Abstract
Paratuberculosis (Johne's disease) is a chronic debilitating disease of domestic and wild ruminants. However, widespread point-of-care testing is infrequent due to the lack of a robust method. The isothermal recombinase polymerase amplification (RPA) technique has applied for rapid diagnosis. Herein, RPA combined with a lateral flow dipstick (LFD) assay was developed to estimate DNA from Mycobacterium avium subsp. paratuberculosis. First, analytical specificity and sensitivity of the RPA-nfo primer and probe sets were assessed. The assay successfully detected M. paratuberculosis DNA in 30 min at 39℃ with a detection limit of up to eight copies per reaction, which was equivalent to that of the real-time quantitative polymerase chain reaction (qPCR) assay. The assay was specific, as it did not amplify genomes from five other Mycobacterium spp. or five pathogenic enteric bacteria. Six hundred-twelve clinical samples (320 fecal and 292 serum) were assessed by RPA-LFD, qPCR, and enzyme-linked immunosorbent assay, respectively. The RPA-LFD assay yielded 100% sensitivity, 97.63% specificity, and 98.44% concordance rate with the qPCR results. This is the first report utilizing an RPA-LFD assay to visualize and rapidly detect M. paratuberculosis. Our results show this assay should be a useful method for the diagnosis of paratuberculosis in resource-constrained settings.
Keywords: Mycobacterium avium subsp. paratuberculosis, isothermal detection, lateral flow dipstick, paratuberculosis, recombinase polymerase amplification
Introduction
Paratuberculosis (Johne's disease) caused by Mycobacterium avium subsp. paratuberculosis is a chronic debilitating disease of domestic and wild ruminants. The main characteristics of paratuberculosis are chronic granulomatous enteritis and refractory diarrhea [10,21]. It can result in significant economic loss to animal husbandry due to increased susceptibility to other diseases, reduced production performance, and premature elimination [5]. In China, cattle, sheep, and special economic animals, such as domestic sika deer, red deer, and alpaca have been infected [13]. For the diagnosis of paratuberculosis, the enzyme-linked immunosorbent assay (ELISA) is the national standard method in China. In addition, ELISA and the real-time quantitative polymerase chain reaction (qPCR) assay are the most widely used in paratuberculosis screening. Paratuberculosis antibody-positive animals can be detected in most provinces of China, and the seroprevalence of antibodies against M. paratuberculosis was reported as 11.7% (121/1,038) at the herd level [21]. However, the positive rate for small farms is higher than that for large farms [21].
There is currently no effective vaccine to prevent paratuberculosis; at the same time, there is a lack of corresponding prevention and control measures in China. Individual cattle showing diarrhea and emaciation are screened at some cattle farms, and some farmers seek an active way of eliminating or controlling the disease through the elimination of infected animals. At present, six endemically infected countries have developed a plan for prevention and control [6]. Quick diagnosis could facilitate control; however, widespread point-of-care testing is infrequent due to the absence of a robust control method. The primary focus of the work reported here was to develop a rapid, sensitive, on-site testing method that combined recombinase polymerase amplification (RPA) with a lateral flow dipstick (LFD) assay for use in the specific detection of M. paratuberculosis in the field [1,8,11].
Obtaining a quick diagnosis of an M. paratuberculosis infected animal is a prerequisite for paratuberculosis prevention, and developing a rapid and sensitive molecular diagnostic technique for use in paratuberculosis eradication is of significant importance. The use of culture methods to detect viable bacteria is labor-intensive, time-consuming, and has longer turnaround times than many molecular tests. Although detection technology of molecular biology assays, such as PCR and qPCR assays, have shown high specificity and sensitivity, these methods require a professional diagnostic laboratory, thermal cycling equipment, and experienced operators. Access to such diagnostic applications is limited in poor and/or disease epidemic areas, especially in developing countries. In the absence of such diagnostic equipment, it is difficult to make an accurate diagnosis of the animal's disease at point-of-care locations. Therefore, developing a point-of-care-located, rapid diagnostic method would be very helpful for paratuberculosis eradication or control.
Recently, RPA, an isothermal technique for DNA amplification emerged as a novel molecular technology for rapid, low-resource use diagnostics. It includes three kinds of detection methods for testing the DNA amplification result [14]. In addition, an RPA-LFD combination is more beneficial for clinical point-of-care diagnoses. At present, RPA assays have been established and reported for the rapid detection of several pathogens [1,11,18]. Moreover, a real-time RPA assay for detecting M. paratuberculosis DNA has also been developed [7]. However, an RPA-LFD assay for M. paratuberculosis detection has not been established. In this study, we developed a sensitive and specific RPA-LFD assay to detect DNA from M. paratuberculosis by using nucleic acid isolated from clinical fecal samples.
Materials and Methods
Ethics statement
Fecal samples were collected and handled with good animal practices as required by the Chinese Regulations of Laboratory Animals (Ministry of Science and Technology of People's Republic of China, 20110108). The animal study proposal was approved by the Experimental Animal Ethics Committee of Shandong Normal University (approval No. 20160901). Animal owners provided oral consent as per the national ethical regulations.
Strains and clinical samples
The reference strain M. paratuberculosis (Table 1) was purchased from the BeNa Culture Collection (BNCC, China) and grown on media following the instructions provided by BNCC. Between September 2016 and September 2017, 320 individual fecal samples and 292 individual serum samples were collected from 10 different dairy farms located in ten distinct geographic regions of Shandong province, China. The herds were selected at random and herd size varied between 200 and 800 animals. There was no history of paratuberculosis in these farms and no results of previous serological tests. Approximately 10% of the animals (adult cows over 24 months old showing diarrhea and emaciation) were selected for sampling. All fecal samples were collected by local field clinical veterinarians via per-rectal drags and were kept at 4℃ for up to 48 h for DNA extraction. Serum samples were collected from cattle tail vein by using a 10 mL sterile syringe and 5 mL were kept in a coagulant tube. The genomic DNA of the reference strain and clinical samples was extracted by using a bacterial genome DNA extraction kit and a stool DNA kit (Tiangen Biotech, China). Specific extraction steps were carried out in accordance with the manufacturer's instructions. DNA extracted from field fecal samples was tested by RPA-LFD and qPCR assays, serum samples were tested by ELISA. During analysis, the results from two different assays were compared.
Table 1. Strains used for specificity testing.
*These strains were purchased from the BeNa Culture Collection (China) a biotechnology research institute. †These strains were preserved in our laboratory.
Generation of DNA standard
The extracted DNA of M. paratuberculosis was amplified using the 2× EasyTaq PCR Supermix kit (Beijing TransGen Biotech, China). Forward primer, 5′-ATCAGCGCGGCAC GGCTCTTG-3′, and reverse primer, 5′-CGGGTAGTTACCG CGGCGAAG-3′, were used to amplify 632 nucleotides of the IS900 gene of M. paratuberculosis (691–1322 of GenBank accession No. S74401.1; National Center for Biotechnology Information, USA). The PCR temperature profile was as follows: initial activation at 94℃ for 3 min, 35 cycles of 94℃ for 30 sec, 58℃ for 30 sec, and 72℃ for 30 sec, and a final extension step of 72℃ for 10 min. The amplified fragment was ligated into the plasmid pEASY-T3 cloning vector by using the pEASY-T3 cloning kit (Beijing TransGen Biotech) and designated as pEASY-T3-MAP. The pEASY-T3-MAP standard DNA was extracted by using the TIANpure mini plasmid kit (Tiangen Biotech) and measured by using a NanoPhotometer P-Class P360 (Implen, Germany). The DNA copy number was calculated as described previously [19]. The DNA standard was then stored at 20℃ until needed.
RPA-nfo primer and probe design
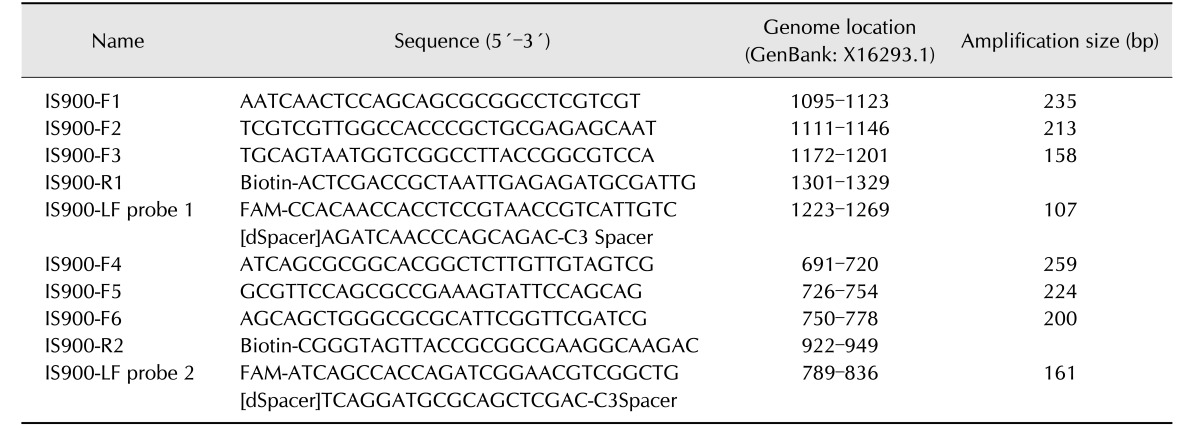
The primer and lateral flow probe for the RPA-lateral flow (RPA-nfo) reaction were designed on the basis of the content of the appendix to the manual for the TwistAmp reaction kit (TwistDx, UK). Molecular biology detection genes are generally based on the IS900 gene sequence for M. paratuberculosis; therefore, six combinations of candidate primers (6 forward and 2 reverse) and two probes were designed according to this gene sequence (Table 2). A specificity analysis was performed by using BLAST and the amplification locus of a 632 nucleotide region indicated 100% identity (query coverage 100%) with that of the M. paratuberculosis IS900 gene. There were no matches to other bacteria.
Table 2. Recombinase polymerase amplification primers and probes designed in this study.
FAM, 6-carboxyfluorescein; dSpacer, exonuclease site; C3 Spacer, a polymerase extension blocking site.
The detail sequence and the modification information are displayed in Table 2. Synthesis and modifications of the oligonucleotides were accomplished by Sangon Biological Engineering, China. Initially, the RPA-nfo reaction system was optimized. The 50 µL reaction volume was prepared according to the instructions for the TwistAmp nfo kit (TwistDX); the volumes of primer and probe (10 µM) were adjusted in accordance with the manufacturer's recommendations. Amounts of rehydration buffer (29.5 µL), DNA template (2 µL), and 280 nM magnesium acetate (2.5 µL) were kept constant. The beginning of the reaction was controlled by the magnesium acetate. All test samples were incubated for a prototypical 30 min at 37℃; the heating equipment was a thermostatic water tank (Shanghai Jing Hong Laboratory, China) unless otherwise noted. The RPA products were purified by using MiniBEST DNA fragment purification kit (ver. 4.0; TaKaRa, China). The size of the RPA oligonucleotides was detected by performing 2% agarose gel electrophoresis (Agarose Broad Range; Beijing TransGen Biotech) with ethidium bromide staining. Because specific amplification was essential for the primer and probe properties, the specificity of primer and probe was first resolved by performing agarose-gel electrophoresis. When the lateral flow probe is cut by the nfo nuclease at the dSpacer position, the probe is transformed into a primer and acts by priming the polymerase extension. Therefore, there were two bands visible in the electrophoresis results.
Lateral flow dipstick assay
Visualization of RPA-nfo amplicons was carried out by using LFD assays (HybriDetect; MileniaBiotec, Germany), with the specific assay steps performed according to HybriDetect's instructions. Briefly, 2 µL of the RPA-nfo product and 98 µL of the HybriDetect assay buffer were blended adequately in a 200 µL centrifuge tube. Subsequently, the LFD was directly dipped into the product-buffer mixture. The visual result should be observed within 5 min.
RPA-nfo conditions and optimization
In order to obtain optimal primer and probe conditions, various combinations were assessed and tested by examining the detected specificity and sensitivity. Next, the incubation temperature and duration of the RPA-nfo reaction were evaluated according to the supplier's (TwistDX) instructions and as reported previously [11]. The tested temperature range was 20℃ to 50℃, and time range tested was 1 to 35 min.
Testing the specificity and sensitivity of the RPA-LFD assay
The specificity of the RPA-LFD assay was determined by using DNA from the bacterial strains listed in Table 1. The genomic DNA of M. paratuberculosis and nuclease-free water were used as the positive and negative controls, respectively, in every run. All bacteria species were provided by BNCC and grown on media by following the instructions given by the BNCC. The analytical sensitivities of the RPA-LFD and qPCR assays were tested in a template with 10-fold serial dilutions of plasmid standard DNA from 4 × 106 to 4 genome copies per microliter.
qPCR assay
The qPCR assay for M. paratuberculosis was performed as described previously [9]. Forward primers M-F (5′-CCACAACCACCTCCGTAACC-3′) and reverse primers M-R (5′-CGCTAATTGAGAGATGCGATTG-3′) were used to amplify a 100 base pair sequences in the 1223–1322 regions of the IS900 gene. The reaction was prepared as a 20 µL reaction volume containing 2× SYBR Green Premix, the forward and reverse primers (10 mM, 0.7 µL each), and 2 µL of DNA template. The following thermal cycling parameters were used: initial denaturation at 95℃ for 30 sec, followed by 40 cycles of 95℃ for 5 sec, and 60℃ for 20 sec. Melting curves parameters were: 95℃ for 5 sec, then 1 cycle of 60℃ for 1 min, and 95℃ for 5 sec followed by a final cooling step at 50℃ for 30 sec. The results were analyzed by using gene scanning software version 1.5 (Roche, Germany). After qPCR, melting curves were analyzed to confirm the specificity of the amplified product.
Detection of M. paratuberculosis antibody
In order to compare the performance of the RPA-LFD and ELISA assays, 292 serum samples were tested for the presence of antibodies to M. paratuberculosis by using a commercial antibody test kit (IDEXX, USA). Specific operation steps followed the manufacturer's instructions.
Statistical analysis
The experimental data were stored in a Microsoft Excel 2007 spreadsheet (Microsoft, USA). Statistical analysis was performed by using SPSS software (ver. 16.0; SPSS, USA). The independent-samples t-test was used to evaluate the results. For all analyses, p < 0.05 was considered to indicate significance. The diagnostic performance of the RPA-LFD, qPCR, and ELISA assays were assessed by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), 95% confidence interval, and coincidence rate as described previously [15].
Results
Screening of M. paratuberculosis RPA-nfo primer and probe
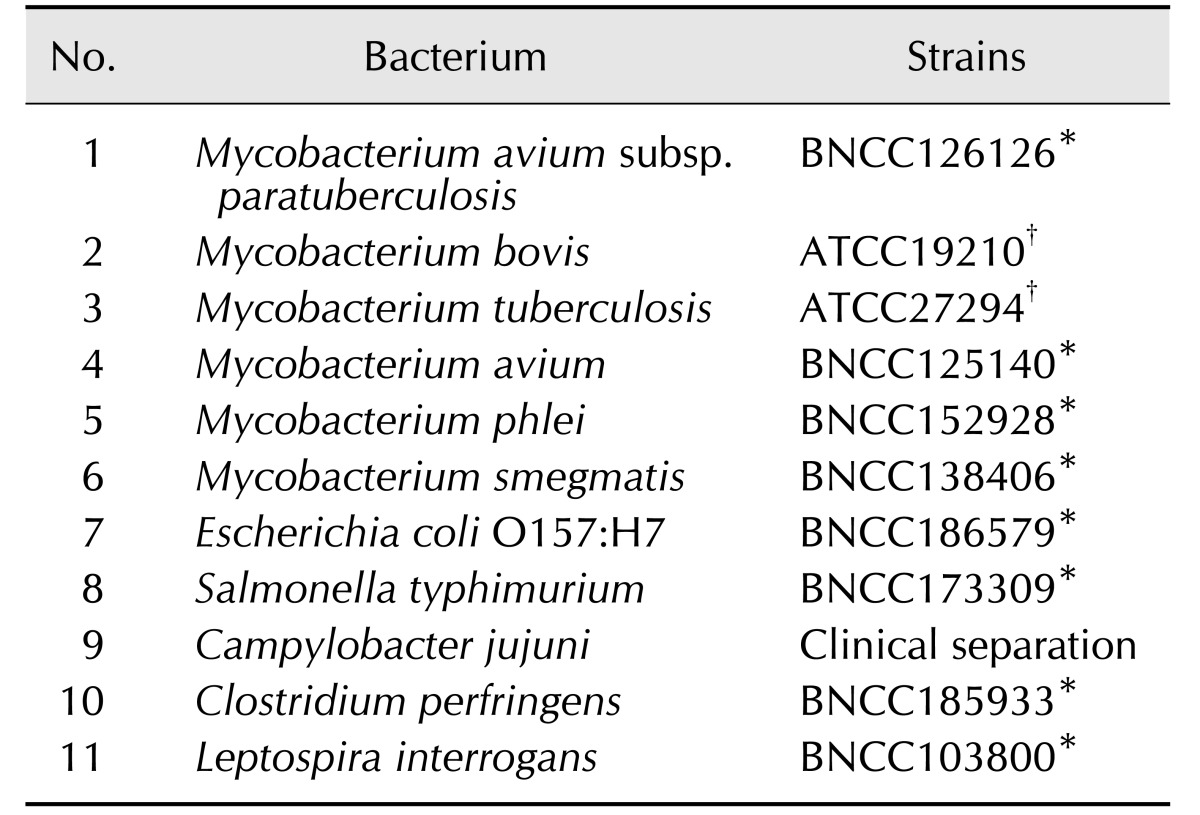
The specificities and sensitivities of the RPA-nfo primer and probe sets were assessed by agarose gel electrophoresis. The various primer pairs and LF (TwistAmp reaction kit; TwistDx, UK)-probes combinations were classified according to specificity and product yield. The performance of the primer and probe in electrophoresis is demonstrated by non-specific amplification (panel A in Fig. 1). In same-primer pairs and LF-probe sets, the second group strip was the brightest in the LFD, and it took the shortest time in the test zone position (panel B in Fig. 1). Due to resource constraints, one set (IS900-F2-IS900-R1 with IS900-LF probe 1) was chosen for subsequent evaluations (Table 2).
Fig. 1. Screening of Mycobacterium paratuberculosis recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) primer and probe. (A) The products of the RPA-nfo reaction were detected by agarose-gel electrophoresis from six primer and probe combinations (F1-LF1-R1, F2-LF1-R1, F3-LF1-R1, F4-LF2-R2, F5-LF2-R2, and F6-LF2-R2; Table 2). (B) ‘a’ shows results of six RPA-nfo reactions on LFD and the DNA template from M. paratuberculosis genomic DNA; and ‘b’ shows negative control (DNase-free water) results for the corresponding combination of primers and probe. Lane 1, F1-R1 (235 bp); Lane 2, F2-R1 (213 bp); Lane 3, F3-R1 (158 bp); Lane 4, F4-R2 (259 bp); Lane 5, F5-R2 (224 bp); Lane 6, F6-R2 (200 bp); M, molecular weight standard (DNA marker 1,000).
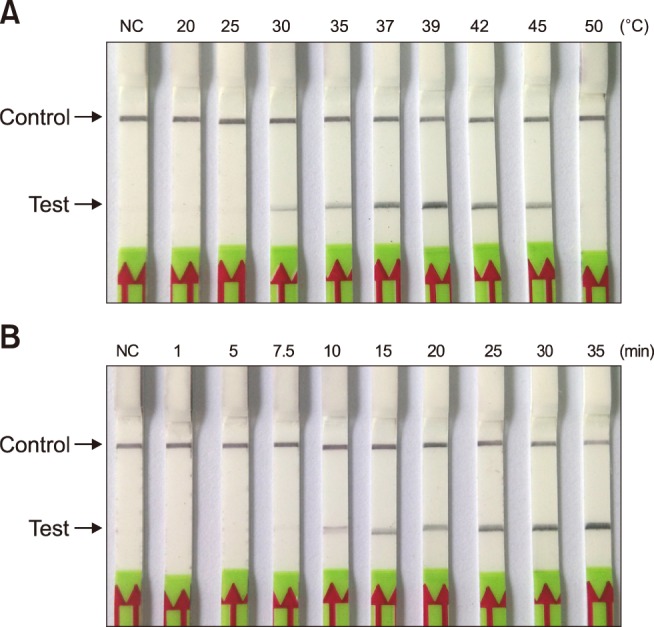
Optimization of RPA-nfo reaction temperature and time
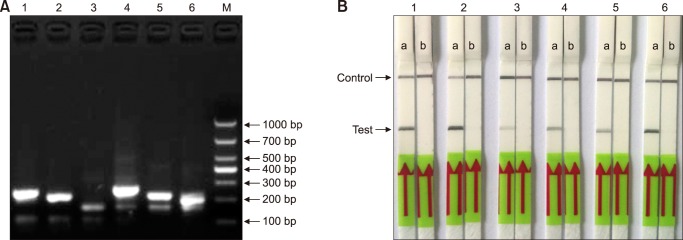
In order to determine the optimum reaction temperature for the M. paratuberculosis RPA-LFD assay, a temperature range of 20℃ to 50℃ was assessed through the reaction of 30 min. The results indicated that the reaction could be effectively completed over a wide interval of temperatures from 30℃ to 45℃. However, the reaction band was brightest at a reaction temperature between 37℃ and 42℃ (panel A in Fig. 2), indicating the optimal temperature range for amplification efficiency. Therefore, in subsequent RPA-LFD assays, the selected reaction temperature was 39℃. The best reaction time was evaluated over a duration scale of 1 to 35 min. The results showed that a distinct band in the test zone position was visible with 10 to 35 min reaction durations. However, the stripe was very weak at 10 min (panel B in Fig. 2). Based on the results, the time of incubation was set at 30 min for subsequent RPA-LFD assay testing.
Fig. 2. Optimization of incubation temperature and reaction time for the Mycobacterium paratuberculosis recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) assay. (A) Amplification performance of RPA-LFD assay was effective within the range of 35℃ to 45℃. (B) Determination of optimum reaction time. After 10 min of amplification reaction, the test line was clearly visible on the LFD strip; NC, negative control (DNase-free water).
Specificity and sensitivity of M. paratuberculosis RPA-LFD assay
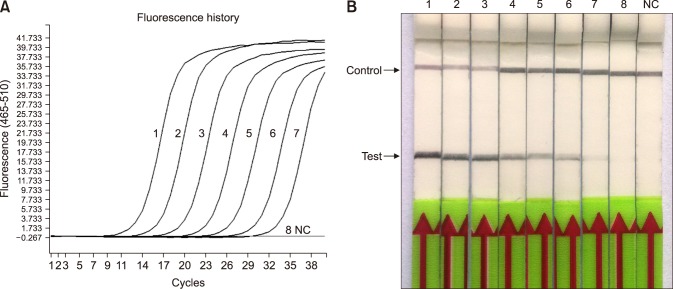
The specificity of the RPA-LFD assay was tested by using DNA extracted from a number of other pathogens that present similarly in the clinic (Table 2). The RPA-LFD assay did not detect the genomic DNA of those 10 bacteria; only the M. paratuberculosis genomic DNA produced a positive result on the LFD strip (Fig. 3). A gradient dilution of M. paratuberculosis plasmid standard DNA (4 × 106 to 4 genome copies per microliter) was used to evaluate the sensitivities of the RPA-LFD and qPCR assays. The results showed that they all were capable of detecting 8 copies per reaction standard DNA (Fig. 4); thus, they had the same threshold detection level.
Fig. 3. Specificity of the recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) assay. The DNA extracted was detected from six strains Mycobacterium and five strains of other bacterial pathogens causing cattle diarrhea; no cross-reactions were detected. Lane 1, Mycobacterium avium subsp. paratuberculosis; Lane 2, Mycobacterium bovis; Lane 3, Mycobacterium tuberculosis; Lane 4, Mycobacterium avium; Lane 5, Mycobacterium phlei; Lane 6, Mycobacterium smegmatis; Lane 7, Escherichia coli O157:H7; Lane 8, Salmonella typhimurium; Lane 9, Campylobacter jujuni; Lane 10, Clostridium perfringens; Lane 11, Leptospira interrogans; NC, negative control (DNase-free water).
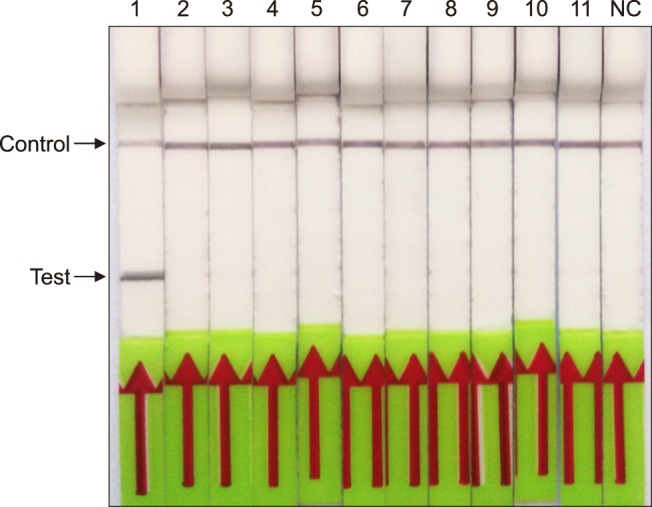
Fig. 4. Comparison of sensitivities of the recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) and quantitative polymerase chain reaction (qPCR) assays. Molecular sensitivity test results of the two assays obtained by using 10-fold serially diluted DNA as a template. (A) Results by qPCR. (B) Result by RPA-LFD. Lanes 1–8, a gradient dilution Mycobacterium paratuberculosis plasmid standard DNA from 4 × 106 to 4 copies per microliter; NC, negative control (DNase-free water).
Performance of RPA-LFD assay on fecal and serum samples
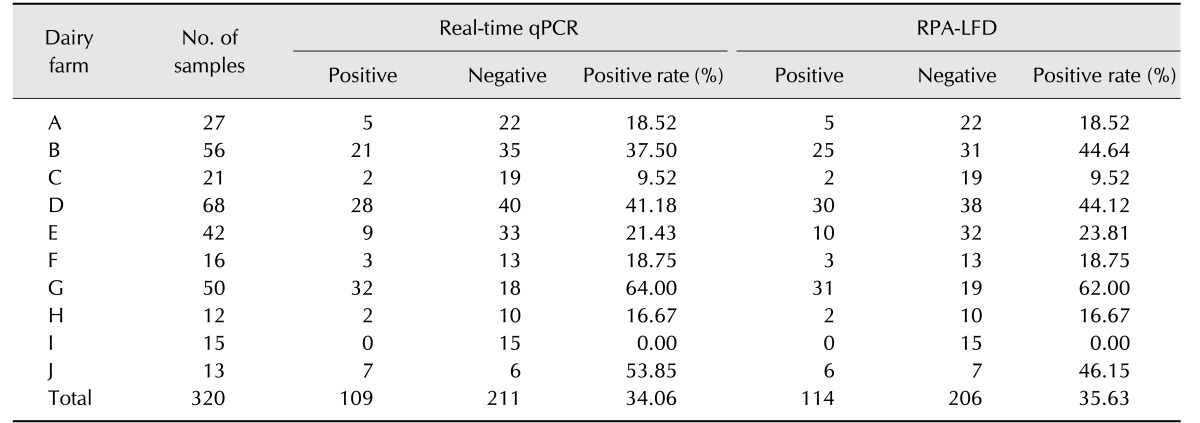
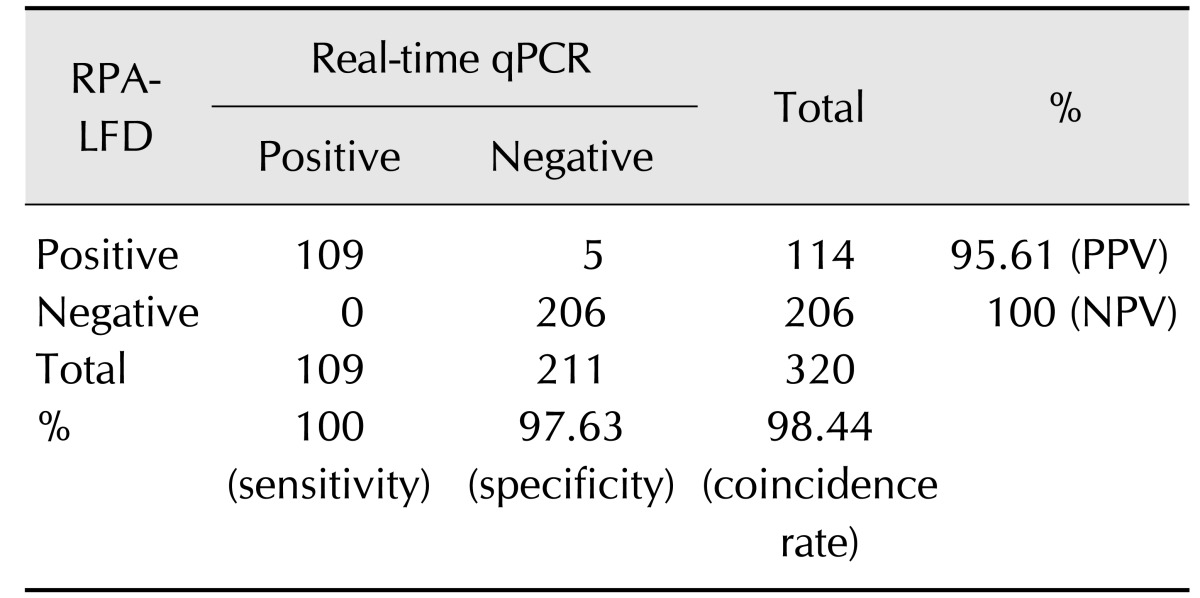
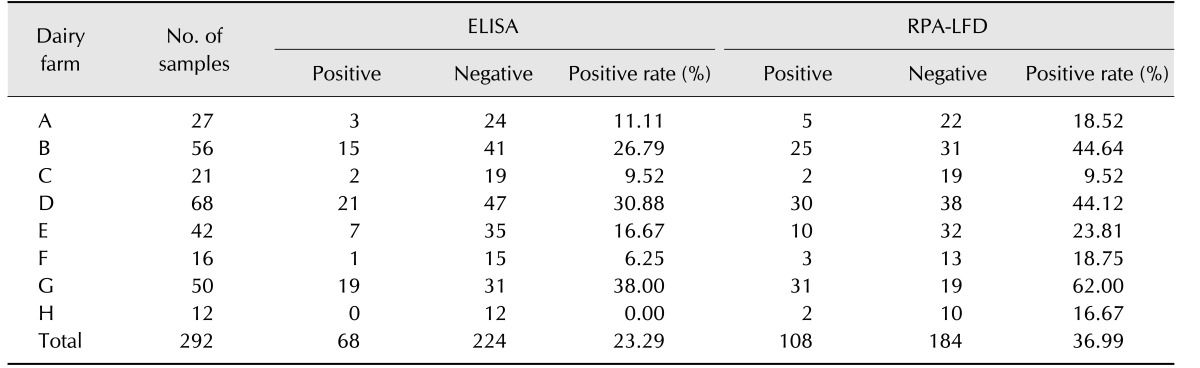
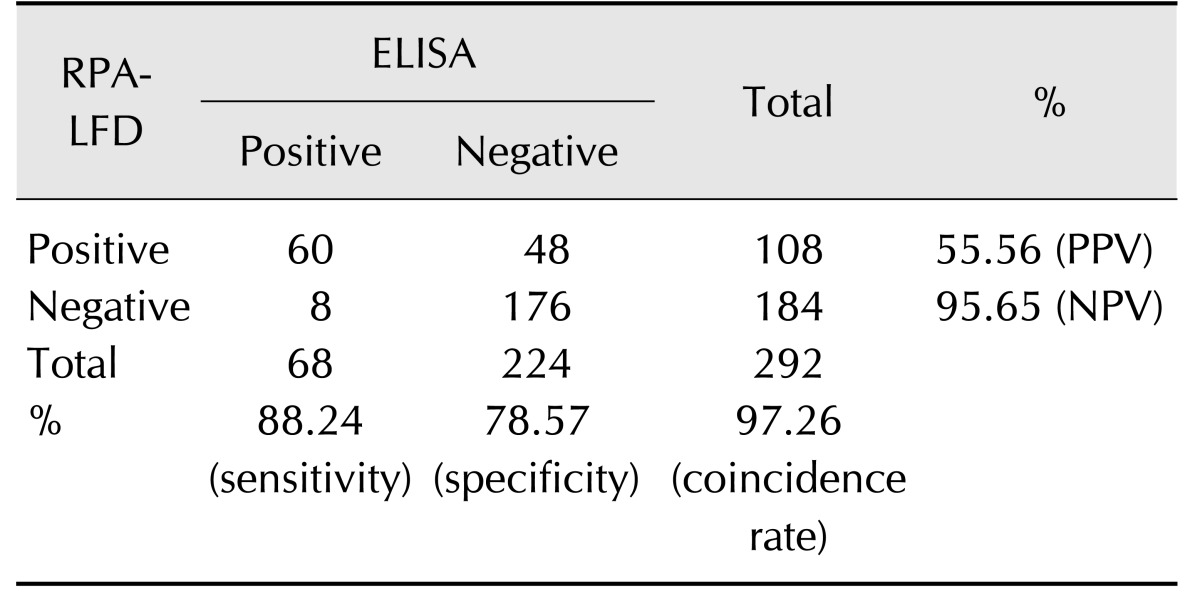
In order to compare the performance of RPA-LFD and qPCR assays, 320 field fecal samples were examined in the same sample set. The RPA-LFD assay had a slightly lower sensitivity than that of the qPCR assay (Table 3). There were 114 positives in the 320 samples in the RPA-LFD assay, and the positive rate was 35.63%. The positive rate of the qPCR assay was 34.06% (109/320) in the same sample set. The results from both assays were not significantly different based on independent-samples t-test results (t [18] = 0.031, p = 0.976; p > 0.05). The RPA-LFD assay yielded 100% sensitivity, 97.63% specificity, and a 98.44% concordance rate with the qPCR assay (Table 4). The results indicate that RPA-LFD and qPCR assays have very almost the same detection performance. Finally, the serum samples of 292 cattle were screened by the RPA-LFD assay and ELISA, respectively. The positive rate of the ELISA assay was 36.99% (108/292); however, the RPA-LFD assay only detected 23.29% (68/292) positive rate in the same sample set (Table 5). The performances of the RPA-LFD assay and ELISA assay are summarized in Table 6, it shown the RPA-LFD assay yielded 88.24% sensitivity, 78.57% specificity, and a 95.65% NPV with the ELISA assay. Based on the results, the RPA-LFD assay should be used to test field samples in the future.
Table 3. Comparison of Mycobacterium avium subsp. paratuberculosis RPA-LFD and real-time quantitative polymerase chain reaction (qPCR) assay results for fecal samples.
RPA-LFD, recombinase polymerase amplification-lateral flow dipstick.
Table 4. Specificity, sensitivity, and predictive value of RPA-LFD and quantitative polymerase chain reaction (qPCR) assays for diagnosing Mycobacterium avium subsp. paratuberculosis infection.
RPA-LFD, recombinase polymerase amplification-lateral flow dipstick; PPV, positive predictive value; NPV, negative predictive value.
Table 5. Comparison of Mycobacterium avium subsp. paratuberculosis ELISA and RPA-LFD assay results for fecal and serum samples.
ELISA, enzyme-linked immunosorbent assay; RPA-LFD, recombinase polymerase amplification-lateral flow dipstick.
Table 6. Specificity, sensitivity, and predictive value of ELISA and RPA-LFD assay for diagnosing Mycobacterium avium subsp. paratuberculosis infection.
ELISA, enzyme-linked immunosorbent assay; RPA-LFD, recombinase polymerase amplification-lateral flow dipstick; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Paratuberculosis has become a common pathogen in dairy farms [21]. However, widespread point-of-care testing is infrequently performed due to the lack of a robust method. In addition, little molecular epidemiological data for China has been reported; therefore, an epidemiological survey of M. paratuberculosis should be performed by on-site molecular diagnostic assays. Because the specificity of the IS900 insertion sequence is very strong in Mycobacterium and 15 to 20 copies are present in the M. paratuberculosis genome, it is commonly used as a molecular diagnostic identification feature in M. paratuberculosis testing [2].
Unlike PCR and loop-mediated isothermal amplification (LAMP) technologies, there is no software available to design suitable RPA-nfo primers and a lateral flow probe [4]. Once candidate primer and a lateral flow probe have been defined according to the appendix to the TwistAmp reaction kit manual, their relative performances can be assessed and compared by performing agarose-gel electrophoresis and LFD. Recent studies have shown that the performance of primers can be assessed by electrophoresis by using an RPA-basic kit [12,17]. However, some studies have reported that only one band is shown in agarose-gel electrophoresis by RPA-nfo assay [16,19]; thus, the results are inconsistent with the reaction principle of the RPA-nfo assay.
The results of this study show that the amplification performance of RPA-LFD assay is very stable over the range of 30℃ to 45℃. This makes the assay suitable for on-site field diagnosis purposes. Moreover, the RPA-LFD assay has high specificity; the primer sequences and probe have been analyzed by using BLAST in GenBank, and there is no cross-reaction with other bacteria. A panel of bacteria, including Mycobacterium and other major bacteria causing cattle diarrhea, were tested by using PRA-LFD assay, which demonstrated that amplification is restricted to the Mycobacterium tuberculosis complex strains. Recent study has shown that the sensitivities of the RPA-LFD and qPCR methods are essentially identical [19]. Our results demonstrated that qPCR assay was slightly higher than that of RPA-LFD; similarly, Yang et al. [20] reported that qPCR assay was a little higher than RPA-LFD. As enzyme proteins can affect electrophoresis, the various RPA amplification products should be purified when they are analyzed by agarose gel electrophoresis. However, this greatly increases the time and procedures used in detection. Recent studies indicate that the detection of an RPA product by the LFD assay has higher sensitivity than that of agarose-gel electrophoresis [3,11,17].
In conclusion, to confirm the diagnostic suitability of an M. paratuberculosis RPA-LFD assay, the same sample (n = 320) set was determined by RPA-LFD and qPCR assays. The results showed that the RPA-LFD assay can achieve the same detection efficiency as that of qPCR; therefore, the RPA-LFD method for detection of M. paratuberculosis should be considered an effective molecular technology-based assay for rapid, low-resource diagnostics. The DNA purification technology in the RPA-LFD assay can be performed independent of a laboratory; thus, this simple, on-site diagnosis assay can be widely used both in developing countries with resource-constrained settings and in field detection everywhere.
Acknowledgments
This work was partially supported by grants from an earmarked fund for the China Agriculture Research System (CARs-37, Hongbin He), the Taishan Scholar and Distinguished Experts (Hongbin He), the Shandong Province Natural Science Foundation (ZR2015PC007, Guimin Zhao), the National Natural Science Fund of China (31502064, 31672556). Shandong Major Agricultural Application Technology Innovation Project (Hongbin He, Hongmei Wang), and the Primary Research & Development Plan of Shandong Province (2015GNC113006, 2016GNC113006).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Abd El, Patel P, Faye O, Thaloengsok S, Heidenreich D, Matangkasombut P, Manopwisedjaroen K, Sakuntabhai A, Sall AA, Hufert FT, Weidmann M. Recombinase polymerase amplification assay for rapid diagnostics of dengue infection. PLoS One. 2015;10:e0129682. doi: 10.1371/journal.pone.0129682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaubey KK, Gupta RD, Gupta S, Singh SV, Bhatia AK, Jayaraman S, Kumar N, Goel A, Rathore AS, Sahzad, Sohal JS, Stephen BJ, Singh M, Goyal M, Dhama K, Derakhshandeh A. Trends and advances in the diagnosis and control of paratuberculosis in domestic livestock. Vet Q. 2016;36:203–227. doi: 10.1080/01652176.2016.1196508. [DOI] [PubMed] [Google Scholar]
- 3.Crannell ZA, Castellanos-Gonzalez A, Irani A, Rohrman B, White AC, Richards-Kortum R. Nucleic acid test to diagnose cryptosporidiosis: lab assessment in animal and patient specimens. Anal Chem. 2014;86:2565–2571. doi: 10.1021/ac403750z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daher RK, Stewart G, Boissinot M, Bergeron MG. Recombinase polymerase amplification for diagnostic applications. Clin Chem. 2016;62:947–958. doi: 10.1373/clinchem.2015.245829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia AB, Shalloo L. The economic impact and control of paratuberculosis in cattle. J Dairy Sci. 2015;98:5019–5039. doi: 10.3168/jds.2014-9241. [DOI] [PubMed] [Google Scholar]
- 6.Geraghty T, Graham DA, Mullowney P, More SJ. A review of bovine Johne's disease control activities in 6 endemically infected countries. Prev Vet Med. 2014;116:1–11. doi: 10.1016/j.prevetmed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Hansen S, Schäfer J, Fechner K, Czerny CP, Abd El. Development of a recombinase polymerase amplification assay for rapid detection of the Mycobacterium avium subsp. paratuberculosis. PLoS One. 2016;11:e0168733. doi: 10.1371/journal.pone.0168733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou P, Wang H, Zhao G, He C, He H. Rapid detection of infectious bovine rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. 2017;13:386. doi: 10.1186/s12917-017-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou P, Zhao G, He C, Wang H, He H. Biopanning of polypeptides binding to bovine ephemeral fever virus G1 protein from phage display peptide library. BMC Vet Res. 2018;14:3. doi: 10.1186/s12917-017-1315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husakova M, Dziedzinska R, Slana I. Magnetic separation methods for the detection of Mycobacterium avium subsp. paratuberculosis in various types of matrices: a review. Biomed Res Int. 2017;2017:5869854. doi: 10.1155/2017/5869854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersting S, Rausch V, Bier FF, von Nickisch-Rosenegk M. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar J. 2014;13:99. doi: 10.1186/1475-2875-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Liu HX, Zhang L, Hou XX, Wan KL, Hao Q. A novel isothermal assay of Borrelia burgdorferi by recombinase polymerase amplification with lateral flow detection. Int J Mol Sci. 2016;17:E1250. doi: 10.3390/ijms17081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng QF, Li Y, Yang F, Yao GZ, Qian AD, Wang WL, Cong W. Seroprevalence and risk factors of Mycobacterium avium subspecies paratuberculosis infection in domestic sika deer in China. Trop Anim Health Prod. 2015;47:999–1003. doi: 10.1007/s11250-015-0819-2. [DOI] [PubMed] [Google Scholar]
- 14.Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4:e204. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L, Zhang H, Hou P, Wang H, Zhao G, Xia X, He H. Development and preliminary application of an indirect ELISA to detect infectious bovine rhinotracheitis virus using recombinant glycoprotein D of IBRV strain SD. Kafkas Univ Vet Fak Derg. 2016;22:503–509. [Google Scholar]
- 16.Sun K, Xing W, Yu X, Fu W, Wang Y, Zou M, Luo Z, Xu D. Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasit Vectors. 2016;9:476. doi: 10.1186/s13071-016-1745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu PA, Shiu JS, Lee SH, Pang VF, Wang DC, Wang PH. Development of a recombinase polymerase amplification lateral flow dipstick (RPA-LFD) for the field diagnosis of caprine arthritis-encephalitis virus (CAEV) infection. J Virol Methods. 2017;243:98–104. doi: 10.1016/j.jviromet.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Ke Y, Wang X, Ren H, Liu W, Lu H, Zhang W, Liu S, Chang G, Tian S, Wang L, Huang L, Liu C, Yang R, Chen Z. Development and evaluation of a rapid and sensitive EBOV-RPA test for rapid diagnosis of Ebola virus disease. Sci Rep. 2016;6:26943. doi: 10.1038/srep26943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Qin X, Wang G, Jin J, Shang Y, Zhang Z. Development of an isothermoal amplification-based assay for rapid visual detection of an Orf virus. Virol J. 2016;13:46. doi: 10.1186/s12985-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Qin X, Zhang W, Li Y, Zhang Z. Rapid and specific detection of porcine parvovirus by isothermal recombinase polymerase amplification assays. Mol Cell Probes. 2016;30:300–305. doi: 10.1016/j.mcp.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Yue R, Liu C, Barrow P, Liu F, Cui Y, Yang L, Zhao D, Zhou X. The isolation and molecular characterization of Mycobacterium avium subsp. paratuberculosis in Shandong province, China. Gut Pathog. 2016;8:9. doi: 10.1186/s13099-016-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]