Abstract
Lipoxygenases (LOXs) have been implicated as central players in ferroptosis, a recently characterized cell death modality associated with the accumulation of lipid hydroperoxides: the products of LOX catalysis. To provide insight on their role, human embryonic kidney cells were transfected to overexpress each of the human isoforms associated with disease, 5-LOX, p12-LOX, and 15-LOX-1, which yielded stable cell lines that were demonstrably sensitized to ferroptosis. Interestingly, the cells could be rescued by less than half of a diverse collection of known LOX inhibitors. Furthermore, the cytoprotective compounds were similarly potent in each of the cell lines even though some were clearly isoform-selective LOX inhibitors. The cytoprotective compounds were subsequently demonstrated to be effective radical-trapping antioxidants, which protect lipids from autoxidation, the autocatalytic radical chain reaction that produces lipid hydroperoxides. From these data (and others reported herein), a picture emerges wherein LOX activity may contribute to the cellular pool of lipid hydroperoxides that initiate ferroptosis, but lipid autoxidation drives the cell death process.
Short abstract
Lipoxygenases are not essential for the execution of ferroptosis, but may play a role in its initiation by contributing to the cellular pool of lipid hydroperoxides that promote lipid autoxidation.
Introduction
Lipid peroxidation has long been implicated in a staggering array of human pathologies.1 However, only recently has a direct connection between lipid peroxidation and a specific mode of cell death been recognized. Ferroptosis—coined for this modality—was introduced in 2012.2−7 It was initially ascribed to the caspase-independent cell death resulting from glutathione depletion by the system xc– inhibitor erastin.8 Glutathione depletion starves glutathione peroxidase 4 (Gpx4) of its reducing cosubstrate, preventing the detoxification of (phospho)lipid hydroperoxides (LOOH) by reduction to the corresponding (phospho)lipid alcohols (LOH). Ferroptosis can also be induced by pharmacological inhibition of Gpx4 (e.g., with RSL3),9 or via inducible deletion of the gene encoding Gpx4.10 Changes to lipid metabolism that increase the pool of oxidizable polyunsaturated (phospho)lipid or which disrupt iron homeostasis also sensitize cells to ferroptosis.11,12
The formation of cellular LOOH is known to occur by two primary mechanisms: an iron-catalyzed spontaneous peroxyl radical-mediated chain reaction called autoxidation,13 and an enzyme-mediated process catalyzed by (non-heme) iron-dependent lipoxygenases (LOXs) (Figure 1A).14 Several reports have implicated LOXs (the 15-LOX-1 isoform, in particular) as a key regulator in ferroptotic cell death.15,16 Evidence for the involvement of LOX in ferroptosis is based largely on the observation that pharmacological inhibition of LOX is cytoprotective.15−17 Although it has also been demonstrated that cells are resistant to ferroptosis when LOX activity is knocked down using siRNAs,16 only pharmacological inhibition of LOX has been shown to rescue gpx4–/– knockout mice;10 disruption of the gene encoding 15-LOX-1 (alox15) failed to do so.18
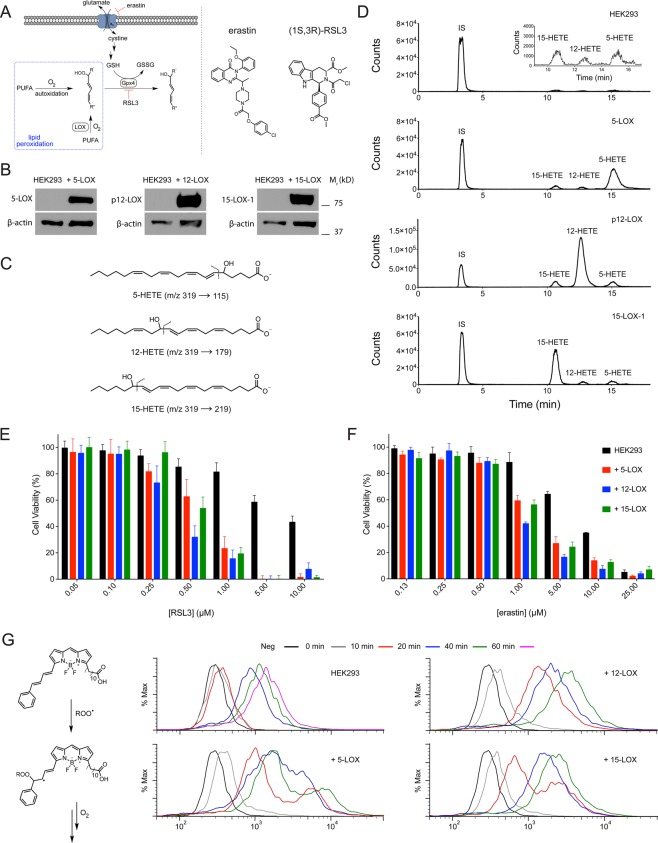
Figure 1.
(A) Formation of cellular lipid hydroperoxides (LOOH) occurs primarily by iron-accelerated free radical autoxidation and LOX-catalyzed oxidation of polyunsaturated fatty acids. (B) Overexpression of 5-LOX, platelet 12-LOX, and 15-LOX-1 in HEK293 cells. (C) MS/MS transitions for the 5-, 12-, and 15-hydroxyeicosatetraenoic acids (HETEs) produced by the overexpressed LOXs. HETEs were analyzed following reduction of the corresponding hydroperoxyeicosatetraenoic acids (HPETEs) with TCEP. (D) LC/MS/MS chromatograms of organic extracts of cell lysates following incubation with 70 μM arachidonic acid for 10 min. (E, F) RSL3- and erastin-induced ferroptosis in each cell line following 4 and 24 h incubation, respectively. Data represent the mean ± SD of 3 independent experiments (P < 0.0001 and P ≤ 0.001 for LD50 values of RSL3 and erastin as determined by one-way ANOVA followed by Dunnett’s multiple comparisons test). (G) Flow cytograms of C11-BODIPY-treated cells supplemented with 70 μM AA.
The identification of specific molecular and genetic inducers of ferroptosis enabled the Stockwell and Conrad groups to identify ferrostatin-1 (Fer-1)19 and liproxstatin-1 (Lip-1)17 as potent inhibitors of ferroptosis from high-throughput screens—the former based on GSH depletion with erastin and the latter by gpx4 knockout with Cre-recombinase. The potencies of Lip-1 and Fer-1 compare very favorably to cytoprotection afforded by known LOX inhibitors such as zileuton (5-LOX inhibitor), PD146176 (15-LOX-1 inhibitor), and NDGA (a pan-LOX inhibitor). We recently demonstrated that while Fer-1 and Lip-1 are poor inhibitors of 15-LOX-1, they are excellent inhibitors of lipid peroxidation (autoxidation) owing to their reactivity as radical-trapping antioxidants (RTAs),20 compounds which reduce chain-carrying peroxyl radicals.21 In fact, both Fer-1 and Lip-1 are more effective RTAs in lipid bilayers than α-TOH, Nature’s premier lipophilic RTA.22 This observation is fully consistent with their greater potency as inhibitors of ferroptosis compared to α-TOH.20 Moreover, highly reactive designer RTAs, including lipophilic naphthyridinols and diarylamines, are potent inhibitors of ferroptosis.23,24 Thus, it follows that either LOX inhibition or RTA activity can subvert ferroptosis, suggesting that both LOX-catalyzed lipid oxygenation and lipid autoxidation contribute to the execution of ferroptosis and preventing one or the other can subvert it. Alternatively, the LOX inhibitors that have been demonstrated to suppress ferroptosis may simply do so as RTAs. Although it is known that zileuton, NDGA, and other redox-active LOX inhibitors have “antioxidant” properties,25 to the best of our knowledge, their reactivity as RTAs have yet to be investigated.
Given the confounding observations surrounding the role of LOX in the execution of ferroptosis (vide supra), we have generated cells that have been stably transfected to overexpress each of the three isoforms most commonly associated with disease (5-LOX, platelet-type 12-LOX, and 15-LOX-1) and characterized both their sensitivity to ferroptosis-inducing compounds and the ability of LOX inhibitors and RTAs to rescue them from cell death. These data suggest that although LOX activity may sensitize cells to ferroptosis by contributing to the cellular pool of hydroperoxides, lipid autoxidation clearly drives cell death.
Results
LOX Overexpression Sensitizes Cells to Ferroptosis
To provide insight on the potential role of different LOX isoforms in the regulation of ferroptosis, LOX-overexpressing HEK293-derived cell lines were generated by stable transfection with recombinant pcDNA3/alox5, alox12, and alox15 constructs.26 Overexpression of each LOX was verified by immunoblotting (Figure 1B), and enzymatic activity was confirmed by determination of the corresponding arachidonic acid oxygenation products (hydroperoxyeicosatetraenoic acids, HPETEs) by UPLC/ESI-MS/MS (Figure 1C,D). LOX expression was not detected in the wild-type cells, and LOX overexpression did not affect Gpx4 levels (Figure S1A–C).
The sensitivity of the LOX-overexpressing cells to ferroptosis was assessed by Gpx4 inhibition with (1S,3R)-RSL39 or GSH depletion with erastin.8 The transfected cell lines were sensitized to ferroptosis induced by either agent, albeit to slightly different extents (Figure 1E,F). LOX overexpression had a more dramatic effect on RSL3-induced ferroptosis, dropping the LD50 from 6.8 μM in the wild-type cells to 0.6, 0.4, and 0.5 μM in the 5-LOX-, p12-LOX-, and 15-LOX-1-overexpressing cells, respectively. Erastin treatment was characterized by LD50s of 6.6 μM in the wild-type cells and 1.4, 0.9, and 1.7 μM in the 5-LOX-, p12-LOX-, and 15-LOX-1-overexpressing cells, respectively. A similar sensitization of the LOX-overexpressing cells to the purported Gpx4 inhibitor ML21027−29 was also observed (Figure S1D).
Insight on the origin of the sensitization of the transfected cells to ferroptosis is evident in the chromatograms shown in Figure 1D. Although LOX overexpression results in an abundance of the specific regioisomeric H(P)ETE derived from LOX catalyzed oxygenation of AA, the amount of nonspecific regioisomeric H(P)ETEs also increase relative to the nontransfected cells. It should be noted that several other regioisomers (i.e., 8-, and 11-H(P)ETE) are also formed in these reactions (Figure S1E–G). Thus, the higher sensitivity of LOX-overexpressing cells to ferroptosis may be ascribed to an initial increase in the concentration of specific LOOH which can subsequently decompose to yield initiating species (alkoxyl and/or hydroxyl radicals by the Fenton reaction) for nonenzymatic lipid peroxidation (autoxidation). To corroborate that the LOX overproducing cells experience a higher rate of (nonregiospecific) lipid autoxidiation concomitant with the increase in regiospecific LOOH formation, C11-BODIPY was used to report on the formation of autoxidation chain-carrying lipid peroxyl radicals.30 Indeed, as is shown in Figure 1G, C11-BODIPY oxidation was significantly higher in LOX-overexpressing cells compared to the nontransfected cells, suggesting that, even in the presence of functional Gpx4, the LOX-overexpressing cells undergo a higher rate of lipid peroxidation. This difference was further exaggerated when Gpx4 was inhibited using RSL3 (Figure S1H–K).
LOX-Overexpressing Cells Are Rescued by Some LOX Inhibitors and All Good RTAs
Given the foregoing results, we sought to delineate the role of LOX-catalyzed lipid peroxidation and autoxidation in ferroptosis by quantitating the potency of both LOX inhibitors (Figures 2A–C, S2A–E) and RTAs (Figures 2D–F, S2F–J) in a systematic manner.
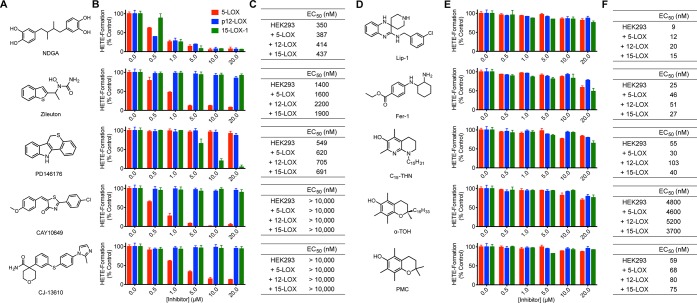
Figure 2.
Structures of some LOX inhibitors (A), their isoform-selectivity (B), and potency as inhibitors of RSL3-induced ferroptosis in the four different cell lines (C). Structures of common RTAs (D), their activity as inhibitors of the various LOX isoforms (E), and potency as inhibitors of RSL3-induced ferroptosis in the four different cell lines (F).
The pan-LOX inhibitor NDGA was an effective inhibitor of RSL3 induced cell death across all LOX-overproducing cell lines (EC50 = 387–437 nM). Interestingly, it was similarly potent in the wild-type cells that were devoid of LOX activity. Perhaps more surprisingly, zileuton, a 5-LOX-selective inhibitor, was not only a moderately potent inhibitor of ferroptosis in 5-LOX overproducing cells (EC50 = 1.6 μM), but it was similarly potent in the nontransfected cells as well as in cells transfected to overproduce p12-LOX and 15-LOX-1. Investigations with PD146176, a 15-LOX-1 selective inhibitor, produced similar results, in that the compound was equally potent in all cell lines. In contrast, 5-LOX inhibitors CAY10649 and CJ-1361031,32 were ineffective inhibitors of ferroptosis in all cell lines.33 From these results, it is clear that LOX inhibitory activity is not at all correlated with antiferroptotic potency.
Lip-1 and Fer-1, the archetypal ferroptosis inhibitors, rescued the transfected cells from RSL3-induced ferroptosis with very similar potencies to those determined in the wild-type cells. For example, in the wild-type cells the EC50 for Lip-1 was 8.9 nM, while it was 12, 20, and 15 nM for the 5-LOX, p12-LOX, and 15-LOX-1 overproducing cells, respectively. Similar results were obtained with Fer-1. Recent results from our group have demonstrated that both Lip-1 and Fer-1 inhibit ferroptosis via their RTA activity, which is excellent in lipid bilayers.20 Consistent with the foregoing results, α-tocopherol (α-TOH) and the improved aza-tocopherol analogue (C15-THN) were also similarly potent in each of the cell lines.
It should be noted that these results are inconsistent with the suggestion that the antiferroptotic activity of α-TOH is due to its ability to inhibit 15-LOX-1 (by inserting its lipophilic side chain into the active site and precluding access of the substrate).15 To provide further insight on this point, 2,2,7,8-pentamethyl-6-chromanol (PMC, Figure 2D), a truncated analogue of α-TOH lacking the lipophilic side chain, was also assayed and was shown to be an excellent inhibitor of ferroptosis in all cell lines, suggesting that the lipophilic side chain of α-TOH is not only unnecessary for its inhibitory activity, but also reduces its potency (e.g., EC50 = 59 nM in the nontransfected cells compared to 4.8 μM for α-TOH). These results are fully consistent with the greater RTA activity of PMC compared to α-TOH in lipid bilayers, which can be ascribed to improved dynamics.34 Furthermore, we found that O-methylated α-TOH (α-TOMe) was not only devoid of LOX inhibitory activity (Figure S3C), but was unable to rescue any of the cells from ferroptosis at concentrations up to 10 μM (Figure S3D). These results are consistent with a previous report wherein it was shown that α-TOH does not inhibit 15-LOX-1 at concentrations where it is effective at subverting ferroptosis.20
Cytoprotective LOX Inhibitors Are Also Good RTAs
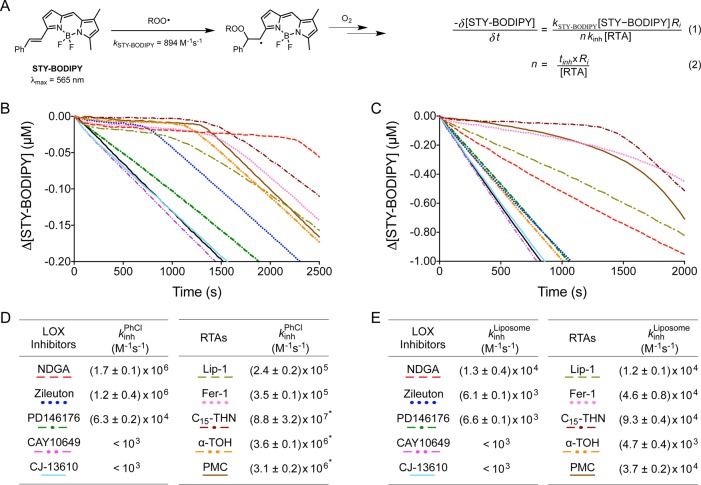
The ability of isoform-selective LOX inhibitors to inhibit ferroptosis in all cell lines with similar potency led us to speculate whether these compounds have off-target effects that underlie their cytoprotective properties. In particular, we wondered if the cytoprotective LOX inhibitors were RTAs, while those LOX inhibitors devoid of antiferroptotic activity were not RTAs. Thus, we determined the RTA activity of NDGA, zileuton, PD146176, CAY10649, and CJ-13610 in inhibited autoxidations of cumene,35 as well as in egg phosphatidylcholine unilammelar liposomes.20,24 Reaction progress was monitored by the consumption of STY-BODIPY, a highly absorbing autoxidizable cosubstrate (Figure 3A). The rate constant for peroxyl radical-trapping (or the inhibition rate constant, kinh) was determined from the initial (inhibited) rate of dye consumption as in eq 1, whereas the reaction stoichiometry (how many peroxyl radicals are trapped by each molecule of RTA) is given from the length of the inhibited period as in eq 2. The autoxidation traces are shown in Figures 3B and 3C, with the kinetic data tabulated below them.
Figure 3.
STY-BODIPY (A) serves as the signal carrier in inhibited autoxidations, enabling determination of inhibition rate constants (kinh) and stoichiometries (n) for reactions of inhibitors with chain-carrying peroxyl radicals (eqs 1 and 2, respectively). Coautoxidations of cumene (3.6 M) and STY-BODIPY (10 μM) initiated by AIBN (6 mM) in chlorobenzene at 37 °C (black) and inhibited by 2 μM of NDGA, zileuton, PD146176, CAY10649, CJ-13610, Lip-1, Fer-1, C15-THN, α-TOH, and PMC (B). Coautoxidations of egg phosphatidylcholine liposomes (1.0 mM) and STY-BODIPY (10 μM) suspended in phosphate-buffered saline (10 mM) at pH 7.4 initiated by MeOAMVN (0.2 mM) at 37 °C (black) and inhibited by 2 μM of NDGA, zileuton, PD146176, CAY10649, CJ-13610, Lip-1, Fer-1, C15-THN, α-TOH, and PMC (C). Inhibition rate constants obtained from coautoxidations of cumene (D) or phosphatidylcholine liposomes (E) with STY-BODIPY at 37 °C. *Rate constants were determined from styrene/PBD-BODIPY co-autoxidations, see ref. (20).
The results for the known RTAs are fully consistent with previous reports. They are good to excellent inhibitors of cumene autoxidation (Figure 3B, Figure S3A), with kinh varying from as low as 2.4 × 105 M–1 s–1 for Lip-1 to as high as 8.8 × 107 M–1 s–1 for C15-THN. The cytoprotective LOX inhibitors NDGA, zileuton, and PD146176 clearly display reactivity as RTAs, ranging from moderately reactive (PD146176, kinh = 6.3 × 104 M–1 s–1) to highly reactive (kinh = 1.7 × 106 and 1.2 × 106 M–1 s–1 for NDGA and zileuton, respectively). On the other hand, the LOX inhibitors which were devoid of antiferroptotic activity were unable to inhibit the autoxidation of cumene. The reactivity of NDGA, zileuton, and PD146176 is explained by the presence of labile phenolic O–H bonds in NDGA,36 the hydroxamic acid O–H bond in zileuton,37 and arylamine N–H bond in PD146176. In contrast, CAY10649 and CJ-13610 do not possess labile H-atoms that could reduce autoxidation chain-carrying peroxyl radicals.
Similar results were obtained in the more biologically relevant model system of egg phosphatidylcholine liposomes (Figure 3C, Figure S3A), albeit where the differences in RTA activity between the compounds were suppressed due to limited diffusion and H-bonding interactions.34,38 All cytoprotective compounds had apparent kinh ranging from 4.7 × 103 M–1 s–1 to 9.3 × 104 M–1 s–1. Again, α-TOMe (Figure S3E,F) and the LOX inhibitors which were devoid of antiferroptotic activity (CAY10649 and CJ-13610) were unreactive.
Only Arachidonic Acid Deuterated at All Bis-Allylic Positions Protects Cells from Ferroptosis
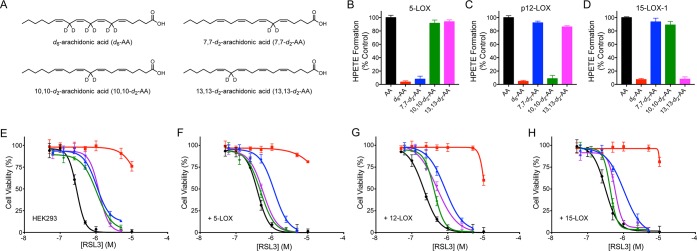
It was recently shown that cells grown in media supplemented with 11,11-d2-linoleic acid (d2-LA) are refractory to ferroptosis induced with either erastin or RSL3.16 Following on this observation, if LOX-catalyzed oxidation of AA—its native substrate—plays a key role in ferroptosis execution, cells grown in media supplemented with AA labeled at the bis-allylic positions from which each LOX isoform regiospecifically abstracts a H-atom should be protected from ferroptosis. The use of regiospecifically labeled substrates39 as isoform-selective inhibitors of LOX (Figure 4A, Figure S4A,B) removes any uncertainty regarding off-target effects of inhibitors or interactions of inhibitors with the agents used to induce ferroptosis. As expected, 7,7-d2-AA inhibited 5-LOX activity, but not p12-LOX or 15-LOX-1 activity (Figure 4B), 10,10-d2-AA inhibited p12-LOX activity, but not 5-LOX or 15-LOX-1 activity (Figure 4C), and 13,13-d2-AA inhibited 15-LOX-1 activity, but not 5-LOX or p12-LOX activity (Figure 4D). These results are consistent with the very large kinetic isotope effects reported for LOX-catalyzed oxidation of AA by p12-LOX and 15-LOX-1.40
Figure 4.
(A) Structures of deuterated arachidonic acids. HPETE formation in cells overexpressing 5-LOX (B), p12-LOX (C), and 15-LOX-1 (D) incubated with 70 μM AA (black), 7,7,10,10,13,13-d6-AA (red), 7,7-d2-AA (blue), 10,10-d2-AA (green), and 13,13-d2-AA (magenta). RSL3-induced ferroptosis in nontransfected HEK293 cells (E) and transfected cells overexpressing 5-LOX (F), p12-LOX (G), and 15-LOX-1 (H) preincubated with 40 μM arachidonic acid (black, ●), 7,7-d2-AA (blue, ▲), 10,10-d2-AA (green, ◆), 13,13-d2-AA (magenta, ▼), and 7,7,10,10,13,13-d6-AA (red, ■).
Despite their ability to specifically inhibit a given LOX isoform, the addition of the d2-AAs had no significant cytoprotective effect on the corresponding LOX-overexpressing cells (Figures 4E–H). However, consistent with the observation that the addition of d2-LA is cytoprotective against ferroptosis, a d6-AA where all abstractable (bis-allylic) H-atoms were replaced with deuterium atoms was strongly cytoprotective. This observation is consistent with the large kinetic isotope effects reported for hydrocarbon autoxidation,41 and specifically H-atom transfer from polyunsaturated lipids.42
Induction of Ferroptosis Does Not Require Specific Hydroperoxide(s)
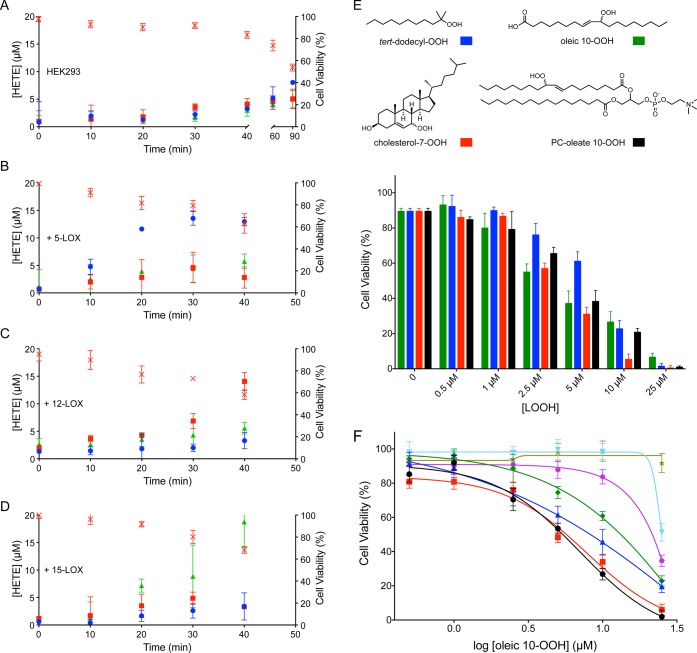
Given the foregoing, we wondered if the H(P)ETEs produced by LOX activity simply contribute to the cellular pool of LOOH to accelerate initiation of massive (nonspecific) autoxidation that drives cell death. Interestingly, when the concentrations of 5-, 12-, and 15-H(P)ETE were monitored in the four cell lines postinduction with RSL3 (Figure 5A–D), the concentration of total LOOH peaked at ca. 20 μM, suggesting that, once a critical threshold of LOOH is achieved, ferroptosis is triggered. To corroborate that the overall concentration of hydroperoxide is far more relevant than its structure, we investigated if cells were similarly sensitized to RSL3 induced ferroptosis upon addition of exogenous LOOH. Four compounds that would not be formed endogenously by LOX catalysis were investigated: 10-hydroperoxy oleic acid (oleic 10-OOH), phosphatidylcholine esterified 10-hydroperoxyoleate (PC-oleate-10-OOH), cholesterol-7-hydroperoxide, and a generic abiotic lipophilic hydroperoxide (tert-dodecyl-OOH). The data in Figure 5F show that the exogenous LOOH sensitize the cells to RSL3 induced ferroptosis to a similar extent. Moreover, the concentration of added LOOH necessary to achieve cell death is strikingly similar to the amount of HPETE produced endogenously in the LOX-overexpressing cells that is associated with cell death. A similar lack of structural dependence on the ability of hydroperoxides to sensitize cells to RSL3-induced cell death was also observed in Pfa1 mouse embryonic fibroblasts (MEFs). These cells have been studied extensively and have been reported to be highly sensitive to ferroptosis.6,17 Similar to HEK293 cells, the MEFs do not express detectable amounts of 5-LOX, p12-LOX, or 15-LOX-1 (Figure S5A–C) and, consistent with their greater sensitivity to ferroptosis (compare LD50s of 38 and 113 nM for RSL3 and erastin, respectively, to 6.8 and 6.6 μM in HEK293 cells, Figure S5D), lower concentrations of added hydroperoxide sensitized them to cell death (Figure S5F).
Figure 5.
Formation of 5-HETE (blue, ●), 12-HETE (red, ■), and 15-HETE (green, ▲) in nontransfected HEK293 cells (A), and cells overexpressing 5-LOX (B), p12-LOX (C), and 15-LOX-1 (D) treated with 5 μM RSL3 and 40 μM arachidonic acid. Cell viability is overlaid on each graph (red ×). (E) Sensitization of wild-type cells to ferroptosis with 0.5 μM RSL3 in the presence of various concentrations of exogenously prepared hydroperoxides: oleic 10-OOH (green), cholesterol-7-OOH (red), tert-dodecyl-OOH (blue), or PC-oleate 10-OOH (black). (F) Viability of HEK293 cells exposed to oleic-10-OOH in the presence of 0.5 μM RSL3 (black, ●) inhibited with 3.1 (red, ■), 6.3 (blue, ▲), 12.5 (green, ◆), 25 (magenta, ●), 50 (cyan, ▼), or 100 (yellow, ★) nM Lip-1.
If LOX catalysis serves simply to supply hydroperoxides to initiate massive autoxidation that drives ferroptosis execution, it follows that as long as there is a sufficiently high concentration of RTA to limit the propagation of autoxidation, higher concentrations of LOOH—endogenous or exogenous—will be tolerated by the cell. This appears to be the case, an example of which is shown in Figure 5F wherein the concentration of oleic hydroperoxide added to wild-type cells was increased in the presence of increasing amounts of Lip-1. While the entire cell population is dead upon incubation with 25 μM oleic 10-OOH, cell viability was increased in a dose-dependent manner with added Lip-1, with full viability being restored at 100 nM Lip-1. It is important to note that Lip-1 does not react directly with oleic 10-OOH under these conditions (Figure S3G).
Discussion
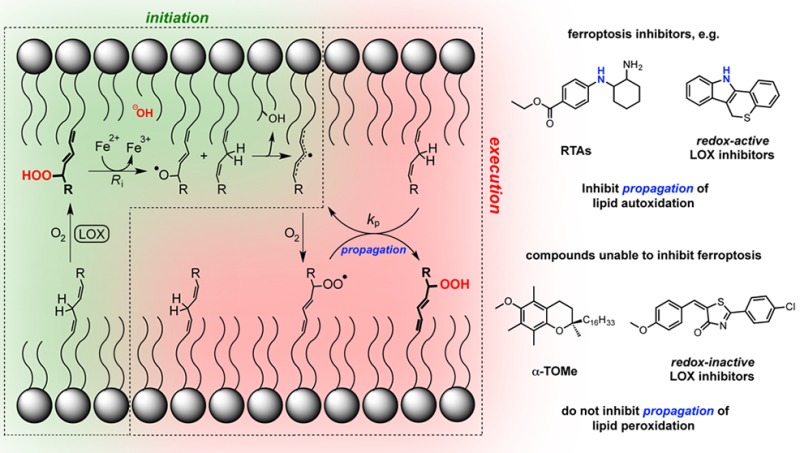
Polyunsaturated fatty acids are integral structural components of biological membranes and serve as precursors to a diverse array of signaling molecules that are essential to maintaining cellular and tissue homeostasis as well as the control of cell proliferation and differentiation. The unsaturation that imparts the molecular geometry necessary to contribute to both a dynamic lipid bilayer and structural diversity of signaling molecules makes PUFAs particularly sensitive to the same deleterious reactions responsible for the degradation of all hydrocarbon-derived products: autoxidation. Autoxidation is the free radical chain reaction that formally inserts a molecule of oxygen into the C–H bond of an organic substrate to yield a hydroperoxide. The hydroperoxide can undergo several possible reactions, including dehydration to yield a carbonyl compound or acid-catalyzed Hock fragmentation to yield a pair of carbonyl compounds (or their equivalent).43,44 In a biological context, lipid-derived carbonyls have long been directly implicated in cell death through caspase-dependent apoptotic processes,45 but only recently have lipid hydroperoxides themselves been implicated directly in regulated cell death, via ferroptosis.2
Lipoxygenase-catalyzed oxidation of PUFAs—primarily arachidonic acid—have been proposed to steer cells toward ferroptosis.15,16 However, the foregoing results clearly demonstrate that although LOX-catalyzed oxygenation of AA may sensitize cells to ferroptosis (induced by the most commonly employed agents RSL3 and erastin),33it is not strictly required. Accordingly, although the inhibition of LOX catalysis cannot rescue cells from ferroptosis induced by the most common ferroptosis-inducing agents, radical-trapping antioxidants can, implying a central role for autoxidation in the execution of ferroptosis.
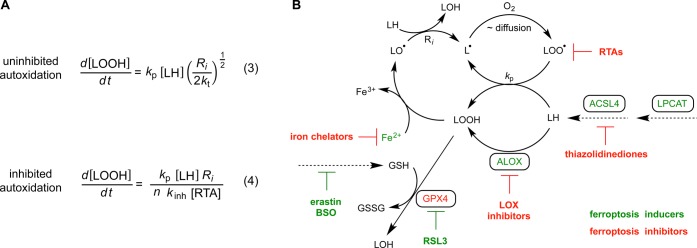
The well-established kinetics of hydrocarbon autoxidation46,47 enable the rationalization of many observations associated with the mechanism of ferroptosis. The expression that generally describes these kinetics—which was first derived from experiments in homogeneous organic solution, but which has also been shown to apply in lipid bilayers48—is shown in eq 3 in Figure 6A. It indicates that the rate of hydroperoxide formation is proportional to the concentration of the substrate (L–H), the rate constant for H-atom abstraction from the substrate by a chain-carrying peroxyl radical (kp), the rate of initiation (Ri), and the rate constant for termination of the chain reaction by the reaction of two chain-carrying peroxyl radicals (kt). Since autoxidation is, in a sense, auto-catalytic, i.e. the reaction product can initiate new chain reactions (Figure 6B), the rate of initiation (Ri) isn't constant, but increases with the conversion of lipids to their corresponding hydroperoxides. Thus, any process that contributes to the cellular pool of LOOH—such as LOX catalysis—will increase Ri and therefore the overall rate, sensitizing cells to ferroptosis. In contrast, gene products that decrease the overall pool of LOOH, such as Gpx4, will decrease Ri and suppress ferroptosis. Moreover, any process that increases the pool of autoxidizable lipid (i.e., [LH]), such as acyl-CoA synthetase long chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3), will increase the overall rate and sensitize cells to ferroptosis activity. In contrast, supplementing cells with less autoxidizable lipids (e.g., D-PUFAs) or with inhibitors of ACSL4 or LPCAT3,12 will suppress ferroptosis.
Figure 6.
Kinetic expressions for uninhibited and inhibited lipid autoxidation (A). Schematic demonstrating the interplay between small molecule and enzymatic inducers and inhibitors of ferroptotic cell death associated with the accumulation of (phospho)lipid hydroperoxides (B).
In homogeneous solution and lipid bilayers, the kinetics of the inhibited autoxidation of hydrocarbons is generally described by eq 4 in Figure 6A.47,48 Thus, compounds with a sufficiently high rate constant (kinh) or stoichiometry (n) for reactions with peroxyl radicals (that do not generate a chain-initiating or chain-propagating radical) will inhibit autocatalytic LOOH formation. These RTA compounds can be either highly reactive or have a high radical-trapping capacity or, ideally, both. α-TOH, Nature’s major lipophilic RTA,22 is only moderately reactive in lipid bilayers,34,48 but its radical-trapping capacity can be bolstered by its regeneration by coantioxidants, such as ascorbate and reduced co-Q10.49,50 The aza-TOH analogue, C15-THN, is much more reactive in lipid bilayers and is also regenerable by coantioxidants,23 which translates to high potency in cells. Lip-1 and Fer-1 also have good activity in lipid bilayers. It remains unclear whether these compounds are regenerable, but it would seem that they have higher radical-trapping capacity in lipid bilayers than either α-TOH or C15-THN, possibly due to the formation of nitroxides,20 which can act as catalytic RTAs.51 The LOX inhibitors NDGA, zileuton, and PD146176 also clearly display this activity, explaining why they inhibit ferroptosis in all four cell lines with similar potencies. Most importantly, LOX inhibitors devoid of any RTA activity (CAY10649 and CJ-13610) do not. It should be noted that other LOX inhibitors have been reported to inhibit ferroptosis, specifically, baicalein,16,52,53 CDC,16 and AA-861.16,52 We have confirmed that these compounds also have significant RTA activity (see Figure S6A–D), which can be ascribed to the labile H-atoms found on their respective redox-active functionalities: CDC contains a lipophilic catechol, baicalein a lipophilic pyrogallol, and AA-861 is a lipophilic quinone analogous to coenzyme Q10 that can be reduced in situ to yield a hydroquinone.25 These results serve to caution investigators to avoid making conclusions regarding the involvement of LOX-catalyzed lipid oxidation in cell death on the basis of inhibition by (redox-active) LOX inhibitors, including (but not limited to) NDGA,53−55 zileuton,16,56,57 PD146176,10,15,16,55,58,59 bacalein,16,52,53,59 CDC16 and AA-861.16
Since hydrocarbon autoxidation can be autocatalytic, the rate of initiation (Ri) is not constant, but ever-increasing, depending on the LOOH concentration and the availability of low valent metals (e.g., Fe2+) and/or other one-electron-reducing agents capable of converting hydroperoxides to chain-initiating species (i.e., hydroxyl and/or alkoxyl radicals). As Ri increases, the cell’s intrinsic RTA defenses are rapidly depleted and, with the accumulation of LOOH left unabated, cell death is inevitable. In HEK293 cells (transfected to overexpress LOX or not) the LOOH concentration at which this takes place appears to be ca. 20 μM. This “threshold” is likely to vary with cell type, metabolic state, and/or specific culture conditions that can affect either Ri or the intrinsic RTA complement/activity of the cell. Indeed, we have shown that cells that are more sensitive to ferroptosis, such as the well-studied mouse embryonic fibroblasts,17 are characterized by a significantly lower threshold, and supplementing cells with additional RTA can significantly raise this threshold.60
Although the results presented above establish autoxidation as the central process in the execution stage of ferroptosis, it must be stressed that the events leading up to it—events that enable the initial buildup of LOOH to a level where Ri will overwhelm a cell’s limited intrinsic RTA capacity—remain unclear. It is at this initiation stage that LOXs may play a role, and their expression levels—as well as those of proteins/enzymes that may be associated with their activity (e.g., PEBP1, a scaffolding protein that was recently reported to assist 15-LOX-1 in acquiring phosphatidylethanolamine-esterified AA for oxygenation,61Figure S7)—may contribute to the sensitivity of a given cell type to ferroptosis. It is also at this stage where pharmacological intervention to suppress lipid oxidation with LOX inhibitors or deuterated PUFAs62 may be useful in pathological contexts. Consistent with this view, 5-LOX-overexpressing cells passaged in the presence of CJ-13610 (the non-redox-active 5-LOX selective inhibitor) were desensitized to RSL3-induced ferroptosis, while the p12-LOX- and 15-LOX-1-overexpressing cells remained sensitized (Figure S4C,D). Although RTA treatment is clearly effective in subverting ferroptosis in cell culture, it targets the downstream process (autoxidation), and once the RTA is exhausted, if the underlying condition remains (e.g., ineffective Gpx4 and/or GSH insufficiency), the cells will die. Indeed, Gpx4–/– cells were viable in α-TOH-enriched media (passaged 20 times), but underwent cell death within 24 h in its absence.12In vivo, where accumulation of initiating LOOH may be slower, and induction of ferroptosis correspondingly less acute, LOX inhibition may ultimately prove effective.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canada Foundation for Innovation. D.A.P. and R.S. also acknowledge the support of the Canada Research Chairs and the NSERC Post-Graduate Scholarships programs, respectively.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00589.
Experimental protocols, Western blots, UPLC–MS/MS traces, ML210 sensitization and inhibition data, flow cytograms, dose–response curves, α-TOMe control experiments, 12-LOX inhibition by deuterated-AA, additional experiments in Pfa1 mouse embryonic fibroblasts, and cell viability of all cells in the presence of CJ-13610 (PDF)
Author Contributions
D.A.P. designed and directed the research, and R.S. performed all experimental work and contributed to project design. R.S. and D.A.P. wrote the manuscript. M.S.S. provided the deuterated polyunsaturated fatty acids and discussed associated experimental results.
The authors declare the following competing financial interest(s): M.S.S. owns shares in Retrotope, Inc.
This article published February 7, 2018 with an error in a chemical structure in Figure 1. The corrected Figure published February 9, 2018.
Supplementary Material
References
- Finkel T.; Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Dixon S. J.; et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S.; Stockwell B. R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M.; Angeli J. P. F.; Vandenabeele P.; Stockwell B. R. Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discovery 2016, 15, 348–366. 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli J. P. F.; Shah R.; Pratt D. A.; Conrad M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. 10.1016/j.tips.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Stockwell B. R.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnhead H. O.; Vandenabeele P.; Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death?. Cell Death Differ. 2017, 24, 1991–1998. 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda N.; et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 865–869. 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S.; Stockwell B. R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A.; Wurst W.; Bornkamm G. W.; Schweizer U.; Conrad M. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Dixon S. J.; et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.; Xu L.; Porter N. A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Haeggström J. Z.; Funk C. D. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5898. 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- Kagan V. E.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S.; et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E4966–75. 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli J. P.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brütsch S. H.; et al. Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the Alox15 gene does not rescue such knock-in mice. Antioxid. Redox Signaling 2015, 22, 281–293. 10.1089/ars.2014.5967. [DOI] [PubMed] [Google Scholar]
- Skouta R.; et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J. Am. Chem. Soc. 2014, 136, 4551–4556. 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilka O.; et al. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold K. U.; Pratt D. A. Advances in radical-trapping antioxidant chemistry in the 21st century: a kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. 10.1021/cr500226n. [DOI] [PubMed] [Google Scholar]
- Burton G. W.; Ingold K. U. Vitamin-E - Application of the Principles of Physical Organic-Chemistry to the Exploration of Its Structure and Function. Acc. Chem. Res. 1986, 19, 194–201. 10.1021/ar00127a001. [DOI] [Google Scholar]
- Li B.; et al. Besting vitamin E: sidechain substitution is key to the reactivity of naphthyridinol antioxidants in lipid bilayers. J. Am. Chem. Soc. 2013, 135, 1394–1405. 10.1021/ja309153x. [DOI] [PubMed] [Google Scholar]
- Shah R.; Margison K.; Pratt D. A. The Potency of Diarylamine Radical-Trapping Antioxidants as Inhibitors of Ferroptosis Underscores the Role of Autoxidation in the Mechanism of Cell Death. ACS Chem. Biol. 2017, 12, 2538–2545. 10.1021/acschembio.7b00730. [DOI] [PubMed] [Google Scholar]
- Czapski G. A.; Czubowicz K.; Strosznajder R. P. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol. Rep. 2012, 64, 1179–1188. 10.1016/S1734-1140(12)70914-3. [DOI] [PubMed] [Google Scholar]
- Nair D. G.; Funk C. D. A cell-based assay for screening lipoxygenase inhibitors. Prostaglandins Other Lipid Mediators 2009, 90, 98–104. 10.1016/j.prostaglandins.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Weiwer M.; et al. Bioorganic & Medicinal Chemistry Letters. Bioorg. Med. Chem. Lett. 2012, 22, 1822–1826. 10.1016/j.bmcl.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. S.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V. S.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib Y. M. A fluorometric method for measurement of peroxyl radical scavenging activities of lipophilic antioxidants. Anal. Biochem. 1998, 265, 290–298. 10.1006/abio.1998.2931. [DOI] [PubMed] [Google Scholar]
- Hofmann B.; et al. A class of 5-benzylidene-2-phenylthiazolinones with high potency as direct 5-lipoxygenase inhibitors. J. Med. Chem. 2011, 54, 1943–1947. 10.1021/jm101165z. [DOI] [PubMed] [Google Scholar]
- Horrillo R.; et al. Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor. J. Pharmacol. Exp. Ther. 2007, 323, 778–786. 10.1124/jpet.107.128264. [DOI] [PubMed] [Google Scholar]
- Similar results were obtained when ferroptosis was induced with ML-210. Specifically, while zileuton and PD146176 were able to rescue all cells from ML210-induced cell death, CJ-13610 did not rescue the 5-LOX-overexpressing cells (see Supporting Information for the data, Figure S1D and Table S1).
- Niki E.; Noguchi N. Dynamics of antioxidant action of vitamin E. Acc. Chem. Res. 2004, 37, 45–51. 10.1021/ar030069m. [DOI] [PubMed] [Google Scholar]
- Haidasz E. A.; Van Kessel A. T. M.; Pratt D. A. A Continuous Visible Light Spectrophotometric Approach To Accurately Determine the Reactivity of Radical-Trapping Antioxidants. J. Org. Chem. 2016, 81, 737–744. 10.1021/acs.joc.5b02183. [DOI] [PubMed] [Google Scholar]
- Floriano-Sánchez E.; et al. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radical Res. 2006, 40, 523–533. 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- Končić M. Z.; Barbarić M.; Perković I.; Zorc B. Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules 2011, 16, 6232–6242. 10.3390/molecules16086232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay L. R. C.; Edwards C. E.; Vinqvist M. R. Media effects on antioxidant activities of phenols and catechols. J. Am. Chem. Soc. 1999, 121, 6226–6231. 10.1021/ja990878u. [DOI] [Google Scholar]
- Fomich M. A.; et al. Full Library of (Bis-allyl)-deuterated Arachidonic Acids: Synthesis and Analytical Verification. ChemistrySelect 2016, 1, 4758–4764. 10.1002/slct.201600955. [DOI] [Google Scholar]
- Wecksler A. T.; Jacquot C.; van der Donk W. A.; Holman T. R. Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid. Biochemistry 2009, 48, 6259–6267. 10.1021/bi802332j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchalski H.; Levonyak A. J.; Xu L.; Ingold K. U.; Porter N. A. Competition H(D) Kinetic Isotope Effects in the Autoxidation of Hydrocarbons. J. Am. Chem. Soc. 2015, 137, 94–97. 10.1021/ja511434j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberson C. R.; et al. Unusual Kinetic Isotope Effects of Deuterium Reinforced Polyunsaturated Fatty Acids in Tocopherol-Mediated Free Radical Chain Oxidations. J. Am. Chem. Soc. 2014, 136, 838–841. 10.1021/ja410569g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski Z. A. M.; Pratt D. A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817–2825. 10.1021/acs.joc.7b00152. [DOI] [PubMed] [Google Scholar]
- Porter N. A. Mechanisms for the autoxidation of polyunsaturated lipids. Acc. Chem. Res. 1986, 19, 262. 10.1021/ar00129a001. [DOI] [Google Scholar]
- Ji C.; Amarnath V.; Pietenpol J. A.; Marnett L. J. 4-hydroxynonenal induces apoptosis via caspase-3 activation and cytochrome c release. Chem. Res. Toxicol. 2001, 14, 1090–1096. 10.1021/tx000186f. [DOI] [PubMed] [Google Scholar]
- Ingold K. U. Inhibition of the Autoxidation of Organic Substances in the Liquid Phase. Chem. Rev. 1961, 61, 563–589. 10.1021/cr60214a002. [DOI] [Google Scholar]
- Burton G. W.; Ingold K. U. Autoxidation of Biological Molecules 1. The Antioxidant Activity of Vitamin-E and Related Chain-Breaking Phenolic Antioxidants Invitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. 10.1021/ja00411a035. [DOI] [Google Scholar]
- Barclay L.; Ingold K. U. Autoxidation of Biological Molecules.2. The Autoxidation of a Model Membrane - a Comparison of the Autoxidation of Egg Lecithin Phosphatidylcholine in Water and in Chlorobenzene. J. Am. Chem. Soc. 1981, 103, 6478–6485. 10.1021/ja00411a036. [DOI] [Google Scholar]
- Doba T.; Burton G. W.; Ingold K. U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim. Biophys. Acta, Lipids Lipid Metab. 1985, 835, 298–303. 10.1016/0005-2760(85)90285-1. [DOI] [PubMed] [Google Scholar]
- Shi H.; Noguchi N.; Niki E. Comparative study on dynamics of antioxidative action of alpha-tocopheryl hydroquinone, ubiquinol, and alpha-tocopherol against lipid peroxidation. Free Radical Biol. Med. 1999, 27, 334–346. 10.1016/S0891-5849(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Haidasz E. A.; et al. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. 10.1021/jacs.6b00677. [DOI] [PubMed] [Google Scholar]
- Xie Y.; et al. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Probst L.; Dächert J.; Schenk B.; Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. 10.1016/j.bcp.2017.06.112. [DOI] [PubMed] [Google Scholar]
- Abrams R. P.; Carroll W. L.; Woerpel K. A. Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol. 2016, 11, 1305–1312. 10.1021/acschembio.5b00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer M. J.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; et al. The 5-Lipoxygenase Inhibitor Zileuton Confers Neuroprotection against Glutamate Oxidative Damage by Inhibiting Ferroptosis. Biol. Pharm. Bull. 2015, 38, 1234–1239. 10.1248/bpb.b15-00048. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Li X.; Zhang X.; Kang R.; Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1338–1343. 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- Matsushita M.; et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintoku R.; et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- It stands to reason that cancer cells, which are generally characterized to have increased oxidative metabolism and have been shown to be more sensitive to ferroptosis, also have suppressed thresholds.
- Wenzel S. E.; et al. PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 2017, 171, 628–641.e26. 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S.; et al. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radical Biol. Med. 2012, 53, 893–906. 10.1016/j.freeradbiomed.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.