ABSTRACT
The MafB transcription factor is expressed in pancreatic α and β cells during development but becomes exclusive to α cells in adult rodents. Mafb-null (Mafb−/−) mice were reported to have reduced α- and β-cell numbers throughout embryonic development. To further analyze the postnatal function of MafB in the pancreas, we generated endocrine cell-specific (MafbΔEndo) and tamoxifen-dependent (MafbΔTAM) Mafb knockout mice. MafbΔEndo mice exhibited reduced populations of insulin-positive (insulin+) and glucagon+ cells at postnatal day 0, but the insulin+ cell population recovered by 8 weeks of age. In contrast, the Arx+ glucagon+ cell fraction and glucagon expression remained decreased even in adulthood. MafbΔTAM mice, with Mafb deleted after pancreas maturation, also demonstrated diminished glucagon+ cells and glucagon content without affecting β cells. A decreased Arx+ glucagon+ cell population in MafbΔEndo mice was compensated for by an increased Arx+ pancreatic polypeptide+ cell population. Furthermore, gene expression analyses from both MafbΔEndo and MafbΔTAM islets revealed that MafB is a key regulator of glucagon expression in α cells. Finally, both mutants failed to respond to arginine, likely due to impaired arginine transporter gene expression and glucagon production ability. Taken together, our findings reveal that MafB is critical for the functional maintenance of mouse α cells in vivo, including glucagon production and secretion, as well as in development.
KEYWORDS: MafB, pancreatic islet, α cell, glucagon, PP cell, F cell
INTRODUCTION
The pancreas is a secretory organ containing two major glands, an exocrine gland for digestive enzymes and an endocrine gland for pancreatic hormones. The endocrine pancreas is composed of small clusters of cells called the islets of Langerhans, which include insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, pancreatic polypeptide (PP)-producing F cells (also known as PP cells), and ghrelin-producing ε cells. In particular, insulin and glucagon regulate glucose homeostasis: blood glucose levels decrease in response to insulin and increase in response to glucagon. Failure of this hormonal balance due to β-cell dysfunction causes hyperglycemia, a key feature of diabetes, which often accompanies α-cell impairment (1–3). Therefore, understanding pancreatic α- and β-cell biology produces deeper insights into hormonal regulation. Pancreatic islet development and specification, including α- and β-cell differentiation, are governed by various important transcription factors (4, 5). For example, Pdx1, a pancreatic progenitor marker, drives all pancreatic lineages. Ngn3, an endocrine progenitor marker, initiates pancreatic endocrine cell fates. Pax4 and Arx promote β- and α-cell specification and differentiation, respectively (4, 5). Finally, MafA is required for β-cell maturation and functional maintenance (6–8), and MafB plays a decisive role in α cells, although MafB is also involved in both α- and β-cell development during pancreas morphogenesis (9–11).
MafB, a member of the large Maf subfamily of basic leucine zipper (b-Zip) transcription factors, is first detected in the mouse embryonic pancreas from embryonic day 10.5 (7, 9) and becomes exclusively confined to α cells within 2 weeks of birth (8). In α cells, MafB activates glucagon gene expression through the G1 element of the glucagon promoter region (9); hence, glucagon is generally accepted as an α-cell marker (4, 5). Previous work demonstrated that Mafb knockout (Mafb−/−) mouse embryos exhibit reduced α- and β-cell numbers with delayed onset of insulin expression in β cells, indicating the role of MafB in α- and β-cell differentiation (11). However, because the Mafb−/− mutation in these mice was neonatal lethal due to defective respiratory rhythm (12), the postnatal function of MafB in pancreatic islets has thus far remained unknown. A recent study of pancreas-wide Mafb-deficient (MafbΔpanc) mice revealed that insulin-positive (insulin+ [Ins+]) and glucagon+ (Glu+) cell numbers from postnatal day 1 (P1) neonates were also decreased (10). Interestingly, both cell types were restored by 2 weeks of age in these mutants, rendering α cells dysfunctional in response to low glucose levels and arginine stimulation in vitro (10). These results suggest that MafB is required only for maintaining α-cell function and not for glucagon production per se, implying that glucagon expression is compensated for by other factors. Given that MafB is a glucagon gene activator (9) and is heavily enriched in mature α cells (13), these results were intriguing. Additionally, frequent reports of diabetic patients displaying abnormal glucagon regulation highlight the importance of understanding α-cell physiology (1–3). Therefore, to confirm the postnatal role of MafB in pancreatic islet cells, we generated two types of conditional-knockout mice: endocrine cell-specific Mafb knockout (MafbΔEndo) and tamoxifen (TAM)-dependent Mafb knockout (MafbΔTAM) mice.
Here, we address an alternative in vivo role of MafB in postnatal pancreatic α cells. Both MafbΔEndo and MafbΔTAM mice failed to express glucagon in α cells, leading to low basal plasma glucagon levels. Moreover, Mafb deficiency disrupted glucagon secretory responses to α-cell stimuli in both mutants. Therefore, our findings demonstrate that MafB is critical for glucagon production during α-cell development and for α-cell functional maintenance in adult mice.
RESULTS
Embryonic deletion of Mafb in endocrine cells results in postnatal decreases in both Ins+ and Glu+ cell populations.
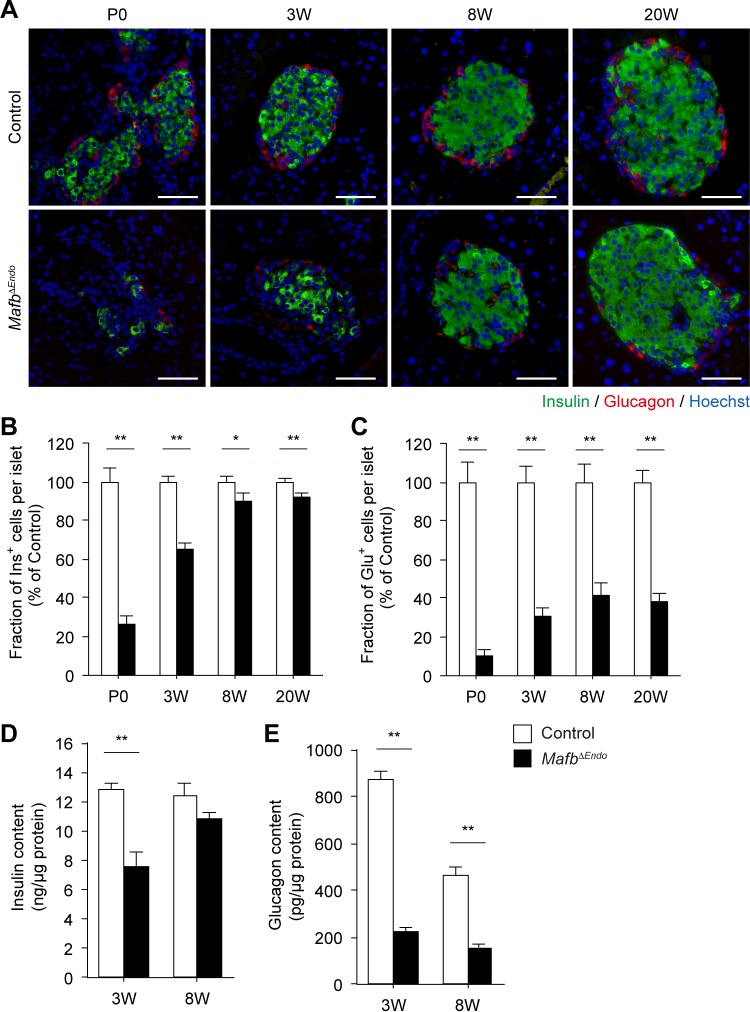
To address the physiological function of MafB in postnatal pancreatic islets, we generated endocrine cell-specific Mafb-deficient mutant Mafbf/f::Neurogenin3 (Ngn3)-Cre (MafbΔEndo) mice. We first performed immunohistochemical studies on pancreatic sections to explore the effects of Mafb loss on the postnatal development of pancreatic endocrine cells by examining insulin and glucagon protein expression. At P0, the fractions of Ins+ and Glu+ cells in MafbΔEndo islets were significantly reduced compared with Mafbf/f control mice (Fig. 1A to C) (control versus MafbΔEndo, Ins+, 100% ± 6.9% versus 25.9% ± 4.3%; Glu+, 100%± 10.0% versus 10.7% ± 2.3%). Interestingly, the reduced population of Ins+ cells in MafbΔEndo pancreata recovered to nearly control levels as the mice aged (Fig. 1A and B) (control versus MafbΔEndo, 3 weeks, 100% ± 2.6% versus 65.4% ± 3.1%; 8 weeks, 100% ± 2.5% versus 89.9% ± 4.0%; 20 weeks, 100% ± 1.8% versus 92.0% ± 2.2%). However, the fraction of Glu+ cells in MafbΔEndo islets remained significantly reduced throughout postnatal development to 20 weeks of age compared with control groups (Fig. 1A and C) (control versus MafbΔEndo, 3 weeks, 100% ± 8.3% versus 30.5% ± 4.4%; 8 weeks, 100% ± 9.7% versus 41.2% ± 7.1%; 20 weeks, 100% ± 6.2% versus 38.5% ± 4.4%), while islet architecture and total islet cell numbers were unaffected (see Fig. S1A in the supplemental material). To confirm the reduction in insulin and glucagon production, we measured total insulin and glucagon contents in whole pancreata from 3- and 8-week-old animals (Fig. 1D and E). As expected, the insulin content in MafbΔEndo pancreata was significantly reduced compared with control pancreata at 3 weeks of age but improved to approximately control levels at 8 weeks of age (Fig. 1D) (control versus MafbΔEndo, 3 weeks, 12.8 ± 0.5 versus 7.6 ± 1.0 ng/μg protein; 8 weeks, 12.4 ± 0.8 versus 10.8 ± 0.5 ng/μg protein), consistent with the restoration of the Ins+ cell population (Fig. 1B). In contrast, the glucagon content in MafbΔEndo pancreata was severely compromised at both 3 and 8 weeks of age, with no sign of recovery to control levels (Fig. 1E) (control versus MafbΔEndo, 3 weeks, 869.7 ± 37.8 versus 220.8 ± 25.0 pg/μg protein; 8 weeks, 467.1 ± 30.5 versus 157.9 ± 16.3 pg/μg protein). Of note, this α-cell abnormality of MafbΔEndo mice did not affect the animals' growth, as the pancreas weight and body weight were both unaltered (see Fig. S1B and C in the supplemental material). These results suggest that the loss of Mafb during embryogenesis affects pancreatic endocrine cell development at early postnatal periods, leading to decreased populations of both Ins+ and Glu+ cells. However, only the α-cell defect persists into adulthood.
FIG 1.
Embryonic deletion of Mafb in endocrine cells decreases the population of both Ins+ and Glu+ cells postnatally. (A) Immunostaining of insulin (green) and glucagon (red) in MafbΔEndo and control (Mafbf/f) pancreata from P0 and 3-, 8-, and 20-week-old (W) animals. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. (B and C) Fractions of Ins+ (B) and Glu+ (C) cells within islets in MafbΔEndo and control pancreata (n ≥ 3). All values were normalized to age-matched controls. *, P < 0.05; **, P < 0.01. (D and E) Pancreatic insulin (D) and glucagon (E) contents in MafbΔEndo and control pancreata from 3- and 8-week-old animals (n ≥ 4). The hormone content was normalized to the protein concentration. Means and SEM are shown. **, P < 0.01.
Endocrine cell-specific Mafb deficiency at the embryonic stage delays insulin production in β cells but suppresses α-cell development after birth.
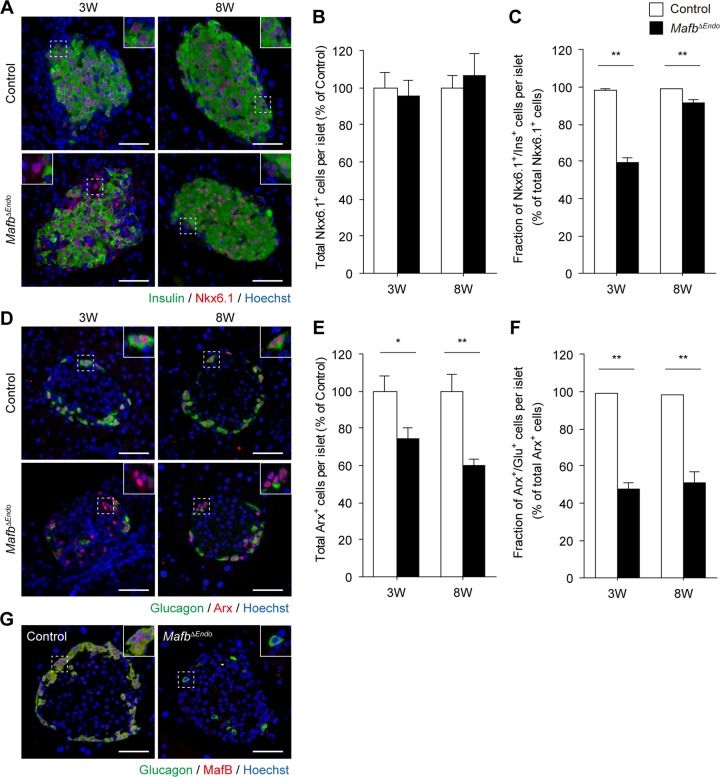
To more precisely investigate the role of MafB in postnatal islet cell development, we performed immunofluorescence staining to examine the expression of β- and α-cell fate markers that characterize cell identity. Pancreas sections from 3- and 8-week-old mice were costained for either insulin and Nkx6.1 in β cells (14) or glucagon and Arx in α cells (15) (Fig. 2A and D). The total Nkx6.1+ cell population remained unchanged, suggesting that Mafb ablation does not affect β-cell lineage differentiation (Fig. 2A and B) (control versus MafbΔEndo, 3 weeks, 100% ± 8.1% versus 95.3% ± 9.2%; 8 weeks, 100% ± 6.8% versus 106.6% ± 12.0%). At 3 weeks of age, only 60% of Nkx6.1+ cells expressed insulin in MafbΔEndo pancreata, whereas almost all Nkx6.1+ cells from control pancreata were also positive for insulin (Fig. 2A and C) (control versus MafbΔEndo, 98.4% ± 0.6% versus 59.2% ± 3.0%). However, this defect of decreased insulin was minimized by 8 weeks of age, at which point Ins+ β cells (Nkx6.1+ Ins+) accounted for greater than 90% of the total Nkx6.1+ cell population (Fig. 2A and C) (control versus MafbΔEndo, 99.0% ± 0.2% versus 91.7% ± 1.4%). These results suggest that embryonic Mafb deficiency in pancreatic islets causes delayed insulin production in β cells without affecting cell fate differentiation. Measurement of fasting blood glucose levels and glucose metabolism by intraperitoneal glucose tolerance test further supported our findings of delayed β-cell development. MafbΔEndo mice showed higher fasting blood glucose levels at P0, which were corrected to the control level by 8 weeks of age; delayed glucose tolerance in 4-week-old MafbΔEndo mice recovered to the control level at 8 weeks (see Fig. S2A to C in the supplemental material).
FIG 2.
Endocrine cell-specific Mafb deficiency at the embryonic stage delays insulin production in β cells but suppresses α-cell development after birth. (A) Insulin (green) and Nkx6.1 (red) immunoreactivity in MafbΔEndo and control (Mafbf/f) pancreata from 3- and 8-week-old animals. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. The insets are enlargements of the boxed areas. (B) Total Nkx6.1+ cells per islet normalized to age-matched controls (n ≥ 4). (C) Fraction of Ins+ β cells among the total Nkx6.1+ cell population in MafbΔEndo and control pancreata (n ≥ 4). **, P < 0.01. (D) Immunofluorescence of glucagon (green) and Arx (red) in MafbΔEndo and control pancreata from 3- and 8-week-old animals. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. (E) Total Arx+ cells per islet normalized to age-matched controls (n ≥ 4). *, P < 0.05; **, P < 0.01. (F) Fraction of Glu+ α cells among the total Arx+ cell population in MafbΔEndo and control pancreata (n ≥ 4). **, P < 0.01. (G) Costaining of glucagon (green) and MafB (red) in 8-week-old MafbΔEndo and control mice. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. Means and SEM are shown.
Conversely, the total Arx+ cell population in MafbΔEndo mice was reduced at both ages (Fig. 2D and E) (control versus MafbΔEndo, 3 weeks, 100% ± 8.5% versus 74.1% ± 6.6%; 8 weeks, 100% ± 9.0% versus 59.7% ± 3.8%). Similarly, 3-week-old MafbΔEndo mice displayed a considerably smaller population of Glu+ α cells (Arx+ Glu+), with fewer than 50% of Arx+ cells expressing glucagon (Fig. 2D and F) (control versus MafbΔEndo, 98.7% ± 0.3% versus 47.5% ± 3.6%). In contrast to β cells, this small population of Glu+ α cells was sustained even at 8 weeks of age without any sign of improvement (Fig. 2D and F) (control versus MafbΔEndo, 98.0% ± 0.6% versus 51.4% ± 6.0%). Thus, these findings demonstrate that Mafb ablation suppresses not only glucagon production but also the α-cell lineage, indicating that MafB plays a significant role in α-cell development. Interestingly, the remaining Glu+ α cells in the MafbΔEndo pancreas did not express MafB (Fig. 2G), suggesting an alternative glucagon production pathway independent of MafB, which could account for the minimal glucagon content level (Fig. 1E).
Loss of Mafb in adult mice impairs glucagon expression in mature α cells without affecting α-cell identity.
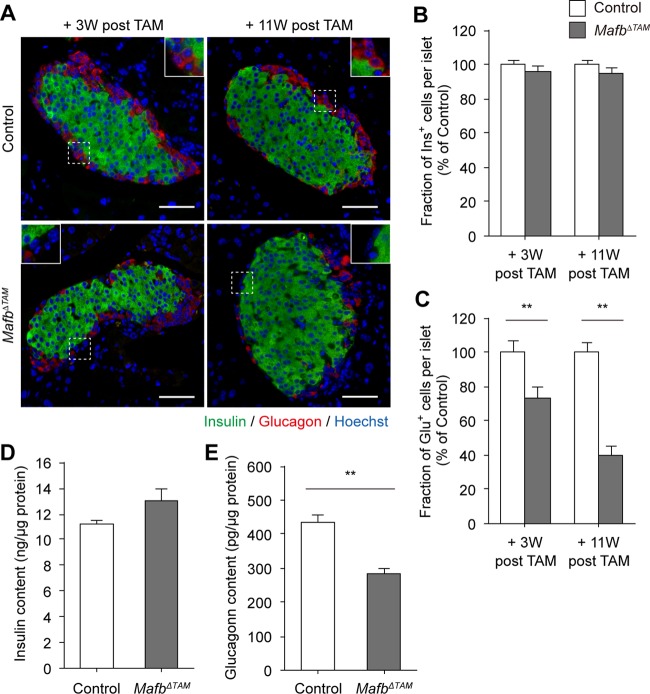
Because MafB is a known regulator of glucagon transcription (9), we hypothesized that the suppression of glucagon expression is due to the deletion of Mafb rather than to the reduced Arx+ cell population in MafbΔEndo mice. To verify the effects of Mafb ablation on glucagon production, we generated conditional Mafb knockout (MafbΔTAM) mice after pancreatic islet cell maturation by tamoxifen (TAM) injection at 5 weeks of age. Immunohistochemistry revealed no significant changes in the organization of Ins+ and Glu+ cells in MafbΔTAM islets compared with Mafbf/f control islets (Fig. 3A). Consistent with the undetectable MafB expression in adult β cells (8, 13), we did not observe any differences in the Ins+ cell population (Fig. 3A and B) (control versus MafbΔTAM, 3 weeks post-TAM, 100% ± 2.6% versus 96.2% ± 2.5%; 11 weeks post-TAM, 100% ± 1.8% versus 95.1% ± 2.9%), and therefore, no differences in fasting blood glucose and plasma insulin levels (data not shown). In contrast, the percentage of Glu+ cells in MafbΔTAM islets was drastically reduced at 3 weeks post-TAM injection and was further reduced by 11 weeks postinjection (Fig. 3A and C) (control versus MafbΔTAM, 3 weeks post-TAM, 100% ± 6.6% versus 73.3% ± 6.0%; 11 weeks post-TAM, 100% ± 6.0% versus 40.0% ± 4.7%). Additionally, as anticipated, insulin content levels were not altered by Mafb deletion in TAM-injected MafbΔTAM pancreata 3 weeks postinjection (Fig. 3D) (control versus MafbΔTAM, 11.2 ± 0.4 versus 13.0 ± 0.9 ng/μg protein), whereas glucagon levels were approximately 65% of control levels (Fig. 3E) (control versus MafbΔTAM, 437.3 ± 18.6 versus 283.8 ± 17.6 pg/μg protein). Taken together, these results indicate that Mafb deficiency in adult mice alters hormone expression in α cells but not in β cells, demonstrating that sustained MafB function is required for normal glucagon production by α cells from postnatal development to maturity.
FIG 3.
Loss of Mafb in adult mice impairs glucagon expression in mature α cells. (A) Immunostaining of insulin (green) and glucagon (red) in MafbΔTAM and control (Mafbf/f) pancreata from mice 3 and 11 weeks post-TAM injection. TAM was injected at 5 weeks of age. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. The insets are enlargements of the boxed areas. (B and C) Fraction of Ins+ (B) and Glu+ (C) cells among islets in MafbΔTAM and control pancreata (n ≥ 4). All the values were normalized to age-matched controls. **, P < 0.01. (D and E) Pancreatic insulin (D) and glucagon (E) contents in MafbΔTAM and control pancreata from mice 3 weeks post-TAM injection (n = 6). Hormone contents were normalized to the total protein concentration. **, P < 0.01. Means and SEM are shown.
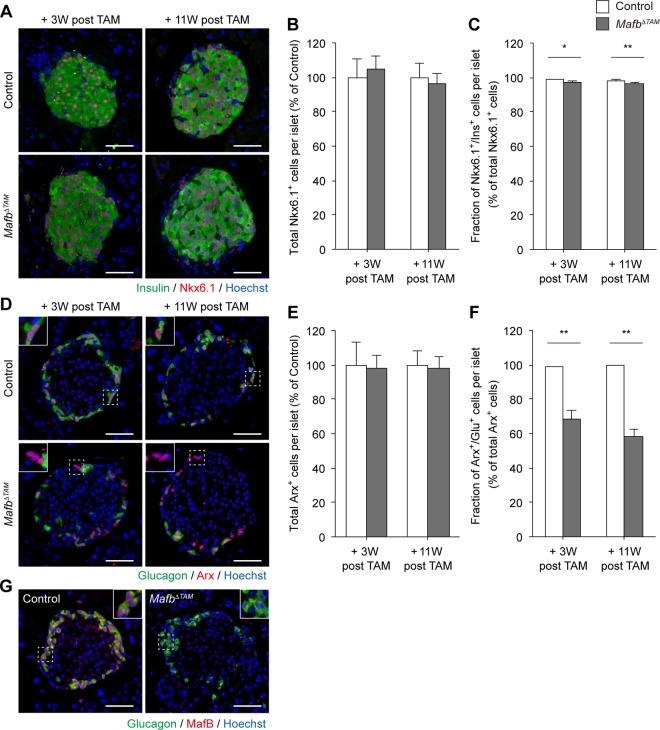
We further investigated whether Mafb ablation in adult mice influences islet cell identity. At 3 and 11 weeks post-TAM injection, the total Nkx6.1+ cell population in MafbΔTAM pancreata did not differ from that in the control (Fig. 4A and B) (control versus MafbΔTAM, 3 weeks post-TAM, 100% ± 10.9% versus 104.5% ± 7.9%; 11 weeks post-TAM, 100% ± 8.4% versus 96.4% ± 5.7%). In addition, almost all Nkx6.1+ cells expressed insulin, albeit at slightly lower levels in MafbΔTAM pancreata than in the controls (Fig. 4A and C) (control versus MafbΔTAM, 3 weeks post-TAM, 98.8% ± 0.4% versus 97.2% ± 0.5%; 11 weeks post-TAM, 98.4% ± 0.4% versus 96.4% ± 0.5%). These results suggest that MafB has no significant effects on mature β cells. Conversely, although the total Arx+ cell population was similar to that of the control (Fig. 4D and E) (control versus MafbΔTAM, 3 weeks post-TAM, 100% ± 13.1% versus 98.1% ± 7.4%; 11 weeks post-TAM, 100% ± 8.1% versus 98.4% ± 6.6%), a marked decrease in the Glu+ α-cell population was noted (Fig. 4D and F) (control versus MafbΔTAM, 3 weeks post-TAM, 98.6% ± 0.4% versus 68.8% ± 4.4%; 11 weeks post-TAM, 99.4% ± 0.3% versus 58.3% ± 4.1%). This reduced proportion of Glu+ α cells was not caused by α-cell apoptosis, as confirmed by negative signals in the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (see Fig. S3A in the supplemental material). Furthermore, the total islet cell number in MafbΔTAM pancreata remained unchanged (see Fig. S3B in the supplemental material), as was observed in MafbΔEndo mice. However, a minimum level of glucagon expression was also observed even in the absence of MafB in MafbΔTAM pancreata (Fig. 4G), consistent with that in MafbΔEndo mice, which suggests the presence of a MafB-independent pathway for glucagon production. Overall, our results indicate that MafB is critical for maintaining α-cell function in adult mice, confirming the principal role of MafB in glucagon expression.
FIG 4.
Adult onset ablation of Mafb does not affect α-cell identity. (A) Insulin (green) and Nkx6.1 (red) immunoreactivity in MafbΔTAM and control (Mafbf/f) pancreata from mice 3 and 11 weeks post-TAM injection. TAM was injected at 5 weeks of age. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. (B) Total Nkx6.1+ cells per islet normalized to age-matched controls (n ≥ 4). (C) Fraction of Ins+ β cells among the total Nkx6.1+ cell population in MafbΔTAM and control pancreata (n ≥ 4). *, P < 0.05; **, P < 0.01. (D) Immunofluorescence of glucagon (green) and Arx (red) in MafbΔTAM and control pancreata from mice 3 and 11 weeks post-TAM injection. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. The insets are enlargements of the boxed areas. (E) Total Arx+ cells per islet normalized to age-matched controls (n ≥ 4). (F) Fraction of Glu+ α cells among the total Arx+ cell population in MafbΔTAM and control pancreata (n ≥ 4). **, P < 0.01. (G) Costaining of glucagon (green) and MafB (red) in MafbΔTAM and control mice 3 weeks post-TAM injection. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. Means and SEM are shown.
MafB expression during embryonic development favors α-cell lineage commitment by suppressing F-cell differentiation.
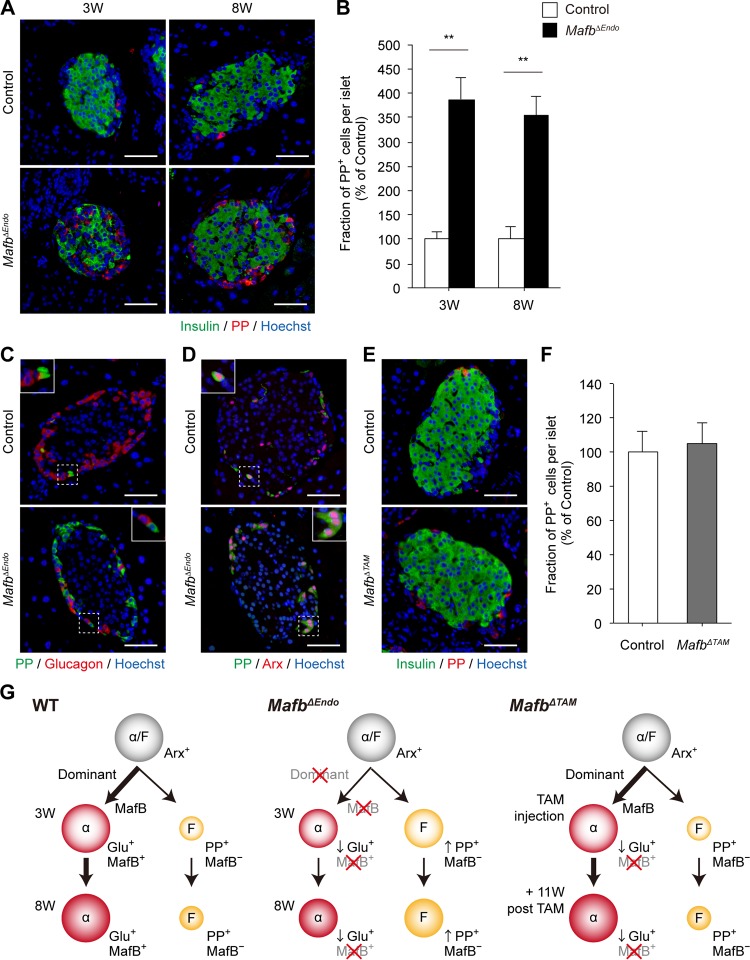
Because, MafB affects the development of both α and β cells, whose precursors are shared with F and δ cells, respectively, we determined to examine MafB's involvement in the other islet cell types. We first performed immunostaining of somatostatin (Som)-producing δ cells and pancreatic polypeptide (PP)-producing F cells to verify any changes in the cell population. In both 3- and 8-week-old MafbΔEndo pancreata, Som+ cell populations remained unaffected (see Fig. S4A and B in the supplemental material). However, the PP+ cell population was increased approximately 3.5-fold in comparison to the control at both ages (Fig. 5A and B) (control versus MafbΔEndo, 3 weeks, 100% ± 16.6% versus 387.7% ± 45.9%; 8 weeks, 100% ± 26.4% versus 352.1% ± 39.2%). Because F cells share a common precursor with α cells, to investigate whether the increased PP+ cell population comes from cell fate change induced by Mafb deletion in α cells, we conducted immunohistochemistry by costaining with glucagon/PP or Arx/PP antibodies. Surprisingly, the majority of PP expression did not merge with glucagon signal (Fig. 5C) but consistently merged with Arx expression regardless of genotypes (Fig. 5D), suggesting that PP+ cells are mainly generated from Arx+ Glu− cells. Although PP+ Glu+ double-positive cells were rarely observed, as previously reported (16), this subpopulation remained unaltered in both groups, excluded from the source of the Arx+ PP+ cell population. More strikingly, the significant 3.5-fold increase in the PP+ cell population was equivalent to the decrease in the Glu+ cell fraction (Fig. 1C), suggesting that the diminished glucagon-producing cell population could be compensated for by an increased PP-producing cell population. These differences (approximately 3.5-fold change) were consistently observed in both 3- and 8-week-old MafbΔEndo mice, whereas MafbΔTAM mice 11 weeks post-TAM injection had no change in any cell population, including δ cells (data not shown) and F cells (Fig. 5E and F) (control versus MafbΔEndo, 100% ± 12.5% versus 105.0% ± 9.9%). Given that the total islet cell numbers did not change (see Fig. S1A in the supplemental material), increased numbers of PP+ cells could have differentiated from Arx+ Glu− precursor cells. In sum, these results indicate that the population change is probably not attributable to cell fate conversion but rather to alterations of cell fate specification during the developmental stage, suggesting the importance of MafB in α-cell lineage specification due to suppressing F-cell fate differentiation (Fig. 5G).
FIG 5.
The PP+ cell population is increased in MafbΔEndo pancreata. (A) Immunostaining of insulin (green) and PP (red) in MafbΔEndo and control (Mafbf/f) pancreata from 3- and 8-week-old animals. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. (B) Fraction of PP+ cells within islets in MafbΔEndo and control pancreata (n ≥ 4). **, P < 0.01. (C and D) Investigation of cell fate conversion by costaining PP (green) with glucagon (C) (red) or Arx (red) (D) antibodies in MafbΔEndo and control pancreata from 8-week-old animals. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. The insets are enlargements of the boxed areas. (E) Insulin (green) and PP (red) immunoreactivity in MafbΔTAM and control pancreata from mice 11 weeks post-TAM injection. TAM was injected at 5 weeks of age. Nuclei were stained with Hoechst 33342. Scale bars, 50 μm. (F) Fraction of PP+ cells within islets in MafbΔTAM and control pancreata (n ≥ 4). (G) Model of α-cell and F-cell lineage specification from α/F precursor cells in wild-type (WT), MafbΔEndo, and MafbΔTAM mutants. Means and SEM are shown.
MafB is a key regulator of glucagon gene expression.
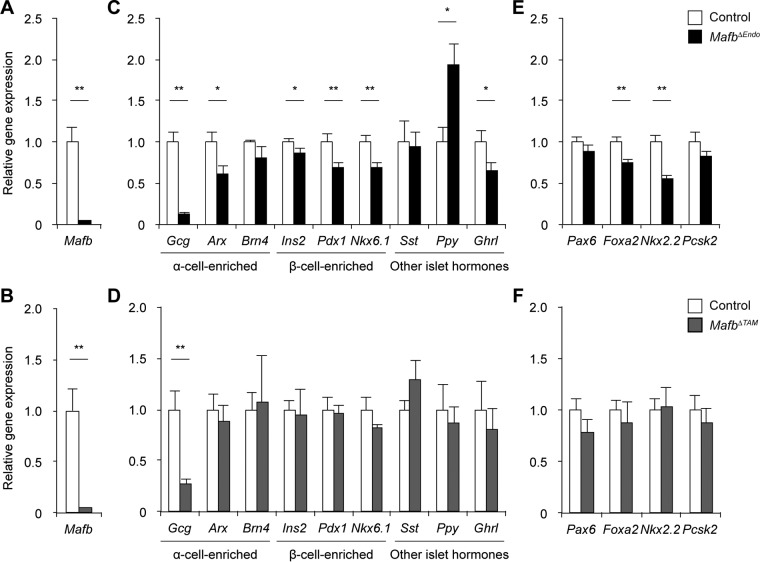
To explore MafB-regulated genes involved in α- and β-cell differentiation, as well as hormone production and secretion, we performed real-time quantitative PCR (qPCR) on pancreatic islet cDNA. Islets were isolated from 10-week-old MafbΔEndo mice and from TAM-injected MafbΔTAM mice at 3 weeks postinjection. The mRNA expression analysis revealed the successful deletion of Mafb in both mutant models (Fig. 6A and B) and the consequent decrease in glucagon gene (Gcg) expression (Fig. 6C and D). MafbΔEndo islets exhibited a marked decrease in Arx transcription that was equivalent to the reduced number of Arx+ cells observed via immunohistochemistry (Fig. 2E), while a moderate decrease in the expression of Brn4, another gene that is highly enriched in α cells (α-cell-enriched gene) (13), was also noted (Fig. 6C). In addition, the expression of β-cell-enriched genes (i.e., Ins2, Pdx1, and Nkx6.1) (13) was significantly lower in MafbΔEndo islets (Fig. 6C), which might explain the partial recovery of insulin production indicated by the slight reduction in the Ins+ cell population and insulin content (Fig. 1B and D). Thus, these results support our conclusion that Mafb deficiency during embryogenesis suppresses α-cell development and glucagon expression but delays β-cell terminal differentiation. Moreover, the increase in the PP+ cell population in MafbΔEndo mice was further supported by an upregulated Ppy mRNA level in the islets (Fig. 6C). Interestingly, MafbΔEndo islets also showed decreased expression of the ghrelin gene (Ghrl) (Fig. 6C). Given that some ghrelin+ cells are reported to express MafB in wild-type embryos and the neonatal mouse pancreas (10, 17), Mafb depletion at the embryonic stage may have affected ghrelin+ cell subpopulation development. Conversely, MafbΔTAM islets did not display any differences in the expression of α- and β-cell-enriched genes (i.e., Arx, Brn4, Ins2, Pdx1, and Nkx6.1) or any other islet hormone genes (i.e., Sst, Ppy, and Ghrl) (Fig. 6D) (13), consistent with the immunostaining results (Fig. 3B, 4B and E, and 5F). These data confirm our finding that Mafb ablation in adult mice impairs glucagon expression in α cells but does not affect other islet cells.
FIG 6.
MafB is a key regulator of glucagon gene expression. Gene expression comparisons were performed by qPCR in pancreatic islets isolated from 10-week-old MafbΔEndo (A, C, and E) and MafbΔTAM (B, D, and F) mice (n ≥ 5) 3 weeks post-TAM injection. TAM was injected at 5 weeks of age. The Mafb gene (A and B), islet hormone and cell identity genes (C and D), and glucagon production/α-cell differentiation regulatory genes (E and F) were analyzed. The primers are listed in Table 1. *, P < 0.05; **, P < 0.01. Means and SEM are shown.
In light of previous work showing the complete recovery of glucagon expression upon Mafb deletion (10), we wanted to address a potential compensatory mechanism of glucagon production in the absence of Mafb. To this end, we further inspected gene expression levels of other transcription factors known to regulate glucagon and/or α-cell differentiation, including Pax6 (18), Foxa2 (19, 20), Nkx2.2 (21), and the peptide-processing gene Pcsk2 (22). Two of four glucagon/α-cell regulatory genes (i.e., Foxa2 and Nkx2.2) were significantly reduced in MafbΔEndo islets, whereas the other two genes (i.e., Pax6 and Pcsk2) exhibited a trend toward reduced expression compared with controls (Fig. 6E). This finding suggests that Mafb deletion in developing endocrine cells leads to the downregulation of other glucagon/α-cell regulators, which in turn exacerbates glucagon reduction. Nevertheless, we cannot exclude the possibility of an effect of β cells, as these factors are also highly conserved in β cells (13) and because MafbΔEndo islets showed a decreased trend of β-cell-enriched gene expression (Fig. 6C). However, in MafbΔTAM islets, none of the glucagon/α-cell regulator genes were affected (Fig. 6F), suggesting that MafB in adult mice is the major controller of α-cell functional maintenance independently of other glucagon/α-cell regulators, such as Pax6, Foxa2, Nkx2.2, and Pcsk2. If these unaffected genes represented α-cell populations, then this could explain the residual glucagon production in Mafb-deficient (MafbΔEndo and MafbΔTAM) animals (Fig. 1E and 3E).
Mafb deletion disrupts glucagon secretion in response to α-cell stimuli.
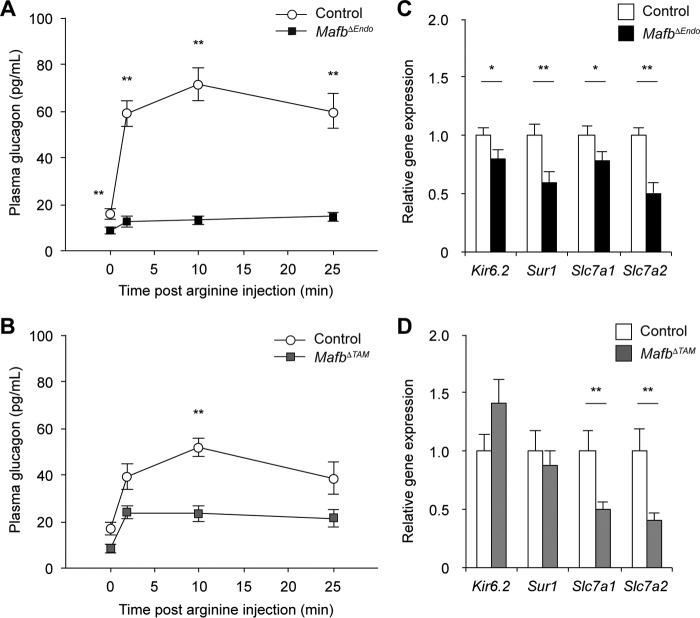
To address the functional competence of Mafb-deficient α cells, we analyzed glucagon secretion in response to amino acid stimulation (23) in both groups of mutant mice. Eight-week-old MafbΔEndo and MafbΔTAM male mice at 3 weeks post-TAM treatment were injected with 1 mg/g l-arginine intraperitoneally after overnight fasting (Fig. 7A and B). In the basal state, plasma glucagon levels in both mutant groups were significantly lower than in control groups (Fig. 7A and B) (control versus MafbΔEndo, 15.7 ± 2.1 versus 8.6 ± 1.3 pg/ml; control versus MafbΔTAM, 17.0 ± 2.8 versus 8.3 ± 1.8 pg/ml). These results are presumed to reflect the constantly observed reduced glucagon production phenotype and suggest the importance of MafB in maintaining the basal glucagon production/secretion. After arginine stimulation, a rapid increase in plasma glucagon levels was observed within 2 min and peaked at 10 min after arginine administration in all the control groups (Fig. 7A and B). However, both groups of Mafb-deficient mice failed to secrete glucagon to the control level, although MafbΔTAM mice exhibited slightly higher levels (Fig. 7A and B). Moreover, plasma glucagon peaked at 2 min after arginine injection in both mutants and remained unaltered (Fig. 7A and B), suggesting possible defects in the cellular machinery that regulates glucagon secretion. To test whether arginine uptake via cationic amino acid transporters and/or the glucagon-secreting machinery was affected, real-time qPCR was performed on isolated pancreatic islets from 10-week-old MafbΔEndo and MafbΔTAM mice 3 weeks post-TAM injection (Fig. 7C and D). Since β cells were persistently affected by Mafb ablation in MafbΔEndo mice, both cationic amino acid transporter genes (i.e., Slc7a1 and Slc7a2) and ATP-sensitive potassium (KATP) channel subunit genes (i.e., Kir6.2 and Sur1) involved in glucagon secretion (23) were downregulated (Fig. 7C). However, MafbΔTAM islets exhibited significant reductions in Slc7a1 and Slc7a2 but not Kir6.2 and Sur1 (Fig. 7D). Therefore, impaired arginine-stimulated glucagon secretion likely reflects reduced cationic amino acid transporter gene expression in both mutants and can be partially attributed to reduced KATP channel subunits in MafbΔEndo mice. Overall, these data suggest that Mafb ablation leads to insufficient glucagon secretion under basal and stimulated conditions, supporting the role of MafB in α-cell development and function.
FIG 7.
Mafb deletion impairs glucagon secretion upon α-cell stimulation. (A and B) Glucagon secretion was measured after intraperitoneal injection of l-arginine (1 mg/g) in 8-week-old MafbΔEndo (n ≥ 8) (A) and MafbΔTAM (B) male mice (n ≥ 4) 3 weeks post-TAM administration. TAM was injected at 5 weeks of age. **, P < 0.01. (C and D) qPCR analysis of glucagon secretion machinery gene expression was performed on pancreatic islets isolated from 10-week-old MafbΔEndo (C) and MafbΔTAM (D) mice (n ≥ 5) 3 weeks post-TAM injection. *, P < 0.05; **, P < 0.01. Means and SEM are shown.
DISCUSSION
In the present study, we demonstrate that MafB regulates glucagon production and secretion in postnatal α cells in vivo by using two different mouse models: endocrine cell-specific (MafbΔEndo) and TAM-dependent (MafbΔTAM) Mafb knockout mice. Both MafbΔEndo and MafbΔTAM mice exhibited decreased populations of Glu+ α cells (Fig. 1C and 3C) and, consequently, reduced glucagon production (Fig. 1E and 3E) and secretion (Fig. 7A and B). Although α-cell fate was not changed, embryonic Mafb deletion further reduced the Arx+ cell population (Fig. 2E) and increased the PP+ cell population to compensate for decreased α-cell differentiation (Fig. 5B). Moreover, both groups of mutant mice failed to respond to arginine (Fig. 7A and B), potentially due to the compromised expression of cationic amino acid transporter genes involved in arginine uptake (Fig. 7C and D). Therefore, our findings clearly demonstrate the contribution of MafB to α-cell development at the neonatal stage and to the maintenance of α-cell function during adulthood in vivo.
Consistent with previous reports (10, 11), Mafb ablation only delayed β-cell development, given that MafB is not expressed in postnatal β cells (8, 13). However, our observations in α cells consistently disagreed with the prior work of Conrad et al. (10). Pancreas-specific Mafb conditional-knockout (Mafbf/f::Pdx1-Cre MafbΔpanc) mice recover Glu+ α cells by 2 weeks of age and islet glucagon content by 8 weeks of age (10). This discrepancy may reflect the use of mice with different genetic backgrounds in the present study, whereas the difference in the Cre drivers used is unlikely to have contributed, because we also generated a Mafb conditional-knockout mouse with Pdx1-Cre (24), which exhibited the same phenotype as MafbΔEndo mice (data not shown). Indeed, the Mafbf/f mice used in the present study (25, 26) and by Conrad et al. (10, 27) were generated in mouse embryonic stem (ES) cells from different genetic backgrounds, e.g., C57BL/6J and 129S4/SvJae, which may explain the phenotypic variations.
Nevertheless, the recovery of α-cell numbers and glucagon content in MafbΔpanc mice (10) led us to explore the secondary effects on glucagon production in the absence of Mafb, which indeed was constantly observed in both groups of mutant mice (Fig. 2G and 4G). Various transcription factors have been reported to regulate glucagon expression in α cells, including MafB (9), c-Maf (28), Pax6 (18), Foxa1 and Foxa2 (19, 20), NeuroD1 (29), Isl1 (30), and Brn4 (31). However, MafB and Brn4 are the only two factors known to be α cell specific, whereas the genes encoding the other factors are expressed in both α and β cells (13). Interestingly, a previous study in Brn4-null mice observed no significant impact on glucagon gene expression, synthesis, and secretion, suggesting that Brn4 is dispensable for glucagon regulation (32). Conversely, the relationship between MafB and glucagon expression has been clearly addressed (33, 34). For example, either overexpression of Arx in pancreatic progenitor cells or deletion of Pdx1 in β cells leads to an increase in the α-cell population, which is frequently associated with MafB and glucagon expression (33, 34). This finding raises the possibility that MafB dominates the regulation of α-cell activity, including glucagon production and secretion, although a minimal level of glucagon is maintained through a MafB-independent pathway. In the MafbΔTAM islet gene analysis, Pax6, Foxa2, Nkx2.2, and Pcsk2 mRNA levels remained unchanged, with no indication of rescuing depleted glucagon gene expression (Fig. 6F). Thus, Mafb ablation is exclusively responsible for glucagon reduction, supporting our hypothesis that MafB is the principal transcriptional activator of the glucagon gene in α cells.
Interestingly, the population of PP+ cells was increased at the cost of decreasing the population of α cells. At early pancreas morphogenesis, α/F precursor cells (Arx+) preferentially differentiate toward the α-cell lineage, while a small number of cells are designated for a F-cell fate (15, 33). However, in MafbΔEndo mice, a significant population of Arx+ Glu− cells are differentiated into PP-producing cells at 3 weeks of age, and their fate is maintained throughout the developmental stage until 8 weeks of age (Fig. 5A to D). These results suggest that α/F precursor cells might have been favored to promote F-cell lineage differentiation in the absence of MafB, indicating a potential role of MafB in the early α-cell fate decision during islet development by inhibiting F-cell differentiation (Fig. 5G).
Although α-cell dysfunction is specifically addressed in the work of Conrad et al., the specific glucagon-secretory machinery affected by Mafb ablation was not identified (10). In both of our groups of mutant mice, cationic amino acid transporter genes (i.e., Slc7a1 and Slc7a2) were significantly decreased (Fig. 7C and D), suggesting that impaired arginine-stimulated glucagon secretion is likely caused by reduced arginine transporter gene expression. Based on the text-mining application from SABiosciences and the UCSC Genome Browser, Foxa2 is predicted to target these transporter genes. However, Foxa2 expression was not reduced in our qPCR analysis results (Fig. 6F), indicating that the reduction in Slc7a1 and Slc7a2 expression is independent of Foxa2 and suggesting an alternative mechanism for the regulation of cationic amino acid transporter gene expression.
Understanding α-cell functional activity will not only increase our knowledge of islet physiology but will also have translational implications, given that the regulation of glucagon secretion and action has been shown to ameliorate diabetes symptoms (35, 36). Extremely high plasma glucagon concentrations are observed in insulin-deficient states, such as type 1 diabetes, advanced type 2 diabetes, and diabetic ketoacidosis (2, 37). Therefore, inhibiting glucagon signaling may potentially reduce diabetic hyperglycemia, as demonstrated in animal studies. For example, mice with either a disrupted glucagon receptor (38, 39) or defective glucagon synthesis (40, 41) tend to display reduced fasting blood glucose levels and improved glucose tolerance compared with control mice, even without treatment. Furthermore, glucagon receptor knockout mice are resistant to diabetes upon streptozotocin (STZ)-induced β-cell destruction (42). In addition, recent work using a peptide-based glucagon receptor antagonist reported improved metabolic control of genetically or directly induced obesity-related diabetes in mice (43). Therefore, our discovery may potentiate glucagon regulation in diabetes through Mafb gene regulation.
Unexpectedly, MafbΔEndo β cells exhibited residual effects, which were supposedly caused by delayed β-cell terminal differentiation, with β-cell populations, insulin content, and β-cell transcript levels all minimally but consistently reduced (Fig. 1B and D and 6C). These results potentially highlight the supporting role of MafB in postnatal β-cell development. A recent study by van der Meulen et al. introduced the novel concept of a “virgin β-cell” subpopulation that contains functionally immature β cells characterized as Ins+ MafB+ Ucn− (44). This minor virgin β-cell subpopulation can transdifferentiate into functional β cells and is found in both mouse and human islets (44). Therefore, the authors concluded that MafB-expressing virgin β cells may be the source for β-cell regeneration through transdifferentiation in adult islets (44). Moreover, another study by Cheng et al. demonstrated β-cell regeneration from a novel mesenchymal cell population after nearly total ablation of preexisting β cells by a high dose of STZ in adult rodents (45). Interestingly, these newly formed β cells expressed MafB and vimentin but not Pdx1 and Nkx6.1 and were thus immature. However, the cells gradually acquired functional maturity and became bona fide β cells (45). These findings suggest that β-cell regeneration is preceded by MafB expression in β cells, which is lost upon functional maturity. Thus, the role of MafB in facilitating β-cell development cannot be neglected.
In contrast to mice, MAFB expression in human β cells is retained postnatally (13, 46), thereby implicating human MAFB in β-cell maintenance and/or activity. Notably, severe reductions in MAFB levels are observed in human type 2 diabetic islets, suggesting that β-cell failure is associated with low MAFB expression (47). In addition, in vitro work using a human β-cell line (EndoC-βH1) also demonstrated that MAFB is required for glucose-stimulated insulin secretion in human β cells, indicating that mature human β cells require MAFB for their functional activity (48). However, clinical reports of patients carrying MAFB mutations did not include their blood glucose levels or HbA1c status (49–51), creating the impression that these patients are normoglycemic. Further investigation will be required to clarify the role of MAFB in human β cells.
In conclusion, our findings indicate that MafB is critical for glucagon production in α cells to maintain development and function. Therefore, this study yields insights into islet physiology in glucagon regulation through MafB in α cells, thereby providing a platform for understanding glucagon control under pathophysiologic conditions.
MATERIALS AND METHODS
Animals.
Mice were maintained under specific-pathogen-free conditions in the Laboratory Animal Resource Center at the University of Tsukuba, Ibaraki, Japan. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Tsukuba. Endocrine cell-specific and TAM-dependent Mafb knockout mice were generated by crossing Mafbf/f mice (25, 26) with either Ngn3-Cre (52) or CAGG-CreER (stock number 004682; The Jackson Laboratory) (53) transgenic mice. Mafbf/f mice were generated in a C57BL/6J strain background, as described previously (25, 26). The Ngn3-Cre transgenic mouse strain was kindly provided by Shosei Yoshida (Division of Germ Cell Biology, National Institute for Basic Biology, Japan). In this study, Mafbf/f::Ngn3-Cre and Mafbf/f::CAGG-CreER mice are referred to as MafbΔEndo and MafbΔTAM mice, respectively. Mafbf/f littermates were used as controls. To activate the Cre recombination system in the CAGG-CreER strain, 5-week-old MafbΔTAM mice and their control groups were injected intraperitoneally with 75 mg of TAM/kg for 5 consecutive days (54). TAM (Sigma) was first dissolved in ethanol and then mixed with corn oil as described previously (55).
Immunohistochemistry.
Pancreatic tissues were fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C, processed, and embedded in paraffin. Then, 2-μm sections were sliced and prepared according to standard methods. For nuclear protein staining, sections were soaked in 0.3% Triton X-100–PBS solution, followed by heat-induced epitope retrieval using target retrieval solution (Dako) and a pressure cooker. All the sections were blocked in appropriate sera for 1 h at room temperature and incubated overnight at 4°C with the following primary antibodies: guinea pig anti-insulin (1:500; ab7842; Abcam), rabbit antiglucagon (1:2,000; 2760; Cell Signaling), guinea pig anti-glucagon (1:1,000; M182; TaKaRa), rabbit anti-Arx (1:250; a generous gift from Kunio Kitamura and Kenichirou Morohashi, Kyushu University, Japan) (56), mouse anti-Nkx6.1 (1:250; F55A10; Developmental Studies Hybridoma Bank), rabbit anti-MafB (1:100; IHC-00351; Bethyl), rabbit anti-pancreatic polypeptide (1:1,000; ab113694; Abcam), goat anti-pancreatic polypeptide (1:50; ab77192; Abcam), and rabbit antisomatostatin (1:50; 18-007; Zymed). Antigens were visualized using appropriate secondary antibodies conjugated to Alexa Fluor 488 or 594 (1:1,000; Life Technologies), and nuclei were labeled with Hoechst 33342 (Molecular Probes). Tissue specimens were mounted with Fluoromount (Diagnostic BioSystems). All images were acquired on a fluorescence microscope (Biorevo BZ-9000; Keyence).
Cell counting.
Following immunofluorescence staining, the different cell types in each islet were manually counted in the islet microscopy images. For each cell type, 20 to 40 representative islets from 3 to 6 mice per group were counted. To calculate the fraction of hormone+ cells within islets, the number of hormone+ cells per islet was manually determined using ImageJ software and divided by the total number of Hoechst+ nuclei from the same islet (percent hormone+ cells/islet Hoechst+ nuclei) and then normalized to the control group. The fractions of Nkx6.1+ Ins+ and Arx+ Glu+ cells were determined by dividing the number of double-positive cells per islet by the total numbers of Nkx6.1+ (Nkx6.1+ Ins− and Nkx6.1+ Ins+) and Arx+ (Arx+ Glu− and Arx+ Glu+) cells, respectively. Total Nkx6.1+ and Arx+ cell counts were normalized to their corresponding controls.
Measurement of pancreatic insulin and glucagon contents.
Whole pancreata were collected from 3- and 8-week-old MafbΔEndo and MafbΔTAM mice 3 weeks post-TAM injection and homogenized in ice-cold acetic-ethanol buffer (1.5% HCl in 75% ethanol) as previously described (57). Total insulin and glucagon contents in pancreatic tissue extracts were measured via enzyme-linked immunosorbent assay (ELISA) (Morinaga Mouse Insulin ELISA kit, M1102; Mercodia Glucagon 10 μl ELISA kit, 10-1281-01) according to the manufacturer's instructions. Each pancreatic content was normalized by the total protein concentration per sample, which was determined with Bradford reagent (Thermo Fisher Scientific).
Isolation of pancreatic islets.
Pancreatic islets were isolated from 10-week-old MafbΔEndo and MafbΔTAM mice 3 weeks post-TAM injection as described previously, with slight modifications (58). Briefly, after clamping the common bile duct at the intestine, the pancreas was inflated with 1 mg/ml collagenase type V (Wako) diluted in Krebs-Ringer bicarbonate (KRBH) buffer (129.4 mM NaCl, 5.2 mM KCl, 1.3 mM KH2PO4, 1.3 mM MgSO4, 2.7 mM CaCl2, 24.8 mM NaHCO3, 10 mM HEPES, pH 7.4). The distended pancreas was transferred into a collecting tube with additional fresh collagenase solution and then incubated at 37°C for 20 min with gentle shaking. After the second wash with ice-cold KRBH buffer containing 0.5% bovine serum albumin (BSA), islets were hand picked under a stereomicroscope.
qPCR.
Total RNA was extracted in Isogen (Nippon Gene) from pancreatic islets isolated from 10-week-old MafbΔEndo and MafbΔTAM mice 3 weeks post-TAM injection. cDNA was synthesized according to the protocol of the QuantiTect reverse transcription kit (Qiagen). Real-time qPCRs were performed in duplicate on a Thermal Cycler Dice real-time system (TaKaRa) with SYBR green PCR master mix (TaKaRa). The expression of all target genes was normalized to Hprt. The primers used in this study are listed in Table 1.
TABLE 1.
Primer sequences for real-time qPCR
| Gene | Primer sequence (5′→3′) |
|
|---|---|---|
| Forward | Reverse | |
| Hprt | TTGTTGTTGGATATGCCCTTGACTA | AGGCAGATGGCCACAGGACTA |
| Mafb | TGAATTTGCTGGCACTGCTG | AAGCACCATGCGGTTCATACA |
| Gcg | AGGGACCTTTACCAGTGATGT | AATGGCGACTTCTTCTGGGAA |
| Arx | TCCGGATACCCCACTTAGCTT | GACGCCCCTTTCCTTTAAGTG |
| Brn4 | CTCGCCGCACACTAACCAT | GCTCCAGCATACCGCTCAC |
| Ins2 | GCTTCTTCTACACACCCATGTC | AGCACTGATCTACAATGCCAC |
| Pdx1 | TTCCCGAATGGAACCGAGC | GTAGGCAGTACGGGTCCTCT |
| Nkx6.1 | CAGACCCACGTTCTCTGGAC | TGACCTGACTCTCCGTCATCC |
| Sst | GAGCCCAACCAGACAGAGAA | GAAGTTCTTGCAGCCAGCTT |
| Ppy | TACTGCTGCCTCTCCCTGTT | CCAGGAAGTCCACCTGTGTT |
| Ghrl | GAAGCCACCAGCTAAACTGC | GCCTGTCCGTGGTTACTTGT |
| Pax6 | ATATGTCGACAGCTCCAGCATGCAGAAC | TGCCCAGAATTTTACTCACACAA |
| Foxa2 | GAGCACCATTACGCCTTCAAC | AGGCCTTGAGGTCCATTTTGT |
| Nkx2.2 | ATGTCGCTGACCAACACAAA | TCACCGGACAATGACAAGGA |
| Pcsk2 | AATGACCCCTACCCATACCC | GAGGAGGCTTCGATGATGTC |
| Kir6.2 | GTAGGGGACCTCCGAAAGAG | TGGAGTCGATGACGTGGTAG |
| Sur1 | CTGGTCCTCAGCAGCACAT | GGAACTCTTGGGACGAGACA |
| Slc7a1 | ATCGGTACTTCAAGCGTGGC | CCATGGCTGACTCCTTCACG |
| Slc7a2 | ATGGCTTTACAGGGACGTTG | GCGTTAAAGCTGCAGAAACC |
Arginine-stimulated glucagon secretion.
Eight-week-old MafbΔEndo MafbΔTAM male mice 3 weeks post-TAM injection and the corresponding controls were fasted for 16 h overnight. On the following morning, 1 mg/g of l-arginine (Sigma) was injected intraperitoneally, and venous blood was collected at 0, 2, 10, and 25 min postinjection in heparinized tubes (Drummond Scientific Company). Plasma glucagon levels were determined using the Mercodia Glucagon 10-μl ELISA kit (10-1281-01).
Statistical analysis.
All data are presented as means and standard errors of the mean (SEM). For statistical significance between MafB mutants and controls, a minimum of three biological replicates were analyzed using Welch's t test, and a P value of <0.05 was considered significant. To compare longitudinal data from the arginine-stimulated glucagon secretion test, P values were calculated using Welch's t test, followed by Holm's correction for multiple comparisons.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Yoshida and K. Kitamura, as well as K. Morohashi, for their generous gifts of Ngn3-Cre transgenic mice and rabbit anti-Arx antibody, respectively. We also thank M. Ojima for technical assistance; A. Tagami, R. Fujii, S. Fuseya, and R. Suzuki for helping with the cell count analysis; and T. Kunath and R. Koshida for proofreading the manuscript.
This work was supported by JSPS Kakenhi grants JP26221004 and 16H06276.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00504-17.
REFERENCES
- 1.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. 1970. Studies of pancreatic α cell function in normal and diabetic subjects. J Clin Invest 49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunning BE, Gerich JE. 2007. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 3.Menge BA, Grüber L, Jørgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. 2011. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 60:2160–2168. doi: 10.2337/db11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez CM, Goodyer WR, Kim SK. 2012. Deconstructing pancreas developmental biology. Cold Spring Harb Perspect Biol 4:a012401. doi: 10.1101/cshperspect.a012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Meulen T, Huising MO. 2015. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J Mol Endocrinol 54:R103–R117. doi: 10.1530/JME-14-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. 2005. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. 2006. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol 293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. 2010. MafA and MafB regulate genes critical to β-cells in a unique temporal manner. Diabetes 59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. 2006. MafB: an activator of the glucagon gene expressed in developing islet α- and β-cells. Diabetes 55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- 10.Conrad E, Dai C, Spaeth J, Guo M, Cyphert HA, Scoville D, Carroll J, Yu W-M, Goodrich LV, Harlan DM, Grove KL, Roberts CT, Powers AC, Gu G, Stein R. 2016. The MAFB transcription factor impacts islet α-cell function in rodents and represents a unique signature of primate islet β-cells. Am J Physiol Endocrinol Metab 310:E91–E102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. 2007. MafB is required for islet β cell maturation. Proc Natl Acad Sci U S A 104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchi B, Kelly LM, Viemari J-C, Lafon I, Burnet H, Bévengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH. 2003. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci 6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 13.Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. 2014. The transcriptional landscape of mouse β cells compared to human β cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. 2000. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of β-cell formation in the pancreas. Development 127:5533–5540. [DOI] [PubMed] [Google Scholar]
- 15.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. 2003. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YH, Sun MJ, Jiang M, Fu BY. 2009. Immunohistochemical localization of glucagon and pancreatic polypeptide on rat endocrine pancreas: coexistence in rat islet cells. Eur J Histochem 53:81–85. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura W, Rowan S, Salameh T, Maas RL, Bonner-Weir S, Sell SM, Sharma A. 2008. Preferential reduction of β cells derived from Pax6-MafB pathway in MafB deficient mice. Dev Biol 314:443–456. doi: 10.1016/j.ydbio.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. 1997. Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature 387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaestner KH, Katz J, Liu Y, Drucker DJ, Schütz G. 1999. Inactivation of the winged helix transcription factor HNF3α affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev 13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier BR, Schwitzgebel VM, Zaiko M, Mamin A, Ritz-Laser B, Philippe J. 2002. Hepatic nuclear factor-3 (HNF-3 or Foxa2) regulates glucagon gene transcription by binding to the G1 and G2 promoter elements. Mol Endocrinol 16:170–183. doi: 10.1210/mend.16.1.0752. [DOI] [PubMed] [Google Scholar]
- 21.Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. 1998. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic β cells. Development 125:2213–2221. [DOI] [PubMed] [Google Scholar]
- 22.Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, Steiner DF. 2001. Severe defect in proglucagon processing in islet α-cells of prohormone convertase 2 null mice. J Biol Chem 276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 23.Quesada I, Tudurí E, Ripoll C, Nadal A. 2008. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 24.Gu G, Dubauskaite J, Melton DA. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457. [DOI] [PubMed] [Google Scholar]
- 25.Tran MTN, Hamada M, Nakamura M, Jeon H, Kamei R, Tsunakawa Y, Kulathunga K, Lin Y-Y, Fujisawa K, Kudo T, Takahashi S. 2016. MafB deficiency accelerates the development of obesity in mice. FEBS Open Bio 6:540–547. doi: 10.1002/2211-5463.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shichita T, Ito M, Morita R, Komai K, Noguchi Y, Ooboshi H, Koshida R, Takahashi S, Kodama T, Yoshimura A. 2017. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med 23:723–732. doi: 10.1038/nm.4312. [DOI] [PubMed] [Google Scholar]
- 27.Yu W-M, Appler JM, Kim Y-H, Nishitani AM, Holt JR, Goodrich LV. 2013. A Gata3-Mafb transcriptional network directs post-synaptic differentiation in synapses specialized for hearing. Elife 2:e01341. doi: 10.7554/eLife.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosmain Y, Avril I, Mamin A, Philippe J. 2007. Pax-6 and c-Maf functionally interact with the α-cell-specific DNA element G1 in vivo to promote glucagon gene expression. J Biol Chem 282:35024–35034. doi: 10.1074/jbc.M702795200. [DOI] [PubMed] [Google Scholar]
- 29.Dumonteil E, Laser B, Constant I, Philippe J. 1998. Differential regulation of the glucagon and insulin I gene promoters by the basic helix-loop-helix transcription factors E47 and BETA2. J Biol Chem 273:19945–19954. doi: 10.1074/jbc.273.32.19945. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Drucker DJ. 1995. The LIM domain homeobox gene isl-1 is a positive regulator of islet cell-specific proglucagon gene transcription. J Biol Chem 270:12646–12652. doi: 10.1074/jbc.270.21.12646. [DOI] [PubMed] [Google Scholar]
- 31.Hussain MA, Lee J, Miller CP, Habener JF. 1997. POU domain transcription factor brain 4 confers pancreatic α-cell-specific expression of the proglucagon gene through interaction with a novel proximal promoter G1 element. Mol Cell Biol 17:7186–7194. doi: 10.1128/MCB.17.12.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain MA, Miller CP, Habener JF. 2002. Brn-4 transcription factor expression targeted to the early developing mouse pancreas induces ectopic glucagon gene expression in insulin-producing β cells. J Biol Chem 277:16028–16032. doi: 10.1074/jbc.M107124200. [DOI] [PubMed] [Google Scholar]
- 33.Collombat P, Hecksher-Sørensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. 2007. Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression. J Clin Invest 117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, Stoffers DA, Edlund H, Matschinsky F, Stein R, Stanger BZ. 2014. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab 19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger RH, Cherrington AD, Wollheim C, Wang Z, Unger R, Friedman J. 2012. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandoval DA, D'Alessio DA. 2015. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 95:513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 37.Müller WA, Faloona GR, Unger RH, Faloona G, Unger R, Derot M. 1973. Hyperglucagonemia in diabetic ketoacidosis. Am J Med 54:52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 38.Parker JC, Andrews KM, Allen MR, Stock JL, McNeish JD. 2002. Glycemic control in mice with targeted disruption of the glucagon receptor gene. Biochem Biophys Res Commun 290:839–843. doi: 10.1006/bbrc.2001.6265. [DOI] [PubMed] [Google Scholar]
- 39.Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hancock AS, Du A, Liu J, Miller M, May CL. 2010. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol 24:1605–1614. doi: 10.1210/me.2010-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi Y. 2011. Metabolic impact of glucagon deficiency. Diabetes Obes Metab 13:151–157. doi: 10.1111/j.1463-1326.2011.01456.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. 2011. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Harte FPM, Franklin ZJ, Irwin N. 2014. Two novel glucagon receptor antagonists prove effective therapeutic agents in high-fat-fed and obese diabetic mice. Diabetes Obes Metab 16:1214–1222. doi: 10.1111/dom.12360. [DOI] [PubMed] [Google Scholar]
- 44.van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dólleman S, Liu S, Ackermann AM, Cáceres E, Hunter AE, Kaestner KH, Donaldson CJ, Huising MO. 2017. Virgin β cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 25:911–926.e6. doi: 10.1016/j.cmet.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng Y, Kang H, Shen J, Hao H, Liu J, Guo Y, Mu Y, Han W. 2015. β-Cell regeneration from vimentin+/MafB+ cells after STZ-induced extreme β-cell ablation. Sci Rep 5:11703. doi: 10.1038/srep11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. 2012. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. 2013. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scoville DW, Cyphert HA, Liao L, Xu J, Reynolds A, Guo S, Stein R. 2015. MLL3 and MLL4, ethyltransferases bind to the MAFA and MAFB transcription factors to regulate islet β-cell function. Diabetes 64:3772–3783. doi: 10.2337/db15-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dworschak GC, Draaken M, Hilger A, Born M, Reutter H, Ludwig M. 2013. An incompletely penetrant novel MAFB (p.Ser56Phe) variant in autosomal dominant multicentric carpotarsal osteolysis syndrome. Int J Mol Med 32:174–178. doi: 10.3892/ijmm.2013.1373. [DOI] [PubMed] [Google Scholar]
- 50.Mehawej C, Courcet J-B, Baujat G, Mouy R, Gérard M, Landru I, Gosselin M, Koehrer P, Mousson C, Breton S, Quartier P, Le Merrer M, Faivre L, Cormier-Daire V. 2013. The identification of MAFB mutations in eight patients with multicentric carpo-tarsal osteolysis supports genetic homogeneity but clinical variability. Am J Med Genet A 161:3023–3029. doi: 10.1002/ajmg.a.36151. [DOI] [PubMed] [Google Scholar]
- 51.Park JG, Tischfield MA, Nugent AA, Cheng L, Di Gioia SA, Chan W-M, Maconachie G, Bosley TM, Summers CG, Hunter DG, Robson CD, Gottlob I, Engle EC. 2016. Loss of MAFB function in humans and mice causes Duane syndrome, aberrant extraocular muscle innervation, and inner-ear defects. Am J Hum Genet 98:1220–1227. doi: 10.1016/j.ajhg.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima YI. 2004. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol 269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi S, McMahon AP. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 54.Nagao M, Cheong CW, Olsen BR. 2016. Col2-Cre and tamoxifen-inducible Col2-CreER target different cell populations in the knee joint. Osteoarthr Cartil 24:188–191. doi: 10.1016/j.joca.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metzger D, Chambon P. 2001. Site- and time-specific gene targeting in the mouse. Methods 24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 56.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K. 2002. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet 32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- 57.im Walde SS, Dohle C, Schott-Ohly P, Gleichmann H. 2002. Molecular target structures in alloxan-induced diabetes in mice. Life Sci 71:1681–1694. doi: 10.1016/S0024-3205(02)01918-5. [DOI] [PubMed] [Google Scholar]
- 58.Lacy PE, Kostianovsky M. 1967. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.