Abstract
Infections with Streptococcus pneumoniae are a major health burden. Glycoconjugate vaccines based on capsular polysaccharides (CPSs) successfully protect from infection, but not all pneumococcal serotypes are covered with equal potency. Marketed glycoconjugate vaccines induce low levels of functional antibodies against the highly invasive serotype 1 (ST1), presumably due to the obscuring of protective epitopes during chemical activation and conjugation to carrier proteins. Synthetic oligosaccharide antigens can be designed to carry linkers for site-selective protein conjugation while keeping protective epitopes intact. Here, we developed an efficacious semisynthetic ST1 glycoconjugate vaccine candidate. A panel of synthetic oligosaccharides served to reveal a critical role of the rare aminosugar, 2-acetamido-4-amino-2,4,6-trideoxy-d-galactose (d-AAT), for ST1 immune recognition. A monovalent ST1 trisaccharide carrying d-AAT at the nonreducing end induced a strong antibacterial immune response in rabbits and outperformed the ST1 component of the multivalent blockbuster vaccine Prevenar 13, paving the way for a more efficacious vaccine.
Short abstract
A synthetic trisaccharide antigen confers antibacterial immunity against the highly invasive S. pneumoniae serotype 1 that is insufficiently covered by marketed vaccines.
Introduction
Infections with Streptococcus pneumoniae cause more than 1.6 million deaths per year.1 Although pneumococci asymptomatically colonize the respiratory tracts of healthy individuals, infections readily occur in risk groups, including young children, elderly adults, and people with underlying infections by influenza and human immunodeficiency viruses.2−4 Pneumococcal disease comprises noninvasive (e.g., pneumonia and otitis media) and invasive (meningitis and bacteremia) symptoms and is the largest vaccine-preventable cause of death in children under 5 years of age.5 More than 90 different pneumococcal serotypes are known that are distinguished based on their capsular polysaccharide (CPS) structure.6 Most recent pneumococcal vaccines contain CPSs from ten (Synflorix) or 13 (Prevenar 13) of the most prevalent serotypes.
As CPSs alone are relatively weak immunogens, conjugation to a carrier protein, such as the nontoxic diphtheria toxin mutant CRM197, results in the formation of long-lasting, polysaccharide-directed immunological memory that, in many cases, protects from disease.7 However, chemical activation and protein conjugation of a CPS during conventional vaccine manufacture inevitably results in glycan modification that risks the destruction of immunogenic epitopes.8,9
Several serotypes are challenging to target by vaccination, as levels of the functional anti-CPS antibodies elicited by marketed vaccines and detected by opsonophagocytic killing (OPK) of bacteria, are less than ideal.10−13 Among these serotypes, serotype 1 (ST1) is particularly virulent and a major cause of meningitis in sub-Saharan Africa, calling for the development of a better glycoconjugate vaccine.14 Synthetic oligosaccharides representing ST1 CPS and related polysaccharides have been generated by us and others.15−19 These saccharides can be furnished site-selectively with functional groups for conjugation, circumventing many of the drawbacks associated with isolated polysaccharide antigens.15 Here, we report the development of an efficacious semisynthetic glycoconjugate vaccine against ST1. We identify a free amino group in the ST1 repeat unit as an epitope that is recognized by the immune system. Using conjugation chemistry that keeps this amine intact yielded a glycoconjugate vaccine that greatly outperformed the ST1 component in Prevenar 13 in immunization experiments in rabbits.
Results and Discussion
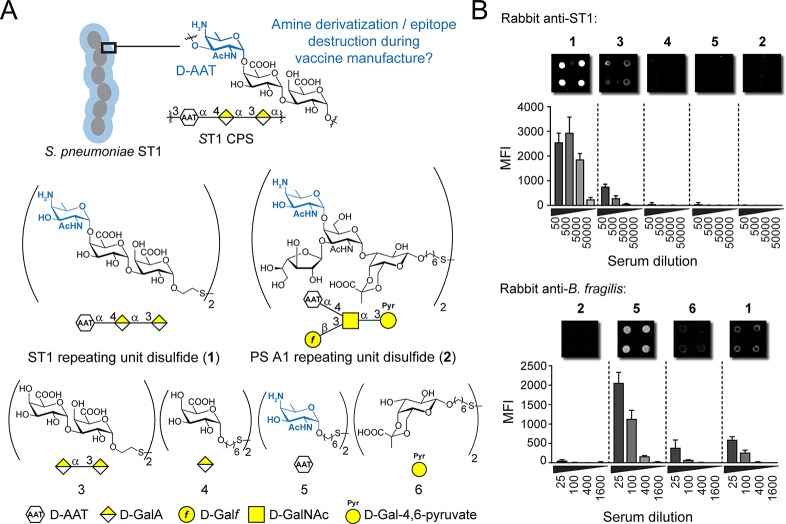
ST1 CPS is special even for a bacterial polysaccharide because it harbors the unusual monosaccharide, 2-acetamido-4-amino-2,4,6-trideoxy-d-galactose (d-AAT), that contains a free amine (Figure 1A). Since the manufacturing of marketed glycoconjugate vaccines uses reductive amination (Prevenar 13) or 1-cyano-4-dimethylaminopyridine activation chemistry (Synflorix) that reacts with free amines,20,10,11 we hypothesized that chemical derivatization of a certain fraction of the d-AAT moieties may lead to reduced vaccine efficacy. Furthermore, polysaccharides are depolymerized during manufacturing, for instance by sodium periodate-mediated diol oxidation,10 which may lead to an additional reduction of efficacy. To test these hypotheses, we used a panel of thiol-containing synthetic oligosaccharides resembling ST1 CPS or closely related Bacteroides fragilis PS A1 CPS that selectively react with suitable electrophiles while leaving the d-AAT amino group intact (Figure 1A and Scheme S1).15 Synthetic oligosaccharides 1–6 were spotted on maleimide-functionalized glycan microarray slides and incubated with ST1 or PS A1-directed antisera (Figure 1B). Antibodies contained in a rabbit-derived ST1 typing serum bound to trisaccharide 1, confirming our previous observations.15 In contrast, disaccharide 3, missing the d-AAT moiety, was bound to a much lower extent, and neither galacturonic acid (GalA) 4 nor d-AAT alone (5) or the PS A1 repeating unit (2) was bound. An anti-B. fragilis serum that recognizes PS A1 polysaccharide bound d-AAT, but not synthetic PS A1 trisaccharide 2.15 Pyruvate-containing galactose 6 and the d-AAT-terminating ST1 tetrasaccharide (1) were bound to a lower extent. These results indicate that d-AAT is important for immune recognition of ST1 glycans embedded in a glycan backbone, while PS A1 recognition relies solely on d-AAT.
Figure 1.
d-AAT is essential for polysaccharide immune recognition. (A) Structure of ST1 CPS and synthetic oligosaccharides. d-AAT is highlighted in blue. (B) Glycan microarray analysis of ST1- (upper panel) and PS A1-directed (lower panel) antisera. Bars represent the mean + SD of eight replicate spots from one representative out of at least two independent experiments. MFI, mean fluorescence intensity.
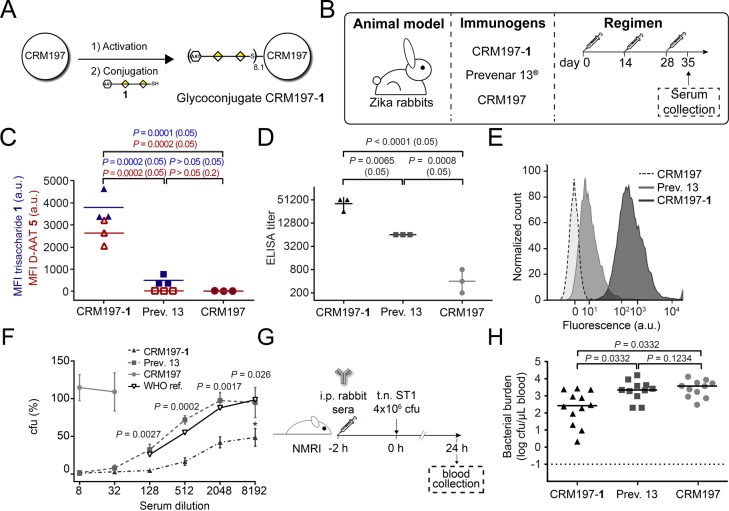
Trisaccharide 1 harbors d-AAT at the nonreducing end of the saccharide chain, exposed for antibody recognition. Thus, we reasoned that 1 should serve as an effective vaccine antigen against ST1. A glycoconjugate was generated by reacting the thiol group of 1 with bromoacetate-activated CRM197 (Figures 2A and S1).15,21 Rabbits were immunized three times with a full dose of Prevenar 13 or an equivalent dose (based on the amount of glycan) of CRM197-1 glycoconjugate adsorbed to the adjuvant aluminum hydroxide, and their sera were compared with sera from rabbits immunized with CRM197 alone in a different experiment (Figure 2B).22 The semisynthetic CRM197-1 glycoconjugate evoked a significantly higher immune response against trisaccharide 1 and d-AAT 5 as well as ST1 CPS than Prevenar 13 or CRM197 alone (Figure 2C,D). The recognition of d-AAT by serum antibodies was detected at a slightly earlier time point than the recognition of di- or mono-GalA 3 and 4 and was correlated with the binding of trisaccharide 1 and ST1 CPS (Figure S2A, B, D–H). Thus, semisynthetic CRM197-1 induces a d-AAT-dependent immune response against native ST1 CPS that is more robust than that of the polysaccharide-based, multivalent Prevenar 13. Antibody responses against the carrier protein CRM197, as well as a spacer construct of bovine serum albumin conjugated to galactose, did not differ substantially between Prevenar 13 and CRM197-1 groups (Figure S2C).
Figure 2.
Evaluation of a CRM197-1 glycoconjugate vaccine in rabbits. (A) Conjugation of trisaccharide 1 to CRM197. (B) Immunization parameters and regimen. (C) Immune response against synthetic saccharides 1 and 5 as assessed by glycan microarray. Data are individual values from n = 3 rabbits per group and median. Statistical analysis was performed by one-way ANOVA and one-tailed Mann–Whitney U test (hyphenated). (D) Immune response against ST1 CPS as assessed by polysaccharide ELISA. Data are individual values from n = 3 rabbits and geometric mean titers ± geometric SD. Statistical analysis was performed by one-way ANOVA and one-tailed Mann–Whitney U test (hyphenated) of log-transformed titers. (E) Binding of pooled immune sera to ST1 bacteria as assessed by flow cytometry. The x-axis is shown in a biexponential scale. (F) Opsonophagocytic killing of ST1 bacteria by pooled immune sera. Data are mean ± SD of cfu relative to control of triplicate samples from one out of three independent experiments. Statistical analysis was performed by an unpaired, one-tailed t test between CRM197-1 and Prevenar 13 groups. (G) Passive immunization regimen. (H) Blood bacterial load of mice passively immunized with pooled rabbit sera (day 35) and transnasally infected with live ST1. Data are individual values from n = 11–12 mice and median. Statistical analysis was performed by one-way ANOVA with Dunn’s post hoc test. The dotted line depicts the detection limit.
We next assessed the antibacterial properties of sera against CRM197-1 in vitro and in vivo. Flow cytometry revealed that antibodies in sera from CRM197-1-immunized rabbits bound better to ST1 bacteria than sera from Prevenar 13-immunized rabbits (Figures 2E and S2I). Bacterial binding correlated with serum OPK capacities. Pooled serum from CRM197-1 vaccinated rabbits induced significantly higher killing of ST1 bacteria by the phagocyte cell line HL-60 than serum from Prevenar 13 immunized rabbits (Figure 2F). Furthermore, CRM197-1 exhibited a killing efficiency markedly higher than that of serum 007sp, a reference serum recognized by the World Health Organization to assess pneumococcal vaccine efficacy.23 Anti-CRM197 serum did not bind ST1 bacteria. We noted that opsonophagocytosis induced by CRM197-1 antiserum, but not by Prevenar 13 antiserum, plateaus at a killing ratio of 50%. It has been found that antibodies can lead to protection by either opsonophagocytosis or agglutination of bacteria.24,25 Agglutination results in bacterial clumping that may impair colony counting in the OPK assay. Indeed, we found that sonication of OPK samples before plating increases colony count at high (>2048-fold) serum dilutions of CRM197-1 antisera (Figure S3A), but not Prevenar 13 antisera (Figure S3B). Sonication does not change colony counts at lower dilutions and, hence, does not influence our findings on the efficacy of CRM197-1.
The protective capacity of ST1-directed immune sera in vivo was then assessed by a passive immunization experiment (Figure 2G). Mice pretreated with pooled CRM197-1 rabbit serum and transnasally infected with ST1 pneumococci showed fewer colony-forming units (cfu) in blood (Figure 2H) and lung homogenates (Figure S4A) than mice pretreated with sera from Prevenar 13 or CRM197-immunized rabbits. While analysis of bronchoalveolar lavage fluid did not show a difference in bacterial load (Figure S4B), the clinical parameters, body weight and body temperature, clearly revealed that antiserum against CRM197-1, but neither Prevenar 13 nor CRM197, attenuated infection by ST1 bacteria (Figure S4C,D)
Semisynthetic CRM197-1 glycoconjugate is a highly efficacious vaccine candidate against ST1 that outperformed the ST1 component in the multivalent vaccine Prevenar 13 in our immunization experiments. The manufacturing process of Prevenar 13 likely obscures the immunologically important d-AAT motif. To illustrate that d-AAT is highly immunogenic, we assessed the immune response toward that monosaccharide. To this end, we synthesized a version of conjugation-ready d-AAT with a shorter linker than molecule 5 to reduce potential linker-associated immunogenicity (molecule S3, Scheme S1). A CRM197-S3 glycoconjugate invoked specific d-AAT-directed antibody responses in mice when co-formulated with Freund’s adjuvant (Figure S5). Antibodies from one mouse cross-reacted with synthetic ST1 and PS A1 oligosaccharides 1 and 2, highlighting the immunogenic nature of that monosaccharide. The neoglycoconjugate CRM197-1 maintains the key epitope unchanged. CRM197-1 induces antibodies recognizing d-AAT in the context of a full ST1 repeating unit that are essential for the induction of an antibacterial immune response, as illustrated by our in vivo challenge experiment. While our rabbit immunization experiments produced highly robust antibacterial immunity, active immunization experiments with CRM197-1 in mice did not produce a reproducible, polysaccharide-directed immune response (data not shown). This finding is in line with our work on other S. pneumoniae serotypes,22 and it indicates that mice may not be a reliable animal model for semisynthetic glycoconjugate vaccines against pneumococci.
We directly compared the immunogenicity of CRM197-1 with that of the marketed vaccine, Prevenar 13. The outcome of such a comparison may be influenced by technical difficulties in reproducing the precise formulation used in Prevenar 13, including the used adjuvant and the presence of other saccharide antigens. We have previously shown that a semisynthetic glycoconjugate vaccine based on a negatively charged oligosaccharide retains immunogenicity when formulated either individually or in conjunction with Prevenar 13.22 Furthermore, CRM197-1 and Prevenar 13 elicit a comparable immune response against the carrier protein, CRM197 (Figure S2C), to indicate that both vaccines are, in fact, comparable.
The semisynthetic glycoconjugate CRM197-1 is advancing in preclinical development for inclusion in semisynthetic vaccines covering multiple serotypes. Further translation to clinical evaluation will require optimization of the chemical synthesis of the ST1 repeating unit 1. With the advancement of procedures to generate building blocks26 and to stereoselectively install glycosidic bonds,27 scalability of the synthetic process is not estimated to be a major obstacle en route to an efficacious, semisynthetic ST1 vaccine.
Acknowledgments
The authors thank K. Hofmann for valuable help during passive immunization experiments and E. Settels for MALDI/TOF-MS measurements and excellent technical support. We are grateful to S. Hammerschmidt (Universität Greifswald) for pneumococcal strains and D. Kasper (Harvard Medical School) for antiserum against B. fragilis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00504.
Synthesis of 5, S-3, and 3; conjugation of 1; immunogenicity of CRM197-1; CRM197-1 and Prevenar 13 antisera; bacterial burden and clinical parameters of mice immunized with CRM197-1, Prevenar 13, or CRM197; conjugation of d-AAT S3 to CRM197; experimental methods; and characterization of new compounds (PDF)
Author Present Address
⊥ Department of Chemistry, Stanford University, 380 Roth Way, Stanford, CA 94305, USA.
Author Present Address
∥ Bacterial Vaccine Discovery Team, Janssen Pharmaceuticals of Johnson & Johnson, Bioscience Park Leiden, Zernikedreef 9, 2333 CK Leiden, Amsterdam Area, Netherlands.
Author Present Address
# Vaxxilon Deutschland GmbH, Magnusstraße 11, 12489 Berlin, Germany.
Author Contributions
B.S., K.R., C.A., M.W., C.L.P., and P.H.S. designed the research. B.S., K.R., P.K., and A.W. performed experiments. B.S. and K.R. performed statistical analyses. B.S., K.R., M.W., C.L.P., and P.H.S. wrote the paper with input from all authors.
The authors thank the Max Planck Society, the German Federal Ministry of Education and Research (BMBF), and the Körber Foundation for generous financial support. This work was supported by a Kekulé doctoral fellowship by the Fonds der Chemischen Industrie (to B.S.), the German Ministry of Education and Research (e:Med/CAPSyS 01ZX1304B to M.W.), and the Deutsche Forschungsgemeinschaft (SFB-TR84, C3 and C6 to M.W. and C8 to P.H.S.).
The authors declare the following competing financial interest(s): This work has been the subject of the patent Synthetic vaccines against Streptococcus pneumoniae, PCT/EP2014/064407, held by the Max Planck Gesellschaft zur Förderung der Wissenschaften e.V. covering synthetic ST1 saccharides. B.S. is an inventor on said patent. C.L.P. is an inventor on said patent and has a significant financial interest in Vaxxilon AG, a company that is developing semisynthetic glycoconjugate vaccines and has exclusively licensed the patent stated above. He is an employee of the German daughter company of Vaxxilon AG called Vaxxilon Deutschland GmbH and has Phantom Stock Options in the parent company. C.A. is an inventor on the patent stated above. P.H.S. is an inventor on the patent stated above and has a significant financial interest in Vaxxilon AG. He is the scientific founder, a member of the board, and a shareholder and acts as a consultant for Vaxxilon AG. K.R., A.W., P.K., and M.W. declare no relevant competing interests.
Supplementary Material
References
- O’Brien K. L.; Wolfson L. J.; Watt J. P.; Henkle E.; Deloria-Knoll M.; McCall N.; Lee E.; Mulholland K.; Levine O. S.; Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009, 374, 893–902. 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- Örtqvist Å.; Hedlund J.; Kalin M. Streptococcus pneumoniae: Epidemiology, Risk Factors, and Clinical Features. Semin. Respir. Crit. Care Med. 2005, 26, 563–574. 10.1055/s-2005-925523. [DOI] [PubMed] [Google Scholar]
- Joseph C.; Togawa Y.; Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir. Viruses 2013, 7, 105–113. 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M. C.; Madhi S. A. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum. Vaccines Immunother. 2012, 8, 161–173. 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. L.; Rudan I.; Liu L.; Nair H.; Theodoratou E.; Bhutta Z. A.; O’Brien K. L.; Campbell H.; Black R. E. Global burden of childhood pneumonia and diarrhoea. Lancet 2013, 381, 1405–1416. 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerling J. P. In Streptococcus pneumoniae: Molecular biology and mechanisms of disease; Tomasz A., Ed.; Mary Ann Liebert: New Rochelle, NY, 2000; pp 81–114. [Google Scholar]
- Hütter J.; Lepenies B. Carbohydrate-Based Vaccines: An Overview. Methods Mol. Biol. 2015, 1331, 1–10. 10.1007/978-1-4939-2874-3_1. [DOI] [PubMed] [Google Scholar]
- Schumann B.; Anish C.; Pereira C. L.; Seeberger P. H. In Biotherapeutics: Recent Developments Using Chemical and Molecular Biology, RSC Drug Discovery Series No. 36; Jones L., McKnight A. J., Eds.; RSC Publishing: Cambridge, 2013; Chapter 3, pp 68–104. [Google Scholar]
- Poolman J.; Frasch C.; Nurkka A.; Käyhty H.; Biemans R.; Schuerman L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin. Vaccine Immunol. 2011, 18, 327–336. 10.1128/CVI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assessment Report for Prevenar 13. European Medicines Agency: London, 2009.
- Assessment Report for Synflorix. European Medicines Agency: London, 2009.
- Mackenzie G. A.; Hill P. C.; Jeffries D. J.; Hossain I.; Uchendu U.; Ameh D.; Ndiaye M.; Adeyemi O.; Pathirana J.; Olatunji Y.; Abatan B.; Muhammad B. S.; Fombah A. E.; Saha D.; Plumb I.; Akano A.; Ebruke B.; Ideh R. C.; Kuti B.; Githua P.; Olutunde E.; Ofordile O.; Green E.; Usuf E.; Badji H.; Ikumapayi U. N. A.; Manjang A.; Salaudeen R.; Nsekpong E. D.; Jarju S.; Antonio M.; Sambou S.; Ceesay L.; Lowe-Jallow Y.; Jasseh M.; Mulholland K.; Knoll M.; Levine O. S.; Howie S. R.; Adegbola R. A.; Greenwood B. M.; Corrah T. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect. Dis. 2016, 16, 703–711. 10.1016/S1473-3099(16)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck R. W. Jr.; Gurtman A.; Rubino J.; Smith W.; van Cleeff M.; Jayawardene D.; Giardina P. C.; Emini E. A.; Gruber W. C.; Scott D. A.; Schmöle-Thoma B. Randomized, Controlled Trial of a 13-Valent Pneumococcal Conjugate Vaccine Administered Concomitantly with an Influenza Vaccine in Healthy Adults. Clin. Vaccine Immunol. 2012, 19, 1296–1303. 10.1128/CVI.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner B. D.; Mueller J. E.; Yaro S. African meningitis belt pneumococcal disease epidemiology indicates a need for an effective serotype 1 containing vaccine, including for older children and adults. BMC Infect. Dis. 2010, 10, 22. 10.1186/1471-2334-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann B.; Pragani R.; Anish C.; Pereira C. L.; Seeberger P. H. Synthesis of conjugation-ready zwitterionic oligosaccharides by chemoselective thioglycoside activation. Chem. Sci. 2014, 5, 1992–2002. 10.1039/C3SC53362J. [DOI] [Google Scholar]
- Wu X.; Cui L.; Lipinski T.; Bundle D. R. Synthesis of monomeric and dimeric repeating units of the zwitterionic type 1 capsular polysaccharide from Streptococcus pneumoniae. Chem. Eur. J. 2010, 16, 3476–3488. 10.1002/chem.200902460. [DOI] [PubMed] [Google Scholar]
- Christina A. E.; van den Bos L. J.; Overkleeft H. S.; van der Marel G. A.; Codee J. D. C. Galacturonic Acid Lactones in the Synthesis of All Trisaccharide Repeating Units of the Zwitterionic Polysaccharide Sp1. J. Org. Chem. 2011, 76, 1692–1706. 10.1021/jo102363d. [DOI] [PubMed] [Google Scholar]
- Pfister H. B.; Mulard L. A. Synthesis of the zwitterionic repeating unit of the O-antigen from Shigella sonnei and chain elongation at both ends. Org. Lett. 2014, 16, 4892–4895. 10.1021/ol502395k. [DOI] [PubMed] [Google Scholar]
- Emmadi M.; Kulkarni S. S. Recent advances in synthesis of bacterial rare sugar building blocks and their applications. Nat. Prod. Rep. 2014, 31, 870–879. 10.1039/C4NP00003J. [DOI] [PubMed] [Google Scholar]
- Reinert R.; Paradiso P.; Fritzell B. Advances in pneumococcal vaccines: the 13-valent pneumococcal conjugate vaccine received market authorization in Europe. Expert Rev. Vaccines 2010, 9, 229–236. 10.1586/erv.10.6. [DOI] [PubMed] [Google Scholar]
- Buskas T.; Li Y.; Boons G. J. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chem. Eur. J. 2004, 10, 3517–3524. 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- Schumann B.; Hahm H. S.; Parameswarappa S. G.; Reppe K.; Wahlbrink A.; Govindan S.; Kaplonek P.; Pirofski L.; Witzenrath M.; Anish C.; Pereira C. L.; Seeberger P. H. A Semisynthetic Streptococcus pneumoniae Serotype 8 Glycoconjugate Vaccine. Sci. Transl. Med. 2017, 9, eaaf5347. 10.1126/scitranslmed.aaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt D.; Plikaytis B. D.; Akkoyunlu M.; Antonello J.; Ashton L.; Blake M.; Burton R.; Care R.; Durant N.; Feavers I.; Fernsten P.; Fievet F.; Giardina P.; Jansen K.; Katz L.; Kierstead L.; Lee L.; Lin J.; Maisonneuve J.; Nahm M. H.; Raab J.; Romero-Steiner S.; Rose C.; Schmidt D.; Stapleton J.; Carlone G. M. Establishment of a New Human Pneumococcal Standard Reference Serum, 007sp. Clin. Vaccine Immunol. 2011, 18, 1728–1736. 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M.; Gohil S.; Coleman J. R.; Manix C.; Pirofski L.-a. Antibodies to Streptococcus pneumoniae Capsular Polysaccharide Enhance Pneumococcal Quorum Sensing. mBio 2011, 2, e00176-11. 10.1128/mBio.00176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio K.; Manix C.; Guimaraes A. J.; Nosanchuk J. D.; Pirofski L. Aggregation of Streptococcus pneumoniae by a Pneumococcal Capsular Polysaccharide-Specific Human Monoclonal IgM Correlates with Antibody Efficacy In Vivo. Clin. Vaccine Immunol. 2010, 17, 713–721. 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christina A. E.; Blas Ferrando V. M.; Overkleeft H. S.; Codee J. D. C.; van der Marel G. A.; de Bordes F.; Spruit W. A. Multigram-scale synthesis of an orthogonally protected 2-acetamido-4-amino-2,4,6-trideoxy-D-galactose (AAT) building block. Carbohydr. Res. 2012, 356, 282–287. 10.1016/j.carres.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Schumann B.; Parameswarappa S. G.; Lisboa M. P.; Kottari N.; Guidetti F.; Pereira C. L.; Seeberger P. H. Nucleophile-Directed Stereocontrol Over Glycosylations Using Geminal-Difluorinated Nucleophiles. Angew. Chem. Int. Ed. 2016, 55, 14431–14434. 10.1002/anie.201606774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.