Abstract
Background
Activating EGFR mutations, HER2, and HER3 are implicated in lung cancer; however, with the exception of EGFR gene amplification in lung adenocarcinoma harboring EGFR mutations, their involvement in disease progression during the early stages is poorly understood. In this paper, we focused on which receptor is correlated with lung adenocarcinoma progression in the presence or absence of EGFR mutation from stage 0 to IA1.
Methods
HER2 and HER3 expression and activating EGFR mutations in surgically resected lung adenocarcinoma exhibiting ground glass nodules on chest computed tomography and re‐classified to stage 0 and IA1 were examined by immunohistochemistry and peptide nucleic acid‐locked nucleic acid PCR clamp method, respectively.
Results
HER2 and HER3 expression was detected in 22.2% and 86.1% of samples, respectively. The frequency of EGFR mutation was 45.7% and was not significantly different between stage 0 and IA1 (40.0% and 48.0%, respectively), suggesting that EGFR mutation does not correlate with cancer progression from stage 0 to IA1. HER2 expression also did not correlate to progression. However, not only the frequency, but also the intensity of HER3 expression was increased in stage IA1 lung adenocarcinoma, particularly in lung adenocarcinoma without EGFR mutation.
Conclusion
HER3 tends to be intensively expressed during the progression of lung adenocarcinoma without EGFR mutation from carcinoma in situ to invasive carcinoma.
Keywords: EGFR mutation, HER2, HER3, lung adenocarcinoma
Introduction
EGFR, HER2, HER3, and HER4 are members of the ErbB receptor family.1, 2, 3, 4, 5, 6 These receptors form homodimer or heterodimer and activate downstream signaling that induces cell growth, differentiation, and carcinogenesis.5 EGF, transforming growth factor, amphiregulin, heparin‐binding EGF‐like growth factor, betacellulin, and epigen are ligands for EGFR; epiregulin is a ligand for both EGFR and HER4; heregulin (neuregulin 1 and neuregulin 2) is a ligand for HER3 and HER4; and HER2 has no ligand.7 These receptors play important roles in survival, proliferation, angiogenesis, and metastasis in many kinds of cancers.7, 8 In 2004, an activating EGFR mutation was reported to render lung cancer cells sensitive to gefitinib, an EGFR‐tyrosine kinase inhibitor (TKI).9, 10 The common activating mutations of EGFR are exon 19 deletion and exon 21 point mutation (L858R). Since the discovery that an activating EGFR mutation may render cancer cells sensitive to EGFR‐TKIs, many studies on mutations in lung cancer have been conducted and have yielded diverse results. For instance, the activating mutant EGFR with exon 19 deletion induces prolonged downstream signaling and enables transformation in vitro,11, 12 whereas both exon 19 deletion and L858R induce lepidic cell growth and form bronchoalveolar carcinoma that is sensitive to erlotinib, an EGFR‐TKI.13, 14 In lung cancers with activating EGFR mutations, mutant EGFR can cooperate with either HER2 or HER3 and activate downstream signaling, such as anti‐apoptotic signaling.15 Yatabe et al. reported that EGFR mutations are implicated in the development of terminal respiratory unit types of lung adenocarcinoma, and that amplification of mutant EGFR may be correlated to tumor progression from in situ lung adenocarcinoma to invasive adenocarcinoma.16
To evaluate if HER2 or HER3 expression is implicated in lung adenocarcinoma progression, we focused on surgically resected stage 0 or IA1 lepidic predominant lung adenocarcinomas with or without EGFR mutations that exhibited ground glass nodules (GGNs) on chest computed tomography (CT). In this study, stage 0 or IA1 lung carcinoma were defined according to the International Association for the Study of Lung Cancer (IASLC) 8th Edition Tumor Node Metastasis (TNM) Classification of Lung Cancer.17 Stage 0 (Tis) adenocarcinoma refers to carcinoma in situ; stage IA1 lung adenocarcinoma includes minimally invasive (T1mi) adenocarcinoma exhibiting < 3 cm of a predominately lepidic pattern with < 5 mm invasion in any one focus, and T1a invasive adenocarcinoma, defined as a tumor with no more than 1 cm at the greatest dimension and unable to be classified as Tis or T1mi. These stages have neither lymph node nor distant metastasis. This type of lung adenocarcinoma reportedly harbors EGFR mutations at frequency of 61%.18 We compared EGFR mutations and HER2 and HER3 expression between stage 0 in situ lung adenocarcinoma and stage IA1 lung adenocarcinoma including minimally invasive and invasive adenocarcinoma.
Methods
Patient population and characteristics
Records of patients with lung adenocarcinoma exhibiting GGN on chest CT and a lepidic histologic pattern that was surgically resected and classified as stage IA with T1N0M0 in 2009 were retrospectively evaluated. All patients signed written informed consent before participating in this study. The Medical Ethics Committee of Osaka International Cancer Institute (previously named the Medical Ethics Committee of Osaka Medical Center for Cancer and Cardiovascular Diseases until March 2017) approved the study, which was performed in accordance with the Helsinki Declaration. Adenocarcinoma reclassified into stage 0 (Tis) in situ adenocarcinoma or IA1 including minimally invasive (T1mi) and invasive adenocarcinoma (T1a) according to the IASLC 8th edition of the TNM Classification of Lung Cancer was used in this study.17 Gender, age, smoking history (Brinkmann’s index), and the greatest dimension of predominately lepidic pattern measured as tumor size were evaluated. Additionally, the five‐year overall survival (OS) data for stage 0 and IA1 was calculated.
Analysis of EGFR mutation
EGFR mutation was evaluated at LSI Medience Corporation using a peptide nucleic acid‐locked nucleic acid PCR clamp method.19 HER2 and HER3 expression was evaluated via immunohistochemistry at SRL Co. Ltd.
Immunohistochemistry
Immunohistochemistry was performed using the automated staining system, Ventana NX20 (Roche Diagnostics K.K., Tokyo, Japan) at SRL Co. Ltd. Rabbit polyclonal anti‐HER2 (Dako Denmark A/S, Glostrup, Denmark) and rabbit polyclonal anti‐HER3 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies were used. These antibodies were detected using an iVIEW DAB Detection Kit (Roche Diagnostics K.K.). Immunohistochemistry samples without anti‐HER2 or anti‐HER3 antibodies were used as negative controls. Protein expression was evaluated using light microscopy with low (×100), intermediate (×200), and high (×400) magnifications.
Positive expression was defined as strong expression of the examined protein in > 30% of lung cancer cells compared to adjacent lung epithelial cells. Expression was defined as 1+, 2+ and 3+ in cases of weak and membrane staining, complete but moderate membrane staining, and strong and complete staining, respectively.
Statistical analysis
Statistical analysis was performed using EZR.20 Baseline patient characteristics were compared using Fisher’s exact test for categorical variables (e.g. a ratio of men to women) and Mann–Whitney U test for continuous variables (e.g. age, Brinkman index, and tumor diameter). The frequency of EGFR mutations and HER2 and HER3 expression between adenocarcinoma in situ (stage 0) and others, including minimally invasive and invasive adenocarcinomas (stage IA1), were compared using Fisher’s exact test. The intensity of HER2 or HER3 expression between the two groups was compared using Mann–Whitney U test. To evaluate the impact of the intensity of HER3 expression on progression from stage 0 to IA1, subgroup analysis was performed between subgroups with or without EGFR mutations using a Mann–Whitney U test.
Results
Patient characteristics
The clinical and histopathological data are summarized in Table 1. A total of 36 cases of stage IA lung adenocarcinoma were reclassified into 10 cases of stage 0 in situ adenocarcinoma (Tis) and 26 cases of stage IA1 adenocarcinoma, including 15 cases of minimally invasive carcinoma (T1mi) and 11 cases of invasive carcinoma (T1a). None of the patients received EGFR‐TKIs before surgery. Five men and five women had stage 0 disease, whereas 10 men and 16 women had stage IA1. The median ages in stage 0 and IA1 groups were 62 and 68 years, respectively. There was no statistically significant difference in the Brinkman index in these groups. The mean diameter of adenocarcinoma in situ was 15.4 ± 7.1 mm (mean ± standard deviation), whereas that of minimally invasive and invasive adenocarcinoma was 20.7 ± 5.7 mm (mean ± standard deviation). Therefore, primary lesions in lung adenocarcinoma in situ with pure GGN (stage 0) have a smaller diameter than the invasive type (stage IA1).
Table 1.
Patient characteristics
| Characteristic | Reclassified stage | Statistical analysis | |
|---|---|---|---|
| Stage 0 | Stage IA1 | ||
| Number of cases | 10 | 26 | |
| Gender | |||
| Female | 5 | 15 | |
| Male | 5 | 11 | NS (P = 0.709) |
| Median age (range) | 62.5 (20–66) | 65.5 (20–83) | NS (P = 0.229) |
| Smoking history (median Brinkmann index) | 120 (0–720) | 53(0–2000) | NS (P = 0.769) |
| Mean tumor size (±SD) | 15.4 ± 7.1 mm | 20.7 ± 5.7 mm | P < 0.05 (0.001) |
| Five‐year survival rate | 100% | 95.5% | |
NS, not significant; SD, standard deviation.
One patient with stage IA1 died 11 months after surgical resection as a result of another (secondary) pleomorphic lung cancer. Postoperative five‐year follow‐up for four patients with stage IA1 was not obtained. No postoperative recurrence has been observed after five years of follow‐up. The five‐year OS rate was 100% for stage 0 and 95.5% for stage IA1 (Table 1).
EGFR mutation and HER2 and HER3 expression in stage 0–IA1 lung adenocarcinoma
EGFR mutation status was examined in the patient cohort. As shown in Table 2, PCR was successful for 35 patients and unsuccessful in one patient. EGFR mutations were detected in 16 (48.6%) of the 35 patients. Five of these cases had exon 19 deletions, and 11 had L858R. Nineteen cases lacked EGFR mutation. The frequency of EGFR mutation between adenocarcinoma in situ and invasive adenocarcinoma was not significant (40.0% and 48.0%, respectively).
Table 2.
EGFR mutation and HER2 and HER3 expression in stage 0–IA1 lung adenocarcinoma
| Reclassified stage Statistical analysis | |||
|---|---|---|---|
| Mutation/Expression | Stage 0 | Stage 1A1 | |
| EGFR mutation | |||
| Unsuccessful | 0 | 1 | |
| Negative | 6 | 13 | |
| Positive | 4 | 12 | NS (P = 0.723) |
| Exon 19 deletion | 1 | 4 | |
| L858R | 3 | 8 | |
| HER2 | Negative | 9 | 19 |
| Positive | 7 | Frequency: NS (P = 0.397) | |
| 1+ | 1 | 5 | |
| 2+ | 0 | 2 | Intensity: NS (P = 0.769) |
| 3 | 0 | 0 | |
| HER3 | Negative | 4 | 1 |
| Positive | 6 | 25 | Frequency: P < 0.05 (0.015) |
| 1+ | 5 | 19 | Intensity: P < 0.05 (0.01) |
| 2+ | 1 | 5 | |
| 3+ | 0 | 1 | |
NS, not significant; SD, standard deviation.
Figure 1 shows images obtained from immunohistochemistry of negative and 2+ expression of HER2 and HER3, respectively. Immunohistochemistry results are summarized in Table 2. HER2 was detectable in 8 out of 36 cases. The frequency of HER2 expression was not significantly different between stage 0 and stage IA1, and its intensity in stage IA1 was not higher than in stage 0 adenocarcinoma. When compared with adjacent lung epithelium cells, HER3 expression was positive in 86.8% of lung cancers tested. The frequency of HER3 expression was 60.0% for stage 0 adenocarcinoma in situ and statistically significantly more frequent (96.1%) in advanced stage (stage IA1). In addition, the intensity of HER3 expression in stage IA1 was greater than in adenocarcinoma in situ (stage 0).
Figure 1.
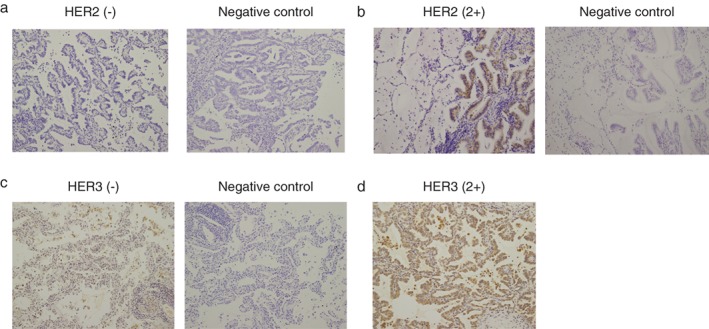
Representative immunohistochemistry images of lung adenocarcinoma (left) versus negative control (immunohistochemistry without anti‐HER2; right). (a) HER2(−), (b) HER2(2+), (c) HER3(−), and (d) HER3 (2+). All images were obtained at 200× magnification.
Meanwhile, there was no statistically significant difference in EGFR status, HER2 expression and intensity, or baseline patient characteristics other than tumor diameter between adenocarcinoma in situ and stage IA1 cancer. We evaluated whether the intensity of HER3 expression might be enhanced in advanced lung adenocarcinoma with active EGFR mutations. As shown in Table 3, subgroup analysis revealed that the intensity of HER3 expression was only enhanced in the group with advanced cancer without any EGFR mutations.
Table 3.
Subgroup analysis of the correlation between HER3 expression level and stage with or without EGFR mutation
| Mutation/Expression | Reclassified stage | Statistical analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 0 | Stage IA1 | ||||||||||
| HER3 expression | — | 1+ | 2+ | 3+ | — | 1+ | 2+ | 3+ | |||
| EGFR mutation negative | 3 | 3 | 0 | 0 | 1 | 8 | 3 | 1 | Intensity: P < 0.05 (0.03) | ||
| EGFR mutation positive | 1 | 2 | 1 | 0 | 0 | 10 | 2 | 0 | Intensity: NS P = 0.689 | ||
NS, not significant; SD, standard deviation.
Discussion
EGFR with activating mutations, including exon 19 deletion and L858R mutation, is a driver oncogene that induces lepidic cell growth and forms bronchoalveolar carcinoma in vivo.13, 14 Although lung cancers with EGFR mutation are initially sensitive to EGFR‐TKIs, they show acquired resistance.9, 10 The most frequent mechanism of acquired resistance is T790M point mutation of EGFR. 21 In some lung cancers with EGFR mutation, HER2 amplification22 causes the resistance, and heregulin, a ligand of HER3, is increased during EGFR‐TKI treatment,23 suggesting that HER2 or HER3 may be implicated in the resistance of lung cancers to EGFR‐TKI treatment. Therefore, HER3 may be a therapeutic target against lung cancer exhibiting EGFR‐TKI resistance.24 The HER3‐targeted antibody, patritumab, combined with erlotinib has been evaluated in non‐small cell lung cancer harboring EGFR‐mutations.25 In addition, the tolerance and efficacy of HER3 target therapy against various kinds of cancers without EGFR mutations, such as breast cancer overexpressing HER2 and head and neck cancer, have also been examined.26, 27 These three studies indicate encouraging efficacy of combination therapies with HER3 target therapies.
On the other hand, HER3 is associated with poor prognosis in gastric cancer.28 As HER3 can homodimerize or heterodimerize with ErbB family receptors, it also might be implicated in the progression or maintenance of the malignant phenotype of non‐small cell lung cancer without EGFR mutations. However, little is known about how HER2 and HER3 impact progression from stage 0 to IA1 of lung cancer without EGFR mutations and lung cancer harboring EGFR mutations when they are not treated with EGFR‐TKIs.
This study focused on an analysis of the implications of HER2 or HER3 in local progression from carcinoma in situ to invasive solid adenocarcinoma. The five‐year OS rate was 100% for stage 0 and 95.5% for stage IA1. Therefore, this study might not be suitable as an analysis of the relationship between recurrence and HER2 or HER3 because the mechanism of lung adenocarcinoma in situ progression may have little influence on the postoperative outcome.
In this study, we observed that the presence or absence of EGFR mutation is not implicated in cancer progression during the early stage, which is consistent with previous data suggesting that amplification rather than EGFR mutation is associated with lung adenocarcinoma progression.16 This result is consistent with reports implicating EGFR in carcinogenesis.13, 14 We observed that the frequency and intensity of HER3 protein expression is higher in stage IA1 than in stage 0 lung cancer. In addition, the intensity of HER3 expression is associated with the progression of lung adenocarcinoma without EGFR mutations. On the other hand, no association was observed between the progression and HER2 by our analysis. Therefore, increased HER3 expression might be implicated in the progression of lung cancer without EGFR mutations. On the other hand, we have previously reported that the neuregulin 1/HER3 pathway activates the AKT signal and induces spheroid culture of primary lung cancer cells, including those harboring wild type EGFR,29 suggesting that HER3 activation may convey a growth advantage even in lung cancer without EGFR mutations.
Further studies are needed to validate whether increased HER3 expression is responsible for cancer progression. Although no correlation between HER3 expression and progression of lung adenocarcinoma harboring EGFR mutations was found, we cannot conclude that HER3 is not implicated in the progression from stage 0 to IA as our analysis only included a small number of cases, which may affect the result with regard to lung cancer with EGFR mutations.
Collectively, our results indicate that HER3 is not only a therapeutic target for EGFR‐TKI resistant lung cancer harboring EGFR mutations, but may also prevent advanced stage lung adenocarcinoma without EGFR mutations from further progression.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We would like to thank all patients for participating in the study. This study was supported in part by a grant from The Osaka Foundation for The Prevention of Cancer and Life‐style related Diseases (Public Interest Incorporated Foundation).
References
- 1. Ullrich A, Coussens L, Hayflick JS et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984; 309: 418–25. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto T, Ikawa S, Akiyama T et al. Similarity of protein encoded by the human c‐erb‐B‐2 gene to epidermal growth factor receptor. Nature 1986; 319: 230–4. [DOI] [PubMed] [Google Scholar]
- 3. Plowman GD, Whitney GS, Neubauer MG et al. Molecular cloning and expression of an additional epidermal growth factor receptor‐related gene. Proc Natl Acad Sci U S A 1990; 87: 4905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plowman GD, Culouscou, Whitney JM, GS e a. Ligand‐specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A 1993; 90: 1746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J 2000;19: 3159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2: 127–37. [DOI] [PubMed] [Google Scholar]
- 7. Appert‐Collin A, Hubert P, Crémel G, Bennasroune A. Role of ErbB receptors in cancer cell migration and invasion. Front Pharmacol 2015; 6: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Luca A, Carotenuto A, Rachiglio A et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol 2008; 214: 559–67. [DOI] [PubMed] [Google Scholar]
- 9. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 10. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 11. Furukawa M, Nagatomo I, Kumagai T et al. Gefitinib‐sensitive EGFR lacking residues 746‐750 exhibits hypophosphorylation at tyrosine residue 1045, hypoubiquitination, and impaired endocytosis. DNA Cell Biol 2007; 26: 178–85. [DOI] [PubMed] [Google Scholar]
- 12. Nagatomo I, Kumagai T, Yamadori T et al. The gefitinib‐sensitizing mutant epidermal growth factor receptor enables transformation of a mouse fibroblast cell line. DNA Cell Biol 2006; 25: 246–51. [DOI] [PubMed] [Google Scholar]
- 13. Ji H, Li D, Chen L et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR‐targeted therapies. Cancer Cell 2006; 9: 485–95. [DOI] [PubMed] [Google Scholar]
- 14. Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down‐regulation of the receptors. Genes Dev 2006; 20: 1496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engelman JA, Jänne PA, Mermel C et al. ErbB‐3 mediates phosphoinositide 3‐kinase activity in gefitinib‐sensitive non‐small cell lung cancer cell lines. Proc Natl Acad Sci U S A 2005; 102: 3788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR‐mutated lung cancer. Cancer Res 2008; 68: 2106–11. [DOI] [PubMed] [Google Scholar]
- 17. Goldstraw P, Chansky K, Crowley J et al The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 18. Yatabe Y, Kosaka T, Takahashi T, Mitsudomi T. EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol 2005; 29: 633–9. [DOI] [PubMed] [Google Scholar]
- 19. Nagai Y, Miyazawa H et al. Genetic heterogeneity of the epidermal growth factor receptor in non‐small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid‐locked nucleic acid PCR clamp. Cancer Res 2005; 65: 7276–82. [DOI] [PubMed] [Google Scholar]
- 20. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non‐small cell lung cancer to gefitinib. N Engl J Med 2005; 352:786–92. [DOI] [PubMed] [Google Scholar]
- 22. Takezawa K, Pirazzoli V, Arcila ME et al. HER2 amplification: A potential mechanism of acquired resistance to EGFR inhibition in EGFR‐mutant lung cancers that lack the second‐site EGFRT790M mutation. Cancer Discov 2012; 2: 922–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yonesaka K, Kudo K, Nishida S et al. The pan‐HER family tyrosine kinase inhibitor afatinib overcomes HER3 ligand heregulin‐mediated resistance to EGFR inhibitors in non‐small cell lung cancer. Oncotarget 2015; 6: 33602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karachaliou N, Lazzari C, Verlicchi A, Sosa AE, Rosell R. HER3 as a therapeutic target in cancer. BioDrugs 2017; 31: 63–73. [DOI] [PubMed] [Google Scholar]
- 25. Nishio M, Horiike A, Murakami H et al. Phase I study of the HER3‐targeted antibody patritumab (U3‐1287) combined with erlotinib in Japanese patients with non‐small cell lung cancer. Lung Cancer 2015; 88:275–81. [DOI] [PubMed] [Google Scholar]
- 26. Mukai H, Saeki T, Aogi K et al. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2‐overexpressing metastatic breast cancer. Cancer Sci 2016; 107: 1465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jimeno A, Machiels JP, Wirth L et al. Phase Ib study of duligotuzumab (MEHD7945A) plus cisplatin/5‐fluorouracil or carboplatin/paclitaxel for first‐line treatment of recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer 2016; 122: 3803–11. [DOI] [PubMed] [Google Scholar]
- 28. Li Q, Zhang R, Yan H et al. Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget 2017; 8: 67140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Endo H, Okami J, Okuyama H et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol 2013; 8: 131–9. [DOI] [PubMed] [Google Scholar]


