The 20th Century witnessed the Industrial Revolution which resulted in social, economic, and health improvements around the world. Unfortunately, these advancements also put Earth’s fresh water supply in peril. The resulting widespread contamination of water with toxic chemicals and heavy metals such as lead and mercury is startling. In developing nations, where environmental protection legislation is minimal, the rapid expansion of manufacturing and mining has pervaded water sources with contaminants endangering billions of people. It is critical to note that developed nations are just as vulnerable; the water crisis in Flint, Michigan, was caused by municipal water pipes leaching lead into drinking water. Most alarmingly, the World Health Organization (WHO) estimates that 2 billion people are without a safely managed drinking-water service.1 Prompted by this critical need to mitigate heavy metal contamination and protect fresh water supplies, Sun et al. designed a metal–organic framework (MOF)/polymer composite, Fe-BTC/PDA, for Hg2+ and Pb2+ capture, which is reported in this issue of ACS Central Science.2
Water remediation research efforts have primarily centered on developing new materials for toxin capture, including, but not limited to, membrane filters, ion exchangers, and sorbents. Adsorption-based materials have received the most attention to date because their chemical functionality can be judiciously designed to selectively capture a target contaminant.3 To be considered a viable purification strategy, a method must be efficient, selective, reusable, and scalable. Most often, a material excels in one of these measures at the expense of other performance standards. The distinguishing feature of this work is that Fe-BTC/PDA excels by all measures without sacrificing performance in any category.
MOFs are a young class of porous crystalline materials with great potential in water remediation applications.4 These materials consist of inorganic nodes and organic linkers which assemble via coordination bonds into multidimensional lattices.5 They have recorded the highest internal surface areas to date, up to 7000 m2/g − the area of a football field contained within the mass of a raisin.6 Additionally, MOF chemical functionality can be easily tuned via several routes of postsynthetic modification to incorporate additional organic functional groups, install catalysts at the node, or occupy the pores with chemical species.7 These materials have already been applied in gas storage and separation, catalysis, chemical sensing, and for heavy metal remediation.8
The MOF/polymer composite, Fe-BTC/PDA, reported by Sun et al. possesses exceptional affinity for Hg2+ and Pb2+ and excels in each of the performance measures, an incredibly rare feat (Figure 1). Cognizant that certain chemical moieties have a high affinity for heavy metal ions, namely, catechols (benzene rings with adjacent hydroxyl groups (−OH)) and amines (−NR3), the authors elected to use polydopamine (PDA), a biocompatible polymer whose backbone is decorated with both catechols and amines.9 In exploiting the porosity and crystallinity of a MOF template, the authors confined short PDA chains within the MOF pores to introduce extrinsic surface area to the traditionally nonporous polymer. Ingeniously chosen, the MOF template, Fe-BTC (also known as MIL-100), contains polymerization-assisting redox active Fe3+ metal sites.10 A range of polymer loadings were achieved, and the composites recorded maximum Hg2+ and Pb2+ uptake capacities of 1634 mg/g and 394 mg/g, respectively.
Figure 1.
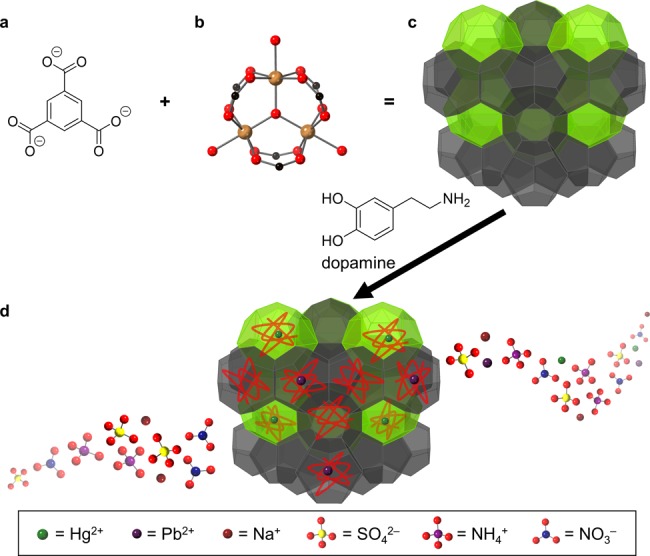
Structure and performance of Fe-BTC/PDA. (a) BTC linker. (b) Fe3+ trimer node. (c) Cage structure of Fe-BTC MOF. (d) Removal of heavy metal contaminants by Fe-BTC/PDA composite.
The composite with 19 mass % PDA, Fe-BTC/PDA-19, was carefully characterized to determine the material’s crystallinity, the extent of polymerization, and the distribution of polymer throughout the framework. Beyond a remarkable uptake capacity for Hg2+ or Pb2+, the composite also exhibits long-term stability when soaked in river and seawater samples for two months. When exposed to river and seawater samples spiked with 1000 ppb of heavy metal analyte, the composite reduced the Pb2+ concentration to 2.2 ppb, well within the mandated drinkable regime of <15 ppb, and the Hg2+ concentration to 8 ppb, only slightly above the drinkable regime of <2 ppb. This exemplifies the composite’s selectivity, given the concentration of interfering cations in these samples was up to 14 000 times that of the target heavy metal ion. Without any significant loss of capacity, the composite can capture analyte and be easily regenerated through multiple cycles extending the material’s lifetime and demonstrating its potential in large-scale purification projects. Furthermore, when exposed to samples with high concentrations of fouling organic species, the composite retains its capacity only showing a slight reduction in kinetics. Most notably, Fe-BTC/PDA-19 reaches saturation within 1 min of exposure to a 1000 ppb solution of either target cation and reduces the concentrations of Hg2+ or Pb2+ to 1.2 and 1.6 ppb, respectively, removing >99.8% of the contaminant. Combined with its capacity and selectivity for Hg2+ or Pb2+ and its exceptional performance in real world water samples, Fe-BTC/PDA’s rapid uptake kinetics highlight its superiority over previously reported materials. Fe-BTC/PDA is a promising candidate for water remediation and will certainly propel the nascent subfield of MOF/polymer composites forward.
The battle to ameliorate heavy metal contamination is far from finished. Sun et al. examined their material in batch-style exposure experiments. To prepare commercially feasible sorbents, academic and industrial teams must consider purification flow processes. For instance, in most water treatment facilities, water is forced through an assortment of filtration steps including packed columns. Establishing design rules to develop materials that perform well when packed into columns or when used as filters would aid in the preparation of new materials. Evaluating structure–property relationships for MOF/polymer composites would certainly facilitate their extension into various remediation sectors such as oxyanion mitigation efforts targeting chromate and dichromate. These findings could be utilized to tailor the selectivity of a material for a single metal independent of speciation (i.e., oxidation state, extent of hydration). While much work remains, Sun et al. set a high standard for Hg2+ or Pb2+ remediation strategies with their material, Fe-BTC/PDA-19, and elucidated the vast potential of MOF/polymer composites for water purification applications.
References
- World Health Organization. Drinking Water Fact Sheet. http://www.who.int/topics/drinking_water/en/.
- Sun D. T.; Reeder W. S.; Moosavi S. M.; Tiana D.; Britt D. K.; Oveisi E.; Queen W. L. Rapid, Selective Heavy Metal Removal from Water by a Metal–Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 10.1021/acscentsci.7b00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. E.; Olin T. J.; Bricka R. M.; Adrian D. D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33 (11), 2469–2479. 10.1016/S0043-1354(98)00475-8. [DOI] [Google Scholar]
- Kobielska P. A.; Howarth A. J.; Farha O. K.; Nayak S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. 10.1016/j.ccr.2017.12.010. [DOI] [Google Scholar]
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402 (6759), 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Farha O. K.; Eryazici I.; Jeong N. C.; Hauser B. G.; Wilmer C. E.; Sarjeant A. A.; Snurr R. Q.; Nguyen S. T.; Yazaydin A. O.; Hupp J. T. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit?. J. Am. Chem. Soc. 2012, 134 (36), 15016–21. 10.1021/ja3055639. [DOI] [PubMed] [Google Scholar]
- Islamoglu T.; Goswami S.; Li Z.; Howarth A. J.; Farha O. K.; Hupp J. T. Postsynthetic Tuning of Metal-Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50 (4), 805–813. 10.1021/acs.accounts.6b00577. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova K. E.; O’Keeffe M.; Yaghi O. M. The chemistry and applications of metal-organic frameworks. Science 2013, 341 (6149), 1230444. 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ai K.; Lu L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114 (9), 5057–5115. 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- Horcajada P.; Surblé S.; Serre C.; Hong D.-Y.; Seo Y.-K.; Chang J.-S.; Greneche J.-M.; Margiolaki I.; Férey G. Synthesis and catalytic properties of MIL-100 (Fe), an iron (III) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. 10.1039/B704325B. [DOI] [PubMed] [Google Scholar]


