Abstract
Understanding the molecular basis of addiction could be greatly aided by using forward genetic manipulation to lengthen the list of candidate genes involved in this complex process. Here, we report that zebrafish exhibit cocaine-induced conditioned place preference. In a pilot screen of 18 F2 generation families of mutagenized fish, we found three with abnormally low responses to cocaine. This behavior was inherited by the F3 generation in a manner that suggests the abnormalities were because of dominant mutations in single genes. Performance profiles in secondary behavioral screens measuring visual dark-adaptation and learning suggest that the defects were the result of mutations in distinct genes that affect dopaminergic signaling in the retina and brain.
Addiction, the compulsive intake of certain substances despite adverse consequences, continues to be a tremendous public health issue, costing billions of dollars per year (1). To understand addiction better and to design therapeutic strategies, several avenues of investigation have been taken to elucidate the genetic bases of addiction-related behaviors. Selective inbreeding of mouse strains displaying differing degrees of addiction-related behaviors has been used to correlate the behavior with particular genetic polymorphisms (2). Although this method has great promise, few strong correlations have been made owing to the time required to generate the large numbers of families necessary. Also, the limited number of inbred stains with a given behavioral phenotype prevents characterization of more than a few genes important in addiction-related behaviors. Transgenics have also been used to correlate specific behaviors with the function of known genes (3). However, background effects and compensation by other related genes can complicate analysis of transgenic mouse models. Furthermore, both methods rely heavily on a candidate approach, requiring that the genes of interest be well characterized ahead of time.
Methods of forward genetics in which the genome is mutagenized, resulting phenotypes are characterized, and underlying genes are subsequently cloned offer the advantage of not needing to know the genes a priori. Indeed, this approach has been used to determine sensitivity to particular substances such as cocaine or ethanol in Drosophila (4). However, the level of behavioral analysis possible in Drosophila is limited by fundamental differences of their central nervous system relative to vertebrates. Forward genetics on a vertebrate displaying complex, addiction-related behavior would be ideal. By virtue of their large clutch size and relatively low maintenance costs, zebrafish (Danio renio) are currently the vertebrate of choice in forward genetics experimentation (5). The level of behavioral analysis possible in these animals is only now being explored.
The role of midbrain dopamine in behaviors related to addiction has been exhaustedly researched (6, 7). Microdialysis, intracerebral injection, lesion, and electrical self-stimulation experiments have all implicated the dopaminergic connection between the ventral tegmental area and the nucleus accumbens as the primary pathway mediating reward in the vertebrate brain. The traditional view of reward is that when a behavior, such as eating or sexual activity, increases dopamine in the nucleus accumbens, the rise in dopamine is translated into motivated activity of the animal such that the behavior is repeated. To date, most drugs of abuse share the commonality of raising dopamine levels in the nucleus accumbens. Cocaine, for example, raises dopamine levels by blocking activity of the dopamine transporter. One way this sensitivity to addictive drugs has been modeled in lower mammals is the conditioned place-preference (CPP) paradigm (8). In this assay, a primary stimulus (i.e., application of drug) is paired to a second stimulus such as a particular set of visual cues. Upon further testing without the primary stimulus, the animal responds to the secondary stimulus alone with an approaching behavior. Experiments by using various selective antagonists have implicated midbrain dopamine as the central mediator of CPP behavior (8).
How dopamine and reward are related to addiction is a matter of controversy. One hypothesis is that individual sensitivity of the midbrain reward pathway to exposure of addictive substances determines the tendency of that individual toward addiction. The altered drug-related behavior of transgenic mice lacking the D2 and D4 dopamine receptors and the dopamine transporter supports this theory (9–11). Furthermore, work by using inbred mouse strains has correlated a chromosomal region close to the D4 dopamine receptor with sensitivity to cocaine and ethanol (2). Bioinfomatic analysis has implicated a polymorphism in the D2 receptor with alcoholism, but these findings remain quite controversial (12). Perhaps a stronger link between dopamine-mediated reward and the tendency toward addiction lies not with the receptors or transporters but in the neuroadaptive components downstream. Dopamine receptors operate in conjunction with G proteins, but the specific neuroadaptive responses of the midbrain and basal forebrain neurons to addictive drugs are largely unknown and difficult to study without candidate genes (13, 14). Forward genetic approaches using zebrafish may prove useful for expanding the list of candidate genes.
Our laboratory uses forward genetics in zebrafish to characterize genes involved in retinal function and development. Male fish are mutagenized by repeated exposure to N-ethyl-nitrosourea and are bred to untreated wild-type females yielding an F1 generation of fish heterozygous for mutations of several genes (5). Outcrossing of the F1 generation provides F2 families heterozygous for a subset of these mutations. We screen for recessive mutations affecting eye function and development in the F3 offspring generated by mating siblings of these F2 families (15, 16) and also F1 and F2 generation fish for dominant mutations affecting retinal function in adults (17). Dopamine, central to the reward pathway in the brain, also regulates retinal function under changing light conditions (18). Dopamine circuitry in the retina shares some commonality with that of the ventral tegmental area–nucleus accumbens pathway, including modulation by γ-aminobutyric acid and opioid peptides (19). Here, we describe a simple behavioral screen for dominant mutations in adult fish affecting dopaminergic pathways using cocaine-induced CPP.
Methods
Animals and Maintenance.
Zebrafish were maintained according to well established protocols (19). The animals were maintained on a constant 14/10-h light/dark cycle at 28.5°C. The animals used in these experiments were F2 families, 8–12 months old, generated from N-ethyl-nitrosourea mutagenized founders as previously described (5, 15–17).
Conditioned Place Preference in Zebrafish.
The testing apparatus is a 2-liter, rectangular tank divided into two halves containing distinct visual cues with a perforated wall that allows complete albeit somewhat impeded movement. After an initial introduction to the apparatus, the fish are tested for base-line preference by calculating the percent time spent on a given side during a 2-min trial. Fish that display abnormal behavior in the apparatus such as deficient or excessive swimming or a base-line preference greater than 70% were not tested further (rarely more than 3 or 4 of 20 fish tested from a given family). The fish are then restricted to the least preferred side and exposed to drug administered by application of a saturated wick. The fish are tested again the next day, and the change in preference, reflective of cocaine's rewarding effect, was obtained by subtracting the base-line percentage from the final value.
Visual Threshold Measurement in Zebrafish.
The behavioral test determining visual threshold was performed as described previously (17). Briefly, a 500-ml Nalgene container with a central post is placed within a rotating cylindrical drum that has a black panel on a white background. When the fish sees the approaching black panel it typically turns away to hide behind the central post. Visual threshold (VT) is determined by varying the light on the drum with neutral density filters and finding the minimal light evoking an escape response. In these experiments, fish were dark-adapted for 30 min, tested for base-line VT, light adapted for 15 min, given cocaine (10 mg/liter), dark-adapted a second time after allowing 10 min for the drug to work, and again tested for VT.
Zebrafish T-Maze.
The fish negotiate an 18-inch long arm and then have a choice of two 12-inch short arms, one of which opens into a large reservoir (9 inches square) that is 2 inches deeper than the rest of the maze. The reservoir contains artificial grass and marbles that offers a favorable habitat for the fish. Most of the fish tested spent the majority of their time in the reservoir once they found it. On the first trial, fish were given 5 min to fully explore the maze, and the time taken to first encounter the reservoir and stay for at least 20 s was recorded. The fish were then given a second trial 3 h later and a final trial 24 h later.
Results
Conditioned Place Preference in Zebrafish.
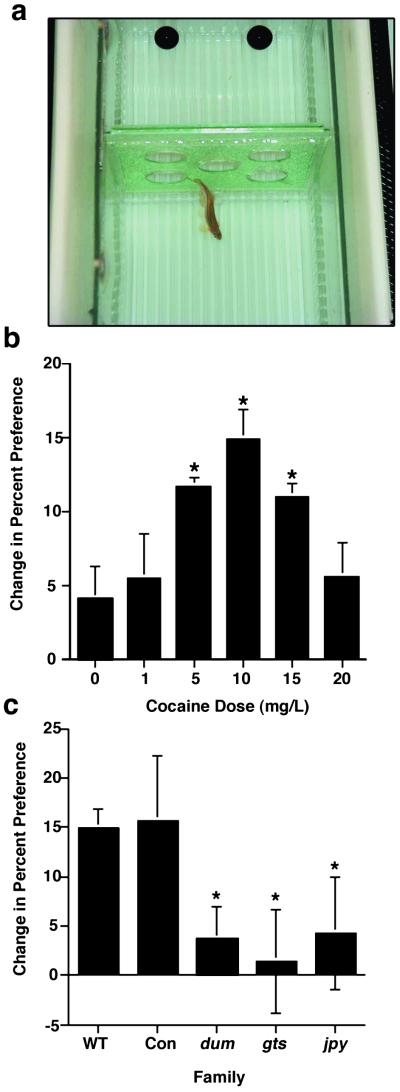
Our behavioral screen for cocaine-induced CPP in zebrafish is similar to that used with other species (Fig. 1a). Initial experiments using only wild-type fish demonstrated a consistent and robust cocaine-induced CPP. Fig. 1b shows results obtained from several families of zebrafish using different doses of cocaine. Maximal results were achieved by using 10 mg/liter cocaine, with 85% of the fish showing a positive change in preference. Lower concentrations elicited a progressively lower response, as did higher concentrations. It is possible that higher concentrations of the drug produce an aversive effect by interacting with the external sensory systems of the fish. There was no sex difference in cocaine-induced CPP at any dose. Lidocaine, which, like cocaine, acts as a local anesthetic but is not rewarding, was used as a control (data not shown). Lidocaine usually induced a change in preference no different from that of untreated controls (5.2 ± 2.6 SEM for six experiments).
Figure 1.
Cocaine-induced CPP in zebrafish. (a) An example of a test subject and apparatus used in these experiments. The response to cocaine is a decided shift in preference for the side of the apparatus in which the fish were exposed to cocaine. (b) This response was dose dependent with a maximal effect at 10 mg/liter. Values are averages of means from multiple experiments conducted at each dose (six families at 0 and 10 mg/liter, three families at the other doses, and a minimum of five fish tested per family). Values at 5, 10, and 15 were significantly higher than both untreated and lidocaine controls (*P < 0.05 by ANOVA). (c) F3 generation fish derived from mutagenized F2 families that showed lower conditioned place preference. Con was a control family derived from normal responders of the same family that produced dum. The F3 families dum, gts, and jpy all show lower cocaine-induced place preference than both wild-type families and the control family (*, P < 0.05 for each compared with wild-type untreated control fish by ANOVA). Error bars represent ± SEM.
Cocaine-induced CPP was used to screen 18 F2 families for abnormal responsiveness to cocaine. Three F2 families were found that had a high proportion (>45%) of members showing an insensitivity to cocaine. The high number of low responders in these F2 families suggests the action of a single dominant mutation. To test this, low responders from each of these families were inbred. These generated the F3 families that were called dumbfish (dum, a92), jumpy (jpy, a108), and goody-two-shoes (gts, a107). A pair of high responding fish from one of these families was also inbred for comparison with its low responding counterparts (Con in Figs. 1–3). The F3 generation from these crosses was raised and examined for CPP. Fig. 1d shows the cocaine-induced CPP for the four F3 families raised from the screen. All F3 generation families derived from F2 low responders displayed abnormally low responsiveness to cocaine, with CPP values lower than or comparable to untreated or lidocaine-treated controls. In contrast, fish from the control F3 generation derived from the same family as dum showed relatively normal cocaine-induced CPP. The proportion of individuals from these F3 families that showed a negative change in preference after treatment with cocaine was 8 of 14 for dum (57%), 6 of 12 for gts (50%), and 5 of 9 for jpy (56%). Clutch size for these families was between 25 and 30 fish, some of which did not perform the assay (20% for dum and gts), and many were tested with lidocaine (about 30%). The jpy family was unusual in that 50% of the fish examined could not be tested because of abnormal behavior in the apparatus. These “jumpy” individuals appeared unduly stressed as evidenced by excessive swimming, surface-rolling, and jumping. This “jumpiness” was also characteristic of the F2 parental family from which these fish were derived.
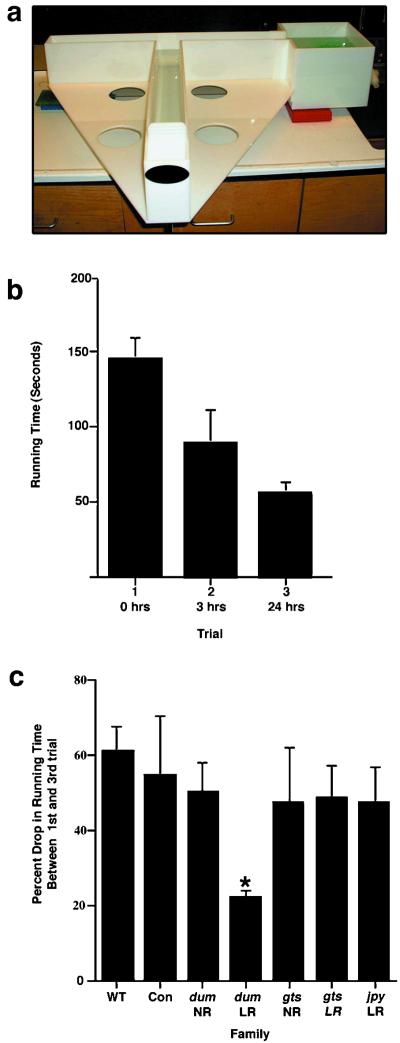
Figure 3.
(a) Cognitive ability of zebrafish was tested by using a T-maze. (b) The time taken in seconds to find the reservoir for 20 wild-type fish was tested in three successive trials. The fish reduced their running time by an average of 60% by the third trial. The behavior in the T-maze of the F3 cocaine-insensitive families was tested. All of the families tested normally with the exception of the dum low-responding family members (dum LR) that displayed very poor learning (*, P < 0.5 compared with wild-type by ANOVA). A minimum of three fish was analyzed from each group, and values were not assigned for fish exhibiting “flunker” or “fast” behavior in their initial trial (see text). Error bars represent ± SEM.
Effects of Cocaine on Visual Sensitivity.
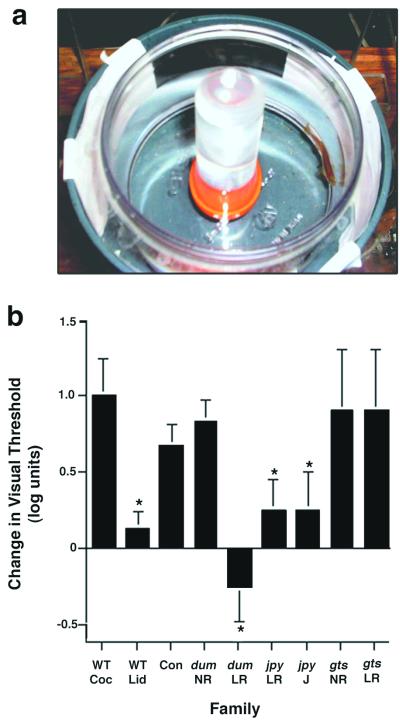
Given that cocaine-induced CPP was abnormal in these families, we wished to further characterize the phenotype. Lowered responsiveness to cocaine suggests an alteration in dopaminergic signaling in the brain. To test this, we examined whether dopaminergic function in the retina is sensitive to cocaine. Dark-adapted visual sensitivity was measured by using an escape response test (17). Cocaine consistently raises the VT of dark-adapted wild-type fish by about a log unit (Fig. 2b); that is, it makes them less sensitive to light. This is not because of an alteration in nonvisual behaviors, as experiments recording the electroretinogram from immobilized fish injected with cocaine yielded similar results; their electroretinogram thresholds were raised by about one log unit (data not shown). In contrast, the VT of fish was unaffected by lidocaine. Fish from each of the F3 families tested for CPP were next tested for visual sensitivity to cocaine. The most striking results were seen with dum; fish in this family that displayed a low response to cocaine in the CPP test (dum LR) were also insensitive to the drug in the visual test (two of the six fish tested actually displayed a lowered VT in response to cocaine). In contrast, normal CPP responders from dum and fish from the control F3 family showed a typical drop in VT of almost a log unit after treatment with cocaine. Family members of jpy that were insensitive to cocaine in the CPP test (jpy LR) also showed lowered responsiveness to the drug in the visual test. As noted above, several members of this family were not testable for CPP because of their erratic behavior. We performed the visual test on three of these “jumpy” fish (jpy J in Fig. 2b) and found that they were as insensitive to cocaine as siblings that displayed low CPP. In contrast, fish from gts displaying both normal and low cocaine-induced CPP (gts NR and gts LR, respectively) showed normal responsiveness in the visual test; that is, their VTs were raised when they were exposed to cocaine.
Figure 2.
Cocaine partially inhibits dark-adaptation. Visual sensitivity was measured by observing the escape response of dark-adapted fish to varying levels of incident light. (a) The apparatus with a fish turning to avoid the moving black panel. (b) Cocaine induces a log-unit drop in visual sensitivity of dark-adapted wild-type fish, whereas lidocaine has no effect. Fish from the control family (Con) showed normal cocaine sensitivity. Fish from dum and jpy that showed normal cocaine-induced CPP (NR) also had normal sensitivity to the drug in the visual test. In contrast, fish from dum and jpy families that showed low cocaine-induced CPP (LR) were also insensitive to the visual effect of the drug. Also shown are “jumpy” fish from the jpy family (jpyJ) with undeterminable CPP but relative insensitivity to cocaine in the visual test. Fish from the gts family showing both normal and low cocaine-induced CPP (NR and LR) show normal sensitivity to the drug in the visual test (*, P < 0.05 when compared with wild-type fish treated with cocaine as measured by ANOVA, with a minimum of four fish from each group). Error bars represent ± SEM.
Testing of Learning and Memory in Zebrafish.
Lack of responsiveness to cocaine in the CPP test might also reflect a defect in the learning or memory capacity of these fish. To test the cognitive ability of our cocaine-insensitive fish, we fashioned a simple T-maze test (Fig. 3a). The time in seconds for wild-type fish to find the reservoir is shown in Fig. 3b. The fish took on average about 140 s to find the reservoir initially; however, individuals varied somewhat in their performance. The initial time appeared to be dependent on the stress levels of the fish. A few fish (5%) were removed from further analysis because they never left the long arm of the maze. These “flunkers” would, however, find the reservoir in subsequent trials if they were chased into it during the first trial. Other fish (10%) displayed bottom-oriented, stress-related behavior and swam blindly into the reservoir very quickly. The initial stress response of these “fast” fish obscured analysis of learning because in later trials the fish swam in a slower, more relaxed manner. At 3 h, the time taken by most fish to find the reservoir was almost cut in half, and on the third trial at 24 h, most fish displayed a very consistent drop in latency of about 60%.
We next tested our cocaine-insensitive families in the T-maze (Fig. 3c). The numbers represent the percent drop in time required to reach the reservoir between the initial trial and final trial at 24 h. Most of the fish tested behaved normally in the maze in that they learned to find the reservoir at 24 h in about half the time that they took initially. The most notable exception were the dum fish that were insensitive to cocaine (dum LR). The analysis of this group, however, was hampered by a high number of “flunker” fish that failed to find the reservoir initially (three of seven fish tested). Unlike wild-type “flunkers,” these fish would never find the reservoir, even after being chased in during the initial and 3-h test trials. Even with these “flunkers” removed from analysis, the dum cocaine low responders scored considerably worse in the maze than their normal siblings; that is, they showed little improvement in the time required to find the reservoir.
Among the jpy low responders (jpy LR), two of the five fish tested were of the “fast” type described above and plunged into the reservoir in less than 15 s. In subsequent trials, these fish were slower, probably because they were less stressed. We therefore excluded these fish from analysis. In contrast to the low responders of the dum family, the testable low-responding jpy fish performed normally in the maze. All gts fish displayed normal performance in the maze.
Discussion
We have shown that cocaine has specific effects on zebrafish behavior. Although we have not yet measured the levels of cocaine taken up by the bloodstream of the animals tested, we believe that the effects we are reporting are not due solely to interactions of the drug with the external sensory systems of the fish for several reasons. First, the acute responses of the fish to cocaine and another local anesthetic, lidocaine, are different. When treated with cocaine, the fish typically displayed slow circling, low in the water column, with fins more or less extended—indicating arousal. In small groups of fish, cocaine induced a striking increase in aggressive behavior marked by dominance displays and chasing. In contrast, lidocaine did not induce any obvious changes in behavior, except for retraction of the fins, possibly because of irritation of the external sensory systems. Second, cocaine induced a change in CPP, whereas results with lidocaine were no different from those for untreated controls. Finally, cocaine induced a decrease in visual sensitivity, whereas lidocaine did not. This response was probably physiological, rather than behavioral, as the fish were fully capable of avoidance behavior under brighter conditions. Furthermore, electroretinogram recordings confirmed a decrease in visual sensitivity with cocaine. Future CPP experiments performed with dopamine antagonists together with cocaine will serve to further test the assumption that the effects we have seen are mediated by direct action on the zebrafish brain.
The zebrafish central nervous system, although perhaps less complex, is essentially organized like the mammalian. Recent anatomical studies have demonstrated that the tyrosine hydroxylase-positive neurons of the posterior tuberal nucleus project to the basal forebrain in a manner reminiscent of the ventral tegmental area–nucleus accumbens connection in mammals (20). Although it is not yet certain that this circuit governs reward in fish, we have detected c-Fos-like immunoreactivity in the forebrain of cocaine-treated fish (data not shown), and a detailed analysis of the anatomy in both wild-type and candidate mutants is currently being conducted. Certainly, teleosts respond to many of the same instinctive drives that mammals do, probably by using analogous neural substrates. In fact, CPP has been well documented in the closely related goldfish, although not with respect to addictive drugs (21). Zebrafish have been shown to approach areas in which they have been exposed to certain amino acids (byproducts of a favorite food source) and avoid areas in which they have encountered heavy metals (22). It therefore should not be too surprising that they respond so reliably to cocaine. In fact the cocaine-induced CPP in zebrafish is comparable to that reported for mice (23, 24). The genetic manipulation possible with the zebrafish makes it an ideal model organism to study the genetic bases for behaviors related to addiction as well as to stress, memory, and learning.
We have isolated three zebrafish families that vary significantly from wild-type families in that a high proportion of their offspring do not display cocaine-induced CPP. Because the families have a different profile of behavior in secondary screens, we believe that each represents a different mutation. Future experiments will involve making double mutants to test this presumption. It was perhaps somewhat surprising to find three abnormal families in the first 18 screened. However, these ratios are not drastically different from those that we have seen in screens for traits such as night-blindness or developmental eye defects (15–17). Only by further screening will we be able to assess meaningfully the frequency of such mutations.
In all of the families that we have pursued, low responsiveness to cocaine in the F2 generation was inherited by the next generation. In contrast, the control family, raised from normal responders of the F2 generation, displayed normal responsiveness to cocaine in F3 fish. For the other families, the high frequencies of low responders in the F2 generation (50%) and in the F3 generation (55–65%) suggest that the abnormal behavior is probably because of mutations in single genes, although all fall somewhat short of the 75% expected from Mendelian inheritance. It should be remembered that these values probably do not reflect the exact proportion of low responders because each family had a few individuals for whom the change in preference was lower than or equal to controls but not negative. Because of our concerns about variability of control values, the most convenient means of comparison was the number of negative responses. Also, some of the fish in each family were used as controls, whereas others would not perform the test at all. Therefore, the number of fish tested with cocaine is still too low to have complete confidence in their accuracy. This was particularly evident in the jpy family, which, like its F2 parental strain, had a high proportion of fish that could not be tested because of stress-related behavior (prompting the name “jumpy”). Many members of this family with indeterminable responsiveness to cocaine in the CPP test subsequently showed low responsiveness in the visual test. Finally, it is also possible that fish homozygous for the presumptive mutations die at some point during development, thereby lowering the proportion of low responders. What is certain is that there are many more negative responders in these families than in wild-type families.
Because the F3 generation was derived from the inbreeding of two abnormal responders, the possibility that the phenotypes observed is due to several, tightly linked genes or to polymorphisms cannot be excluded. To take full advantage of the clutch size possible for zebrafish, we are currently raising the F4 generation of fish by outcrossing all of the low responders in each family to wild-type fish to determine the frequencies of cocaine insensitivity for several families raised in parallel. Drawing correlations between certain genetic polymorphisms and behavior using inbred strains of mice currently requires selective breeding for at least 20 generations (2). Although the generation time for zebrafish is actually a bit longer than that of mice (3 months), the large clutch size and subsequent number of families available allows a higher degree of confidence in determining genetic relationships to behavior over the same number of generations.
Two of the abnormal families examined (dum and jpy) showed clear cocaine insensitivity in both the CPP and visual assays. That two independent behaviors were affected strongly suggests that some components of dopaminergic signaling, common to both the brain and retina, are abnormal in these families. Cocaine-insensitive members of the two families also behaved differently in the T-maze. That is, low responders in the CPP test from the dum family also exhibited poor maze performance. In fact, some of these low responders never found the reservoir despite being chased in repeatedly. The poor maze performance did not appear to be stress-related or a matter of preference. Rather, we favor the explanation that these fish had a cognitive deficit (hence the name dumbfish). Striatal dopamine has been implicated in certain aspects of learning and memory in mammals, even for nondrug-related behaviors (25). It is possible the presumptive mutation in dum affects a similar mechanism in fish.
In contrast, cocaine-insensitive fish from jpy showed normal cognitive ability in the T-maze. What was different about this family was the high incidence of stress evidenced by their “jumpy” behavior. The “jumpiness” affected performance in the T-maze, with some fish exhibiting “fast” behavior described above. We suspect that the longer times in later trials were attributable to a more relaxed manner of swimming, rather than poor learning. Although we could not correlate jumpiness with low response in the CPP test, the three jumpy fish tested proved insensitive to cocaine in the visual test. Poor performance in the introductory trial for some of these fish might represent an abnormal response to novelty. Both stress and novelty response have been correlated with the onset of amphetamine self-administration in rodents (26). Also, hyperactivity in response to novelty has been reported for transgenic mice lacking the dopamine transporter (11). Perhaps the “fast” behavior of the jpy fish in the T-maze represents an abnormal response to novelty that is somehow related to reward. Of course, not all cocaine-insensitive fish in this family showed the same degree of “jumpiness.” It remains to be seen whether the variation in this phenotype is because of a different mutation, genotype, penetrance, or background. Successive generations of breeding should provide the answer.
The gts family stands in contrast to the other families we have investigated in that all of the low-responding individuals behave normally in both of the secondary screens we have devised. It is possible that the abnormality in these fish is a defect in a component of dopaminergic signaling specific to the midbrain. Alternatively, this family may have a defect in another neural circuit that modulates reward.
The aim of these studies was to find zebrafish families with altered sensitivity to cocaine in hopes of eventually characterizing the underlying genes. Three such families have been identified, each of which seems to have a different mutation leading to distinct behavioral consequence. Our studies suggest that forward genetic screening of zebrafish employing behavioral testing is a promising way to uncover novel genes linked to addictive behavior.
Acknowledgments
We thank William McCarthy, Salvatore Sciascia, and Christian Lawrence for technical support in raising the fish. We also thank Paul Poznauskis and Paul Farabella of the Biological Laboratories machine shop for assistance in building the testing apparatuses. Finally, we are indebted to Barry E. Kosofsky, Department of Neurology at Massachusetts General Hospital, for his critical role during the initial stages of this project and to Steven Hyman of the National Institute of Mental Health for valuable comments on the manuscript. This work was supported by the National Eye Institute and by a supplement from the National Institute on Drug Abuse [RO1 EY00811 (to J.E.D.) and NRSA EY06979 (to T.D.)].
Abbreviations
- CPP
conditioned place preference
- VT
visual threshold
References
- 1.Mark T L, Woody G E, Juday T, Kleber H D. Drug Alcohol Depend. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 2.Crabbe J C, Phillips T J, Buck K J, Cunningham C L, Belknap J K. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- 3.Picciotto M R, Wickman K. Physiol Rev. 1998;78:1131–1163. doi: 10.1152/physrev.1998.78.4.1131. [DOI] [PubMed] [Google Scholar]
- 4.Bainton R J, Tsai L T Y, Singh C M, Moore M S, Neckameyer W S, Heberlein U. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 5.Solnica-Krezel L, Schier A F, Driever W. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise R A. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 7.Wise R A. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 8.Tzschentke T M. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 9.Phillips T J, Brown K J, Burkhart-Kasch S, Wenger C D, Kelly M A, Rubenstein M, Grandy D K, Low M J. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein M, Phillips T J, Bunzow J R, Falzone T L, Dziewezapolski G, Zhang G, Fang Y, Larson J L, McDougall J A, Chester J A, et al. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 11.Giros B, Jaber M, Jones S R, Wrightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 12.Noble E P. Eur Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 13.Self D W, Nestler E J. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 14.Nestler E J, Lansman D. Nature (London) 2001;6822:834–835. doi: 10.1038/35057015. [DOI] [PubMed] [Google Scholar]
- 15.Brockerhoff S E, Hurley J B, Janssen-Beinhold U, Neuhauss S C, Driever W, Dowling J E. Proc Natl Acad Sci USA. 1995;92:10545–10549. doi: 10.1073/pnas.92.23.10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadool J M, Brockerhoff S E, Hyatt G E, Dowling J E. Dev Genet (Amsterdam) 1997;20:288–295. doi: 10.1002/(SICI)1520-6408(1997)20:3<288::AID-DVG11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Dowling J E. Proc Natl Acad Sci USA. 1997;94:11645–11650. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umino O, Dowling J E. J Neurosci. 1991;11:3034–3041. doi: 10.1523/JNEUROSCI.11-10-03034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish, Brachydanio rerio. Eugene, OR: Univ. of Oregon Press; 1995. [Google Scholar]
- 20.Rink E, Wulliman M F. Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- 21.Mattioli R, Nelson C A, Huston J P, Sieler R E. Brain Res Bull. 1998;45:41–44. doi: 10.1016/s0361-9230(97)00287-6. [DOI] [PubMed] [Google Scholar]
- 22.Steele C W. Responses of Zebrafish, Brachidanio rerio, to Behavior-Altering Chemicals. Ann Arbor, MI: Univ. of Michigan Dissertation Services; 1986. [Google Scholar]
- 23.Sora I, Wichems C, Takahashi N, Li X-F, Zeng Z, Revay R, Lesch K-P, Murphy D L, Uhl G R. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sora I, Hall F S, Andrews A M, Itokawa M, Li X-F, Wei H-B, Wichems C, Lesch K-P, Murphy D L, Uhl G R. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berke J D, Hyman S E. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 26.Piazza P V, LeMoal L M. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]