doi: 10.1016/j.eplepsyres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Spike-wave discharges: absence or not, a common finding in common laboratory rats. Epilepsy Curr. 2004;4:176–177. doi: 10.1111/j.1535-7597.2004.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM, Jukkola PI, Kharlamov EA, et al. Long-term video-EEG recordings following transient unilateral middle cerebral and common carotid artery occlusion in Long-Evans rats. Exp. Neurol. 2006;201:495–506. doi: 10.1016/j.expneurol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Shiau DS, Jukkola PI, et al. Effects of age and cortical infarction on EEG dynamic changes associated with spike wave discharges in F344 rats. Exp. Neurol. 2011;232:15–21. doi: 10.1016/j.expneurol.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM. Poststroke epilepsy. In: Pitkanen A, Buckmaster PS, Galanopoulou AS, Moshe SL, editors. Models of Seizures and Epilepsy. 2. San Diego: Elsevier; 2017. pp. 727–741. [Google Scholar]
- Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B135–B141. doi: 10.1093/gerona/55.3.b135. [DOI] [PubMed] [Google Scholar]
- Kharlamov EA, Jukkola PI, Schmitt KL, et al. Electrobehavioral characteristics of epileptic rats following photothrombotic brain infarction. Epilepsy Res. 2003;56:185–203. doi: 10.1016/j.eplepsyres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Lamy C, Domigo V, Semah F, et al. Early and late seizures after cryptogenic ischemic stroke in young adults. Neurology. 2003;60:400–404. doi: 10.1212/wnl.60.3.400. [DOI] [PubMed] [Google Scholar]
- Lin RC, Polsky K, Matesic DF. Expression of gamma-aminobutyric acid immunoreactivity in reactive astrocytes after ischemia-induced injury in the adult forebrain. Brain Res. 1993;600:1–8. doi: 10.1016/0006-8993(93)90394-3. [DOI] [PubMed] [Google Scholar]
- Loiseau J, Loiseau P, Duch CB, et al. A survey of epileptic disorders in Southwest France: seizures in elderly patients. Ann. Neurol. 1990;27:232–237. doi: 10.1002/ana.410270304. [DOI] [PubMed] [Google Scholar]
- Loiseau P. Pathologic processes in the elderly and their association with seizures. In: Rowan AJ, Ramsay RE, editors. Seizures and epilepsy in the elderly. Butterworth-Heinemann; Boston: 1997. pp. 63–85. [Google Scholar]
- Luhdorf K, Jensen LK, Plesner AM. Etiology of seizures in the elderly. Epilepsia. 1986;27:458–463. doi: 10.1111/j.1528-1157.1986.tb03567.x. [DOI] [PubMed] [Google Scholar]
- Manwani B, Liu F, Xu Y, et al. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg C, Johansson BB. Secondary thalamic lesions after ligation of the middle cerebral artery: an ultrastructural study. Acta Neuropathol. 1996;91:61–66. doi: 10.1007/s004010050392. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Yoshida Y, Okudera T, et al. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology. 1997;204:255–262. doi: 10.1148/radiology.204.1.9205256. [DOI] [PubMed] [Google Scholar]
- Petito CK, Halaby IA. Relationship between ischemia and ischemic neuronal necrosis to astrocyte expression of glial fibrillary acidic protein. Int. J. Dev. Neurosci. 1993;11:239–247. doi: 10.1016/0736-5748(93)90082-o. [DOI] [PubMed] [Google Scholar]
- Petito CK, Morgello S, Felix JC, et al. The two patterns of reactive astrocytosis in postischemic rat brain. J. Cereb. Blood Flow Metab. 1990;10:850–859. doi: 10.1038/jcbfm.1990.141. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Bhimani A, Kuruba R, et al. Prospects of modeling poststroke epileptogenesis. J. Neurosci. Res. 2017;95(4):1000–1016. doi: 10.1002/jnr.23836. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CL, Dinapoli VA, Nagamine T, et al. Influence of age on stroke outcome following transient focal ischemia. J. Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- Scheuer ML, Cohen J. Seizures and epilepsy in the elderly. Neurol. Clinics. 1993;11:787–804. [PubMed] [Google Scholar]
- Schmidt-Kastner R, Szymas J, Hossmann KA. Immunohistochemical study of glial reaction and serum-protein extravasation in relation to neuronal damage in rat hippocampus after ischemia. Neuroscience. 1990;38(2):527–540. doi: 10.1016/0306-4522(90)90048-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Aguirre-Chen C, Saul I, et al. Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res. 2005;1052:28–39. doi: 10.1016/j.brainres.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Soriano MA, Ferrer I, Rodriguez-Farre E, et al. Apoptosis and c-Jun in the thalamus of the rat following cortical infarction. Neuroreport. 1996;7:425–428. doi: 10.1097/00001756-199601310-00012. [DOI] [PubMed] [Google Scholar]
- Tamura A, Tahira Y, Nagashima H, et al. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke. 1991;22:615–618. doi: 10.1161/01.str.22.5.615. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. Seizures and epilepsy in the elderly. Arch. Int. Med. 1997;157:605–617. [PubMed] [Google Scholar]
- Velez L, Selwa LM. Seizure disorders in the elderly. Am. Fam. Physician. 2003;67:325–332. [PubMed] [Google Scholar]
- Wang RY, Wang PSG, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49:27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kumon Y, Ohta S, et al. Protein synthesis inhibitor transiently reduces neuronal death in the thalamus of spontaneously hypertensive rats following cortical infarction. Neurosci. Lett. 1997;233:25–28. doi: 10.1016/s0304-3940(97)00617-4. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kumon Y, Ohta S, et al. Changes in protein synthesis and calcium homeostasis in the thalamus of spontaneously hypertensive rats with focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1998;18:686–696. doi: 10.1097/00004647-199806000-00011. [DOI] [PubMed] [Google Scholar]
- Zelano J. Poststroke epilepsy. Ther. Adv. Neurol. Disord. 2016;9(5):424–435. doi: 10.1177/1756285616654423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.