Abstract
Background
Despite the high incidence of breast cancer worldwide, methods for early non‐invasive diagnosis and sensitive and specific prognostic evaluation remain difficult. In this study, we investigated microwave parameters as a potential non‐invasive approach to detect breast cancer.
Methods
Samples of freshly excised breast tissues (n = 509) from 98 patients were identified as normal, benign tumor, or malignant cancer via histology. Further samples were prepared and the microwave effective dielectric permittivity and effective conductivity were measured every 0.0375 GHz from 0.5 GHz to 8 GHz. These parameters were compared among the breast tissue types.
Results
The effective relative permittivity and effective conductivity at each frequency was significantly higher in breast cancer tissues compared with benign tumors, which in turn was significantly higher than in normal breast tissue. The standard deviation of each parameter was narrowest at ~2.5 GHz in both normal and malignant breast tissues.
Conclusions
The effective dielectric permittivity and effective conductivity, measured via microwave technology, could differentiate breast cancer from normal and benign tumor tissues.
Keywords: Breast cancer, dielectric properties, non‐invasive diagnosis, open‐ended coaxial method
Introduction
Breast cancer is a common cancer among women worldwide, with an estimated 1.3 million new cases and 465 000 deaths annually.1, 2 Thus, breast cancer is a leading cause of cancer mortality.3 Early diagnosis of breast cancer could significantly decrease the mortality rate. Mammography and ultrasound are currently the standard diagnostic tools that have proved successful for the detection of early stage breast cancer. However, these methods are still limited by low sensitivity and specificity, and pose a radiation hazard to the patient.4, 5, 6 Thus, early detection remains a major hurdle in the management of breast cancer, and new non‐invasive diagnostic approaches with higher sensitivity and specificity are urgently needed.
Microwaves refer to electromagnetic radiation with frequencies from 300 MHz to 300 GHz.7 This range is particularly attractive for biomedical imaging and treatment, as it balances spatial resolution and penetration depth. For breast cancer detection specifically, microwaves may be useful because of the differences in the electrical properties of malignant tumor and normal breast cells.8, 9 The differences in microwave properties have been estimated at almost 5–10 times larger.10 Moreover, microwave frequencies are non‐ionizing, with adequate penetration of breast tissue.11 Thus, breast examination with microwaves is harmless and mass surveys may be feasible in the future.
Previous evaluations of microwave frequencies of the dielectric properties of diseased breast tissues, relative to normal, have been limited by small sample sizes, a lack of comparison among different tissue types, and inconsistent results.8, 11 To rectify these issues, we conducted a large‐scale study to characterize the dielectric properties of freshly excised normal, benign tumor, and malignant breast tissues obtained from cancer surgeries.
Methods
Study objective
The Ethics Committee of Sichuan Provincial People’s Hospital approved this study. The study included malignant, benign tumor, and normal breast tissues freshly excised from 98 patients (509 breast samples) obtained during radical mastectomy (malignant tumor and normal breast tissue far away from the tumor) or ectomy of breast fibroadenoma (benign tumor) at Sichuan Provincial People’s Hospital. All specimens were stored in heated, sealed, and insulated containers to minimize desiccation, and transported to the measurement location.
Pathological examination
The pathological examination conformed to the hospital’s standard protocol. Typically, a pathologist paged one of the engineers responsible for conducting the measurements as soon as the specimens were collected during surgery. The time between excision and measurement was five minutes to two hours. To confirm the histological type, samples were first stained with hematoxylin and eosin (H&E). We collected the samples for this study from the pathology department and conducted the microwave measurements within two hours of the surgeries.
Original data acquisition
At the time of collection from the pathology department, the pathologist cut one piece off each specimen. The shape of each piece conformed to the following requirements: at least one side was as flat as possible to avoid an air gap between the sample and the aperture of the coaxial line, and this side was minimally 5–6× the area of the coaxial aperture.12, 13, 14, 15, 16, 17 Specifically, because the aperture was ~10.2 mm2, the edges of the square face of the flat side were > 7.8 mm. The minimum thickness of the sample, from the flat to the opposite side, was 2–3 mm.12, 13, 14, 15, 16, 17
A Vector Network Analyzer (Rohde & Schwarz, Munich, Germany) was used to connect to the coaxial probe to obtain the scattering (S) microwave parameter of each sample (Fig 1). Measurements were designated S1, S2 … Sn. The reference S‐parameter (S0) was measured when the probe was exposed to air. The S‐parameters were measured every 0.0375 GHz at frequencies from 0.5 to 8 GHz. At the time of initial measurement, the S‐parameters comprised data extending from the coaxial probe to the samples.
Figure 1.

Schematic of the measuring system. PC, personal computer; VNA, Vector Network Analyzer.
Data de‐embedding
The data specific to the breast tissue sample had to be de‐embedded from the initial calibrated measurement, which included the coaxial probe (Fig 1). To accomplish this, the initial S‐parameters (S1, S2 … Sn) were saved to ASCII Files (*.CSV) using Vector Network Analyzer viewer software version V1.44 (ZVH viewer, Rohde & Schwarz). The ASCII file data was then imported into MATLAB version 2014 A (MathWorks, Natick, MA, USA). The data relevant to the coaxial probe was deleted from the S‐parameters. The de‐embedded data for the S‐parameters was exported into SnP Files (in this case, S1P).
Modeling
According to microwave theory, any material with an electromagnetic field can transmit microwaves. Therefore, we may consider the samples as comparable to transmission lines, using a series of discrete cells as a model (Fig 2), n in number (in this case n = 2, 4, or 8). The components inductance (L) and capacitance (C) represent energy storage, and resistance (R) and conductance (G) constitute energy loss.7
Figure 2.
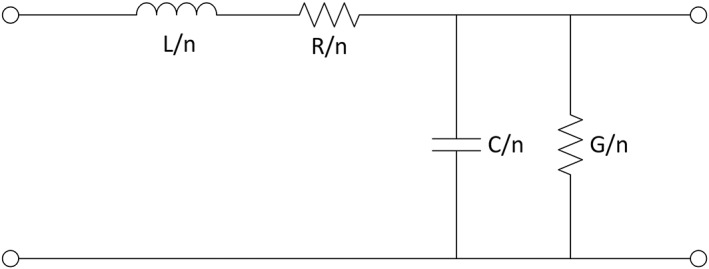
One cell of the transmission line model.
To create the model, the SnP files were imported into the Advanced Design System Version 2015 (ADS; Agilent Technologies, Santa Clara, CA, USA,). In the Advanced Design System, a transmission line model was designed to fit the S‐parameter from each SnP file. A good fit was defined as a model that described the sample well. The L, C, R, and G values for each sample were then recorded.
Measurement results
When the exact values of the model parameters were obtained, we applied them to the formulas of microwave theory:
(1)
where Z * is the complex characteristic impedance of the sample; j is the imaginary unit; ω is the angular frequency; Z 0 is the complex characteristic impedance of air; μ 0 is the permeability of vacuum; μ r is the relative permeability of sample (for the human body, equal to 1); ε 0 is the permittivity of vacuum; and ε r * is the complex relative dielectric permittivity.
Given that Z 0 = 377 Ω, ε 0 = 8.854 × 10−12 F/m, μ 0 = 4π × 10−7 N/A2, and ω = 2π × frequency, we could calculate the complex relative dielectric permittivity, ε r *, of each sample.
According to dielectric physics and microwave theories, we know that:
(2)
where ε′ and ε′′ are the real and imaginary parts of ε *, respectively. Thus, both ε eff (effective dielectric permittivity) and σ eff (effective conductivity) are real numbers. We could then calculate the effective dielectric permittivity and effective conductivity of each sample from the complex relative dielectric permittivity, as a function of frequency.
The mean effective dielectric permittivity and effective conductivity were compared across a frequency range of 0.5–8 GHz for each group of tissue types (normal breast, benign tumor, and breast cancer).
Statistical analysis
All data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Measurement data is shown as mean ± standard deviation and was compared using multivariate repeated‐measures analysis of variance. Statistical significance was considered as P < 0.05.
Results
Histology‐based group criteria
To minimize uncertainty when determining the composition of tissues within the probe’s sensing volume, we established criteria for categorizing tissue groups based on histology slides (Fig 3). Two experienced pathologists obtained a histopathologic diagnosis from 128 breast cancer, 224 normal, and 157 benign tumor tissue samples. The diagnosis was made according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology.
Figure 3.
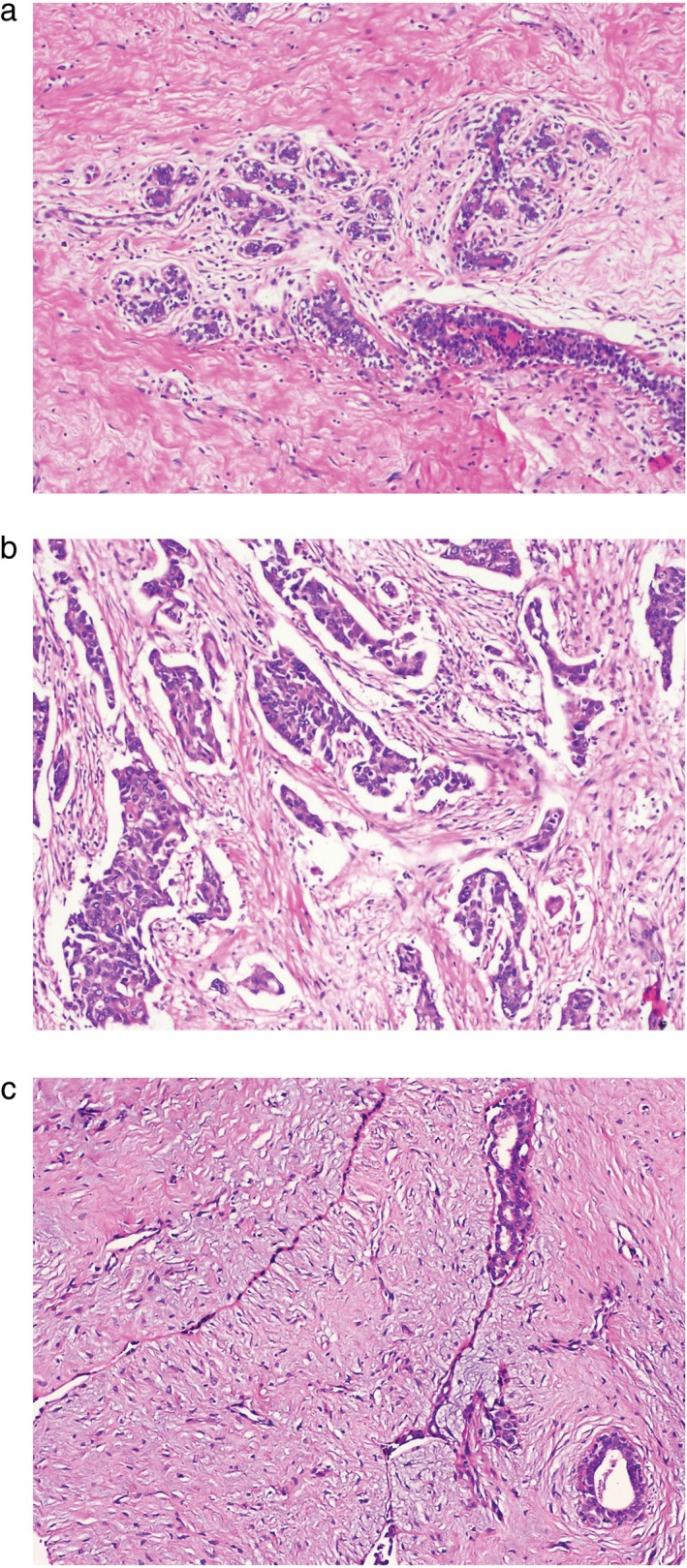
Hematoxylin and eosin staining of (a) normal breast tissue, (b) breast cancer, and (c) benign breast tumor (fibroadenoma).
Effective dielectric permittivity and effective conductivity of breast tissue types as a function of frequency
Breast tissue types (normal, benign tumor, and cancer) could be differentiated according to the effective dielectric permittivity and effective conductivity as a function of frequency (Table 1, Fig 4). The ranges of effective dielectric permittivity of the three tissue types significantly differed, not only between normal breast and breast cancer tissues but also between benign breast tumor and breast cancer tissues (P < 0.05).
Table 1.
Microwave parameters of three breast tissue types at low (0.5 GHz), middle (2 GHz, 4 GHz, 6 GHz), and high (8 GHz) frequencies
| Tissue type | 0.5 GHz | 2GHz | 4GHz | 6GHz | 8 GHz | |
|---|---|---|---|---|---|---|
| ε eff | Normal | 9.070 ± 0.714 | 8.163 ± 0.577 | 7.202 ± 0.428 | 6.569 ± 0.332 | 6.292 ± 0.291 |
| Benign tumor | 24.842 ± 1.829 | 21.664 ± 1.559 | 18.303 ± 1.814 | 16.093 ± 2.214 | 15.120 ± 2.423 | |
| Cancer | 66.696 ± 2.479 | 63.008 ± 2.108 | 59.125 ± 1.802 | 56.551 ± 1.664 | 55.425 ± 1.623 | |
| pa | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| pb | 0.0003 | 0.0002 | 0.0002 | 0.0002 | 0.0002 | |
| σ eff, | Normal | 0.245 ± 0.145 | 0.497 ± 0.069 | 0.758 ± 0.119 | 0.930 ± 0.187 | 1.006 ± 0.219 |
| S/m | Benign tumor | 0.279 ± 0.187 | 0.955 ± 0.122 | 1.669 ± 0.077 | 2.139 ± 0.079 | 2.346 ± 0.877 |
| Cancer | 1.697 ± 0.464 | 4.164 ± 0.074 | 6.774 ± 0.356 | 8.487 ± 0.633 | 9.242 ± 0.755 | |
| pa | 0.0218 | 0.0000 | 0.0011 | 0.0029 | 0.0036 | |
| pb | 0.0243 | 0.0000 | 0.0004 | 0.0011 | 0.0014 |
ε eff, effective dielectric permittivity; σ eff, effective conductivity; pa, cancer group compared with normal group; pb, cancer group compared with benign tumor group.
Figure 4.

Microwave parameters differentiating breast tissue types as a function of frequency. Effective dielectric permittivity and effective conductivity of: (a,b) breast cancer; (c,d) normal breast tissue; and (e,f) benign breast tumor tissue, respectively.  Mean,
Mean,  Mean plus standard deviation (std.),
Mean plus standard deviation (std.),  Mean minus std.
Mean minus std.
The ranges in the effective dielectric conductivity of the three tissue types also significantly differed (Table 1, Fig 4). The effective conductivity showed obvious statistical differences not only between normal breast and breast cancer tissues but also between benign breast tumor and breast cancer tissues (P < 0.05).
It was also noted that for breast cancer and normal breast tissue, the standard deviation in effective conductivity varied over the frequency range (Fig 4): from 0.5 GHz to approximately 2.5 GHz the standard deviation decreased, and then increased from 2.5 to 8 GHz.
Discussion
This study determined whether the dielectric properties of microwaves (i.e. effective dielectric permittivity and effective conductivity) may be used to differentiate normal, benign tumor, and malignant breast tissues. Directly after tumor surgery, specimens from 509 breast samples from 98 patients were obtained, prepared, and the effective dielectric permittivity and effective conductivities were measured over frequencies from 0.5 to 8 GHz. These microwave parameters of each tissue group were compared, with the types referenced to the results of H&E histology. Normal, benign tumor, and malignant breast tissues differed significantly regarding both effective dielectric permittivity and effective conductivity. In addition, the standard deviations of the effective conductivities for normal and breast cancer tissues changed similarly with changes in frequency: for each, the standard deviation decreased gradually from 0.5 to ~2.5 GHz, and then increased gradually from ~2.5 to 8 GHz. Thus, the standard deviation was very narrow at ~2.5 GHz, for both normal and breast cancer tissues, while the mean of each differed significantly from the other. The single frequency, 2.5 GHz may be sufficient to differentiate normal tissues from breast cancer.
Lazebnik et al. showed that contrast in microwave frequency dielectric properties between malignant and normal adipose‐dominated tissues in the breast is considerable, as large as 10:1, but no benign breast lesions were compared.12 Previous studies have shown differences in the electrical properties of malignant and benign breast epithelial cells, and that both permittivity and conductivity of breast cancer are significantly higher than those of normal tissues.14, 18 The values of these properties correlate with frequency, and provide a basis for investigating the mechanisms that underlie malignancy, including cellular proliferation, cytoskeleton, and metabolism. Our results are consistent with previous research. This is partly because normal breast tissue is predominantly adipose tissue, which has lower water content and dielectric properties compared with benign tumors or malignant breast cancer.
One of the challenges of using the dielectric probe concerns the contact between the probe and the tissues. Thus, the method is ideal for soft tissue measurements, as both the probe and the tissues naturally conform to the surface of the open‐ended coaxial line. Accordingly, probes that are suited for hard materials are not recommended for measuring the dielectric properties of breast tissues.
Compared with conventional diagnostic pathology methods for complicated procedures, such as H&E which requires considerable time and high cost, microwave detection offers several attractive advantages, including its easy operation, non‐compressive nature, and low‐cost. Prior researchers have demonstrated the feasibility of microwave imaging for the diagnosis of breast cancer and to monitor neoadjuvant chemotherapy. Hakam et al. found that the ratio of the electrical permittivity of malignant and normal breast tissue measured in the low frequency range was 160:1, while that of breast cancer and fibrocystic breast tissues could be 1.3:1.19 These results suggest that electrical permittivity could be applied to identify breast cancer and normal breast tissues. Meaney et al. reported that the normalized mean conductivity of breast cancer tissue in patients who achieved a complete pathological response to neoadjuvant chemotherapy was significantly different from that of partial responders.20
Microwave technology is very promising for early diagnosis and treatment evaluation of breast cancer. The effective dielectric permittivity and effective conductivity may be able to differentiate breast tissues. In the present study, the results of clinical specimens, with reference to histologically stained samples, showed that the measurement of dielectric properties is helpful for detecting breast cancer. The data was obtained with low radiation and high resolution and provided a large amount of information.
This study is limited, in that the specimens included both adipose and fibroconnective tissues, which may affect the dielectric properties.12 We did not reference the parameters associated with these tissues, and such details need to be investigated.
In this study, freshly excised tissues were histological stained and used as the gold standard reference of tissue type (normal, benign tumor, and breast cancer). These were then compared, with regard to microwave effective dielectric permittivity and effective conductivities, at frequencies from 0.5 to 8 GHz, with 201 sample points. The effective dielectric permittivity was negatively associated with frequency in all tissue types, while the effective conductivity was positively associated. However, each of the parameters at each frequency was significantly higher in breast cancer tissue compared with benign tumor tissue, which in turn was significantly higher than in normal breast tissue. Based on these differences, a good differential diagnosis of breast cancer may be achieved. These results indicate that these microwave parameters may be a useful novel method to detect breast cancer non‐invasively, harmlessly, and accurately.
Disclosure
No authors report any conflict of interest.
References
- 1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24: 2137–50. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis CE, Fedewa SA, Goding Sauer SA et al Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 2016; 66: 31–42. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–49. [DOI] [PubMed] [Google Scholar]
- 4. Saslow D, Boetes C, Burke W et al American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57: 75–89. [DOI] [PubMed] [Google Scholar]
- 5. Kriege M, Brekelmans CT, Boetes C et al Efficacy of MRI and mammography for breast‐cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351: 427–37. [DOI] [PubMed] [Google Scholar]
- 6. Spanu A, Sanna D, Chessa F et al The clinical impact of breast scintigraphy acquired with a breast specific gamma‐camera (BSGC) in the diagnosis of breast cancer: Incremental value versus mammography. Int J Oncol 2012; 41: 483–9. [DOI] [PubMed] [Google Scholar]
- 7. Pozar DM. Microwave Engineering, 4th edn. Wiley, Hoboken NJ: 2012. [Google Scholar]
- 8. Ng EY, Sree SV, Ng KH, Kaw G. The use of tissue electrical characteristics for breast cancer detection: A perspective review. Technol Cancer Res Treat 2008; 7: 295–308. [DOI] [PubMed] [Google Scholar]
- 9. Fear EC, Hagness SC, Meaney PM, Okoniewski M et al Enhancing breast tumor detection with near‐field imaging. IEEE Microw Mag 2002; 3: 48–56. [Google Scholar]
- 10. El‐Shenawee M. Electromagnetic imaging for breast cancer research. Proceedings of the 2011 I.E. Radio and Wireless Symposium, 16–19 Jan 2011, Phoenix, AZ. IEEE, Piscataway, NJ. [Google Scholar]
- 11. Hassan AM, El‐Shenawee M. Review of electromagnetic techniques for breast cancer detection. IEEE Rev Biomed Eng 2011; 4: 103–18. [DOI] [PubMed] [Google Scholar]
- 12. Lazebnik M, Popovic D, McCartney L et al A large‐scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys Med Biol 2007; 52: 6093–115. [DOI] [PubMed] [Google Scholar]
- 13. Lazebnik M, McCartney L, Popovic D et al A large‐scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys Med Biol 2007; 52: 2637–56. [DOI] [PubMed] [Google Scholar]
- 14. Meaney PM, Gregory A, Epstein N et al Microwave open‐ended coaxial dielectric probe: Interpretation of the sensing volume re‐visited. BMC Med Phys 2014; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meaney PM, Williams BB, Geimer SD et al A coaxial dielectric probe technique for distinguishing tooth enamel from dental resin. Adv Biomed Eng Res 2015; 3: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karni T, Pappo I, Sandbank J et al A device for real‐time, intraoperative margin assessment in breast conservation surgery. Am J Surg 2007; 194: 467–73. [DOI] [PubMed] [Google Scholar]
- 17. Cenanovic A, Martius S, Kilian A et al. Nondestructive complex permittivity determination of glass material with planar and convex surface. Proceedings of the 6th German Microwave Conference, 14–16 Mar 2011, Darmstadt, Germany. MTT/AP Joint Chapter in the IEEE Germany Section.
- 18. Kaufman Z, Paran H, Haas I et al Mapping breast tissue types by miniature radio‐frequency near‐field spectroscopy sensor in ex‐vivo freshly excised specimens. BMC Med Imaging 2016; 16: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abd El‐Ha R, Khalil S, Mahani R. Dielectric and FT‐Raman spectroscopic approach to molecular identification of breast tumor tissues. Spectrochim Acta A Mol Biomol Spectrosc 2015; 151: 208–12. [DOI] [PubMed] [Google Scholar]
- 20. Meaney PM, Kaufman PA, Muffly LS et al Microwave imaging for neoadjuvant chemotherapy monitoring: Initial clinical experience. Breast Cancer Res 2013; 15: R35. [DOI] [PMC free article] [PubMed] [Google Scholar]


