Abstract
Background
The quality of life (QoL) of patients with end-stage renal disease (ESRD) is very poor, plausibly due to both psychosocial and medical factors. This study aimed to determine the relationship among psychosocial factors, medical factors, and QoL in patients with ESRD undergoing hemodialysis (HD).
Methods
In total, 55 male and 47 female patients were evaluated (mean age, 57.1 ± 12.0 years). The QoL was evaluated using the Korean version of World Health Organization Quality of Life Scale-Abbreviated Version. The psychosocial factors were evaluated using the Hospital Anxiety and Depression Scale, Multidimensional Scale of Perceived Social Support, Montreal Cognitive Assessment, Pittsburgh Sleep Quality Index, and Zarit Burden Interview. The medical factors were assessed using laboratory examinations. Correlation and canonical correlation analyses were performed to investigate the association patterns.
Results
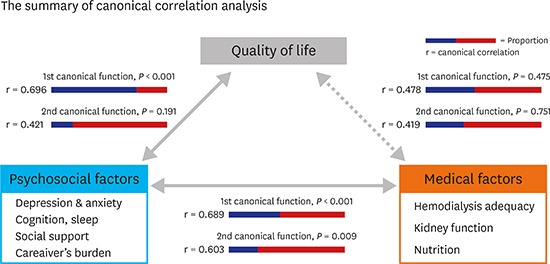
The QoL was significantly correlated with the psychosocial factors, and to a lesser extent with the medical factors. The medical and psychosocial factors were also correlated. The canonical correlation analysis indicated a correlation between QoL and psychosocial factors (1st canonical correlation = 0.696, P < 0.001; 2nd canonical correlation = 0.421, P = 0.191), but not medical factors (1st canonical correlation = 0.478, P = 0.475; 2nd canonical correlation = 0.419, P = 0.751). The medical and psychosocial factors were also correlated (1st canonical correlation = 0.689, P < 0.001; 2nd canonical correlation = 0.603, P = 0.009).
Conclusion
Psychosocial factors influence QoL in patients with ESRD, and should thus be carefully considered when caring for these patients in clinical practice.
Keywords: Psychosocial Factor, Medical Factor, Quality of Life, End-stage Renal Disease, Canonical Correlation Analysis
Graphical Abstract
INTRODUCTION
End-stage renal disease (ESRD) is a serious public health problem associated with poor quality of life (QoL).1,2 In Korea, the number of patients with ESRD undergoing dialysis has been rapidly growing in recent years, especially in elderly patients. The total number of patients with renal replacement therapy (RRT) in Korea was 75,042 in 2013. The prevalence of RRT was 1,446.4 patients per million population.3 The concept of health-related QoL (HRQoL) of the World Health Organization (WHO) is defined as the subjective assessment of the impact of disease and its treatment across the physical, psychological, and social domains of functioning and well-being.4 Low HRQoL scores in dialysis patients are strong independent predictors of hospitalization and mortality.5
The treatment of ESRD is challenging and lengthy, and requires to improve QoL in addition to survival.6 However, many patients with ESRD do not report improvement in their QoL after they initiate the hemodialysis (HD).7,8,9 Thus, we assume that factors other than medical factors are implicated in the QoL of patients with ESRD. Indeed, emotional and social factors may also influence QoL. However, the psychosocial factors are often overlooked in the medical field. Thus, we investigated the impact of both psychosocial and medical factors on QoL in patients with ESRD undergoing HD. There are few studies that have evaluated the medical and psychosocial factors that are associated with HRQoL in HD patients. However, most studies have used Short-Form 36 (SF-36) or EuroQoL-5 Dimensions (Eq-5D) to measure HRQoL. Moreover, only few studies evaluated the association between HRQoL and psychosocial factors and used multivariate analysis for a comprehensive understanding.
The present study used the WHO Quality of Life Scale-Abbreviated Version (WHOQOL-BREF) to measure QoL in patients with ESRD. Furthermore, we used the canonical correlation analysis to analyze comprehensively the psychosocial and medical factors; indeed, QoL can be explained clearly by multivariate rather than univariate analysis.
METHODS
Patients and study design
Out of 155 patients with ESRD, undergoing HD at the Daegu Catholic University Medical Center between September and October 2013, we selected those who could complete the questionnaires individually. Patients were excluded if they were receiving HD because of acute kidney injury, hospitalized with a psychotic spectrum disorder, or under 20 or over 80 years of age. Finally, QoL was assessed in 102 patients using the WHOQOL-BREF along with measurements of medical and psychosocial factors.
Measurement of QoL
The WHOQOL-100 was developed to perform QoL assessments that would be applicable cross-culturally. Although WHOQOL-100 allows detailed assessment of each individual facet relating to QoL, it may be too lengthy for practical use. Thus, the WHOQOL-BREF Field Trial Version, a shorter version consisting of 26 questions, was developed instead. The WHOQOL-BREF produces a QoL profile and enables the determination of four domain scores: physical health, psychosocial, social, and environmental. There are also two items that are examined separately, namely the individual's overall perception of QoL and health. The four domain scores denote an individual's perception of QoL in each particular domain. They are scaled in a positive direction, with the higher scores denoting higher QoL. Each domain has 4–20 score variations. In this study, the Korean version of WHOQOL-BREF has been used.10,11,12
Medical measurements
Biomarkers for all patients included blood test analyses, body mass index (BMI), causes of ESRD, HD duration, clearance × time/volume (Kt/V), normalized protein catabolic rate (nPCR), and Charlson Comorbidity Index (CCI) score. Blood test analyses included hemoglobin, total cholesterol, calcium, phosphorus, 25-hydroxyvitamin D also known as 25(OH)D, ferritin, and intact parathyroid hormone (iPTH). The Kt/V indicates a function of the dialyzer urea clearance, treatment time, and urea distribution volume, and is commonly used as a marker for dialysis adequacy. The CCI score predicts 10-year mortality for a patient who may have some comorbid conditions. The nPCR is a formula to assess dietary protein intake in dialysis patients, and is reported in grams of urea nitrogen per kilogram per day.
Psychosocial measurements
The psychosocial factors were evaluated using the Hospital Anxiety and Depression Scale (HADS), Multidimensional Scale of Perceived Social Support (MSPSS), Montreal Cognitive Assessment (MOCA), and Pittsburgh Sleep Quality Index (PSQI). Furthermore, the main caregivers were interviewed using the Zarit Burden Interview (ZBI) to evaluate their burden as another psychosocial factor.
The HADS is a 14-item self-report screening scale that was developed to determine the anxiety and depression states in the setting of a non-psychiatric outpatient clinic.13 The HADS consists of a 7-item anxiety subscale (HADS-A) and a 7-item depression subscale (HADS-D). Each item is scored on a 4-point Likert scale, resulting in a maximum subscale score of 21 for anxiety and depression, respectively. The sum of each subscale indicates the level of anxiety and depression (0–7 = normal; 8–10 = borderline abnormal; 11–21 = abnormal).
The MSPSS is a 12-item scale scored using a 7-point scale (1 = strongly disagree to 7 = strongly agree), and measures three subscales corresponding to three sources of support: family, friends, and others.14 This scale is short and easy to understand, and can be performed by individuals who cannot tolerate long questionnaires or who have a limited literacy level. The subscale “others” was defined as the medical team.
The MOCA was developed as a brief screening instrument to detect mild cognitive impairment (MCI). It requires approximately 10 minutes and has a maximum score of 30 points. The MOCA assesses multiple cognitive domains including attention, concentration, executive functions, memory, language, visuospatial skills, abstraction, calculation, and orientation.15 In general, a total score of 22 or lower indicates cognitive impairments. We used the Korean version of the MOCA in the present study.
The PSQI is an instrument used to measure the quality and patterns of sleep. It differentiates ‘poor’ from ‘good’ sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction over the last month. Each of the seven domains is subjectively rated by the patients. The PSQI is scored based on a 4-point Likert scale, where 0 reflects the positive extreme and 3 reflects the negative extreme. A global score of 5 or greater indicates poor sleep.16
The ZBI is a 22-item instrument for measuring the caregiver's perceived burden of providing family care. The 22 items are assessed on a 5-point Likert scale, ranging from 0 (never) to 4 (nearly always). The total score corresponds to the sum of the item scores and ranges from 0 to 88, with higher scores indicating greater burden. The questions focus on the caregiver's health, psychological well-being, finances, social life, and relationship between the caregiver and the patient.17
Ethics statement
The Institutional Review Board of Daegu Catholic University Medical Center approved this study (IRB No. CR-13-076) and all participants provided written informed consent.
Statistical analysis
Statistical analysis was performed using SAS 9.3 Win ver. 6.1 (SAS Institute Inc., Cary, NC, USA). The results were presented as mean ± standard deviation. The associations between QoL and medical and/or psychosocial factors were assessed using correlation and canonical correlation analyses.
Canonical correlation analysis is used to investigate the interrelationships between sets of multiple dependent and independent variables. It is a generalization of multiple regression analysis with more than one set of dependent variables. Therefore, this statistical method enables studying the complex interactions of data from two sets of variables.18 In this study, the analysis was done between three sets of variables, each of which had virtual variants for QoL, medical factors, and psychosocial factors. We developed canonical functions for each analysis in such a way that the cumulative proportion exceeded 70%.
RESULTS
Characteristics of HD patients
A total of 102 patients (55 men, 47 women; mean age, 57.1 ± 12.0 years) receiving HD were recruited for the study. The demographic, medical, and psychosocial data of the 102 patients are listed in Table 1. The mean duration of HD was 36.0 ± 35.9 months. Diabetes mellitus (52.0%) was the most common cause of ESRD, followed by hypertension (21.6%).
Table 1. Demographic, clinical, and psychosocial parameters of the patients.
| Variables | Value (n = 102) | ||
|---|---|---|---|
| Demographics | |||
| Age, yr | 57.1 ± 12.0 | ||
| Male:female | 55 (53.9):47 (46.1) | ||
| Cause of ESRD | |||
| Diabetes mellitus | 53 (52.0) | ||
| Hypertension | 22 (21.6) | ||
| Chronic glomerulonephritis | 12 (11.7) | ||
| Others | 15 (14.7) | ||
| Clinical laboratory parameters | |||
| BMI, kg/m2 | 22.1 ± 3.0 | ||
| Total cholesterol, mg/dL | 137.24 ± 33.01 | ||
| nPCR, g/kg/day | 0.82 ± 0.18 | ||
| Hemoglobin, g/dL | 10.09 ± 1.08 | ||
| Calcium, mg/dL | 8.99 ± 0.76 | ||
| Phosphorus, mg/dL | 5.45 ± 1.91 | ||
| Ferritin, ng/mL | 242.60 ± 223.64 | ||
| 25(OH)D, ng/mL | 16.42 ± 8.94 | ||
| iPTH | 337.05 ± 263.71 | ||
| CCI score | 3.92 ± 1.40 | ||
| Duration of HD, mon | 36.03 ± 35.93 | ||
| Kt/V | 1.44 ± 0.57 | ||
| Psychosocial parameters | |||
| WHOQOL-BREF | |||
| Physical health | 10.05 ± 2.84 | ||
| Psychological | 10.17 ± 2.69 | ||
| Social relationship | 10.65 ± 2.91 | ||
| Environment | 10.44 ± 2.28 | ||
| HADS-A | 6.04 ± 4.17 | ||
| HADS-D | 9.49 ± 4.03 | ||
| MOCA | 19.26 ± 7.78 | ||
| MSPSS | 36.4 ± 9.9 | ||
| PSQI | 8.31 ± 4.65 | ||
| ZBI | 18.62 ± 22.89 | ||
Values are presented as mean ± standard deviation or number (%).
ESRD = end-stage renal disease, BMI = body mass index, nPCR = normalized protein catabolic rate, 25(OH)D = 25-hydroxyvitamin D, iPTH = intact parathyroid hormone, CCI = Charlson Comorbidity Index, HD = hemodialysis, HADS = Hospital Anxiety and Depression Scale, Kt/V = clearance × time/volume, WHOQOL-BREF = world health organization quality of life scale-abbreviated version, HADS-A = Hospital Anxiety and Depression Scale consists of a 7-item anxiety subscale, HADS-D = Hospital Anxiety and Depression Scale consists of a 7-item depression subscale, MOCA = Montreal Cognitive Assessment, MSPSS = Multidimensional Scale of Perceived Social Support, PSQI = Pittsburgh Sleep Quality Index, ZBI = Zarit Burden Interview.
Correlation between the WHOQOL score and the medical factors
First, we investigated the relationship between multiple variants by correlation analysis. Table 2 summarizes the correlation coefficients and the P values, and indicates multiple correlations. The physical health domain was positively correlated with nPCR (r = 0.235, P = 0.022) and phosphorus (r = 0.202, P = 0.045). In the psychological domain, we observed a negative correlation with the HD duration (r = −0.222, P = 0.027), indicating that a lower psychological QoL was associated with longer HD duration. In the social relationship domain, phosphorus (r = 0.236, P = 0.019) and iPTH (r = 0.224, P = 0.026) were positively associated with QoL. In the environment domain, nPCR (r = 0.239, P = 0.02) was positively correlated with QoL.
Table 2. Results of the correlation analysis.
| Variables | DOM1 | DOM2 | DOM3 | DOM4 | HADS-A | HADS-D | MOCA | MSPSS | ZBI | PSQI | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 0.185 | 0.181 | 0.027 | 0.12 | 0.151 | −0.119 | −0.006 | 0.113 | −0.1 | −0.117 | 0.091 |
| (0.067) | (0.074) | (0.794) | (0.237) | (0.134) | (0.237) | (0.95) | (0.264) | (0.327) | (0.247) | (0.368) | |
| HD_DUR | −0.198 | −0.222a | −0.092 | −0.16 | −0.029 | 0.207a | −0.209a | −0.039 | −0.137 | −0.017 | 0.172 |
| (0.05) | (0.027) | (0.364) | (0.114) | (0.777) | (0.039) | (0.037) | (0.701) | (0.176) | (0.87) | (0.086) | |
| CCI | −0.088 | −0.033 | −0.101 | 0.066 | −0.234a | 0.062 | −0.061 | 0.074 | 0.036 | 0.083 | 0.254a |
| (0.388) | (0.745) | (0.319) | (0.515) | (0.019) | (0.541) | (0.545) | (0.462) | (0.721) | (0.409) | (0.011) | |
| Kt/V | −0.036 | −0.026 | 0.191 | 0.117 | −0.122 | −0.009 | −0.185 | −0.001 | −0.103 | 0.138 | 0.094 |
| (0.731) | (0.803) | (0.064) | (0.259) | (0.235) | (0.934) | (0.071) | (0.994) | (0.321) | (0.179) | (0.365) | |
| nPCR | 0.235a | 0.167 | 0.162 | 0.239a | 0.031 | −0.221a | 0.12 | −0.064 | −0.134 | 0.038 | 0.041 |
| (0.022) | (0.105) | (0.116) | (0.02) | (0.764) | (0.03) | (0.244) | (0.535) | (0.195) | (0.712) | (0.694) | |
| Hb | 0.064 | 0.152 | 0.121 | 0.079 | 0.09 | −0.067 | 0.051 | 0.051 | 0.154 | 0.135 | −0.122 |
| (0.527) | (0.134) | (0.233) | (0.438) | (0.371) | (0.508) | (0.617) | (0.616) | (0.128) | (0.182) | (0.225) | |
| Alb | 0.122 | 0.011 | 0.161 | 0.058 | 0.111 | −0.038 | 0.012 | 0.03 | −0.212a | 0.064 | −0.229a |
| (0.228) | (0.915) | (0.111) | (0.567) | (0.27) | (0.705) | (0.908) | (0.771) | (0.035) | (0.526) | (0.022) | |
| Tchol | 0.073 | −0.002 | 0.052 | −0.012 | −0.201a | −0.078 | −0.181 | −0.023 | 0.065 | −0.07 | 0.002 |
| (0.47) | (0.986) | (0.609) | (0.908) | (0.044) | (0.44) | (0.072) | (0.82) | (0.52) | (0.49) | (0.983) | |
| Ca | −0.104 | −0.012 | −0.051 | −0.054 | 0.127 | 0.092 | −0.029 | 0.023 | −0.069 | 0.202a | −0.07 |
| (0.305) | (0.907) | (0.616) | (0.593) | (0.208) | (0.362) | (0.775) | (0.82) | (0.495) | (0.044) | (0.486) | |
| P | 0.202a | 0.139 | 0.236a | 0.096 | 0.04 | −0.062 | 0.261b | 0.07 | −0.107 | 0.207a | −0.255a |
| (0.045) | (0.17) | (0.019) | (0.345) | (0.693) | (0.539) | (0.009) | (0.487) | (0.293) | (0.038) | (0.01) | |
| Ferritin | 0.03 | −0.065 | −0.075 | −0.026 | −0.007 | 0.046 | 0.055 | −0.129 | −0.047 | −0.025 | −0.195 |
| (0.767) | (0.521) | (0.461) | (0.795) | (0.943) | (0.653) | (0.587) | (0.199) | (0.641) | (0.803) | (0.052) | |
| iPTH | 0.169 | 0.103 | 0.224a | 0.052 | 0.005 | −0.081 | −0.073 | 0.154 | −0.09 | 0.132 | −0.065 |
| (0.095) | (0.312) | (0.026) | (0.609) | (0.959) | (0.423) | (0.472) | (0.126) | (0.373) | (0.19) | (0.522) | |
| 25(OH)D | 0.059 | 0.019 | 0.023 | 0.034 | 0.204a | 0.006 | −0.011 | −0.139 | −0.135 | 0.034 | 0.046 |
| (0.564) | (0.854) | (0.822) | (0.737) | (0.042) | (0.955) | (0.912) | (0.168) | (0.183) | (0.74) | (0.647) | |
| DOM1 | −0.263b | −0.464b | 0.25a | 0.287b | −0.15 | −0.259b | −0.161 | ||||
| (0.008) | (0) | (0.012) | (0.004) | (0.138) | (0.009) | (0.108) | |||||
| DOM2 | −0.381b | −0.556b | 0.158 | 0.413b | −0.048 | −0.095 | −0.065 | ||||
| (0) | (0) | (0.114) | (0) | (0.636) | (0.342) | (0.52) | |||||
| DOM3 | −0.292b | −0.381b | 0.057 | 0.397b | −0.107 | −0.029 | −0.09 | ||||
| (0.003) | (0) | (0.569) | (0) | (0.291) | (0.771) | (0.368) | |||||
| DOM4 | −0.215a | −0.39b | 0.197a | 0.312b | −0.104 | −0.212a | −0.032 | ||||
| (0.031) | (0) | (0.048) | (0.002) | (0.302) | (0.034) | (0.754) |
Values are presented by correlation coefficient (P value).
25(OH)D = 25-hydroxyvitamin D, Alb = albumin, Ca = calcium, BMI = body mass index, CCI = Charlson comorbidity index, DOM1 = physical health domain of WHOQOL-BREF, DOM2 = psychosocial domain of WHOQOL-BREF, DOM3 = social relationships domain of WHOQOL-BREF, DOM4 = environmental domain of WHOQOL-BREF, Hb = hemoglobin, HADS = Hospital Anxiety and Depression Scale, HADS-A = Hospital Anxiety and Depression Scale consists of a 7-item anxiety subscale, HADS-D = Hospital Anxiety and Depression Scale consists of a 7-item depression subscale, HD_DUR = duration of dialysis, iPTH = intact parathyroid hormone, Kt/V = clearance × time/volume, MOCA = Montreal Cognitive Assessment, MSPSS = Multidimensional Scale of Perceived Social Support, nPCR = normalized protein catabolic rate, P = phosphorus, PSQI = Pittsburgh Sleep Quality Index, WHOQOL-BREF = World Health Organization Quality of Life Scale-Abbreviated Version, ZBI = Zarit Burden Interview, Tchol = total cholesterol.
aP < 0.05; bP < 0.01.
Subsequently, we performed canonical correlation analysis to investigate concretely the structure of the correlations in a manner similar to multivariate analysis. The results indicated no significant correlation between QoL and medical factors (1st canonical function: r = 0.478, P = 0.475; 2nd canonical function: r = 0.419, P = 0.751; Table 3). Table 4 shows canonical coefficients, correlation loadings and cross correlation loadings for each of functions between QoL and medical factors.
Table 3. Results of the canonical correlation analysis.
| Variable groups | Canonical function | Canonical correlation | Eigen value | Wilk's λ | Proportion | P value |
|---|---|---|---|---|---|---|
| WHOQOL & medical factors | First | 0.478 | 0.294 | 0.542 | 0.438 | 0.475 |
| Second | 0.419 | 0.213 | 0.702 | 0.316 | 0.751 | |
| WHOQOL & psychosocial factors | First | 0.696 | 0.938 | 0.403 | 0.778 | 0.000 |
| Second | 0.421 | 0.215 | 0.781 | 0.178 | 0.191 | |
| Medical factors & psychosocial factors | First | 0.689 | 0.901 | 0.152 | 0.435 | 0.000 |
| Second | 0.603 | 0.572 | 0.289 | 0.294 | 0.009 |
WHOQOL = World Health Organization Quality of Life Scale.
Table 4. Canonical weights and loadings for the functions (WHOQOL score & medical factors).
| Variable groups | Variables | The 1st canonical function | The 2nd canonical function | ||||
|---|---|---|---|---|---|---|---|
| Coefficients | Correlation | Cross correlation | Coefficients | Correlation | Cross correlation | ||
| WHOQOL | DOM1 | 1.098 | 0.892 | 0.427 | −0.017 | 0.387 | 0.162 |
| DOM2 | −0.228 | 0.462 | 0.221 | 0.990 | 0.706 | 0.296 | |
| DOM3 | 0.386 | 0.632 | 0.302 | −0.865 | −0.145 | −0.061 | |
| DOM4 | −0.458 | 0.259 | 0.124 | 0.401 | 0.455 | 0.191 | |
| Medical factors | BMI | 0.104 | 0.241 | 0.115 | 0.317 | 0.451 | 0.189 |
| HD_DUR | −0.462 | −0.281 | −0.134 | −0.450 | −0.459 | −0.193 | |
| CCI | −0.352 | −0.374 | −0.179 | 0.154 | 0.258 | 0.108 | |
| Kt/V | −0.221 | −0.027 | −0.013 | −0.412 | −0.342 | −0.144 | |
| nPCR | 0.050 | 0.363 | 0.173 | 0.656 | 0.279 | 0.117 | |
| Hb | −0.149 | 0.105 | 0.050 | 0.394 | 0.198 | 0.083 | |
| Alb | 0.281 | 0.360 | 0.172 | −0.605 | −0.355 | −0.149 | |
| Tchol | 0.262 | 0.257 | 0.123 | −0.165 | −0.175 | −0.073 | |
| Ca | −0.391 | −0.223 | −0.107 | 0.419 | −0.018 | −0.008 | |
| P | 0.348 | 0.485 | 0.232 | −0.269 | 0.001 | 0.001 | |
| Ferritin | 0.128 | 0.111 | 0.053 | 0.231 | −0.051 | −0.021 | |
| iPTH | 0.563 | 0.460 | 0.220 | 0.068 | −0.155 | −0.065 | |
| 25(OH)D | 0.064 | 0.099 | 0.047 | −0.087 | 0.052 | 0.022 | |
25(OH)D = 25-hydroxyvitamin D, Alb = albumin, Ca = calcium, BMI = body mass index, CCI = Charlson comorbidity index, DOM1 = physical health domain of WHOQOL-BREF, DOM2 = psychosocial domain of WHOQOL-BREF, DOM3 = social relationships domain of WHOQOL-BREF, DOM4 = environmental domain of WHOQOL-BREF, Hb = hemoglobin, HD_DUR = duration of dialysis, iPTH = intact parathyroid hormone, Kt/V = clearance × time/volume, nPCR = normalized protein catabolic rate, P = phosphorus, WHOQOL-BREF = World Health Organization Quality of Life Scale-Abbreviated Version, Tchol = total cholesterol.
Correlation between the WHOQOL score and the psychosocial factors
Table 2 summarizes the significant correlations between the different variables. All the WHOQOL domains were negatively correlated with both HADS-A and HADS-D. The MOCA score was positively correlated with the physical health and environment domains, suggesting that these two domains may be related to the patients' cognition. There were also significantly positive correlations between the MSPSS score and all QoL domains, indicating that support from others can highly influence the patients' QoL. In addition, the physical health and environment domains were negatively correlated with the PSQI score, suggesting that patients who experience poor sleep could have lower physical health and environment quality.
The canonical correlation analysis indicated significant positive correlations between QoL and the psychosocial factors (1st canonical function: correlation = 0.696, P < 0.001; 2nd canonical function: correlation = 0.421, P = 0.191; Table 3). The proportion explaining the variance attributed to a given canonical correlation was 0.778 in the 1st canonical function.
In the canonical cross correlation loading of the 1st canonical function, we found that three variants including HADS-A (−0.394), HADS-D (−0.585), and MSPSS (0.455) contributed to the correlation between QoL and the psychosocial factors (Table 5). This is in line with a previous suggestion by Tabachnick and Fidell19 proposing that the structure coefficient is interpreted as meaningful when its value exceeds 0.3. Thus, higher anxiety and depression scores may be associated with lower QoL, while better support from others results in a higher QoL.
Table 5. Canonical weights and loadings for the functions (WHOQOL score & psychosocial factors).
| Variable groups | Variables | The 1st canonical function | The 2nd canonical function | ||||
|---|---|---|---|---|---|---|---|
| Coefficients | Correlation | Cross correlation | Coefficients | Correlation | Cross correlation | ||
| WHOQOL | DOM1 | 0.082 | 0.742 | 0.516 | −1.031 | −0.552 | −0.232 |
| DOM2 | 0.645 | 0.939 | 0.654 | 0.534 | 0.090 | 0.038 | |
| DOM3 | 0.255 | 0.754 | 0.524 | 0.715 | 0.296 | 0.125 | |
| DOM4 | 0.210 | 0.676 | 0.470 | −0.407 | −0.421 | −0.177 | |
| Psychosocial factors | HADS-A | −0.108 | −0.566 | −0.394 | −0.347 | −0.120 | −0.050 |
| HADS-D | −0.736 | −0.841 | −0.585 | −0.020 | 0.178 | 0.075 | |
| MOCA | 0.035 | 0.266 | 0.185 | −0.704 | −0.521 | −0.219 | |
| MSPSS | 0.482 | 0.654 | 0.455 | 0.198 | 0.199 | 0.084 | |
| ZBI | −0.117 | −0.132 | −0.092 | 0.211 | 0.225 | 0.095 | |
| PSQI | 0.092 | −0.179 | −0.124 | 0.811 | 0.661 | 0.278 | |
| Age | 0.032 | −0.127 | −0.088 | −0.139 | 0.196 | 0.083 | |
DOM1 = physical health domain of WHOQOL-BREF, DOM2 = psychosocial domain of WHOQOL-BREF, DOM3 = social relationships domain of WHOQOL-BREF, DOM4 = environmental domain of WHOQOL-BREF, HADS_A = Hospital Anxiety and Depression Scale consists of a 7-item anxiety subscale, HADS-D = Hospital Anxiety and Depression Scale consists of a 7-item depression subscale, MOCA = Montreal Cognitive Assessment, MSPSS = Multidimensional Scale of Perceived Social Support, PSQI = Pittsburgh Sleep Quality Index, WHOQOL-BREF = World Health Organization Quality of Life Scale-Abbreviated Version, ZBI = Zarit Burden Interview.
Association between the medical and psychosocial factors
The correlation analysis indicated positive and negative relationships between the medical and psychosocial factors. The HD duration was positively correlated with the HADS-D score and negatively correlated with the MOCA score, which indicates that explain the higher depression score and lower cognitive function may be associated with longer HD durations. Phosphorus was correlated with MOCA, PSQI, and age, while CCI was positively correlated with age (Table 2).
The canonical correlation analysis indicated a positive correlation between the medical and psychological factors (1st canonical function: correlation = 0.689, P < 0.001; 2nd canonical function: correlation = 0.603, P = 0.009; Table 3). The proportion of the 1st and 2nd canonical functions was 0.435 and 0.294, respectively.
In the canonical cross correlation loading of the 1st canonical function, we found that only phosphorus (0.360) was implicated in the correlations observed with the medical variants, while both the MOCA score (0.386) and age (−0.560) were implicated in the correlations with the psychosocial variants (Table 6).
Table 6. Canonical weights and loadings for the functions (medical factors & psychosocial factors).
| Variable groups | Variables | The 1st canonical function | The 2nd canonical function | ||||
|---|---|---|---|---|---|---|---|
| Coefficients | Correlation | Cross correlation | Coefficients | Correlation | Cross correlation | ||
| Medical factors | BMI | −0.132 | 0.004 | 0.002 | −0.487 | −0.506 | −0.305 |
| HD_DUR | −0.691 | −0.410 | −0.283 | −0.071 | 0.132 | 0.080 | |
| CCI | −0.297 | −0.373 | −0.257 | 0.433 | 0.379 | 0.229 | |
| Kt/V | −0.310 | −0.173 | −0.119 | 0.229 | 0.222 | 0.134 | |
| nPCR | −0.164 | 0.093 | 0.064 | −0.732 | −0.410 | −0.247 | |
| Hb | 0.251 | 0.358 | 0.246 | 0.134 | 0.060 | 0.036 | |
| Alb | 0.116 | 0.339 | 0.234 | −0.032 | −0.146 | −0.088 | |
| Tchol | −0.250 | −0.171 | −0.118 | 0.180 | 0.277 | 0.167 | |
| Ca | 0.222 | 0.177 | 0.122 | 0.011 | 0.041 | 0.025 | |
| P | 0.464 | 0.522 | 0.360 | 0.449 | 0.059 | 0.035 | |
| Ferritin | 0.285 | 0.159 | 0.109 | −0.105 | 0.107 | 0.065 | |
| iPTH | 0.409 | 0.184 | 0.127 | 0.137 | 0.093 | 0.056 | |
| 25(OH)D | −0.246 | 0.026 | 0.018 | −0.367 | −0.427 | −0.258 | |
| Psychosocial factors | HADS-A | 0.427 | 0.278 | 0.191 | −0.878 | −0.530 | −0.320 |
| HADS-D | −0.345 | −0.207 | −0.143 | 0.566 | 0.267 | 0.161 | |
| MOCA | 0.162 | 0.560 | 0.386 | −0.287 | −0.079 | −0.048 | |
| MSPSS | 0.180 | 0.169 | 0.116 | 0.199 | 0.301 | 0.182 | |
| ZBI | 0.150 | 0.088 | 0.061 | 0.346 | 0.265 | 0.160 | |
| PSQI | 0.303 | 0.299 | 0.206 | 0.380 | 0.346 | 0.208 | |
| age | −0.718 | −0.815 | −0.561 | −0.422 | −0.184 | −0.111 | |
25(OH)D = 25-hydroxyvitamin D, Alb = albumin, Ca = calcium, BMI = body mass index, CCI = Charlson comorbidity index, Hb = hemoglobin, HADS-A = Hospital Anxiety and Depression Scale consists of a 7-item anxiety subscale, HADS-D = Hospital Anxiety and Depression Scale consists of a 7-item depression subscale, HD_DUR = duration of dialysis, iPTH = intact parathyroid hormone, Kt/V = clearance × time/volume, MOCA = Montreal Cognitive Assessment, MSPSS = Multidimensional Scale of Perceived Social Support, nPCR = normalized protein catabolic rate, P = phosphorus, PSQI = Pittsburgh Sleep Quality Index, ZBI = Zarit Burden Interview, Tchol = total cholesterol.
DISCUSSION
As evidenced by both the correlation and canonical correlation analyses, there were significant and positive correlations between QoL and the psychosocial factors. On the other hand, although there were some correlations between QoL and the medical factors, these were not consistent. Indeed, we could not validate those correlations using the canonical correlation analysis. The results also indicated some positive correlations between the medical and psychosocial factors.
Although several studies have reported correlations between QoL and medical and psychosocial factors in patients with chronic illnesses, these are still debated to date. Cruz et al.20 suggested that more sociodemographic factors were associated with decreased QoL than medical factors. However, our present results indicated that the medical factors were also strongly implicated.
Spiegel et al.21 suggested that the HRQoL score in patients with ESRD is most affected by medical factors and nutritional biomarkers. However, they demonstrated poor correlations with Kt/V, mineral metabolism indices, and inflammatory markers. Soni et al.22 suggested that anemia and depression are also determinants of HRQoL in patients with chronic kidney disease. In contrast, Mazairac et al.23 reported that widely accepted clinical performance targets that are recommended by the Kidney Disease Outcomes Quality Initiative, including Kt/V, hemoglobin, vascular access, phosphorus, parathyroid hormone, and blood pressure, were not related to the HRQoL score in HD patients. Furthermore, Saad et al.24 demonstrated similar results indicating no significant correlation between medical factors and QoL in patients with ESRD.
Our present findings are in line with previous findings and indicate that the medical factors can influence the psychosocial factors directly, and the QoL indirectly. Thus, evaluating only clinical parameters without taking the psychosocial aspects into account may attenuate the effective prediction of the patients' QoL throughout the course of the disease.
Our results indicated significant correlations between the psychosocial factors and QoL. In particular, correlations with depression, anxiety, and support from others were the most notable. Previous studies showed that anxiety and depression are important aspects in QoL in people with chronic illnesses. Dogan et al.25 reported a correlation among high depression score, low SF-36 score, high anxiety score, and QoL in patients with Huntington's disease. Other studies have also confirmed the relationship between QoL and the social support in patients with Huntington's disease.26 Untas et al.27 suggested that poorer social supports are associated with higher mortality risk, lower adherence to medical care, and poorer physical QoL in HD patients. However, there are also few studies that demonstrate a strong correlation between QoL and other psychosocial factors such as cognition, sleep disturbance in patients with chronic illnesses. In the present study, the low range of depression/anxiety and the high range of support from others were more relevant for QoL than other factors. However, these discrepancies may be the result of differences in ethnicities and customs.
Our results indicated correlations between the medical and psychosocial factors, which were validated using the canonical correlation analysis, particularly in terms of two factors, which is in line with a previous study.28 In particular, the younger age and better cognitive function were proven to influence the medical factors of patients with ESRD. It is worth noting that cognition was important for improving the patients' clinical condition, but was not directly related to their QoL. In addition, we also investigated whether there were correlations between the patients' age and QoL. Our results indicated no correlation in both the correlation and canonical correlation analyses. In contrast, age was related to several medical factors. In a recent study, Laudański et al.29 suggested that younger patients required more ESRD-oriented support to relieve their health-related complaints than older patients. These results indicate that younger patients may also have lower QoL. In contrast, Kutner et al.30 suggested that older patients on dialysis reported more functional disabilities, decreased ability to do the things they would like to do, and lower levels of perceived mastery over their lives; this correlation was further debated in other studies.31,32
Our study had several limitations. There may have been a selection bias when choosing the medical and psychosocial factors, which did not represent all the factors we aimed to investigate. In addition, the sample size was relatively small, and the study was based on results from a single center. Thus, future studies using a larger sample size and multiple centers are still warranted to validate our findings. Despite these limitations, our present study had the advantage of using canonical correlation analysis to validate the correlations between the groups of factors, and using the WHOQOL-BREF score to analyze many aspects of QoL. Furthermore, vigorous and broad investigations to evaluate the patients' characteristics were performed.
In conclusion, this study suggested that there is a strong correlation between psychosocial factors and QoL in patients with ESRD undergoing HD. The psychosocial and medical factors were also correlated. However, the medical factors were not correlated with QoL in our present study; thus, further research on this point is still needed. Our findings suggest that medical workers should recognize and treat the psychosocial and medical complications equally. Moreover, active consultation liaison is needed between the nephrologists and psychiatrists to improve QoL in patients with ESRD.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kang GW, Woo J. Data curation: Kang GW, Woo J. Formal analysis: Woo J, Kim K. Investigation: Kang GW, Woo J. Writing - original draft: Kim K, Woo J. Writing - review & editing: Kim K, Kang GW, Woo J.
References
- 1.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation--a meta-analytic review. Nephrol Nurs J. 2010;37(1):37–44. [PubMed] [Google Scholar]
- 2.Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: the Korean National Health and Nutritional Examination Survey 2011–2013. J Korean Med Sci. 2016;31(6):915–923. doi: 10.3346/jkms.2016.31.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin DC. Dialysis registries in the world: Korean Dialysis Registry Kidney. Kidney Int Suppl (2011) 2015;5(1):8–11. doi: 10.1038/kisup.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res. 2000;9(8):887–900. doi: 10.1023/a:1008996223999. [DOI] [PubMed] [Google Scholar]
- 5.Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 6.Joshi VD. Quality of life in end stage renal disease patients. World J Nephrol. 2014;3(4):308–316. doi: 10.5527/wjn.v3.i4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niechzial M, Hampel E, Grobe T, Nagel E, Dörning H, Raspe H. Determinants of the quality of life in chronic renal failure. Soz Praventivmed. 1997;42(3):162–174. doi: 10.1007/BF01300567. [DOI] [PubMed] [Google Scholar]
- 8.Mazairac AH, de Wit GA, Grooteman MP, Penne EL, van der Weerd NC, den Hoedt CH, et al. Effect of hemodiafiltration on quality of life over time. Clin J Am Soc Nephrol. 2013;8(1):82–89. doi: 10.2215/CJN.00010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tel H. Determining quality of life and sleep in hemodialysis patients. Dial Transplant. 2009;38(6):210–215. [Google Scholar]
- 10.The WHOQOL group. The World Health Organization Quality of Life assessment(WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46:1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 11.Min SK, Lee CI, Kim KI, Suh SY, Kim DK. Development of Korean version of WHO quality of life scale abbreviated version (WHOQOL-BREF) J Korean Neuropsychiatr Assoc. 2000;39(3):571–579. [Google Scholar]
- 12.WHOQOL-BREF: introduction, administration, scoring and generic version of the assessment, field trial version. [Updated 1996]. [Accessed November 10, 2017]. http://www.who.int/iris/handle/10665/63529.
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Zimet GD, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. 1990;55(3-4):610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 15.Julayanont P, Nasreddine ZS, Chertkow H. Montreal cognitive assessment (MoCA): concept and clinical review. In: Larner J, editor. Cognitive Screening Instruments: a Practical Approach. London, United Kingdom: Springer; 2013. pp. 111–151. [Google Scholar]
- 16.Buysse DJ, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Seng BK, Luo N, Ng WY, Lim J, Chionh HL, Goh J, et al. Validity and reliability of the zarit burden interview in assessing caregiving burden. Ann Acad Med Singapore. 2010;39(10):758–763. [PubMed] [Google Scholar]
- 18.Kowalski J, Tu XM, Jia G, Perlis M, Frank E, Crits-Christoph P, et al. Generalized covariance-adjusted canonical correlation analysis with application to psychiatry. Stat Med. 2003;22(4):595–610. doi: 10.1002/sim.1332. [DOI] [PubMed] [Google Scholar]
- 19.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 3rd ed. New York, NY: Harper Collins; 1996. [Google Scholar]
- 20.Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso Rde C. Quality of life in patients with chronic kidney disease. Clinics. 2011;66(6):991–995. doi: 10.1590/S1807-59322011000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegel BM, Melmed G, Robbins S, Esrailian E. Biomarkers and health-related quality of life in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2008;3:1759–1768. doi: 10.2215/CJN.00820208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soni RK, Weisbord SD, Unruh ML. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19(2):153–159. doi: 10.1097/MNH.0b013e328335f939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazairac AH, de Wit GA, Grooteman MP, Penne EL, van der Weerd NC, den Hoedt CH, et al. Clinical performance targets and quality of life in hemodialysis patients. Blood Purif. 2012;33(1-3):73–79. doi: 10.1159/000334639. [DOI] [PubMed] [Google Scholar]
- 24.Saad MM, El Douaihy Y, Boumitri C, Rondla C, Moussaly E, Daoud M, et al. Predictors of quality of life in patients with end-stage renal disease on hemodialysis. Int J Nephrol Renovasc Dis. 2015;8:119–123. doi: 10.2147/IJNRD.S84929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dogan E, Eryonucu B, Sayarlioglu H, Agargun MY. Relation between depression, some laboratory parameters, and quality of life in hemodialysis patients. Ren Fail. 2005;27(6):695–699. doi: 10.1080/08860220500242728. [DOI] [PubMed] [Google Scholar]
- 26.Cohen SD. Social Support interventions will improve the quality of life of ESRD patients. Semin Dial. 2013;26(3):262–265. doi: 10.1111/sdi.12064. [DOI] [PubMed] [Google Scholar]
- 27.Untas A, Thumma J, Rascle N, Rayner H, Mapes D, Lopes AA, et al. The associations of social support and other psychosocial factors with mortality and quality of life in the dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol. 2011;6(1):142–152. doi: 10.2215/CJN.02340310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cukor D, Cohen SD, Peterson RA, Kimmel PL. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042–3055. doi: 10.1681/ASN.2007030345. [DOI] [PubMed] [Google Scholar]
- 29.Laudański K, Nowak Z, Niemczyk S. Age-related differences in the quality of life in end-stage renal disease in patients enrolled in hemodialysis or continuous peritoneal dialysis. Med Sci Monit. 2013;19:378–385. doi: 10.12659/MSM.883916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutner NG, Brogan DJ. Assisted survival, aging, and rehabilitation needs: comparison of elderly dialysis patients and age-matched peers. Arch Phys Med Rehabil. 1992;73(4):309–315. doi: 10.1016/0003-9993(92)90001-d. [DOI] [PubMed] [Google Scholar]
- 31.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Predictors of poor outcome in chronic dialysis patients: the Netherlands Cooperative Study on the Adequacy of Dialysis. Am J Kidney Dis. 2000;35(1):69–79. doi: 10.1016/s0272-6386(00)70304-0. [DOI] [PubMed] [Google Scholar]
- 32.Hestin D, Frimat L, Hubert J, Renoult E, Huu TC, Kessler M. Renal transplantation in patients over sixty years of age. Clin Nephrol. 1994;42(4):232–236. [PubMed] [Google Scholar]


