Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) has become prevalent worldwide in the last decade. However, the recent prevalence of NAFLD in adolescents has not yet been investigated in Korea.
Methods
Data were obtained from 1,416 participants aged 10–18 years from the Korea National Health and Nutrition Examination Survey conducted in 2010 and 2015. Systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, waist circumference (WC), body mass index (BMI), fasting glucose, total cholesterol, high-density lipoprotein (HDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT) level, waist-to-height ratio (WHtR), and pediatric NAFLD fibrosis index (PNFI) were analyzed.
Results
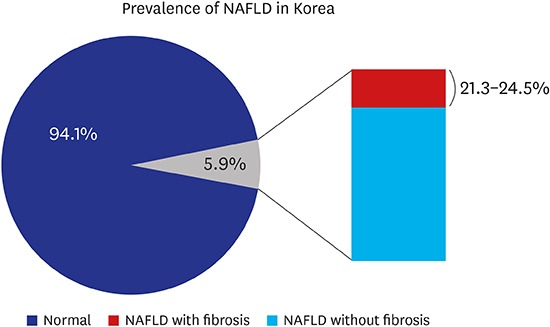
SBP, weight, WC, BMI, WHtR, and total cholesterol level were significantly higher in 2015 than in 2010. Prevalence of NAFLD (BMI ≥ 85th percentile plus ALT > 30 U/L for boys and ALT > 19 U/L for girls) were 4.7% in 2010 and 5.9% in 2015 (P = 0.360). Using various cutoffs for the ALT level (> 40, > 30, > 25.8 U/L for boys and >22.1 U/L for girls) NAFLD prevalence rates were 3.0%, 4.1%, and 5.5% in 2010; 2.9%, 5.0%, and 7.1% in 2015, respectively (P = 0.899, 0.469, and 0.289). Boys had a higher SBP, DBP, height, weight, WC, BMI, WHtR, fasting glucose, total cholesterol, ALT, and lower HDL level than girls. The probability of liver fibrosis using the PNFI varies between 21.3% and 24.5% among NAFLD participants (P < 0.001).
Conclusion
The Korean society needs to quickly control the increasing prevalence of NAFLD in adolescents and reduce its complications.
Keywords: Nonalcoholic Fatty Liver Disease, Estimated, Prevalence, Korea, Adolescents
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as steatosis in > 5% of hepatocytes, and it includes a wide spectrum of fatty liver disease, ranging from bland steatosis to nonalcoholic steatohepatitis (NASH). Additionally, it is the most common cause of liver disease in adults and children in the United States.1 NASH is a serious form of NAFLD that can develop into varying degrees of fibrosis. NASH occurs in 25% of patients with NAFLD, and of these, 10%–20% of cases may progress to severe fibrosis or cirrhosis.2,3,4 NAFLD has emerged as a significant cause of liver disease worldwide, even in developing economies in the last decade.5 Due to its association with obesity, the prevalence of NAFLD in Korean adolescents might have increased.6
The serum alanine aminotransferase (ALT) level is commonly used in clinical practice and research to determine the prevalence of NAFLD in public, as it is widely available, minimally invasive, and inexpensive to evaluate.1,7 However, the serum ALT level can be normal for children with histological NAFLD, thus there is a significant limitation of using the serum ALT level to screen for NAFLD.1,8 Moreover, determining an ALT cutoff for NAFLD varies widely across different laboratories and populations. Despite these limitations, Park et al.9 set an ALT cutoff value > 40 U/L to estimate the prevalence of NAFLD in Korean adolescents in 1998. Fraser et al.10 and Strauss et al.11 defined an abnormal ALT level as > 30 U/L using National Health and Nutrition Examination Survey (NHANES) data to estimate the prevalence of adolescents with NAFLD in the Unites States. Recently, the Screening ALT in Elevation in Today's Youth (SAFETY) study showed that the upper limit of the ALT level is set too high to reliably detect chronic liver disease, including NAFLD,12 suggesting ALT levels of 25.8 U/L for boys and 22.1 U/L for girls among the 95th percentile in healthy weight, metabolically normal, liver disease-free pediatric participants of the NHANES from 1999 to 2006. Recent trends in the prevalence of NAFLD among adolescents in the United States were assessed with these ALT levels as the upper normal limits.13 However, even though ALT cutpoints for defining elevation has not been specified, Expert Committee and North American Society of Pediatric Gastroenterology, Hepatology and Nutrition guidelines recommended the use of serum ALT levels to screen for NAFLD.13,14,15 Further, Kim et al.16 proposed ALT can be used as indicator of liver disease such as NAFLD, alcoholic liver disease, and metabolic liver disease. Thus, the criteria for ALT used to determine the prevalence of NAFLD may vary across ethnicity, age and sex.
Park et al.17 used ALT levels > 30 U/L for boys and > 19 U/L for girls to predict cardiovascular risk in Korean adolescents. We used these ALT cutoffs to evaluate the prevalence of NAFLD in Korean adolescents because it is the only data available for Korean adolescents even with such a limitation.
Identifying and validating potential novel, noninvasive biomarkers to define NAFLD with fibrosis in children is promising. Liver histology is required to confirm a diagnosis of NAFLD, and it is challenging to determine the accurate prevalence of adolescents with NAFLD in general populations.15 As a liver biopsy is not easy to perform in every pediatric patient, many noninvasive methods for adults have been used to evaluate fibrosis in pediatric patients. Further, the pediatric NAFLD fibrosis index (PNFI) was developed to predict fibrosis in patients with NAFLD, and it seems to predict fibrosis in children with NAFLD.18 PNFI has been introduced as a method to access fibrosis less precisely in European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition guideline, which is a disadvantage, but it is the only method that can be used to predict fibrosis as a method with data examined by Korean NHANES (KNHANES).15,19 A PNFI score ≥ 9 has a positive predictive value of 98.5 (confidence interval 91.8 to 100), and it can be used to rule in liver fibrosis without performing a liver biopsy.18
The purpose of this study was to analyze data from the 2010 and 2015 KNHANES to estimate the current prevalence of NAFLD among Korean adolescents using various noninvasive methods.
METHODS
Data
Data from KNHANES conducted in 2010 and 2015 by the Korea Centers for Disease Control and Prevention (KCDC) were used in this study. KNHANES is a cross-sectional and nationally representative survey based on the stratified, multi-stage probability samples of Korean households representing the civilian non-institutionalized population.
Subject selection
Data of total 16,338 participants from the 2010 and 2015 KNHANES were included. Participants aged < 10 or > 18 years, those who were hepatitis B surface antigen-positive, and participants who had missing data were excluded. Finally, 1,416 participants (776 boys and 640 girls) aged 10–18 years from the 2010 and 2015 KNHANES were included (Fig. 1).
Fig. 1.
Total 1,416 participants (776 boys and 640 girls) aged 10–18 years from the 2010 and 2015 KNHANES.
KNHANES = Korean National Health and Nutrition Examination Survey, HBsAg = hepatitis B virus surface antigen.
Measurement of anthropometric and laboratory variables
Height was measured to the nearest 0.1 cm using a stadiometer (SECA 225; Seca GmbH & Co. KG, Hamburg, Germany), and weight was measured to the nearest 0.1 kg using a balance beam scale (GL-600-20; G-Tech International, Uijungbu, Korea). Waist circumference (WC) was measured to the nearest 0.1 cm using a measuring tape (SECA 200; Seca GmbH & Co. KG). The body mass index (BMI) and waist-to-height ratio (WHtR) were calculated from the measured height and weight of participants (kg/m2 and waist [cm]/height [cm] × 100, respectively). The BMI percentile status was categorized by age and sex, according to 2007 Korean children-specific growth charts. Levels of cholesterol, triglyceride (TG), high-density lipoprotein (HDL), aspartate aminotransferase (AST), and ALT were measured enzymatically using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in 2010 and Hitachi Automatic Analyzer 7600-210 (Hitachi) in 2015. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured to the nearest 2 mmHg using a mercury sphygmomanometer (Baumanometer Desk model 0320 in 2010 or Baumanometer Wall Unit 0850 in 2015; W.A. Baum, Copiague, NY, USA), and blood pressure was calculated as the mean of three successive readings. For comparison purposes, the upper normal limit of the ALT level was categorized into 4 groups: sex-specific cutoffs for Korean adolescents, ALT > 30 U/L for boys and ALT > 19 U/L for girls17; sex-specific cutoffs for adolescents in the United States, > 25.8 U/L for boys and > 22.1 U/L for girls13; both sexes, > 40 U/L9; and both sexes, > 30 U/L.10 Additionally, the BMI was categorized into 4 groups: BMI ≥ 85th, ≥ 90th, ≥ 95th, and ≥ 97th percentile for age and sex. We examined the prevalence of suspected NAFLD by the ALT level and BMI category. The PNFI was calculated as:
lp = −6.539 × loge[age (years)] + 0.207 × waist (cm) + 1.957 × loge[TGs (mg/dL)] − 0.074
The linear predictor was transformed into the PNFI using the following equation, and a PNFI score ≥ 9 was considered as fibrosis in participants with suspected NAFLD18:
Statistical analysis
All statistical analyses were performed using SPSS, version 23.0 (IBM Corp., Armonk, NY, USA). Sampling weight factors representative of the Korean population were used because of the complex survey design of the KNHANES. Participants' anthropometry values and laboratory values were expressed as means and standard errors; t-tests were used to compare continuous variables between 2010 and 2015, and sexes. The χ2 test and Pearson correlation coefficients were used to analyze categorical variables, such as the prevalence of NAFLD and fibrosis. For all analyses, P values < 0.05 were considered statistically significant.
Ethics statement
The KNHANES survey (http://knhanes.cdc.go.kr/) was approved by the Institutional Review Board (IRB) of the KCDC (2010-02CON-21-C and 2015-01-02-6C). Written informed consent was obtained from all participants prior to the survey.
RESULTS
Characteristics of study participants in 2010 and 2015
Table 1 represents the weighted sample of 1,416 participants (776 boys and 640 girls) analyzed in this study. The SBP, weight, WC, BMI, WHtR, fasting glucose level, cholesterol level, and HDL level were significantly increased from 2010 to 2015. Levels of TG, AST, and ALT were also increased from 2010 to 2015, but they were not significantly different between the years.
Table 1. Participants characteristics in KNHANES 2010 and 2015.
| Characteristics | KNHANES | P value | |
|---|---|---|---|
| 2010 | 2015 | ||
| Age, yr | 14.223 ± 0.143 | 14.416 ± 0.127 | 0.314 |
| SBP, mmHg | 107.170 ± 0.551 | 109.048 ± 0.495 | 0.011 |
| DBP, mmHg | 66.966 ± 0.446 | 66.456 ± 0.369 | 0.378 |
| Height, cm | 161.540 ± 0.515 | 161.830 ± 0.540 | 0.698 |
| Weight26 | 54.476 ± 0.564 | 57.182 ± 0.662 | 0.002 |
| Waist, cm | 69.610 ± 0.445 | 72.742 ± 0.490 | < 0.001 |
| BMI, kg/m2 | 20.665 ± 0.153 | 21.599 ± 0.190 | < 0.001 |
| WHtR | 43.132 ± 0.266 | 44.969 ± 0.283 | < 0.001 |
| Glucose, mg/dL | 88.857 ± 0.303 | 91.435 ± 0.297 | < 0.001 |
| Cholesterol, mg/dL | 157.185 ± 1.169 | 161.544 ± 1.248 | 0.011 |
| HDL, mg/dL | 49.254 ± 0.399 | 51.307 ± 0.499 | 0.001 |
| TG, mg/dL | 84.897 ± 2.537 | 87.830 ± 2.696 | 0.428 |
| AST, U/L | 19.141 ± 0.334 | 19.962 ± 0.656 | 0.265 |
| ALT, U/L | 15.314 ± 0.619 | 16.554 ± 1.230 | 0.368 |
Data presented as mean ± standard error.
KNHANES = Korean National Health and Nutrition Examination Survey, SBP = systolic blood pressure, DBP = diastolic blood pressure, BMI = body mass index, WHtR = waist-to-height ratio, HDL = high-density lipoprotein, TG = triglyceride, AST = aspartate aminotransferase, ALT = alanine aminotransferase.
Changes in the ALT level and BMI
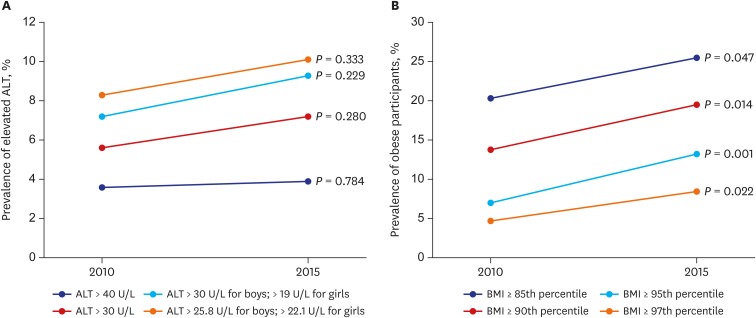
The ALT level among Korean adolescents in 2010 and 2015 changed by 7.2% to 9.3% (P = 0.229) using cutoffs of an ALT level > 30 U/L for boys and > 19 U/L for girls; by 8.3% to 10.1% (P = 0.333) using cutoffs of an ALT level > 25.8 U/L for boys and > 22.1 U/L for girls; by 3.6% to 3.9% (P = 0.784) using a cutoff of an ALT level > 40 U/L for both sexes; and by 5.6% to 7.2% (P = 0.280) using a cutoff of an ALT level > 30 U/L for both sexes (Fig. 2A). An elevated BMI was seen in 2010 and 2015, increasing by 20.3% to 25.5% (P = 0.047) using a BMI ≥ 85th percentile; by 13.8% to 19.5% (P = 0.014) using a BMI ≥ 90th percentile; by 7.0% to 13.2% (P = 0.001) using a BMI ≥ 95th percentile; and by 4.7% to 8.4% (P = 0.022) using a BMI ≥ 97th percentile (Fig. 2B).
Fig. 2.
ALT and BMI among Korean adolescents. (A) Prevalence of elevated ALT with 4 different criteria. (B) Prevalence of obese with 4 different BMI criteria.
ALT = alanine aminotransferase, BMI = body mass index.
Prevalence of NAFLD with various cutoffs of the ALT level and BMI
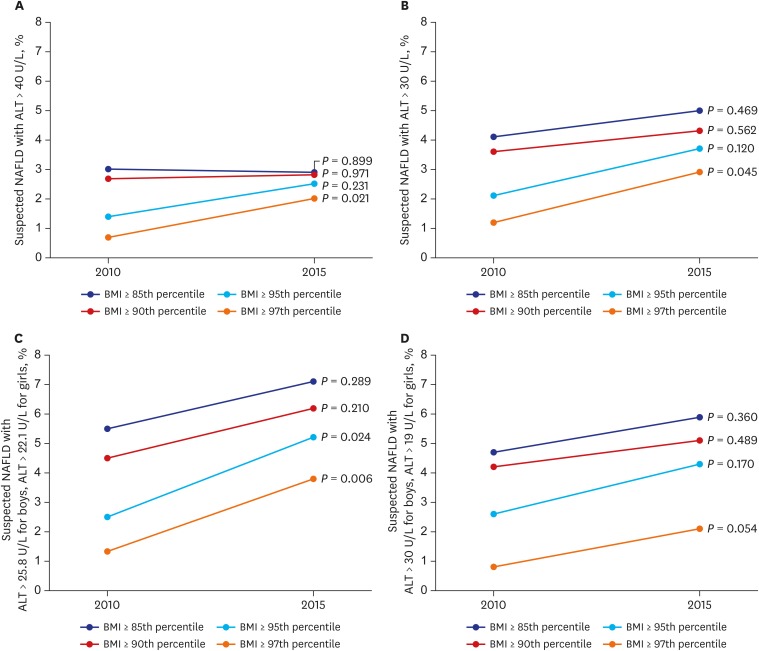
The prevalence of NAFLD was estimated in Korean adolescents by an elevated BMI plus an elevated ALT level according to the different categories. Prevalences of an ALT level > 40 U/L plus an elevated BMI by 4 categories are shown in Fig. 3A. The prevalence changed by 3.0% to 2.9% using a BMI ≥ 85th percentile (P = 0.899); by 2.7% to 2.8% using a BMI ≥ 90th percentile (P = 0.971); by 1.4% to 2.5% using a BMI ≥ 95th percentile (P = 0.231); and by 0.7% to 2.0% using a BMI ≥ 97th percentile (P = 0.021). Prevalences of an ALT level > 30 U/L plus an elevated BMI by 4 categories are shown at Fig. 3B. The prevalence changed by 4.1% to 5.0% using a BMI ≥ 85th percentile (P = 0.469); by 3.6% to 4.3% using a BMI ≥ 90th percentile (P = 0.562); by 2.1% to 3.7% using a BMI ≥ 95th percentile (P = 0.120); and by 1.2% to 2.9% using a BMI ≥ 97th percentile (P = 0.045). Prevalences of an ALT level > 25.8 U/L for boys and > 22.1 U/L for girls13 plus an elevated BMI by 4 categories are shown in Fig. 3C. Prevalences changed by 5.5% to 7.1% using a BMI ≥ 85th percentile (P = 0.289); by 4.5% to 6.2% using a BMI ≥ 90th percentile (P = 0.210); by 2.5% to 5.2% using a BMI ≥ 95th percentile (P = 0.024); and by 1.3% to 3.8% using a BMI ≥ 97th percentile (P = 0.006). Prevalences of an ALT level > 30 U/L for boys and > 19 U/L for girls plus an elevated BMI by 4 categories are shown in Fig. 3D. Prevalences changed by 4.7% to 5.9% using a BMI ≥ 85th percentile (P = 0.289); by 4.2% to 5.1% using a BMI ≥ 90th percentile (P = 0.489); by 2.6% to 4.3% using a BMI ≥ 95th percentile (P = 0.170); and by 0.8% to 2.1% using a BMI ≥ 97th percentile (P = 0.054).
Fig. 3.
Suspected adolescent NAFLD in Korea with different BMI and ALT criteria. (A) obese + ALT > 40 U/L; (B) obese + ALT > 30 U/L; (C) obese + ALT > 25.8 U/L for boys and > 22.1 U/L for girls; (D) obese + ALT > 30 U/L for boys and > 19 U/L for girls.
NAFLD = nonalcoholic fatty liver disease, BMI = body mass index, ALT = alanine aminotransferase.
Fibrosis in participants suspected of having NAFLD
The PNFI was calculated in participants suspected of having NAFLD. Estimated prevalences of fibrosis were 21% with an ALT level > 30 U/L for boys and > 19 U/L for girls (P < 0.001); 21.3% with an ALT level > 30 U/L for boys and > 19 U/L for girls (P < 0.001); 23% with an ALT level > 40 U/L for both sexes (P < 0.001); and 24.5% with an ALT level > 30 U/L for both sexes (P < 0.001).18,20
Characteristics of study participants by sex
A description of the weighted sample of 776 boys and 640 girls is provided in Table 2. The SBP, height, weight, waist, cholesterol level, AST level, and ALT level were significantly higher in boys than in girls (P < 0.001). Also, DBP (P = 0.020), BMI (P = 0.005), WHtR (P = 0.041), fasting glucose level (P = 0.005) were higher in boys, and the HDL level was lower in boys than in girls (P < 0.001). Similarly, the prevalence of NAFLD was higher in boys than in girls. Additionally, the prevalence of NAFLD in boys has not changed since 2010 to 2015 (Fig. 4A). However, the prevalence of NAFLD in girls has generally increased from 2010 to 2015 (Fig. 4B).
Table 2. Participants characteristics in KNHANES by sex.
| Characteristics | KNHANES | P value | |
|---|---|---|---|
| Male | Female | ||
| Age, yr | 14.335 ± 0.109 | 14.202 ± 0.135 | 0.404 |
| SBP, mmHg | 110.505 ± 0.518 | 105.098 ± 0.444 | < 0.001 |
| DBP, mmHg | 67.297 ± 0.408 | 66.078 ± 0.379 | 0.020 |
| Height, cm | 165.279 ± 0.526 | 157.439 ± 0.362 | < 0.001 |
| Weight26 | 59.105 ± 0.615 | 51.698 ± 0.541 | < 0.001 |
| Waist, cm | 73.124 ± 0.473 | 68.558 ± 0.440 | < 0.001 |
| BMI, kg/m2 | 21.400 ± 0.173 | 20.718 ± 0.173 | 0.005 |
| WHtR | 44.316 ± 0.288 | 43.543 ± 0.259 | 0.041 |
| Glucose, mg/dL | 90.531 ± 0.303 | 89.421 ± 0.293 | 0.005 |
| Cholesterol, mg/dL | 155.120 ± 1.286 | 163.880 ± 1.191 | < 0.001 |
| HDL, mg/dL | 49.294 ± 0.425 | 51.220 ± 0.434 | 0.001 |
| TG, mg/dL | 85.806 ± 2.537 | 86.706 ± 2.274 | 0.772 |
| AST, U/L | 21.302 ± 0.604 | 17.410 ± 0.211 | < 0.001 |
| ALT, U/L | 19.395 ± 1.149 | 11.741 ± 0.266 | < 0.001 |
Data presented as mean ± standard error.
KNHANES = Korean National Health and Nutrition Examination Survey, SBP = systolic blood pressure, DBP = diastolic blood pressure, BMI = body mass index, WHtR = waist-to-height ratio, HDL = high-density lipoprotein, TG = triglyceride, AST = aspartate aminotransferase, ALT = alanine aminotransferase.
Fig. 4.
Estimated prevalence of NAFLD by sex in Korea defined using overweight (BMI ≥ 85th percentile) plus various cutoffs for elevated ALT. (A) boys; (B) girls.
NAFLD = nonalcoholic fatty liver disease, BMI = body mass index, ALT = alanine aminotransferase.
DISCUSSION
Since diagnosis of NAFLD requires a biopsy or imaging study, the diagnosis by simple blood tests requires many assumptions and difficulties. Although there have been studies to determine the prevalence of NAFLD in liver biopsies of children who have died in unexpected accidents, it is difficult to carry out biopsy for all adolescents.21 In the past, there were numbers of studies that attempted to determine the prevalence of NAFLD with a simple ALT elevation. In Korea, there was also a study in which showed the prevalence of NAFLD in 1998 was 3.2% in Korea, defined as NAFLD as ALT over 30. Hyun et al.22 suggested metabolic syndrome and NAFLD can be main cause of ALT elevation in Korea. Further, adolescent obesity seemed to increase by years in many study.23 However, there was no study to determine the prevalence of NAFLD by obesity plus ALT elevation. It was thought that NAFLD would also increase through such data, but it was impossible to confirm it. Therefore, we tried to define the NALFD using the most widely used BMI over 85 percentile plus elevated ALT in Korean adolescents to predict the prevalence. In this study, we tried to define NAFLD based on obesity plus elevated ALT through hypothesis, but it is also problematic that there is no defining elevated BMI and ALT criteria have not been specified. So, in this paper, we tried to define NAFLD based on various BMI and ALT.
The prevalence of NAFLD in Korean adolescents in the general population is difficult and unclear to assess accurately due to a lack of validated Korean-specific noninvasive diagnostic methods. Similar to other study, SBP, BMI, weight, and WHtR of adolescents increased in 2015 compared to 2010.24 In addition, fasting blood sugar and cholesterol were significantly increased. TG was increased but not significant.24,25
According to our results, prevalence of NAFLD among Korean adolescents were 5.9% in 2015 by an ALT level > 30 U/L for boys and > 19 U/L for girls, and 7.1% in 2015 by an ALT > 25.8 U/L for boys and > 22.1 U/L for girls. Additionally, the prevalence seemed to increase, especially for girls, since 2010 to 2015. Previous studies on estimating the prevalence of NAFLD among Korean adolescents were conducted using an ALT level > 40 U/L for criteria of NAFLD. If we had set the cutoff of ALT as > 40 U/L to estimate the prevalence of NAFLD in Korean adolescents, the prevalence would be 2.9% in 2015, indicating a decrease of 3.2% in 1998. However, it is doubtful that the prevalence of NAFLD has decreased from 1998 to 2015. Further, NAFLD prevalence can be predicted more accurately if the upper normal limit of BMI and the ALT level is modified for NAFLD in Korean adolescents. About 1/5 of patients suspected of having NAFLD also seem to have liver fibrosis regardless of the criteria.
This study had some limitations. First, the upper normal values of ALT for Korean adolescents were unavailable, so further study will be needed in the future to estimate the prevalence of NAFLD more meaningfully. Second, the Korean-specific growth chart used in this study was made in 2007, thus this growth chart needs to be updated. Third, liver-specific laboratory tests, such as the levels of albumin, γ-glutamyl transferase, prothrombin time, etc., should be included in further KNHANESs. Fourth, the use of hepatotoxic medications was unavailable for participants in the KNHANES, and alcohol consumption in adolescents was not included in these data. These limitations may have resulted in an overestimation of the true prevalence of NAFLD in Korean adolescents. However, despite these limitations, this is the first large-scale study to estimate the prevalence of NAFLD with various methods in the general population of Korean adolescents.
In conclusion, we estimated that 296,865 and 62,341 of Korean adolescents in 2015 had NAFLD and NAFLD with fibrosis, respectively. According to our findings, the Korean society must to be prepared to quickly control the increasing prevalence of NAFLD and reduce its complications.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kang Y. Data curation: Kang Y. Formal analysis: Kang Y. Investigation: Kang Y. Methodology: Koh H. Software: Kim S, Park S. Validation: Kim S, Park S. Writing - original draft: Kang Y. Writing - review & editing: Kang Y, Kim S, Park S, Koh H.
References
- 1.Suchy FJ, Sokol RJ, Balistreri WF. Liver Disease in Children. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2014. [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19(3):210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 6.Lee HJ, Kim SH, Choi SH, Lee JS. The association between socioeconomic status and obesity in Korean children: an analysis of the fifth Korea National Health and Nutrition Examination Survey (2010–2012) Pediatr Gastroenterol Hepatol Nutr. 2017;20(3):186–193. doi: 10.5223/pghn.2017.20.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh H, Jun DW, Saeed WK, Nguyen MH. Non-alcoholic fatty liver diseases: update on the challenge of diagnosis and treatment. Clin Mol Hepatol. 2016;22(3):327–335. doi: 10.3350/cmh.2016.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes. 2008;32(2):381–387. doi: 10.1038/sj.ijo.0803711. [DOI] [PubMed] [Google Scholar]
- 9.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82(5):1046–1051. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 10.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133(6):1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136(6):727–733. [PubMed] [Google Scholar]
- 12.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357–1364. 1364.e1–1364.e2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162(3):496–500.e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 15.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) J Pediatr Gastroenterol Nutr. 2017;64(2):319–334. doi: 10.1097/MPG.0000000000001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC, Public Policy Committee of the American Association for the Study of Liver Disease Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 17.Park HK, Hwang JS, Moon JS, Lee JA, Kim DH, Lim JS. Healthy range of serum alanine aminotransferase and its predictive power for cardiovascular risk in children and adolescents. J Pediatr Gastroenterol Nutr. 2013;56(6):686–691. doi: 10.1097/MPG.0b013e31828b4e67. [DOI] [PubMed] [Google Scholar]
- 18.Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. doi: 10.1186/1741-7015-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54(5):700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 20.Park BH, Yoon JM, Kim JH, Moon JH, Lee YH, Jang SM, et al. Pathologic impact of insulin resistance and sensitivity on the severity of liver histopathology in pediatric non-alcoholic steatohepatitis. Yonsei Med J. 2017;58(4):756–762. doi: 10.3349/ymj.2017.58.4.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 22.Hyun HJ, Shim JJ, Kim JW, Lee JS, Lee CK, Jang JY, et al. The prevalence of elevated alanine transaminase and its possible causes in the general Korean population. J Clin Gastroenterol. 2014;48(6):534–539. doi: 10.1097/MCG.0b013e3182a474d3. [DOI] [PubMed] [Google Scholar]
- 23.Nam HK, Kim HR, Rhie YJ, Lee KH. Trends in the prevalence of extreme obesity among Korean children and adolescents from 2001 to 2014. J Pediatr Endocrinol Metab. 2017;30(5):517–523. doi: 10.1515/jpem-2016-0456. [DOI] [PubMed] [Google Scholar]
- 24.Choi DH, Hur YI, Kang JH, Kim K, Cho YG, Hong SM, et al. Usefulness of the waist circumference-to-height ratio in screening for obesity and metabolic syndrome among Korean children and adolescents: Korea National Health and Nutrition Examination Survey, 2010–2014. Nutrients. 2017;9(3):E256. doi: 10.3390/nu9030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MJ, Boston BA, Oh M, Jee SH. Prevalence and trends of metabolic syndrome among Korean adolescents: from the Korean NHANES Survey, 1998–2005. J Pediatr. 2009;155(4):529–534. doi: 10.1016/j.jpeds.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 26.Arslan N, Büyükgebiz B, Oztürk Y, Cakmakçi H. Fatty liver in obese children: prevalence and correlation with anthropometric measurements and hyperlipidemia. Turk J Pediatr. 2005;47(1):23–27. [PubMed] [Google Scholar]