Abstract
On December 3, 2014, a type O foot-and-mouth disease (FMD) outbreak began in Korea. Although vaccinations were administered, FMD cases increased steadily for five months, and reached 185 cases by April 2015. Most of the affected animals were pigs, which are vulnerable to vaccination. The FMD virus belonged to the South-East Asia (SEA) topotype that had been observed three times in Korea between April 2010 and July 2014. However, the FMD virus isolated in December 2014 had a unique feature; that is, partial deletion of the 5′ non-coding region, a deletion not seen in previous SEA topotype isolates identified in Korea. We conclude that this outbreak included the introduction of a new FMD strain to Korea, and that Korea was now affected by genetically similar FMD virus strains that are related to those from neighboring countries.
Keywords: Korea, control, foot-and-mouth disease, vaccination
Introduction
Foot-and-mouth disease (FMD) is an economically important disease because it is highly contagious and infects many cloven-hoofed animals, and once animals have been infected, culling or stamping out policies are implemented in most countries [13]. The FMD viruses (FMDVs) are classified as small icosahedral viruses in the Aphthovirus genus of the Picornaviridae family. There are seven serotypes of FMDVs (O, A, C, SAT1, SAT2, SAT3, and Asia1) [1]. The O and A serotypes frequently occur globally, but the Asia1 serotype occurs only in Asia. Among these types, the O type is the most widespread [2].
After large-scale type O FMD outbreaks resulted in 153 cases from November 2010 to April 2011, Korea began nationwide administration of vaccinations against the O, A, and Asia1 types of FMDV [10]. With the continuous occurrence of FMD in neighboring countries such as China, Vietnam, and North Korea, FMD occurred twice in Korea between 2000 and 2002 and five times between 2010 and 2014 [8,9,10,11]. This was unexpected due to Korea's strong vaccination policies; regardless, the country experienced two more FMD outbreaks after beginning nationwide vaccinations. In the FMD outbreak of July 2014, the disease only resulted in three cases. However, a type O FMD outbreak resulted in 185 cases over a five-month period from December 2014 through April 2015. Most of the animals affected were pigs, but five cattle farms were also affected.
This study examined the regional distribution of the December 2014 virus as well as the prevalence of antibodies to the viral structural proteins (SP) and nonstructural proteins (NSP) of the FMDV after the outbreak and additional vaccinations. The characteristics and genetic similarities between the FMDVs in Korea and those occurring in neighboring countries were also analyzed.
Materials and Methods
Spatiotemporal analysis of FMD cases in cattle and pigs
We counted the number of FMD cases on cattle and pig farms during the FMD outbreak from December 2014 through April 2015, and the cumulative case numbers were determined. Regional distribution for each month of the outbreak was schematized by undertaking spatiotemporal analysis.
Virus detection and isolation
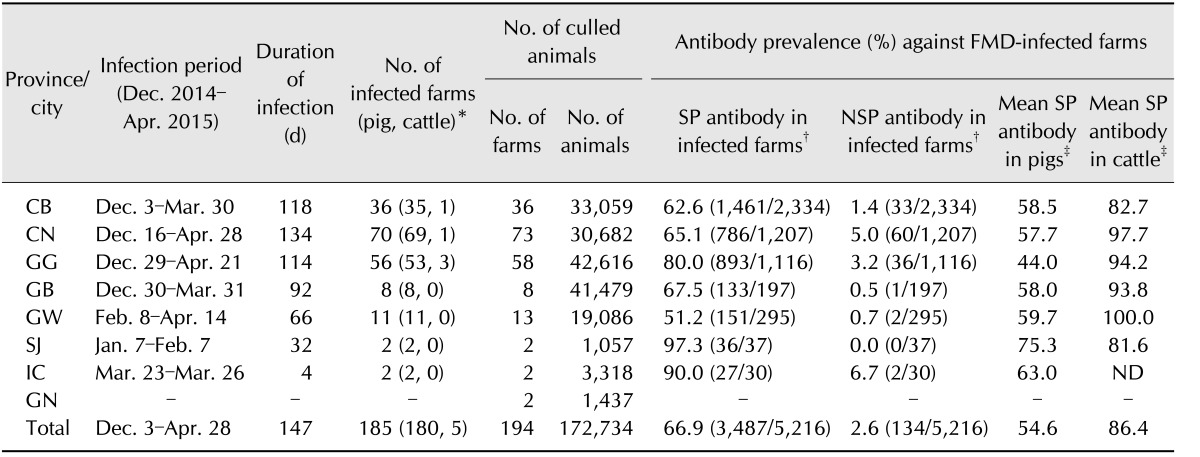
We isolated the virus from the vesicular fluid of the infected animals after several passages with fetal goat tongue cells (ZZ-R cell line) for analysis of serological relationships and full genome sequencing. When clinical signs of FMD were reported in field animals, a local veterinarian was dispatched to diagnose the animal based on a lateral-flow assay (PBM, USA). We amplified the genes by undertaking polymerase chain reaction (PCR), sequenced for genetic analysis of the isolated virus, and submitted the full genome sequence to the National Center for Biotechnology Information (NCBI, USA). The NCBI GenBank accession number for the sequence is KX162590.1. Most of the viruses that were isolated in the outbreak between December 2014 and April 2015 showed similar sequences with approximately 99% nucleotide similarity. The 185 cases FMD outbreak during the study period occurred on cattle and pig farms in Korea (Table 1).
Table 1. Summary of foot-and-mouth disease infection between December 2014 and April 2015 in Korea.
FMD, foot-and-mouth disease; SP, structural proteins; NSP, nonstructural proteins; CB, Chungbuk; CN, Chungnam; GG, Gyeonggi; GB, Gyenogbuk; GW, Gangwon; SJ, Sejong city; IC, Incheon city; GN, Gyenognam; ND, not done. *Diagnosis by real-time polymerase chain reaction, antigen enzyme-linked immunosorbent assay, or lateral-flow assay. †Percentage antibody prevalence (No. of positive/No. of tested). ‡Mean antibody prevalence (positive rate) in Korea, December 2014.
Antibody test by SP enzyme-linked immunosorbent assay (ELISA) and NSP ELISA
The FMD vaccine-induced antibody prevalence (the ratio of determined by using PrioCHECK FMDV type O ELISA (Prionics AG, Switzerland) for SP antibody detection according to the manufacturer's instructions. The ELISA plate absorbance was converted to a percent inhibition (PI) value. When the PI value was 50% or higher, the animals were regarded as antibody positive. Although it has a low correlation with neutralizing antibodies, antibody prevalence determined by SP-O ELISA can be an important index for herd immunity. The NSP ELISA kits (BioNote or Median Diagnostics, Korea) for NSP antibody detection, a signal of possible FMDV infection, were used according to the manufacturer's instructions to assess the possibility of viral circulation. When the NSP kit results from a sample registered as SP positive were also positive, the animal was finally regarded as antibody positive.
Similarity and genetic analysis of outbreak viruses
Analysis of genetic similarity and generation of a phylogenetic tree were conducted by using BioEdit for Windows 95/98/NT/2K/XP/7 [4] and MEGA software (ver. 6.0) [12] with the neighbor-joining method. The percentage of replicates from the tree in which the associated taxa were clustered together in the bootstrap test (1,000 replicates) is shown next to the branches.
Collection of vaccine matching results
When a FMD case occurs in Korea, samples are sent to an FMD reference laboratory in either the UK, Russia, or Argentina or to the vaccine manufacturing company. The vaccine match results are then collected from the appropriate test organization.
Results
Diagnosis of FMD cases
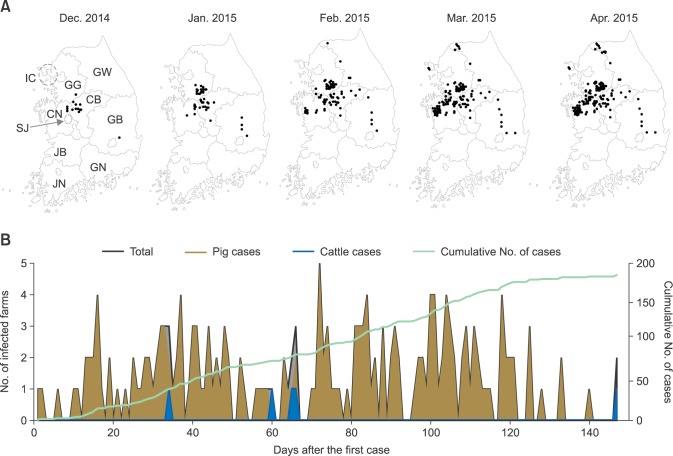
During the December 2014 to April 2015 FMD outbreak, the first case occurred on 3 December 2014 with an FMD case being diagnosed at a large-scale pig farm in Jincheon County, Chungbuk (CB) province, by using a lateral-flow assay, PCR, and sequence analysis. FMD cases continued to be reported until April 28, 2015, when the cumulative number of cases reached 185 (Fig. 1). The FMD cases between December 2014 and April 2015 occurred on 180 pig farms and 5 cattle farms (Table 1). The pigs had typical FMD symptoms such as vesicles on their feet. The cattle also had typical FMD symptoms such as ulceration of the tongue, salivation, and vesicles in the mouth and on the teats. All five cattle farms where the FMD occurred (two cases in January, two cases in February, and one case in April) had native Korean cattle. Only on the farm where cattle FMD first occurred (January 5, in Anseong, Korea) did two cohabitating cattle both test positive for NSP antibodies without exhibiting symptoms. No transmission among animals occurred on other farms.
Fig. 1. Outbreak of foot-and-mouth disease (FMD) in Korea in 2014 and 2015. (A) Monthly progress of FMD cases. GG, Gyeonggi; GW, Gangwon; CN, Chungnam; CB, Chungbuk; GB, Gyeongbuk; GN, Gyeongnam; JN, Jeonnam; JB, Jeonbuk; SJ, Sejong city; IC, Incheon city. (B) Number of detected cases in cattle and pigs, and cumulative case numbers occurring after the first case.
Regional transmission of FMD
FMD cases were observed from the Chungbuk (CB) to the Gyeongbuk (GB) provinces between December 2014 and January 2015, followed by additional outbreaks in the areas where these cases occurred (panel A in Fig. 1). FMD next occurred in Chungnam (CN) and Gyeonggi (GG) provinces in February and then in Gangwon (GW) province (panel A in Fig. 1). Between one and three FMD cases occurred approximately daily until March 2015 when the number of occurrences decreased (panel B in Fig. 1). During the outbreak period, approximately 172,000 infected or suspected pigs were selected and culled for disease control (Table 1). Complete culling of infected or suspected animals under the national vaccination program did not occur because of opposition from private organizations. Therefore, only partial culling of infected animals with clinical signs was conducted.
The animal movement restriction for disease control was lifted on 22 May 2015, and the crisis warning for FMD in Korea was lowered from “alert” to “caution” on 14 May and from “caution” to “attention” on 21 July 2015.
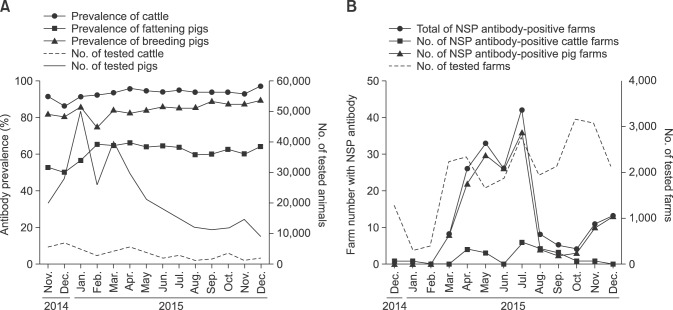
Control by vaccination
Because of the extensive FMD vaccinations performed in Korea in July 2014, the mean positive rate of SP antibody in 2015 (62.5%) was 17.1% higher than that of 2014 (45.4%) in fattening pigs. However, in areas without an FMD outbreak, the antibody prevalence was relatively low. While the antibody prevalence was high in pigs that had been vaccinated twice, it was low in single vaccination areas without an FMD outbreak (Jeonnam, Jeonbuk, Gyeongnam, and Jeju provinces). The SP antibody prevalence in pigs increased in January 2015 due to additional vaccinations being administered after the December 2014 outbreak. The level of antibody prevalence in cattle and breeding pigs was sustained (panel A in Fig. 2). The possibility of viral circulation at several NSP-positive pig farms without clinical signs increased from May through July 2015 (panel B in Fig. 2). Tests for NSP antibody and FMDV antigen after restriction of animal movement for 3 weeks in NSP antibody positive farms should be implemented, and after reexamination for every 3 week, if the positive rate of NSP antibody detection in 16 samples has not increased, and a negative result is obtained in the antigen test, the animal farm's limit on movement control should be lifted. After March 2015, detection of NSP antibodies on pig farms increased, and the occurrence of FMD continued until April. Detected numbers of NSP-positive farms increased from May to July 2015; however, because no farm tested NSP-positive between April and August 2015, it was assumed that there was no increase in the number of NSP-positive farms during that time.
Fig. 2. Antibody prevalence against foot-and-mouth disease in 2014 and 2015 cases in South Korea. (A) Type O antibody prevalence obtained by structural protein (SP) antibody enzyme-linked immunosorbent assay in 2014 and 2015. (B) Detected number of nonstructural protein (NSP) antibody positive farms in 2014 and 2015.
Genetic analysis of FMD virus
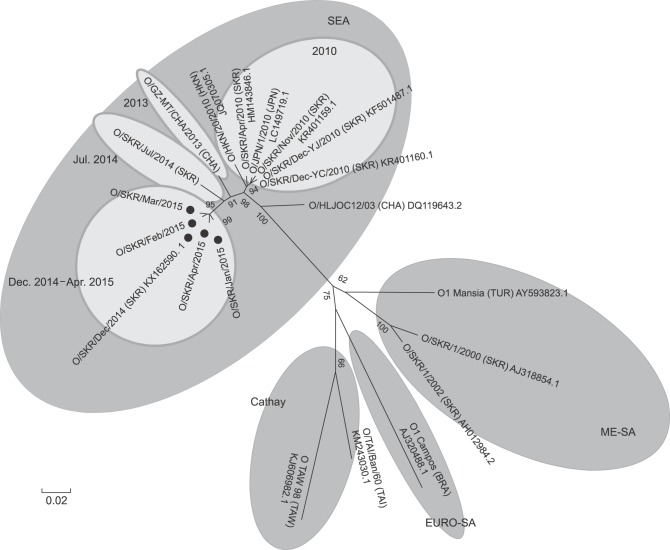
The FMD serotypes that were recently circulating in Asia were primarily the O and A serotypes. Of these types, the Middle East and South Asia (ME-SA) and South-East Asia (SEA) topotypes of the O serotype were responsible for the virus circulation. The FMD viruses isolated in July and December of 2014 in Korea were all O serotype and SEA topotype FMDVs. The FMDVs isolated in April and November of 2010 in Korea were all SEA topotypes (Fig. 3), and their genetic lineages were all Mya-98.
Fig. 3. Comparison of VP1 sequences of vaccine prototypes and foot-and-mouth disease (FMD) viral strains closely related to O/SKR/Dec/2014 with a phylogenetic tree showing viral FMD strains closely related to O/SKR/Dec/2014 (black dot). The O/TAI/Ban/60 strain (GenBank KM243030.1) is a similar strain to O 3039. The National Center for Biotechnology Information GenBank accession numbers for the virus strains are as follows: O/SKR/Apr/2010 (HM143846.1), O/SKR/1/2000 (AJ318854.1), O/SKR/1/2002 (AH012984.2), O/HLJOC12/03 (DQ119643.2), O/JPN/1/2010 (LC149719.1), O/SKR/Nov/2010 (KR401159.1), O/SKR/Dec-YJ/2010 (KF501487.1), O/SKR/Dec-YC/2010 (KR401160.1), O/SKR/Dec/2014 (KX162590.1), O/TAI/Ban/60 (KM243030.1), O TAW 98 (KJ606982.1), O1 Manisa (AY593823.1), O1 Campos (AJ320488.1), and O/GZ-MT/CHA/2013 (KJ646655). SEA, South-East Asia; ME-SA, Middle East and South Asia; CHA, China; HKN, Hong Kong; JPN, Japan; SKR, South Korea; TUR, Turkey; BRA, Brazil; TAI, Thailand; TAW, Taiwan. The scale bar indicates nucleotide substitutions per site.
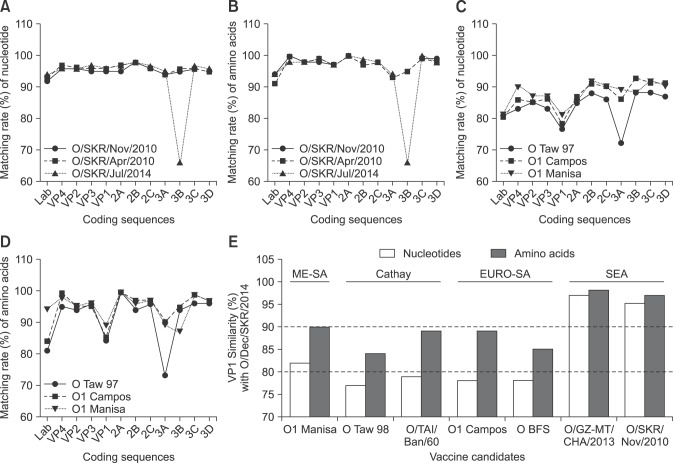
An FMDV present in China (O/GZ-MT/CHA/2013) is most similar to O/SKR/Dec/2014, with a 97.18% similarity among the known viruses (Fig. 4). The FMDV isolate of July 2014 had the next highest similarity to O/SKR/Dec/2014, while O/SKR/Dec/2014 had a 96.87% similarity with the FMDV that occurred in Russia (O/Primorskiy/RUS/2014). In addition, the virus had 96.56% to 96.71% similarity with the FMDVs that occurred in Uiseong and Hapcheon, Korea in July through August 2014 (O/SKR/Jul/2014). It also had 95% to 96% similarity with FMDVs derived from Hong Kong, China, Japan, and Korea in 2010–2013.
Fig. 4. Comparison of complete genome and VP1 sequences of the foot-and-mouth disease (FMD) viral strains closely related to O/SKR/Dec/2014. (A) Nucleotide similarity in FMD viral strains closely related to O/SKR/Dec/2014. (B) Amino acid similarity in FMD viral strains closely related to O/SKR/Dec/2014. (C) Nucleotide similarity between vaccine prototypes and O/SKR/Dec/2014. (D) Amino acid similarity between vaccine prototypes and O/SKR/Dec/2014. (E) Similarity between VP1 nucleotides and amino acids in the coding sequences of vaccine prototypes or wild type strains compared with O/SKR/Dec/2014. The National Center for Biotechnology Information GenBank accession number for the virus strain is as follows: O BFS (AY593816). ME-SA, Middle East and South Asia; SEA, South-East Asia.
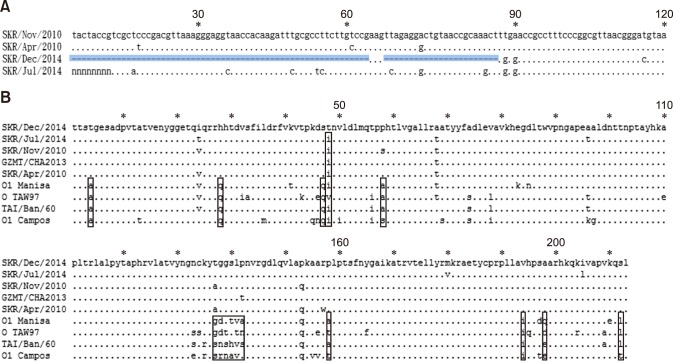
In this study, O/SKR/Dec/2014 was shown to have a unique defect in the 5′ non-coding region (NCR) (Fig. 5) that was not observed in FMDVs isolated between 2010 and 2014. Whether a defect in this region affects the pathogenicity of FMDV is unknown, but the virus can infect both cattle and pigs. Therefore, this virus is thought to be different from the O/SKR/Jul/2014, even though the virus had the highest similarity in the VP1 sequence comparison. Furthermore, in the full-length sequence analysis based on O/SKR/Dec/2014 (Jincheon strain), each encoded sequence that was identified in April 2010, November 2010, and July 2014 had very high similarity (Fig. 4), except for the difference in the 3B1 gene of O/SKR/Jul/2014, which was identified as a unique deletion when compared with the other viruses. The Lpro sequence showed the least similarity to these genes (Fig. 4). After analyzing the VP1 sequence, all three Korean isolates (Apr. 2010, Nov. 2010, and Jul. 2014) were highly similar to O/SKR/Dec/2014 (Fig. 3); however, the nucleotide similarity to O1 Manisa, which was used as a vaccine strain during the December 2014 to April 2015 outbreak, was low (panels C and D in Fig. 4). The difference in the amino acid sequences in O1 Manisa was high for the 3B, 3A, and VP1 proteins (panel D in Fig. 4). Notably, despite the relatively low VP1-amino acid similarity (89%), there was no substantial difference (approximately 5%) in amino acids among the VP4, VP2, and VP3 proteins (Fig. 4) of the virus particle.
Fig. 5. Comparison of unique foot-and-mouth disease (FMD) viral strain sequences closely related to O/SKR/Dec/2014. (A) Alignment of 5′ non-coding region (NCR) among Korean isolates since 2010. The rectangular box represents the deleted region in the 5′ NCR downstream of the poly-C region. (B) Alignment of VP1 sequence among Korean isolates since 2010 and vaccine prototypes. The rectangular box represents the unique region in the matching sequences. National Center for Biotechnology Information GenBank accession numbers for the virus strains are as follows: O/SKR/Apr/2010 (HM143846.1), O/SKR/Nov/2010 (KR401159.1), O/SKR/Dec/2014 (KX162590.1), O/SKR/Jul/2014 (kvcc.hahis.go.kr), O1 Mansia (AY593823.1), O/TAI/Ban/60 (KM243030.1), O TAW 97 (AY593835.1), O1 Campos (AJ320488.1), O/GZ-MT/CHA/2013 (KJ646655).
Serological relationships to FMD vaccine strains
At the start of the FMD outbreak (December 2014), the vaccine used in Korea was a trivalent vaccine with O1 Manisa, A Malaysia97, and Asia1 Shamir. This is a versatile vaccine with universal antigenicity that protects against the three serotypes reported in nearby regions.
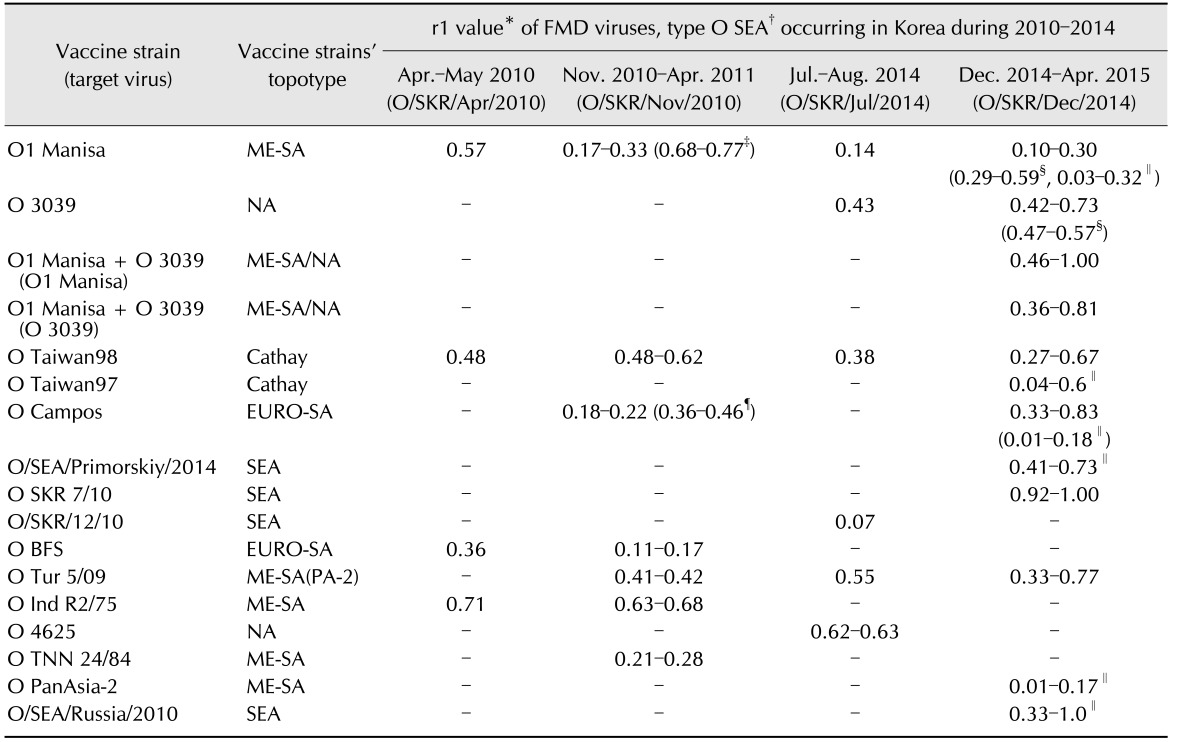
Since February 2015, the O serotype (O1 Manisa + O 3039 or O SKR 7/10) vaccine, which was later used for intensive vaccination programs, was considered more effective because O 3039 (0.42–0.73) and O SKR 7/10 (0.92–1.00) showed higher r1 values than that of O1 Manisa (0.10–0.30) in the vaccine matching test (Table 2) depending on bovine reference sera, urgently. Among the available vaccine strains, O SKR 7/10 had the highest VP1 similarity with O/SKR/Dec/2014, while the amino acid sequences of O1 Manisa, O Taw98, and O1 Campos had relatively low similarities (Fig. 4). The recent FMDVs were analyzed, with limited vaccine matching data, and thought to be genetically similar to those from China and Russia (Figs. 3, 4, 5).
Table 2. Variability and limitation of application by vaccine matching of globally available vaccines for type O FMDV occurring in Korea during 2010–2014.
FMDV, foot-and-mouth disease virus; FMD, foot-and-mouth disease; SEA, South-East Asia; ME-SA, Middle East and South Asia; NA, not available; -, not tested. *Values were calculated by division between the viral titers (serum titer against viruses used for cross virus neutralization test/serum titer against vaccine viruses in the vaccination groups). †Tested by World Reference Laboratory for FMD (Pirbright Institute), UK except an indication note. ‡Tested by MSD Animal Health (FMD vaccine manufacturer). §Tested by Merial Boehringer Ingelheim (FMD vaccine manufacturer). ∥Tested by World Organisation for Animal Health (OIE) Regional Reference Laboratory for FMD (ARRIAH), Russia. ¶Tested by OIE Reference Laboratory for FMD (SENASA), Argentina.
Discussion
FMD vaccines should be analyzed for effectiveness, and those with a broad antigenic spectrum should be selected. Furthermore, FMD antibody prevalence should be maintained in the field through repeated vaccinations. Various serotypes or topotypes of FMD have been identified in Asian countries around Korea, and these viruses are genetically related to those from Korea [7]. In this study, O/SKR/Dec/2014 was determined to have a unique defect in the 5′ NCR that was not present in viruses from 2010–2014. Therefore, this virus is believed to be different from other Korean strains.
In addition, the 3B1 gene was not found in the genomic sequence of O/SKR/Jul/2014 [11]. The FMDV (O/SKR/Jul/2014) that was identified in Uiseong County of Gyeongbuk (GB) province had different genetic features from the viruses (O/SKR/Dec/2014) that were identified in Jincheon County of Chungbuk (CB) in December 2014. The 3B gene sequence of FMDV includes the 3B1, 3B2, and 3B3 genes, which enable proper replication; however, O/SKR/Jul/2014 only contained the 3B2 and 3B3 genes in its genomic sequence [11]. Therefore, the virus did not replicate properly in the cells, and the clinical scores for the animals infected with this virus strain were no higher than those for animals with other field viruses.
There is minimal information available from viral vaccine matching tests, and some countries use vaccine strains that they have developed on their own [3], making the identification of international vaccine strains in Korea difficult.
Despite control measures, the practice of selective culling of only infected animals that had been vaccinated has limited benefits as a control policy in Korea. In the FMD outbreak of December 2014, some of the animals were affected by the SP antibody level resulting from vaccination, but they showed no additional clinical symptoms; moreover, there were also animals with no clinical signs in herds on the same farms.
On the examined cow farms, only one cow was infected, while the rest of the animals were asymptomatic and uninfected. Cattle species are an indicator of FMD circulation as, compared to pigs, cattle are easily affected by a smaller amount of FMDV; in addition, they are sensitive to respiratory infection, which can be used to indicate the effect of vaccines in the field. Such an effect was possible because the antibody prevalence rate from vaccination was high in the cattle (64%–100%); therefore, it appears that infections were not transmitted throughout the cattle farms.
Once the FMDV contaminates the environment, enters farms, and infects several incompletely immunized pigs, it is pointless to administer additional vaccines as much of the virus can be excreted from infected pigs [6]. The FMD infection in Korea occurred primarily in animals whose immunity was not completely developed. Considering that vaccine efficacy is imperfect in pigs, repeated vaccinations (more than twice) are necessary during an outbreak period to ensure sufficient antibodies are induced to protect against FMD infection. Therefore, it is desirable to vaccinate pigs twice, at the 12th and 14th weeks of age, in order to induce immunity and avoid intervention from maternally derived antibodies [6].
There are four possible causes of NSP antibody detection in the absence of clinical signs: infection with FMDV; repeat vaccination against FMD; maternal antibodies from a previous generation; and non-specific reactivity of the serum in the test being used. Nevertheless, detection of NSP antibodies on farms is considered a strong indicator of additional FMD occurrences [5]. In Korea, there are no published reports of actual infections with clinical signs and antigen detection results for NSP-positive farms. Thus, the number of animals that are NSP antibody-positive compared to the numbers in years during the follow-up period should be determined; moreover, antigen tests should be conducted regularly to verify viral circulation. Finally, as a preventive measure against FMD outbreaks and in addition to vaccination protocols, thorough farm biosecurity should be a priority.
Acknowledgments
This research was supported by the Animal and Plant Quarantine Agency (APQA). We thank the staff for FMD control at APQA. We would like to thank WRLFMD (Pirbright Institute), World Organisation for Animal Health (OIE) FMD Reference Laboratories (ARRIAH, SENASA) and FMD vaccine manufacturer (Merial BI, MSD Animal Health) on sharing of vaccine matching data.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Abdela N. Sero-prevalence, risk factors and distribution of foot and mouth disease in Ethiopia. Acta Trop. 2017;169:125–132. doi: 10.1016/j.actatropica.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Brito BP, Rodriguez LL, Hammond JM, Pinto J, Perez AM. Review of the global distribution of foot-and-mouth disease virus from 2007 to 2014. Transbound Emerg Dis. 2017;64:316–332. doi: 10.1111/tbed.12373. [DOI] [PubMed] [Google Scholar]
- 3.Cai C, Li H, Edwards J, Hawkins C, Robertson ID. Meta-analysis on the efficacy of routine vaccination against foot and mouth disease (FMD) in China. Prev Vet Med. 2014;115:94–100. doi: 10.1016/j.prevetmed.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 5.Kitching RP. Clinical variation in foot and mouth disease: cattle. Rev Sci Tech. 2002;21:499–504. doi: 10.20506/rst.21.3.1343. [DOI] [PubMed] [Google Scholar]
- 6.Kitching RP, Alexandersen S. Clinical variation in foot and mouth disease: pigs. Rev Sci Tech. 2002;21:513–518. doi: 10.20506/rst.21.3.1367. [DOI] [PubMed] [Google Scholar]
- 7.Knowles NJ, He J, Shang Y, Wadsworth J, Valdazo-González B, Onosato H, Fukai K, Morioka K, Yoshida K, Cho IS, Kim SM, Park JH, Lee KN, Luk G, Borisov V, Scherbakov A, Timina A, Bold D, Nguyen T, Paton DJ, Hammond JM, Liu X, King DP. Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg Infect Dis. 2012;18:499–501. doi: 10.3201/eid1803.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Lee KN, Kim SM, Lee HS, Ko YJ, Tark DS, Shin YK, Seo MG, Kim B. Reemergence of foot-and-mouth disease, South Korea, 2000–2011. Emerg Infect Dis. 2014;20:2158–2161. doi: 10.3201/eid2012.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Lee KN, Ko YJ, Kim SM, Lee HS, Park JY, Yeh JY, Kim MJ, Lee YH, Sohn HJ, Cho IS, Kim B. Diagnosis and control measures of the 2010 outbreak of foot-and-mouth disease A type in the Republic of Korea. Transbound Emerg Dis. 2013;60:188–192. doi: 10.1111/j.1865-1682.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Lee KN, Ko YJ, Kim SM, Lee HS, Shin YK, Sohn HJ, Park JY, Yeh JY, Lee YH, Kim MJ, Joo YS, Yoon H, Yoon SS, Cho IS, Kim B. Control of foot-and-mouth disease during 2010–2011 epidemic, South Korea. Emerg Infect Dis. 2013;19:655–659. doi: 10.3201/eid1904.121320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Tark D, Lee KN, Lee SY, Ko MK, Lee HS, Kim SM, Ko YJ, Seo MG, Chun JE, Lee MH, Kim B. Novel foot-and-mouth disease virus in Korea, July–August 2014. Clin Exp Vaccine Res. 2016;5:83–87. doi: 10.7774/cevr.2016.5.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada M, Stevenson M, Cogger N, Carpenter T. Evaluation of the control strategy for the 2010 foot-and-mouth disease outbreak in Japan using disease simulation. Transbound Emerg Dis. 2017;64:978–989. doi: 10.1111/tbed.12467. [DOI] [PubMed] [Google Scholar]