Abstract
Working memory is a system by which a limited amount of information can be kept available for processing after the cessation of sensory input. Because working memory resources are limited, it is adaptive to focus processing on the most relevant information. We used a retro-cue paradigm to determine the extent to which monkey working memory possesses control mechanisms that focus processing on the most relevant representations. Monkeys saw a sample array of images, and shortly after the array disappeared, they were visually cued to a location that had been occupied by one of the sample images. The cue indicated which image should be remembered for the upcoming recognition test. By determining whether the monkeys were more accurate and quicker to respond to cued images compared to un-cued images, we tested the hypothesis that monkey working memory focuses processing on relevant information. We found a memory benefit for the cued image in terms of accuracy and retrieval speed with a memory load of two images. With a memory load of three images, we found a benefit in retrieval speed but only after shortening the onset latency of the retro-cue. Our results demonstrate previously unknown flexibility in the cognitive control of memory in monkeys, suggesting that control mechanisms in working memory likely evolved in a common ancestor of humans and monkeys more than 32 million years ago. Future work should be aimed at understanding the interaction between memory load and the ability to control memory resources, and the role of working memory control in generating differences in cognitive capacity among primates.
Keywords: cognitive control, retro-cue, attention, primate
Working memory is a central component of complex human cognitive abilities such as learning, language comprehension, planning, and reasoning (Unsworth & Robison, 2014). It allows for a limited amount of information to be kept available for processing in the absence of sustained sensory input, and the proficiency with which individuals do so is positively correlated with individual differences in scores of fluid intelligence (Baddeley & Hitch, 1974; McElree, 2006; Smith, Jonides, & Koeppe, 1996; Unsworth & Engle, 2007). Because the capacity of working memory is so limited, and because the relevance of information in working memory may change from moment to moment, it is critical to allocate working memory resources so as to process representations that are most relevant for current behavior (Chun, Golomb, & Turk-Browne, 2011; Cowan, 2010; Unsworth & Engle, 2007; Myers, Stokes, & Nobre, 2017). For instance, imagine an organism that has just encoded the spatial location of several food items varying in value. If a higher ranking individual approaches to take the highest value food, the lower ranking individual might benefit by allocating working memory resources to the maintenance and retrieval of the lower-value food items. Thus, the control of working memory resource allocation is adaptive because it increases the utility of working memory.
Human working memory possesses control mechanisms that allocate resources to processing relevant information (Astle & Scerif, 2011; Berryhill, Richmond, Shay, & Olson, 2012; Griffin & Nobre, 2003; Lepsien, Griffin, Devlin, & Nobre, 2005; MacLeod, 1998; Matsukura, Luck, & Vecera, 2007; Sligte, Scholte, & Lamme, 2008), but it is unclear whether nonhuman primate working memory includes similar cognitive control. Because working memory is critical to complex cognitive functions in humans, it is possible that differences in the extent to which humans and nonhuman primates control working memory resources efficiently may explain quantitative and qualitative differences in cognition among primates. For instance, a lack of control over memory resources may result in a working memory system cluttered with irrelevant information, reducing the quality and quantity of information available to control behavior. We assessed whether or not monkey working memory possesses control mechanisms that allow for relevant representations to recieve enhanced processing relative to other representations concurrently in working memory.
Both physiological (Fuster, 1997; Goldman-Rakic, 1995; Miller, Erickson, & Desimone, 1996) and behavioral (Basile & Hampton, 2010, 2013a; Tu & Hampton, 2014) evidence indicates that monkeys engage in short-term maintenance of representations relevant to current behavioral demands. Maintenance of some memories in monkeys is sensitive to competing cognitive load, indicating an active and cognitively demanding process akin to human working memory (Basile & Hampton, 2013). Processes supporting active maintenance in human working memory are thought to be greatly facilitated by verbal mechanisms (Baddeley, 2003; Wright et al., 1990), thus these findings are particularly striking because they suggest qualitative similarity in monkey working memory even in the absence of language.
Directed forgetting paradigms have been used to investigate human working memory control (Bjork & Woodward, 1973; MacLeod, 1998; Sheard & MacLeod, 2005) and have also been modified for use with pigeons (Kendrick, Rilling, & Stonebraker, 1981; Zentall, Roper, & Sherburne, 1995) and monkeys (Roberts, Mazmanian, & Kraemer, 1984; Tu & Hampton, 2014; Washburn & Astur, 1998). Subjects study a sample and a subsequent cue “instructs” them to either remember or forget the studied sample. Nonhuman animals, like humans, often show superior memory following remember cues, indicative of an active working memory control process. However, these findings from nonhumans have been extensively critiqued and may be subject to alternative explanations such as motivational differences from unequal reinforcement on remember and forget trials (see Washburn & Astur, 1998; Zentall, Roper, & Sherburne, 1995). A recent study in monkeys addressed many of these alternative explanations and still found evidence for active control in working memory (Tu & Hampton, 2014). Across several experiments controlling for reward expectation, differential reinforcement, the surprising nature of the probes, and the repeated use of a test following a forget cue, accuracy was significantly lower on memory tests following a forget-cue compared to a remember-cue (Tu & Hampton, 2014). These results extend the findings that monkeys engage in active maintenance (Basile & Hampton, 2013), and suggest that the engagement of this process can be under the control of external stimuli that differentiate between occasions on which memory is or is not beneficial.
After encoding a set of representations, behavioral demands may change, making some representations irrelevant. Working memory efficiency would be enhanced if resources were shifted to focus processing only on relevant representations. The directed forgetting paradigms used with monkeys thus far have not tested whether or not the target of working memory processing can be shifted among representations concurrently in working memory. For instance, the remember-cue used in Tu & Hampton (2014) indicated that all studied items should be remembered, and the forget-cue indicated that none of the studied items should be remembered. Thus, a fundamental question that has not been addressed is whether control mechanisms exist in monkey working memory that selectively allocate resources to the processing of relevant representations over other representations concurrently in working memory. To address this question we used a retro-cue paradigm in which a post-encoding cue identifies a single representation as most relevant among multiple representations encoded into working memory.
Retro-cue paradigms have been used in humans to investigate the ability to shift the target of processing in working memory (Astle & Scerif, 2011; Berryhill, Richmond, Shay, & Olson, 2012; Griffin & Nobre, 2003; Lepsien, Griffin, Devlin, & Nobre, 2005; Matsukura, Luck, & Vecera, 2007; Sligte, Scholte, & Lamme, 2008). Participants were briefly presented with an array of stimuli to encode, and after the offset of the stimuli, a spatial cue indicated to participants which of the stimuli in the previously viewed array would be tested in an upcoming recognition test. Focused processing of the cued item is inferred when participants are either more accurate or respond more quickly on trials in which the cue correctly predicts which item will be tested, compared to trials in which the cue incorrectly predicts which item will be tested, or to neutral trials that do not contain a cue (Astle & Scerif, 2011; Griffin & Nobre, 2003). Similar to the way in which a pre-cue shifts attention to enhance processing of stimuli in the environment (Posner, 1980), the retro-cue is thought to shift attention within working memory to the target item, enhancing the processing of that item, commonly referred to as the retro-cue benefit (Lepsien et al., 2005; Oberauer, 2013).
The two most prominent accounts of how retro-cues cause memory enhancement are the prioritization account and the protection account. The prioritization account maintains that the retro-cue focuses an “attentional spotlight” within working memory to guide the retrieval processes initiated at test (Griffin & Nobre, 2003; Rerko, Souza, & Oberauer, 2014). This shift in attention is most closely associated with faster reaction times to cued items compared to un-cued items, although initiating memory search with the target item could also improve accuracy. By contrast, the protection account posits that a shift in attention to the cued item draws memory resources away from the un-cued representations during the retention interval (Astle, Summerfield, Griffin, & Nobre, 2012; Makovski, Sussman, & Jiang, 2008). The protection account is most closely associated with improved accuracy for the cue item, rather than shorter response latency, but of course, enhancing discriminability of the target item in memory could also speed responding. These two accounts are therefore not mutually exclusive as improvements in latency or reaction time are consistent with both accounts, and may overlap with other explanations of the retro-cue effect (see Souza & Oberauer, 2016).
Although the exact processes invoked by retro-cues are still debated, it is agreed that shifting attention within working memory is a type of cognitive control (Berryhill et al., 2012). Thus, if we observe retro-cue effects in monkeys, this would indicate a previously unknown form of cognitive control in monkeys. The mechanism responsible for enhancing processing of retro-cued items appears to be visual-spatial rather than sub-vocal because the same results are obtained when participants are given a concurrent articulatory suppression task, (Makovski, Jiang, & Shim, 2006). Therefore, the retro-cue paradigm is well-suited for investigating control mechanisms within working memory in monkeys, particularly the specificity with which memory resources can be allocated after encoding multiple representations. Accordingly, we presented monkeys with stimulus arrays varying in cognitive load, and tested whether or not a retro-cue would result in enhanced accuracy, reaction time, or both, for the cued image.
2. Experiment 1
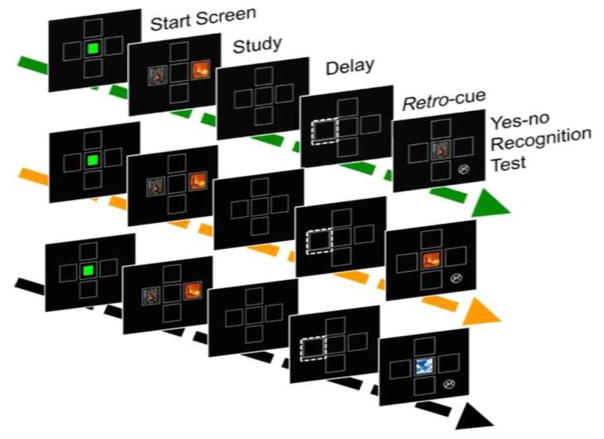
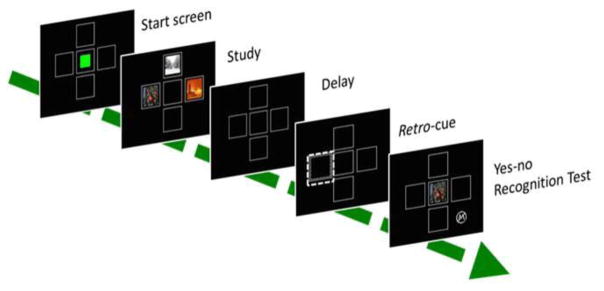
In Experiment 1, we tested whether memory would be enhanced by a spatial retro-cue that predicted which of two studied images would appear in a subsequent recognition memory test. Monkeys were trained on a yes/no recognition paradigm. During the study phase, an array of two images was presented, and during the test phase, monkeys had to decide whether a single test image had been present in the previously studied array. Half of the trials were match trials in which one of the studied images was presented at test, and half of the trials were non-match trials in which a non-studied image was presented at test. Between the offset of the study phase and the onset of the test phase, a retro-cue appeared in the location that had been occupied by one of the studied images. Match trials consisted of two types, congruent-match trials, and infrequent incongruent-match probe trials. Congruent-match trials were match trials in which the studied image previously in the cued location was tested. On incongruent-match probe trials, the image in the location other than the one indicated by the retro-cue was presented at test (Figure 1). Changes in task difficulty can be reflected in changes in either accuracy or response time (Basile & Hampton, 2013b; Hanks, Kiani, & Shadlen, 2014). Subjects may respond slowly to maintain accuracy, or accept deterioration of accuracy while holding response latency relatively constant. Thus, changes in memory processing caused by the retro-cue could manifest as a reaction time effect, accuracy effect, or both. We hypothesized that if the retro-cue focuses processing on the cued image, then we would observe faster reaction time, higher accuracy, or both, for congruent-match trials compared to incongruent-match probe trials.
Figure 1. Retro-cue paradigm.
Matching trials consisted of two types. Congruent-match trials were match trials in which the image previously in the cued location was tested (top). On incongruent-match probe trials the image in the location other than the one indicated by the retro-cue was presented at test (middle). On non-match trials the test image presented had not appeared in the study array (bottom). During the recognition test, touching the image was rewarded if it had appeared in the sample array; touching the non-match symbol was rewarded when the test image had not been present in the sample array. Half of trials were matching trials and half were non-matching trials. On matching trials the retro cue predicted which image would be tested 100% of trials during training, and 92 % of trials during probe testing.
2.1 Methods
2.1.1 Subjects and apparatus
Six adult male rhesus monkeys (Macaca mulatta) were used. Monkeys were pair-housed, received a full daily food ration, and had ad libitum access to water. The monkeys used in this study had extensive experience with recognition memory tasks using touch-screen computers. These tasks consisted of two forced choice and four forced choice match-to-sample paradigms and as well as yes/no memory recognition paradigms (Basile & Hampton, 2013a; Basile & Hampton 2013b). The monkeys had no previous experience with the flashing square stimulus that was used for the attention cue in the current study, and had never been shown multiple images simultaneously to remember. Monkeys were trained and tested in their home cage using a portable testing rig consisting of a 15-inch color LCD touch-sensitive screen (Elo TouchSystems, Menlo Park, CA) operating with a resolution of 1024 X 768 pixels, and two automatic food dispensers (Med Associates, Inc., Stl. Albans, VT) that delivered nutritionally balanced primate pellets (Bio-Serv, Frenchtown, NJ) into food cups below the screen. Each screen was mounted on the front of the monkey’s cage. Testing was controlled by a personal computer with a custom program written in Visual Studio 2013 (Microsoft Corporation). For each monkey, the calories from pellets earned during the day were subtracted from their total food ration, and they were given the balance of their ration in primate chow at the end of the day.
2.1.2 Stimuli
Five 218 X 196 pixel white frames were displayed on a black background. The frames were arranged in a cross pattern, so that a rectangle was in the center with the other four rectangles arranged on each side of the middle rectangle. The frames never went away during a testing session, and they defined the locations in which stimuli appeared (Figure 1). Test stimuli were nine 146 X 139 pixel color photographs of nature scenes that were repeated across trials. The same nine images were used across all experiments reported here. During the recognition test, a white circle with an M crossed out was used as a non-match response, used to indicate when the test image had not been part of the sample array from a given trial.
2.2 Procedure
Experiments were conducted in the monkeys’ home cages. During testing pair-housed monkeys were separated by dividers that allowed limited visual and physical contact, but prevented access to one another’s testing equipment. Each subject received multiple sessions per day, six days a week.
2.2.1 Initial Training
Monkeys were first trained on a yes/no recognition paradigm. Trials began when the monkey touched twice (FR 2) a green “ready” stimulus (100 X 91 pixels) presented in the middle of the center frame. The green ready stimulus then disappeared. During study, two images appeared simultaneously for two seconds, pseudo-randomly allocated to two of the 4 frames surrounding the central frame. The sample images disappeared automatically and were followed by a 1.8 second delay during which only the rectangle frames were on screen. During the recognition phase, an image appeared in the middle frame, in addition to a non-match response that appeared below the right most frame (Figure 1). Touching (FR2) the image in the middle frame was correct if the image had appeared during study on that trial (match trial). Touching (FR2) the non-match icon was correct if the central image was not from the study phase of that trial. Correct responses were rewarded with one banana pellet as well as a positive auditory stimulus. Incorrect responses resulted in a negative auditory stimulus and a seven second time-out during which only the frames were on screen. Correct trials were separated by an inter-trial interval of 5 seconds.
Training sessions consisted of 120 trials, 60 match trials, and 60 non-match trials. Sessions were divided into five 24 trial blocks. Within each block, each of the four study locations contained sample images equally often, and each location was paired with the other three study locations an equal number of times. Images were selected randomly with the constraint that six of the nine images appeared three times per block during the study phase; and three appeared twice. The three images used twice per block were randomly selected at the beginning of each block. The pairing of images for match trials and for non-match trials never repeated within a block. A sample from each location was used as a test image on matching trials three times during each block. On non-match trials, non-studied images were randomly selected to serve as the test image. Monkeys completed a minimum of six sessions and moved to the next phase after completing 75% or more of trials correctly, two sessions in a row.
2.2.2 Concurrent Cue Training 100% predictive
Monkeys were trained with an attention-getting cue that occurred concurrently with the sample image that they were to remember. The cue consisted of a larger frame that bordered one of the sample frames, and the original frame changed from a solid border to a dashed line border (Figure 1). This cue appeared for 150 ms, disappeared for 150 ms, and appeared again for 150 ms creating the perception of a blinking frame. The first blink began 150 ms before the onset of the sample, and the second blink began 150 ms after the onset. The cue predicted which image would be tested on 100% of the matching trials. Once monkeys completed two consecutive sessions above 80 percent, they proceeded to retro-cue training.
2.2.3 100 % predictive Retro-cue training
Trials proceeded as in concurrent cue training, except that instead of a concurrent cue, we used a retro-cue that appeared during the delay period between study and test. Before inserting retro-cue probe trials, we conducted a retro-cue training phase to ensure that they could perform the task accurately with the change from a concurrent cue to a retro-cue. The retro-cue was the same cue as used in concurrent training, and appeared 700 ms after the offset of the sample images. The test image appeared 650 ms after the retro-cue offset (Figure 1). Thus the delay interval between the offset of the study phase and the onset of the test phase was 1800 ms.
Training sessions used the same counter-balancing and consisted of the same number of trials as in initial training with the concurrent cue. Monkeys had to complete at least six sessions and obtain 80 percent accuracy or better in two consecutive sessions to move on to the next phase. Four of six monkeys met this criterion in less than 15 sessions. For the two monkeys that did not, the delay after the retro-cue was shortened to 400 ms such that the overall delay from study to test decreased from 1.8 seconds to 1.5 seconds. With this adjustment, the two monkeys met criterion within 15 additional sessions.
2.2.4 Retro-cue probe trials
Trials were the same as in retro-cue training, with the exception that five of the 60 match trials (8%) were incongruent-match probe trials. On incongruent-match probe trials the un-cued image, rather the cued image, appeared at test. Across 8 sessions, an image from each location served as the probe image two times. Choosing the test image was reinforced appropriately as a correct answer. Monkeys ran eight sessions of this phase, resulting in 40 incongruent-match probe trials and 440 congruent-match trials.
2.3 Results and Discussion
For all experiments, proportion correct scores were converted into d′ scores for analysis. The d′ score is the normalized proportion of hits (selecting the image on matching trials) minus the normalized proportion of false alarms (selecting the image on non-match trials). Because false alarms can only occur on non-match trials, the false alarm rates used in calculating the d′ scores were the same for congruent-match and incongruent-match trials. Using d′ provides an advantage over analyzing proportion correct scores because it controls for response bias that monkeys might have in a yes/no recognition paradigm. Congruent-match and incongruent-match probe trial d′ prime scores were compared with an independent samples t-test. Reaction time data were analyzed using repeated measures ANOVA of mean median reaction times with error trials excluded. All pair-wise comparisons were performed with fishers’ LSD correction.
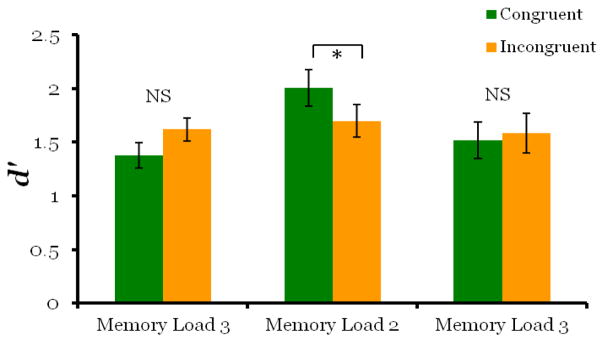
We observed a retro-cue benefit both in terms of accuracy and reaction time. Overall accuracy was high on both match trials (71%) and non-match trials (85%). Monkeys had significantly higher d′ scores on congruent-match trials, when the retro-cue correctly predicted which image appeared at test, than on incongruent-match probe trials, when the un-cued image was tested (Figure 2; t5 = 4.32, p = 0.008).
Figure 2. Monkeys were more accurate on congruent-match that incongruent-match trials in Experiment 1.
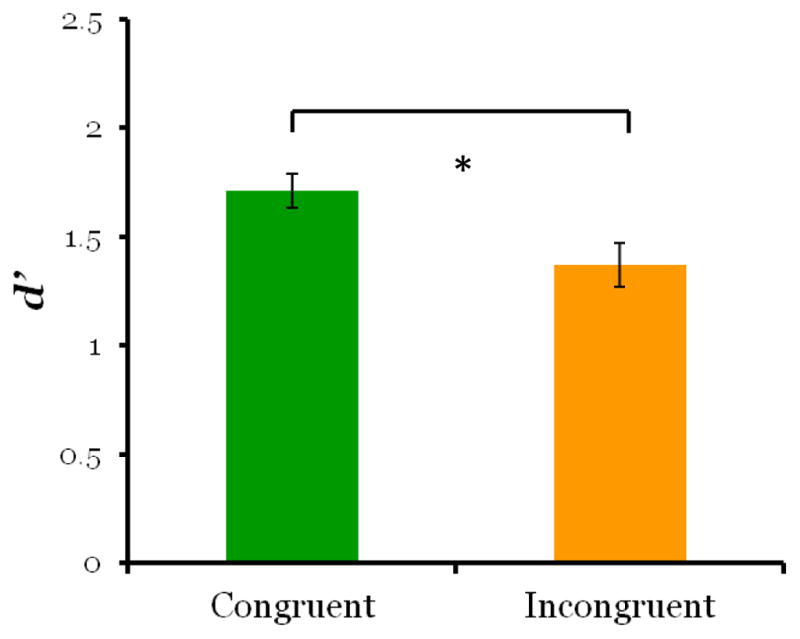
d′ scores were significantly higher for cued items compared to un-cued items. Because the retro-cue occurred after the offset of the sample images, the greater d′ score for cued items suggests that the retro-cue caused reallocation of processing to the cued image in working memory.
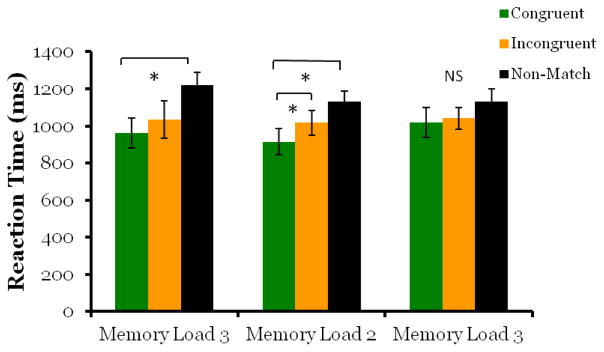
The retro-cue also caused monkeys to respond more quickly to cued images compared to un-cued images (Figure 3; F2,10 = 8.23, p = .008; congruent-match vs. incongruent-match: MD = −143.8, p = .026, congruent-match vs. non-match: MD = −372, p = .015).
Figure 3. Monkeys responded significantly faster to cued images.
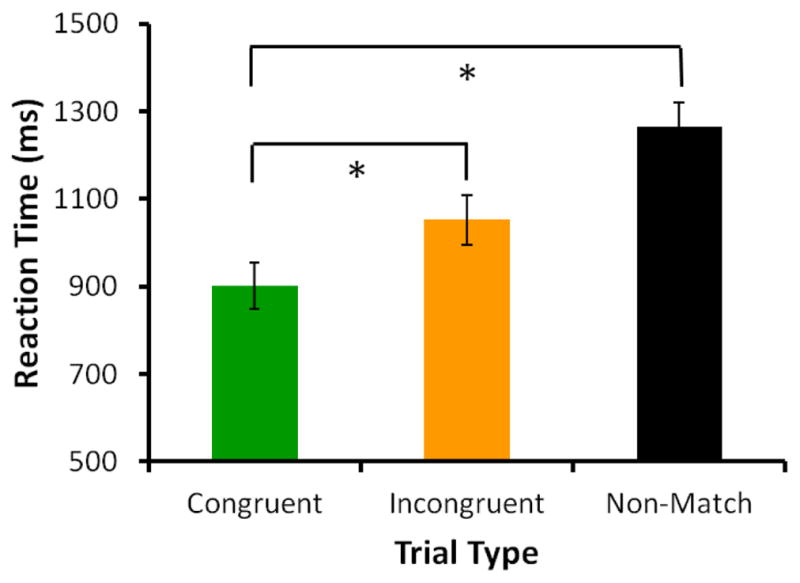
This further suggests that the retro-cue caused enhanced processing of cued images.
Taken together, this behavioral pattern of higher memory performance and faster response times to cued images indicates that after encoding, cued images received enhanced processing in working memory compared to un-cued images. The results obtained here are similar to those obtained by Griffin & Nobre (2003), suggesting that retro-cues can control the focus of processing within working memory in monkeys in a manner similar to what has been observed in humans.
3. Experiment 2
After establishing that the retro-cue benefit is present in monkeys in Experiment 1, we then tested whether the retro-cue responds to manipulations of memory load in the same way that it does in humans. It has been argued that when a sample array is within the limits of working memory capacity, there should be no cost, in terms of accuracy, to remembering un-cued items, and the benefit of reallocating memory resources will manifest itself primarily in the retrieval process, consistent with the prioritization account described earlier (Griffin & Nobre, 2003; Rerko et al., 2014). However when the number of images in the sample array exceeds the capacity of working memory, focusing processing on the cued image results in the un-cued images being eliminated from working memory (Astle et al., 2012; Makovski et al., 2008). Because performance remained above chance for un-cued images in Experiment 1, this suggests that they were not completely removed from working memory. Thus, in Experiment 2 we tested whether adding another image to the stimulus array would result in a greater demand to remove un-cued images from working memory. In Experiment 2a, monkeys performed the same experimental procedure as Experiment 1 with the exception that they were presented with three images in the stimulus array rather than two (Figure 4). We hypothesized that if allocating resources to enhance processing of the cued image results in memory resources being drawn from un-cued images towards the cued image, consistent with the protection account, then the difference in accuracy between congruent-match and incongruent-match probe trials would be larger compared to Experiment 1.
Figure 4. Memory load of three images.
Example of congruent-match retro-cue trial with a memory load of three images
3.1 Experiment 2a
3.1.1 Methods
3.1.1.1 Subjects and apparatus
The same monkeys were used. Testing apparatuses were identical.
3.1.1.2 Stimuli
The same stimuli used in Experiment 1 were used in Experiment 2.
3.1.2 Procedure
3.1.2.1 Training
Monkeys were trained as in Experiment 1 with the exception that three stimuli were presented during study phase instead of two (Figure 4). To meet criterion and move on to probe trials, monkeys had to complete at least six sessions and score above 70 percent correct in two consecutive sessions. The criterion for this phase was lower than Experiment 1 because the task was expected to be more difficult overall.
3.1.2.2 Probe Trials
The proportion of probe trials was the same as Experiment 1. Monkeys completed eight sessions of 120 trials, resulting in 40 total probe trials.
3.1.3 Results and Discussion
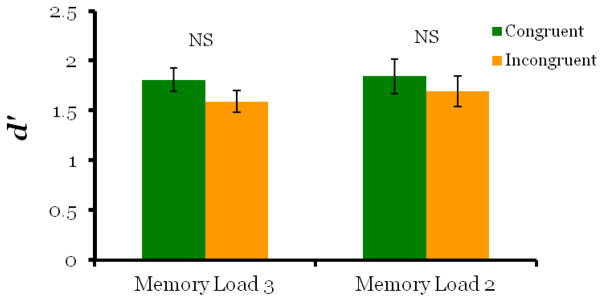
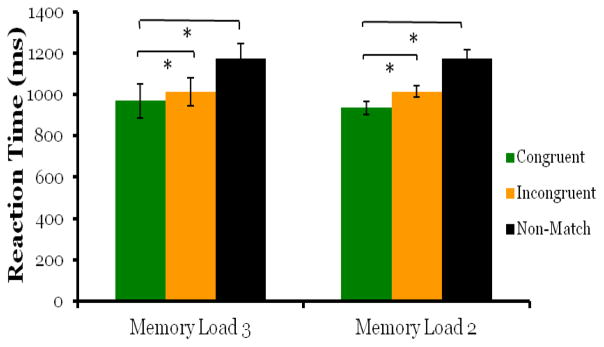
When the memory load was increased to three images, we found no evidence that the retro-cue affected processing in working memory. Overall accuracy on match trials (64%) and non-match trials (81%) was lower than Experiment 1 as expected with increased task difficulty. There was no significant difference in d′ scores for congruent-match and incongruent-match probe trials (Figure 5; t5 = −2.30, p = .07). Although the p value is approaching significance, the direction of the effect was reversed, as scores on incongruent-match probe trials were greater than congruent-match trials. The retro-cue also did not cause cued images to be retrieved faster than un-cued images. Although repeated measures ANOVA indicated a main effect of trial type (F2,10 = 4.92, p = .03), this result was driven entirely by slower response times on non-match trials (M = 1221ms, SD =163ms ) compared to match trials (congruent-match: M = 960ms, SD =198ms; incongruent-match: M = 1035ms, SD =251ms), with pair wise comparisons indicating no significant difference between congruent-match and incongruent-match trials (Figure 6; MD = −73.8, p = .388). These results show that when working memory load was increased to three images, monkeys performed above chance, but the retro-cue did not cause memory resources to be drawn away from un-cued images.
Figure 5. Results of Experiment 2.
In Experiment 2, d′ scores were significantly higher for cued items compared to un-cued items when working memory load was two images (Exp2b) replicating Experiment 1, but this effect was not found when working memory load was three images (Experiments 2a and 2c). These results suggest that sharing memory resources between three images compared to two, may result in insufficient memory strength for the retro-cue to have an effect.
Figure 6. Monkeys only show a retro-cue effect with a memory load of two.
Similar to the d′ scores of Experiment 2, the retro-cue only affected reaction time with a memory load of two images (Exp2b) and not with a memory load of three images (Exp 2a,2c). This further suggests representations in working memory with a memory load of 3 images were not sufficient in strength to be affected by the retro-cue.
The aim of Experiment 2a was to investigate whether increasing memory load would create more of a demand to remove un-cued images from processing in working memory, similar to findings in humans (Astle et al., 2012; Makovski et al., 2008). Monkeys showed no behavioral advantage from the retro-cue in accuracy or reaction time. Because monkeys still performed above chance on both congruent-match and incongruent-match trials, the lack of a retro-cue effect was not due to a failure to encode all three images. It is possible that due to extensive experience with incongruent-match probe trials, the monkeys began to ignore the retro-cue, and process all images equally. To determine whether the failure to see the retro-cue effect in Experiment 2a was caused by the change in memory load, rather than some other factor associated with extensive testing, we determined whether we could recover the retro-cue effect in Experiment 2b. We then also retested for the effect with a memory load of three images in Experiment 2c generating an A-B-A design. If monkeys began to ignore the retro cue in Experiment 2a, then they should continue to do so in Experiment 2b and 2c and we should find no further retro-cue effects. In contrast, if it is the increased memory load that caused the loss of the retro-cue effect, we should see the retro-cue effect again in Experiment 2b, but not in 2c.
3.2 Experiment 2b
3.2.1 Methods
All subjects, stimuli, and procedures were the same as in Experiment 1.
3.2.2 Results and Discussion
We replicated the results of Experiment 1 in Experiment 2b, finding that cued images received enhanced processing in working memory compared to un-cued images. Overall performance for match trials (80%) and non-match trials (85%) both increased from Experiment 2a. Monkeys had significantly higher d′ scores on congruent-match trials, when the cue correctly predicted the test image, than on incongruent-match probe trials (Figure 5; t5 = 6.24, p = 0.002). The retro-cue also caused cued images to be retrieved more quickly, compared to un-cued images, indicated by a main effect of trial type and a significant reaction time difference between congruent-match and incongruent-match trials, replicating the effect seen in Experiment 1 (Figure 6; F2,10 = 22.3, p = .000; congruent-match vs. incongruent-match: MD = −129.3, p = .008, congruent-match vs. non-match: MD = −202, p = .002).
3.3 Experiment 2c
3.3.1 Methods
All subjects, stimuli, and procedures were the same as in Experiment 2a.
3.3.2 Result and Discussion
We replicated the results of Experiment 2a in Experiment 2c, finding no retro-cue effect with a memory load of three images. Overall accuracy for match trials (70%) and non-match trials (80%) dropped with task difficulty. There was no significant difference in d′ scores between congruent-match and incongruent-match probe trials (Figure 5; t5 = −.404, p =.703). The retro-cue also did not have an effect on retrieval speed at test. Repeated measures indicated no main effect of trial type, (Figure 6; F2,10 = 2.7, p =.115), although monkeys were numerically fastest on match trials, (congruent-match trials: M = 1019ms, SD =200ms; incongruent-match trials M = 1040ms, SD =141ms) and slowest on non-match trials (M = 1130ms, SD =176ms).
The purpose of Experiment 2 was to investigate whether increasing memory load would increase the magnitude of the retro-cue effect. However, we failed to find the retro-cue effect at all with a memory load of three images. This absence of the retro-cue effect cannot be explained by practice, as monkeys demonstrate the effect when tested with a memory load of two images, and then fail to do so when tested with three images for a second time. Although overall accuracy was lower when memory load was increased, monkeys still performed well and significantly above chance. This suggests that monkeys encoded all three images at study, and all three images were available for retrieval at test. Therefore, the lack of a retro-cue effect with three images may be due to some qualitative difference in the representations maintained in working memory. Because the retro-cue operates by cueing a location, one intriguing possibility is that as memory load increases, the conjunction of spatial information and visual information is lost in working memory representations such that only visual identity remains. According to this account, the monkeys could still maintain the visual identity of the images but the retro-cue would not be able to enhance processing of any one image due to the loss of the spatial information necessary to determine which image would appear at test. Alternatively, it could be that the as memory load increases, information is lost in both visual and spatial domains such that the overall strength of the memory trace is weaker. If spatial information is sufficiently attenuated, the effect of the retro cue might be too weak to detect with our paradigm. To evaluate whether increasing memory load caused an absolute loss of spatial information or a quantitative deterioration in memory overall we arranged for the retro-cue to occur earlier following the offset of the sample array. We hypothesized that if increasing memory load to three images caused the loss of information about the location of each image, shortening the interval between sample array offset and retro-cue onset would not result in a retro-cue benefit. In contrast, if the increase in memory load resulted in a quantitative deterioration in memory overall, shortening the interval to onset of the retro-cue might result in recovery of the retro-cue effect, because memories would be stronger at the time the retro-cue occurred.
4. Experiment 3
In Experiment 3, we tested whether the retro-cue effect would be sensitive to changes in the strength of representations in working memory. In Experiment 3a we presented monkeys with three images during study and shortened the pre-cue delay compared to Experiment 2, so that the retro-cue would appear earlier, when representations should be stronger (Figure 7). We hypothesized that if the absence of the retro-cue effect with a working memory load of three images was because representations were overall too weak, then presenting the retro-cue earlier would make it more likely for the retro-cue effect to occur. Conversely, in Experiment 3b, we presented monkeys with two images, a condition in which we previously did get a retro-cue effect, but presented the retro-cue after a longer delay than in Experiment 1. We hypothesized that if the strength of the representations is a major determinant of the retro-cue effect, then presenting the cue later when representations are weaker would attenuate the effect of the retro-cue.
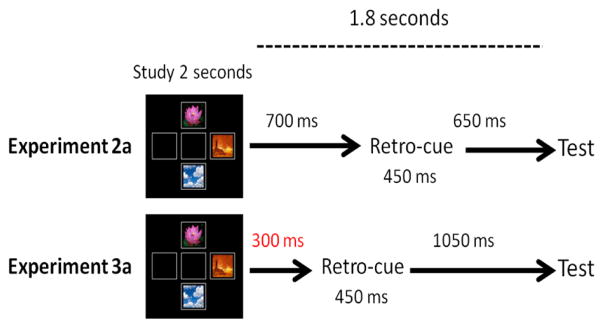
Figure 7. Comparison of Experiment 2a and Experiment 3a.
The pre-cue delay (red) was shortened by 400ms in Exp3a. However, the total time between study and test remained 1.8 seconds in both experiments.
4.1 Experiment 3a
4.1.1 Methods
4.1.1.1 Subjects and apparatus
The same subjects used in Experiment 1 were also used in Experiment 2. In addition, all testing apparatuses were identical to those described in Experiment 1.
4.1.1.2 Stimuli
The same stimuli used in Experiment 1 were used in Experiment 2.
4.1.2 Procedure
4.1.2.1 Training
Monkeys were trained with the same paradigm as Experiment 2a, except that the pre-cue delay was shortened by 400 ms. In Experiment 2a, the retro-cue appeared 700 ms after stimulus offset, while in Experiment 3a, the retro-cue appeared 300 ms after stimulus offset (Figure 7.). Monkeys were trained with a 100 percent predictive cue until reaching a criterion of 70 percent overall accuracy or higher in two consecutive sessions. All monkeys reached criterion within 15 sessions.
4.1.2.2 Probe Trials
The proportion of probe trials was the same as Experiment 1. Monkeys ran eight sessions of 120 trials, resulting in 40 total probe trials.
4.1.3 Results and Discussion
We found evidence indicating that when the delay to the onset of the retro-cue was shortened cued images received enhanced processing in working memory compared to un-cued images, even with a memory load of three. Overall accuracy was high for both match trials (78%) and non-match trials (83%). While monkeys were no more accurate on congruent-match trials (78%) compared to incongruent-match trials (71%) (Figure 9; t5 = .917, p = .401), they did respond more quickly to cued images compared to un-cued images (Figure 10; F2,10 = 22.6, p < .001; congruent-match vs. incongruent-match: MD = −42.5, p = .048, congruent-match vs. non-match: MD = −203.7, p = .003)
Figure 9. Results of Experiment 3.
When the retro-cue was presented earlier than in Experiment 2, with a memory load of three items, or later than in Experiment 2, with a memory load of two items, there was no effect of the retro cue on d′ scores (Experiment 3a and 3b). Because the total time between study and test was held constant, the attenuation of the retro-cue effect from Experiment 1 to 3b supports the hypothesis that representations in working memory must be of sufficient memory strength to be affected by a retro-cue.
Figure 10. Results of Experiment 3.
While there was no retro-cue effect on reaction time in Experiment 2a with a memory load of three items, there was an effect when the retro-cue was presented earlier (Exp3a). Compared to Experiment 1 and 2b with a memory load of two items, when the retro-cue was presented later (Exp3b), the retro-cue effect on reaction time was not attenuated. Because the total time between study and test remained constant between Experiment 2 and 3, the presence of the reaction time effect in Exp 3a supports the hypothesis that representations in working memory must be above a strength threshold for the retro-cue to have an effect.
Because we found an effect on accuracy with a memory load of two images, it is intriguing that we did not observe the same outcome here. The lack of an effect on accuracy does not support the hypothesis proposed in Experiment 2, that increasing memory load would result in un-cued images being removed from working memory. One possible explanation for the lack of an effect on accuracy seen here is that the cognitive process invoked by the retro-cue in monkeys responds differently to an increase in memory load than it does in humans, where it has been proposed that un-cued items are actively removed from working memory (Astle et al., 2012). However, a more conservative explanation, and one we think is more likely, is that changes in both latency and accuracy reflect the same underlying cognitive process. The extent to which both or only one of these dependent measures changes may depend on the extent to which subjects protect accuracy by slowing down their responses, or maximize the speed of responding at the cost of accuracy (Basile & Hampton, 2013; Hanks, Kiani, & Shadlen, 2014). Thus, changes in either latency or accuracy reflect a retro cue effect, and changes in just one of these measures, with the other relatively unaffected may reflect a comparatively small effect. The higher overall accuracy on congruent-match trials in Experiment 3a (78%) compared to Experiment 2a (64%) and 2c (70%) supports the idea that memory strength was greater at the time of the retro-cue onset in Experiment 3a. A retro-cue effect in Experiment 3a, when the memory load was the same as in Experiment 2a and 2c but the delay to the onset of the cue was shortened, supports the hypothesis that stronger memory traces are more subject to control by the retro-cue.
In Experiment 3b, we further tested this hypothesis by presenting two images during study and delaying the retro-cue to appear when representations may be weakened. If the ability of the retro-cue to modify processing within working memory depends on the strength of the representations, presenting the retro-cue later when representations are weaker would make a retro-cue effect less likely.
4.2 Experiment 3b
4.2.1 Methods
4.2.1.1 Subjects and apparatus
Two of the six monkeys used in Experiment 3a became unavailable after the conclusion of the experiment. To maintain the sample size of six, four of the monkeys used in the previous experiments were still tested and two new monkeys were added to the sample. The task-related experience of the new monkeys was the same as that of the other monkeys. The new monkeys were trained through all training phases described in Experiment 1, including probe trials. They did not complete Experiment 2. After the two additional monkeys completed Experiment 1, their data was added to the data from the six monkeys used in Experiment 1 and the same analyses were repeated. Overall accuracy remained high for both congruent-match trials (73%) and non-match trials (85%), and both the effects of accuracy and reaction time replicated (accuracy: t7 = 3.73, p = .007; reaction time, F2,14 = 13.98, p < .001; congruent-match vs. incongruent-match: MD= −149.6, p = .003, congruent-match vs. non-match: MD = −363.6, p = .002). After completing all phases of Experiment 1, the monkeys began Experiment 3b training with the other four monkeys. All testing apparatuses were identical to those described in Experiment 1.
4.2.1.2 Stimuli
The same stimuli used in Experiment 1 were used in Experiment 3.
4.2.2 Procedure
4.2.2.1 Training
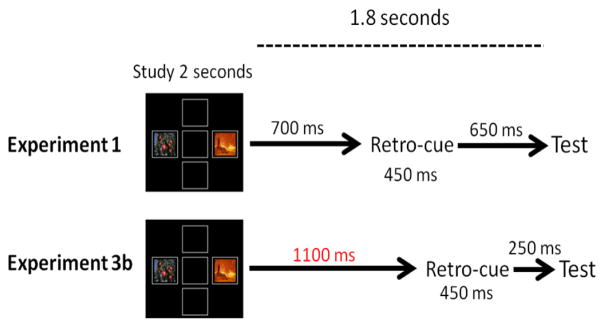
Monkeys were trained with the same paradigm as Experiment 1, with the exception that the retro-cue appeared 400 ms later. In Experiment 1, the retro-cue appeared 700 ms after stimulus offset, while in Experiment 3b, the retro-cue appeared 1100 ms after stimulus offset (Figure 8). Monkeys were trained with a 100 percent predictive cue until reaching a criterion of 80 percent overall accuracy or higher in two consecutive sessions.
Figure 8. Comparison of Experiment 1 and Experiment 3b.
The pre-cue delay (red) was lengthened by 400ms in Exp3b. However, the total time between study and test remained 1.8 seconds in both experiments.
4.2.2.2 Probe Trials
The proportion of probe trials was the same as Experiment 1. Monkeys ran eight sessions of 120 trials, resulting in 40 total probe trials.
4.2.3 Results and Discussion
With a memory load of two images and a delayed onset of the retro-cue we still found evidence that cued images received enhanced processing within working memory. But as in Experiment 3a, this effect was confined to a difference in reaction time, and not accuracy. Overall accuracy for higher than Experiment 2c, for both match trials (72%) and non-match trials (86%), however there was no significant difference between d′ scores for congruent-match and incongruent-match probe trials (Figure 9; t5 = 1.39, p =0.22). Monkeys responded more quickly to cued images than un-cued images (Figure 10; F2,10 = 52.8, p = .000, congruent-match vs. incongruent-match: MD = −79.3, p = .007, congruent-match vs. non-match: MD = −240.6, p = .000).
The lack of an effect on accuracy, combined with a reaction time effect, is similar to the results of Experiment 3a, suggesting that changing the timing of the retro-cue had a similar effect on the magnitude of the retro-cue effect. Furthermore, the difference in reaction time between congruent-match and incongruent-match trials in Experiment 1, where we found both an effect of accuracy and reaction time, is larger than in Experiment 3b where the pre-cue delay was lengthened. If monkeys traded slower response time for better accuracy, we would expect the latency difference to increase between Experiment 1 and 3b. Instead it decreased, but remained significant. The attenuation of the accuracy effect is therefore unlikely to be due to a speed-accuracy tradeoff, but rather a decrease in the overall effectiveness of the retro-cue.
In Experiment 3, we found evidence that the retro-cue effect is sensitive to the memory strength of representations in working memory. In Experiment 3a, we hypothesized that if representations were not of sufficient strength when presented with a working memory load of three images, then providing the cue earlier, when representations are stronger, would make it more likely for a retro-cue effect to occur. Experiment 3a showed that when the retro-cue was presented earlier, a reaction time effect was evident as monkeys responded more quickly to the presence of cued images compared to un-cued images. Compared with the absence of a retro-cue effect in Experiment 2, the effect observed in Experiment 3a where the only parameter changed was presenting the retro-cue earlier, supports the hypothesis that memory representations must be of sufficient strength for the retro-cue to have an effect. This suggests that increasing memory load does not lead to a specific loss of spatial information, but rather to a general deterioration in the quality of memory for the sample array. Conversely, in Experiment 3b, we predicted that with a memory load of two images, a condition in which monkeys previously showed a retro-cue effect, providing the cue later when representations are weaker should attenuate the effect of the retro-cue. Results indicated that the effect of the retro-cue on accuracy was attenuated, but not reaction time. Taken together, the results of Experiment 3 suggest that the ability of retro-cues to focus processing within working memory depends on the strength of the target representations in memory. An interesting avenue of further research will be to investigate what cognitive control mechanisms are available to memory representations of varying strength or resolution.
5. General Discussion
We found that a retro-cue controlled the allocation of working memory resources in monkeys. This effect was reduced when memory load was increased from two images to three images in Experiment 2, and when the delay between offset of the study array and onset of the retro-cue was increased in Experiment 3. Because decreasing the delay interval between study and the appearance of the retro-cue restored the retro-cue effect in Experiment 3a, it appears that a major determinant of the effectiveness of the retro-cue is the strength of the representations in working memory. Our observations of the retro-cue effect demonstrate a new form of cognitive control in monkeys, and indicate a striking similarity in working memory processes between monkeys and humans. Because monkey working memory operates in the absence of verbal mechanisms, this similarity suggests that the ability to control working memory resources evolved before the emergence of language. Our findings also suggests that this form of cognitive control may be more constrained in monkeys compared to humans, perhaps due to differences in the capacity of working memory.
In Experiment 1, we observed significantly higher accuracy and faster reaction times for cued images compared to un-cued images. This effect was consistent across Experiment 1 and Experiment 2b. As the encoding conditions were the same for both cued and un-cued trials, the difference in memory performance indicates that the retro-cue had an effect specifically on working memory processing. The results of Experiment 1 and 2b also parallel those from humans (Astle, Summerfield, Griffin, & Nobre, 2012; Griffin & Nobre, 2003; Makovski & Jiang, 2007). This suggests that monkey working memory, similar to human working memory, possesses control mechanisms that allow for relevant representations to recieve enhanced processing compared to other representations concurrently in working memory.
In Experiment 2, we hypothesized that if focusing memory resources on the cued image results in memory resources being drawn away from un-cued images and toward the cued image, then we should observe even more powerful effects of the retro-cue when the number of images to maintain in working memory increased from two to three. Increasing memory load results in a greater difference in accuracy between cued and un-cued items in humans (Duncan Edward Astle et al., 2012). However, we found that increasing memory load caused the retro-cue effect to disappear. The absence of the effect was not because monkeys failed to encode all three images, as performance remained above chance on both normal and probe trials. Nor was the absence of the effect due to practice, as monkeys showed the effect when memory load was decreased back to two images (Exp 2b), and then failed to show the effect when tested again with a memory load of three images (Exp 2c). Because monkeys were able to encode and retrieve all three images, we suggest that the lack of the retro-cue effect, and thus the lack of memory control, may have been due to a difference in memory strength of the representations in working memory at the time of the retro-cue onset. As monkeys had the same amount of study time allotted for encoding two images as they did three images, one possibility is that monkeys encoded three images with a memory strength strong enough to be recalled at test, but too weak to be affected by a retro-cue.
In Experiment 3, we found support for the hypothesis that memory strength is a critical determinant of the retro-cue effect. The appearance of the retro-cue effect in Experiment 3a indicates that in contrast to Experiment 2, memory representations that were stronger were more responsive to the retro-cue. Furthermore, in Experiment 3b we found that presenting the retro-cue later with a memory load of two images, when representations are presumably weaker, resulted in the attenuation of the retro-cue effect previously seen in Experiment 1 and Experiment 2a. Taken together, the results of Experiment 3 support the hypothesis that control over working memory representations may be confined to mental representations that are of sufficient strength. While this hypothesis fits well with some literature that has documented properties of visual working memory in humans, there are two main schools of thought that differ fundamentally on the interaction between memory load and memory strength.
Slot models suggest that visual working memory (VWM) in humans consists of three or four independent memory “slots” that each store information about a single visual item (Cowan, 2010; Luck & Vogel, 1997; Pashler, 1988; Schmidt, Vogel, Woodman, & Luck, 2002). According to the slot model, increasing the number of items in VWM does not change the resolution, or strength, of each item until memory load exceeds the number of slots. As performance remained above chance in monkeys with a memory load of three items, it was not the case that the representations had been lost from memory. Therefore, our finding of accurate recognition under conditions in which we found no retro-cue benefit are not easily reconcilable with slot models.
The view that working memory consists of a fixed number of independent slots has recently been challenged by studies suggesting that the resolution with which items are maintained depends critically on how many other items are concurrently in memory (Alvarez & Cavanagh, 2004; Awh, Barton, & Vogel, 2007). This resource model of VWM proposes that a single memory resource is shared between items concurrently in working memory. In contrast to the slot model, this model predicts that the resolution, or strength, with which an item is represented is determined by the fraction of total resources allocated to it (Bays, Catalao, & Husain, 2009). Thus, as more items are added to working memory, the resolution of each item decreases as fewer resources are available to maintain each item. Our results map easily to resource models. When we stretched memory resources thinly by either increasing the number of images to be remembered, or by increasing the delay interval, the retro-cue effect was lost or attenuated.
The resource model may explain why there was no effect of the retro-cue on performance when memory load was increased to three items. In the retro-cue paradigm, correctly associating the retro-cue with an item from VWM requires memory for both the visual identity and spatial position of each studied sample. According to the resource model, representations can be maintained at higher resolution with lower memory loads. Therefore, in the current study, a viable explanation of why there was no effect of the retro-cue when three items were maintained in VWM may be that the resolution with which each item was stored was such that the spatial information had been lost by the time of the retro-cue onset.
An interesting avenue for future studies in monkeys will be to investigate how the conjunction of spatial and visual information is maintained, or lost, when working memory load is increased. Previous evidence shows that when birds are presented a visual stimulus in a spatial location, birds that cache seeds prioritize the retention of the spatial features of the stimuli compared to birds that do not cache seeds. This suggests that the spatial and visual information of mental representations can decay independently as a result of processing strategies (Shettleworth & Westwood, 2002). One intriguing possibility is that in response to increased cognitive demand, the monkeys focused processing on the most relevant stimulus features needed to respond correctly. In our case, while spatial location was necessary to gain a benefit from the retro-cue, the most relevant feature at test was visual identity. Therefore, in the higher memory load condition, if memory strength decreased such that the spatial resolution was lost and only visual information was maintained, this would explain why the spatial retro-cue did not control the reallocation of memory resources, but matching performance remained above chance.
Because working memory plays a central role in many complex cognitive abilities associated with human intelligence (Unsworth & Engle, 2007), it is likely that the development of an efficient working memory system was critical in the evolution of human intelligence. Control mechanisms that allow for processing to be focused on information relevant to current demands is one way that the efficiency of working memory can be increased. The results of the current study provide evidence that working memory in monkeys possesses flexible control mechanisms that focus processing of relevant information over other representations concurrently in working memory, similar to humans. The memory control observed in the current study was most robust with a memory load of two images, when the retro-cue was presented 700 ms after stimulus offset. Human memory has been found to be affected by a retro-cue with sample arrays from two to eight items, and when the retro-cue is presented up to 15 seconds after stimulus offset (Astle et al., 2012; Griffin & Nobre, 2003; Matsukura et al., 2007). These results suggest a dramatic difference in the capacity and duration of human and monkey working memory, as has been reported previously (Elmore et al., 2011; Fagot & De Lillo, 2011). While these results add to the growing literature showing similarities in human and monkey working memory (Basile & Hampton, 2011, 2013; Miller et al., 1996; Tu & Hampton, 2014), they also point to at least quantitative differences in the control processes at work in human and nonhuman primate working memory.
Supplementary Material
Acknowledgments
We thank Tara A. Dove-VanWormer for help running subjects. This work was supported by National Science Foundation Grants IOS-1146316 and BCS-1632477, and by the Office of Research Infrastructure Programs, P51OD0111
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aslte D, et al. Orienting attention to locations in mental representations. Journal of Cognitive Neuroscience. 2003;15(8):1176–1194. doi: 10.1162/089892903322598139. 2012. http://doi.org/10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Alvarez G, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science: A Journal of the American Psychological Society/APS. 2004;15(2):106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. http://doi.org/10.1167/2.7.273. [DOI] [PubMed] [Google Scholar]
- Astle DE, Scerif G. Interactions between attention and visual short-term memory (VSTM): What can be learnt from individual and developmental differences? Neuropsychologia. 2011;49(6):1435–1445. doi: 10.1016/j.neuropsychologia.2010.12.001. http://doi.org/10.1016/j.neuropsychologia.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Astle DE, Summerfield J, Griffin I, Nobre AC. Orienting attention to locations in mental representations. Attention, Perception, & Psychophysics. 2012;74(1):146–162. doi: 10.3758/s13414-011-0218-3. http://doi.org/10.3758/s13414-011-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychological Science. 2007;18(7):622–628. doi: 10.1111/j.1467-9280.2007.01949.x. http://doi.org/10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. http://doi.org/10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working memory. The Psychology of Learning and Motivation Advances in Research and Theory. 1974 http://doi.org/10.1016/S0079-7421(08)60452-1.
- Basile BM, Hampton RR. Rhesus monkeys (Macaca mulatta) show robust primacy and recency in memory for lists from small, but not large, image sets. Behavioural Processes. 2010;83(2):183–190. doi: 10.1016/j.beproc.2009.12.013. http://doi.org/10.1016/j.beproc.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Monkeys show recognition without priming in a classification task. Behavioural Processes. 2013a;93:50–61. doi: 10.1016/j.beproc.2012.08.005. http://doi.org/10.1016/j.beproc.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Hampton RR. Recognition errors suggest fast familiarity and slow recollection in rhesus monkeys. Learning & Memory (Cold Spring Harbor, NY) 2013b;20(8):431–7. doi: 10.1101/lm.029223.112. http://doi.org/10.1101/lm.029223.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. Journal of Vision. 2009;9(10):7, 1–11. doi: 10.1167/9.10.7. http://doi.org/10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Richmond LL, Shay CS, Olson IR. Shifting attention among working memory representations: Testing cue type, awareness, and strategic control. The Quarterly Journal of Experimental Psychology. 2012;65(3):426–438. doi: 10.1080/17470218.2011.604786. http://doi.org/10.1080/17470218.2011.604786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The Magical Mystery Four: How Is Working Memory Capacity Limited, and Why? Current Directions in Psychological Science. 2010;19(1):51–57. doi: 10.1177/0963721409359277. http://doi.org/10.1177/0963721409359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore LC, Ji Ma W, Magnotti JF, Leising KJ, Passaro AD, Katz JS, Wright AA. Visual short-term memory compared in rhesus monkeys and humans. Current Biology. 2011;21(11):975–979. doi: 10.1016/j.cub.2011.04.031. http://doi.org/10.1016/j.cub.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot J, De Lillo C. A comparative study of working memory: Immediate serial spatial recall in baboons (Papio papio) and humans. Neuropsychologia. 2011;49(14):3870–3880. doi: 10.1016/j.neuropsychologia.2011.10.003. http://doi.org/10.1016/j.neuropsychologia.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Fuster - Network memory. 1997:1–9. Retrieved from papers2://publication/uuid/E5A118CC-8FAA-46FF-A772-CFBE78069EB5.
- Goldman-Rakic P. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. http://doi.org/10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Griffin IC, Nobre AC. Orienting attention to locations in internal representations. Journal of Cognitive Neuroscience. 2003;15(8):1176–1194. doi: 10.1162/089892903322598139. http://doi.org/10.1162/089892903322598139. [DOI] [PubMed] [Google Scholar]
- Hanks T, Kiani R, Shadlen MN. A neural mechanism of speed-accuracy tradeoff in macaque area LIP. 2014:1–17. doi: 10.7554/eLife.02260. http://doi.org/10.7554/eLife.02260. [DOI] [PMC free article] [PubMed]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. NeuroImage. 2005;26(3):733–743. doi: 10.1016/j.neuroimage.2005.02.026. http://doi.org/10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. Luck 1997- Nature- VWM. 1997;193(1996):1996–1998. http://doi.org/10.1038/36846. [Google Scholar]
- Makovski T, Jiang YV, Shim WM. Interference from filled delays on visual change detection. Journal of Vision. 2006;6(12):1459–70. doi: 10.1167/6.12.11. http://doi.org/10.1167/6.12.11. [DOI] [PubMed] [Google Scholar]
- Makovski T, Sussman R, Jiang YV. Orienting attention in visual working memory reduces interference from memory probes. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(2):369–380. doi: 10.1037/0278-7393.34.2.369. http://doi.org/10.1037/0278-7393.34.2.369. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Luck SJ, Vecera SP. Attention effects during visual short-term memory maintenance: protection or prioritization? Perception & Psychophysics. 2007;69(8):1422–34. doi: 10.3758/bf03192957. http://doi.org/10.3758/BF03192957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElree B. Accessing Recent Events. Psychology of Learning and Motivation - Advances in Research and Theory. 2006;46(6):155–200. http://doi.org/10.1016/S0079-7421(06)46005-9. [Google Scholar]
- Miller EK, Erickson Ca, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. http://doi.org/10.1.1.41.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K. The focus of attention in working memory-from metaphors to mechanisms. Frontiers in Human Neuroscience. 2013 Oct;7:673. doi: 10.3389/fnhum.2013.00673. http://doi.org/10.3389/fnhum.2013.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44(4):369–378. doi: 10.3758/bf03210419. http://doi.org/10.3758/BF03210419. [DOI] [PubMed] [Google Scholar]
- Rerko L, Souza AS, Oberauer K. Retro-cue benefits in working memory without sustained focal attention. Memory & Cognition. 2014;42(5):712–728. doi: 10.3758/s13421-013-0392-8. http://doi.org/10.3758/s13421-013-0392-8. [DOI] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Perception and Psychophysics. 2002;64(5):754–763. doi: 10.3758/bf03194742. http://doi.org/10.3758/BF03194742. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ, Westwood RP. Divided Attention, Memory, and Spatial Discrimination in Food-Storing and Nonstoring Birds, Black-Capped Chickadees ( Poecile atricapilla ) and Dark-Eyed Juncos ( Junco hyemalis ) 2002;28(3):227–241. http://doi.org/10.1037//0097-7403.28.3.227. [PubMed] [Google Scholar]
- Sligte IG, Scholte HS, Lamme VAF. Are there multiple visual short-term memory stores? PLoS ONE. 2008;3(2):2–10. doi: 10.1371/journal.pone.0001699. http://doi.org/10.1371/journal.pone.0001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe Ra. Dissociating Verbal and Spatial Working. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. http://doi.org/10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Tu H, Hampton RR. Control of Working Memory in Rhesus Monkeys ( Macaca mulatta ) Journal of Experimental Psychology: Animal Learning and Cognition. 2014;40(4):467–476. doi: 10.1037/xan0000030. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. http://doi.org/10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Robison MK. Individual differences in the allocation of attention to items in working memory: Evidence from pupillometry. Psychonomic Bulletin & Review. 2014:757–765. doi: 10.3758/s13423-014-0747-6. http://doi.org/10.3758/s13423-014-0747-6. [DOI] [PubMed]
- Wright aa, Cook RG, Rivera JJ, Shyan MR, Neiworth JJ, Jitsumori M. Naming, rehearsal, and interstimulus interval effects in memory processing. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1990;16(6):1043–1059. doi: 10.1037//0278-7393.16.6.1043. http://doi.org/10.1037/0278-7393.16.6.1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.