Abstract

How birds sense the variations in Earth’s magnetic field for navigation is poorly understood, although cryptochromes, proteins homologous to photolyases, have been proposed to participate in this magnetic sensing. Here, in electrochemical studies with an applied magnetic field, we monitor the repair of cyclobutane pyrimidine dimer lesions in duplex DNA by photolyase, mutants of photolyase, and a modified cryptochrome. We find that the yield of dimer repair is dependent on the strength and angle of the applied magnetic field even when using magnetic fields weaker than 1 gauss. This high sensitivity to weak magnetic fields depends upon a fast radical pair reaction on the thymines leading to repair. These data illustrate chemically how cyclobutane pyrimidine dimer repair may be used in a biological compass informed by variations in Earth’s magnetic field.
Short abstract
DNA electrochemistry studies illustrate how thymine dimer repair by photolyase and a truncated cryptochrome depends upon weak magnetic fields and thus may serve as a biological compass.
Introduction
Migratory birds and other animals can detect Earth’s magnetic field to guide navigation, though the mechanisms underlying this magnetic sensing are unclear.1 The two mechanisms proposed to explain the phenomenon of avian magnetoreception are not mutually exclusive and involve sensing using (i) magnetically sensitive radical pairs or (ii) magnetic iron-containing nanoparticles.2 Photolyases are enzymes that repair UV-induced lesions and contain a highly conserved core structure that could be involved in such magnetosensitive radical pair chemistry,3 although experiments exploring the magnetosensitivity of DNA-bound photolyase have not been reported previously. The conserved region contains a redox-active flavin adenine dinucleotide (FAD) cofactor, which absorbs blue light, and carries out electron transfers with pyrimidine dimer lesions via a cavity in the center of a DNA-binding groove.4,5
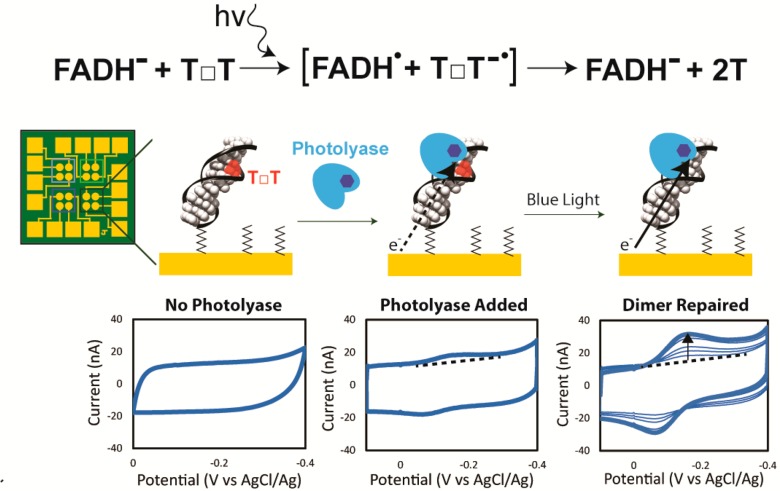
We have developed electrochemical methods to monitor the repair of cyclobutane pyrimidine dimer (CPD) lesions by photolyase.6Escherichia coli photolyase repairs a cyclobutane pyrimidine dimer in a reductive catalytic cycle upon irradiation of the fully reduced flavin cofactor (FADH–) with blue light. CPD lesions form as a result of a photoinduced [2 + 2] cycloaddition between two adjacent pyrimidines, typically thymines, on the same DNA strand and significantly kink duplex DNA. We employ DNA-modified electrodes immersed in aqueous buffer using DNA charge transport (DNA CT) to monitor repair of the CPD lesion within a DNA oligonucleotide duplex. DNA CT relies on charge moving through the internal base pair stack of the DNA duplex, and the efficiency of DNA CT is extremely sensitive to disruptions in base stacking such as those that arise with a CPD lesion.7 Upon repair of the CPD by photolyase, DNA regains its well-stacked structure and is able to support efficient DNA CT to the flavin cofactor. As a result, the repair of CPD lesions by photolyase is monitored as an increase in electrochemical response, because the repair directly improves the yield of DNA-mediated CT between the electrode and flavin. Figure 1 illustrates this electrical monitoring of repair through cyclic voltammetry (CV) performed on one set of gold electrodes modified with duplex DNA containing a CPD, bound by photolyase and irradiated with blue light. When bound to the CPD, the redox-active flavin is apparent at −120 mV versus AgCl/Ag, though the signal is small, owing to the presence of the intervening CPD; upon irradiation, the photolyase repairs the CPD and the signal increases.
Figure 1.
Cyclic voltammetry of thymine dimer repair by photolyase. (Top) Reductive catalytic cycle of the flavin cofactor in photolyase to repair thymine dimers. (Bottom) Cyclic voltammetry on multiplexed chip electrodes modified with 29 bp dsDNA and backfilled with mercaptohexanol. The reaction cartoon on the electrode is shown above with corresponding CV below. (Left) Monolayer of duplex DNA (29 bp), each with a single thymine dimer (red T□T), is scanned anaerobically at 100 mV/s in Tris buffer (50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 10% glycerol, pH 7.5). (Center) Addition of E. coli photolyase (50 μM) shows a small flavin redox peak centered around −100 mV vs AgCl/Ag, which is consistent with the fully reduced flavin. (Right) Irradiation with blue light repairs the thymine dimer over time and increases the yield of charge transferred through the DNA duplex to and from the flavin. After subtracting the background current (dotted line), the area under the reductive peak can be integrated to give the total charge transferred to the flavin.
We can explore directly how a magnetic field affects the DNA repair reaction carried out by photolyase using this electrochemistry in the presence of a magnetic field. Indeed, the electrode serves to orient the DNA and DNA reaction relative to the magnetic field. Previous experiments have monitored changes in the transient absorption spectra of photolyase in the presence of a magnetic field,8 but in the absence of DNA; it is therefore unclear from these experiments how photolyase activity on its DNA substrate is affected by a magnetic field. Indeed, we find a remarkable sensitivity to low strength magnetic fields in this reaction to repair cyclopyrimidine dimers and not only by photolyase but also by a truncated cryptochrome, the protein family thought to be responsible for magnetoreception generally.
Results and Discussion
Effects of Magnetic Fields on the Photolyase Reaction
Multiplexed chips consisting of 16 separate DNA-modified gold electrodes allow for the simultaneous or sequential comparison of four distinct monolayers created under identical conditions with 4-fold redundancy,9 and thus these multiplexed chips allow the evaluation of the effects of magnetic fields on the photolyase reaction in a well-controlled system. Figure 2 shows representative data from a single multiplexed chip where two quadrants were incubated with the same thiolated duplex DNA containing a thymine dimer (T□T); the third quadrant contains duplex DNA with a C:A mismatch intervening between the T□T and the gold surface, and the last quadrant contains duplex DNA with no dimer or mismatches. When photolyase is added to a monolayer of duplex DNA, containing a T□T (29 bp duplexes, ∼8 pmol/cm2) in the absence of an applied magnetic field, irradiation with blue light (405 ± 10 nm) leads to the increase in current for the FADH– redox couple. Importantly, the gold surface uniformly orients the DNA as well as the DNA-bound photolyase. Shining light on an identical monolayer but in the presence of an applied magnetic field, however, leads to a significant decrease in the yield of charge transferred over the same period of time. The lack of signal on the electrode modified with DNA but without the T□T shows that the photolyase binds specifically to its substrate CPD lesion. Furthermore, incorporating a single mismatch significantly decreases the yield of charge transferred to the flavin, indicating that the flavin is reduced and oxidized by charge transferred through the DNA duplex; perturbations to the base stack as occurs with a mismatch are sufficient to decrease DNA CT.
Figure 2.
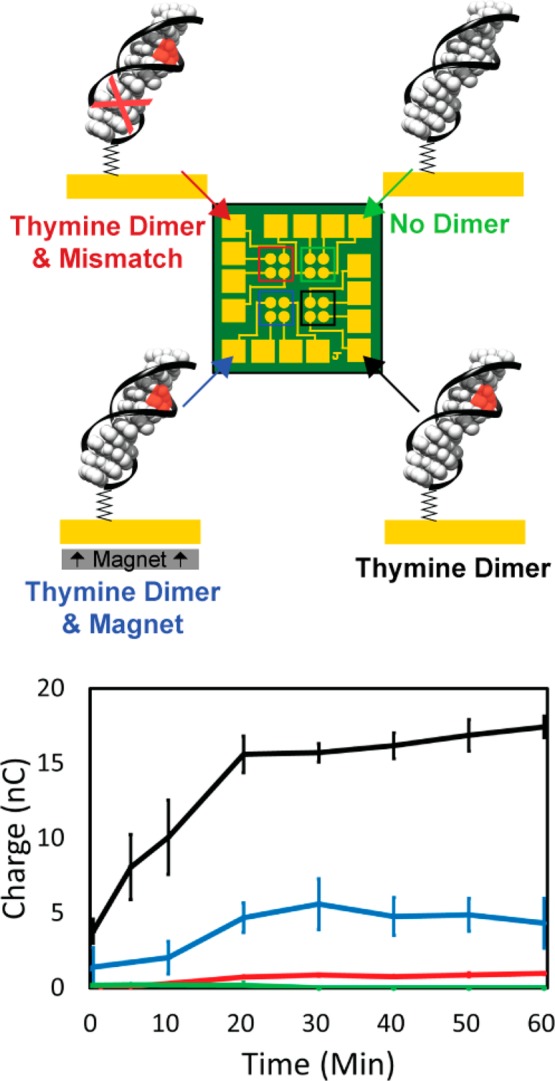
Integrated cyclic voltammetry measurement of a representative multiplexed chip over time irradiated. (Top) Representation of the multiplexed chip and the different duplex DNA monolayers and experimental conditions that were tested. (Bottom) Plot of the area under the reductive peak, which gives the total amount of charge transferred to the flavin, over time irradiated. The color of traces corresponds to the quadrants shown in the representation above. In each case, 50 μM photolyase was added and irradiated with blue light at t = 0 anaerobically in Tris buffer (50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 10% glycerol, pH 7.5). In the green quadrant, the 29 bp dsDNA contained no thymine dimer. In the red quadrant, the 29 bp dsDNA contained a thymine dimer and a C:A mismatch between the dimer and the electrode surface. In the black quadrant, the 29 bp dsDNA contained a thymine dimer. In the blue quadrant, the same 29 bp dsDNA containing a thymine dimer was used as was tested in the black quadrant, but the entire experiment was conducted with a 560 G magnetic field pointing perpendicularly up intersecting the plane of the electrode. Standard error was plotted with n = 4.
Control experiments show that the protein is still active after multiple hours of incubation in a magnetic field and that the protein binds competitively to CPD-containing duplex DNA (Figure S1). There is also no distinguishable difference when adding this CPD-containing duplex DNA in the presence or absence of a magnetic field, suggesting that the magnetic field does not cause a significant change in photolyase affinity for CPD. Assays with a SQUID magnetometer furthermore show that there is no magnetite on the electrode surface that is influencing this chemistry (Figure S2).
The magnetic field influence on the yield of DNA CT depends upon when the magnetic field is applied during the reaction. The presence or absence of an externally applied magnetic field during flavin photoreduction, before the incubation of the protein with the duplex DNA substrate, does not influence the signal during repair (Figure S3). Importantly, removing the magnetic field during repair restores the yield of charge transfer (Figure 3). After repair has been completed, adding a magnetic field has no influence on the yield of charge transferred. Randles–Sevcik analysis (Figure S4) demonstrates that photolyase diffuses away from surface of the DNA-modified electrode when there is no applied magnetic field, associated with repair of the CPD and lowering of the protein affinity, but the photolyase stays bound to the surface with an applied magnetic field. Together, these data indicate that the presence of the magnetic field during repair directly inhibits the efficiency of repair.
Figure 3.
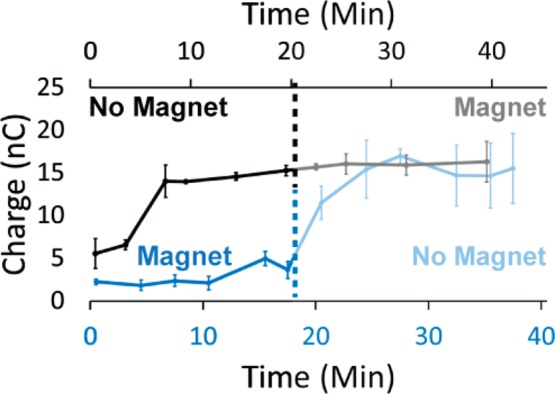
Total amount of charge transferred over time irradiated with varying magnetic field conditions. First, 50 μM photolyase was added to a monolayer of 29 bp dsDNA with T□T and irradiated with blue light (t = 0) anaerobically in the absence (black) or presence (blue) of a 30 G magnetic field applied perpendicularly up intersecting the plane of the electrode. At the time indicated by the dotted line the magnetic field was either applied (gray) or removed (light blue) to switch the magnetic field conditions in a given experiment. Standard error was plotted with n = 4.
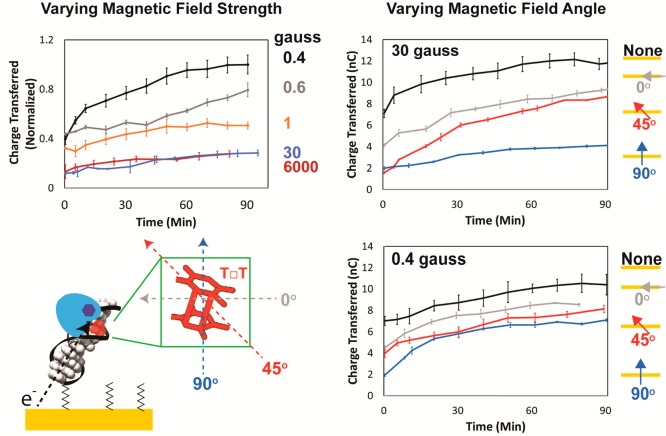
Figure 4 illustrates how the repair efficiency varies with magnetic field strength and angle. Significantly, at weak magnetic fields, the magnetic field strength plays an important role in the efficiency of dimer repair. The background magnetic field during our experiments was measured to be 0.4 G and resulted in the highest yield of repair. However, applying an additional magnetic field perpendicular to the surface as weak as 0.2 G results in diminished yield. Increasing the field strength further decreases the yield, but eventually the effect is saturated; applied fields of 30 and 6000 G result in the same magnitude decrease in yield. As a control, we also examined the enzymatic restriction of an oligonucleotide by HincII; as expected, this reaction is not influenced by the presence of a magnetic field, nor by irradiation (Figure S14).
Figure 4.
Total amount of charge transferred over time irradiated with varying magnetic field strengths and angles. In all experiments photolyase (50 μM) was added to 29 bp dsDNA-modified electrodes containing T□T and then irradiated with blue light (t = 0) anaerobically in Tris buffer (50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 10% glycerol, pH 7.5). (Top left) The background magnetic field was 0.4 G, and the applied field was added to give the total field strength listed to the right of the plot. (Top right) The magnetic field was applied perpendicularly up intersecting the plane of the electrode surface. The magnetic field angle was varied by applying a 30 G field (top right) or 0.4 G field (bottom right) at either a 0°, 45°, or 90° angle relative to the plane of the electrode surface. (Bottom left) The approximate angles at which the magnetic fields intersect thymine dimers are illustrated. The redox potential of the flavin lies negative of the potential of zero charge of the working electrode. At this potential the duplexes line up approximately normal to the electrode surface, meaning that the thymines are approximately parallel to the surface.9 The largest magnetic field effect occurs when the field intersects the dimer perpendicular to the plane of the bases, and the weakest effect occurs when the field is parallel to the plane of the bases. Standard error was plotted with n ≥ 4.
Moreover, the angle of the magnetic field relative to the plane of the electrode significantly influences the yield. A magnetic field perpendicular to the plane of the electrode exhibits the largest effect. Changing the angle of inclination to 45° diminishes the effect, as does applying a field parallel to the plane of the surface. Interestingly, there is no difference in yield observed for a magnetic field pointing perpendicularly up versus perpendicularly down (Figure S5), which suggests that only the angle of the field and not the polarity direction of the field is important.10
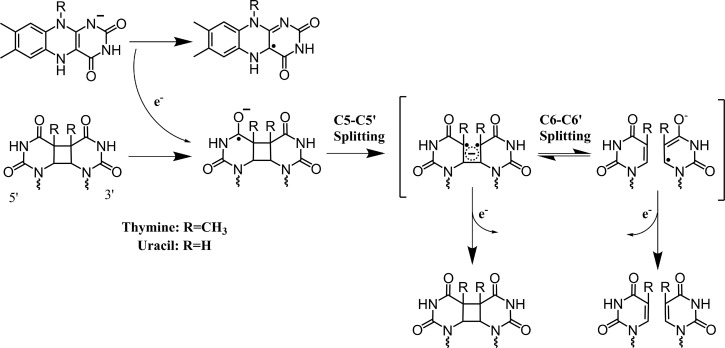
These results clearly illustrate that the CPD reaction is sensitive to low magnetic field strengths and field direction. These data are reminiscent of experiments carried out by N. J. Turro, who established conditions critical for observation of reactions controlled by weak magnetic fields.11 What is required is a competition between two processes: one that is magnetic field dependent and one that is magnetic field independent. Figure 5 illustrates the CPD repair reaction carried out by photolyase.12 Here radical pair formation followed by electron transfer leads either to separation of the two repaired thymines or to futile back electron transfer without repair. Thus, the magnetic field dependent radical pair affects the efficiency of the subsequent bond-breaking repair reaction.
Figure 5.
Radical repair scheme for cyclobutane pyrimidine dimers (CPD) based on previous work.11 Forward electron transfer from the fully reduced flavin results in a radical residing on the CPD. First the C5–C5′ bond splits, followed by either C6–C6′ bond splitting or futile back electron transfer to the CPD state. Following bond splitting, either the radical residing on the pyrimidine can undergo electron return to the flavin, resulting in the completion of the repair process, or the radical can facilitate CPD formation and undergo futile back electron transfer.
Mutations in Photolyase and the CPD Lesion
To examine the factors governing this reaction in more detail, we tested mutants of photolyase that perturb the internal electron transfer pathways. In particular, we would expect mutations that affect the lifetime of the CPD radical pair to be most sensitive to magnetic field effects. Radical pairs that involve both the flavin and dimer radical anion could serve also as magnetically sensitive intermediates in the repair reaction. Photoactivation of FAD initiates electron transfers along a conserved triad of tryptophan residues that gives a flavin radical (FAD•) and a tryptophan radical (TrpH+•) that have been shown through transient absorption spectroscopy to be sensitive to weak applied magnetic fields (in the range of 30–390 G); this range is, however, stronger than the earth’s magnetic field (0.25–0.65 G).3 Multiple photolyase active site mutants were previously characterized using ultrafast spectroscopy to determine the rates of electron transfer and bond breaking steps in CPD repair in the absence of a magnetic field.12−15 The mutant N378C interacts with the flavin and displays slow forward electron transfer from the flavin to the dimer and only slightly reduced electron return from the thymine radical to the flavin. M345A interacts with both the dimer and the flavin and shows increased rates for forward electron transfer and electron return. E274A also interacts with both the flavin and the dimer and has faster electron return but slower forward electron transfer. As shown in Figure 6, we find that both of the mutations near the dimer eliminate magnetosensitivity. In contrast, the N378C mutant retains magnetosensitivity despite having a destabilized flavin radical, which has its redox potential shifted −100 mV relative to WT (Figure S6). The magnetosensitivity of these mutants correlates with slower electron return and does not correlate with forward electron transfer or quantum yield of CPD repair.13 Importantly, these results point to the radical pair on the pyrimidine dimer as the critical player rather than radical pairs that involve the flavin.
Figure 6.
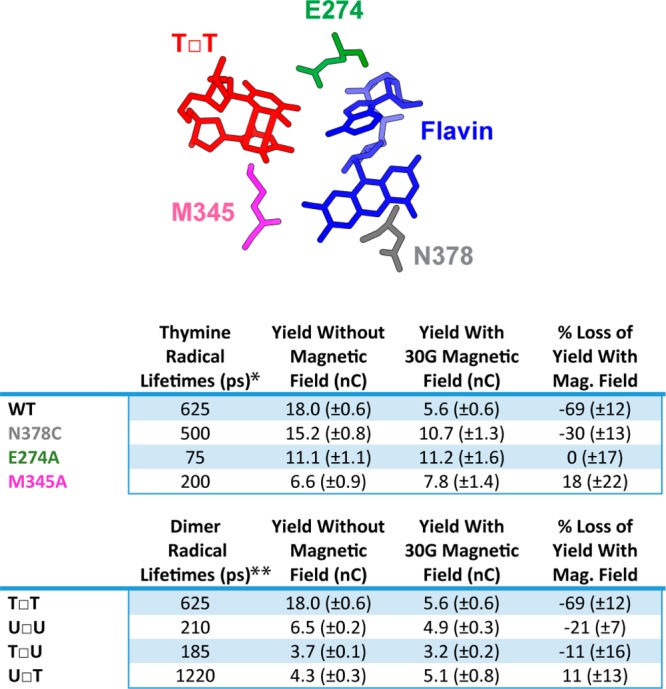
Effect of structural perturbations on magnetosensitivity. (Top) Cartoon showing placement of thymine dimer relative to flavin cofactor and three active site residues in photolyase. Positions of residues adapted from DNA-bound photolyase crystal structure.15 (Middle) Comparison of the yield of charge transferred to different active site mutants with and without 30 G magnetic field perpendicularly intersecting the plane of the electrode surface after 60 min of irradiation with blue light anaerobically in Tris buffer (50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 10% glycerol, pH 7.5). (Bottom) Comparison of the yield of charge transferred to different cyclobutane pyrimidine dimers with and without 30 G magnetic field perpendicularly intersecting the plane of the electrode surface after 60 min of irradiation with blue light. For these dimers U = uracil, T = thymine, and the 5′ position is listed first with the 3′ position second. Standard error was included with n ≥ 6. *Lifetimes of thymine radicals with mutant photolyase were obtained from C. Tan et al.13 **Lifetimes of different dimer radicals were obtained from Z. Liu et al.14
Repair of uracil-containing dimers further reveal the underlying basis for magnetosensitivity. In these experiments the repair of T□T, U□T, T□U, and U□U dimers were monitored with and without an applied magnetic field and are presented in Figure 6. The U□U dimer has diminished but significant magnetosensitivity. Both the U□T and T□U dimers show no significant magnetosensitivity, despite the T□U having a radical lifetime on par with the U□U, and the U□T having a longer radical lifetime than any of the other dimers.14 Together these data argue further that perturbation in the uracil/thymine radical pairs most affect the magnetosensitivity of CPD repair.
These data thus allow us to pinpoint the likely source of magnetosensitivity in photolyase repair: the pyrimidine dimer. Active site mutations near the CPD as well as changes in the CPD structure eliminate magnetosensitivity, but the N378C mutation near the flavin and away from the CPD retains magnetosensitivity. The crucial condition for observation of magnetic field effects is a competition between two processes, and the magnetic field changes their relative favorability.11 As illustrated in Figure 5, our data show that the competition either to maintain or to repair the CPD is shifted toward maintenance with an externally applied magnetic field. The magnetosensitive chemistry serves to perturb the favorability of C6–C6′ splitting that results in repaired CPD and futile back electron transfer that maintains the CPD.13 Rapid singlet–triplet interconversion, as has been observed in biradical species, can change the favorability of bond cleavage versus reformation.16 A second competition occurs after the C6–C6′ splitting because the radical resides on one of the pyrimidines long enough that it may recreate the dimer before it safely returns to the flavin.13,17 However, the limited influence of the N378C mutant on magnetosensitivity suggests that this second process is unlikely to be the primary source of competition. Weak magnetic fields on the order of 10–100 G can influence the equilibrium between singlet and triplet states and change the reaction products of biradical species,18 similar to what occurs during CPD repair on the thymine dimer. It is not immediately clear why CPD repair is still more sensitive than these reactions, but the short radical lifetimes and very fast equilibration between singlet and triplet states may be critical factors. The confinement of the thymine dimer within the photolyase binding pocket may further aid the magnetosensitivity of the radical pair by constraining conformation, thereby stabilizing the relative energy of the singlet and triplet states and minimizing energy changes caused by conformational motion that has previously been shown to overwhelm magnetic field effects. Certainly these data serve to highlight that it is the radical pair on the thymines that leads to the magnetosensitivity.
Reaction of a Truncated Cryptochrome in the Absence and Presence of a Magnetic Field
If it is the radical pair of the thymine dimer that is critical to the biological compass, the relevance to magnetoreception by the homologous cryptochromes becomes difficult to understand. Cryptochromes have been implicated in magnetosensitive processes in both animals and plants.19 Cryptochromes are essentially defined by their homology to photolyases but also by their inability to carry out CPD repair.1,20 Explanations for this inability to repair CPD lesions range from structural differences in the domains of cryptochromes versus photolyases to the possibility that their inability to repair CPD is reliant on special conditions. In cryptochromes, C-terminal extensions appear to block the DNA-binding pocket and could prevent CPD repair except under conditions where the extensions are released.21,22 Few crystal structures of cryptochromes are available, however a crystal structure of Arabidopsis CRY1 photolyase-homologous region, a model of Arabidopsis CRY2 photolyase-homologous region, and a crystal structure of Drosophila cryptochrome show partial conservation of the positively charged groove of photolyase that could allow for DNA to associate with the active site if the C-terminal extension were released from this region.22−26 It is important to note that Arabidopsis CRY2-GFP fusion proteins bind to chromosomes within mitotic cells.12 It is also noteworthy that a single amino acid substitution has previously revealed photolyase activity in Arabidopsis cry1 attributed to stabilization of the reduced flavin; here poor DNA binding was cited as a possible reason for its very low efficiency.27
We therefore tested a truncated Arabidopsis thaliana cryptochrome 1 lacking the C-terminal extension (AtCRY1ΔC) for CPD repair in the presence and absence of an applied magnetic field. The truncated cryptochrome is 509 amino acids in length and lacks the entire C-terminal tail (172 amino acids). The crystal structure of AtCRY1ΔC has also been reported,28 and while it has been shown specifically that the wild type protein does not repair dsDNA,29 there are no reports describing the ability of this truncated protein to repair dsDNA. We therefore tested repair by the truncated protein both in solution and electrochemically. The truncated protein, AtCRY1ΔC, was first incubated with dsDNA containing T□T and irradiated with blue light in aqueous solution. HPLC traces of the DNA before and after incubation show that AtCRY1ΔC repairs T□T similarly to the photolyase (Figure S7). We used matrix assisted laser desorption ionization time-of-flight mass spectroscopy (MALDI-TOF) to characterize the mass of the CPD-containing oligonucleotide before and after incubation with AtCRY1ΔC and found that while the HPLC mobility had changed, the m/z did not shift, as would be expected with dimer repair. We then used a phosphodiesterase to digest the DNA before and after incubation with AtCRY1ΔC. The peak characteristic of the thymine dimer dinucleotide, distinct from the individual nucleotides, was identified using HPLC combined with time-of-flight mass spectrometry, and this peak decreased upon incubation with AtCRY1ΔC, but only in the presence of blue light; this result chemically confirms that the CPD is being repaired by irradiation of the cryptochrome (Figures S12 and S13).
Thymine dimer repair by AtCRY1ΔC was then monitored both electrochemically and in solution in the presence and absence of a magnetic field (Figure 7). When the cryptochrome is added to a monolayer of duplex DNA containing T□T in the absence of an applied magnetic field, irradiation with blue light leads to the increase in current for the FAD redox couple (Figures 7, S8). Shining light on an identical monolayer in the presence of an applied magnetic field, however, leads to a significant reduction in the yield of charge transferred over the same period of time, consistent with the change observed for photolyase. In solution, where the protein bound to DNA is randomly oriented, quantitation of the DNA by digestion following irradiation shows a decrease in the T□T dinucleotide in the absence of a magnetic field, consistent with repair upon irradiation, but a similar decrease is evident also in the presence of the magnetic field (Figure 7). Thus, repair in solution requires irradiation but is insensitive to magnetic field direction. This result contrasts experiments conducted on the DNA-modified electrode, consistent with the idea that protein orientation is needed for magnetosensitivity. Whether such orientation is provided biologically on chromatin requires examination. It should be noted also that the diminished signal on the electrode modified with DNA without thymine dimers shows that AtCRY1ΔC binds preferentially to the CPD lesion (Figure S9). Binding of AtCRY1ΔC to dsDNA containing a CPD lesion was further confirmed using electrophoretic mobility shift experiments (Figure S10).
Figure 7.
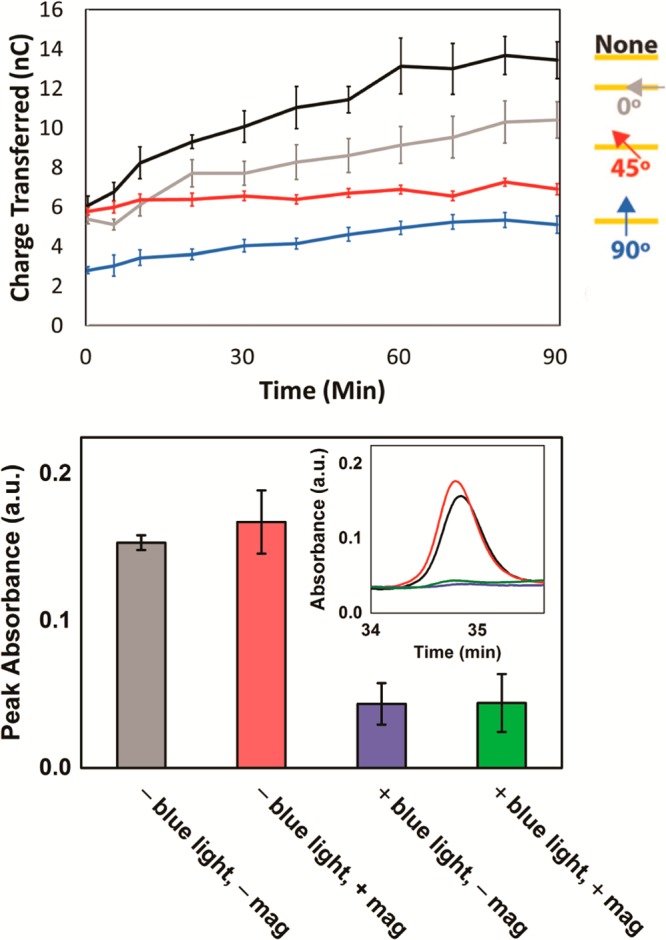
Monitoring CPD repair by cryptochrome (AtCRY1ΔC). (Top) Electrochemical experiments show the total amount of charge transferred to the flavin of AtCRY1ΔC over time irradiated with varying magnetic field angles. 50 μM cryptochrome was added to a monolayer of 29 bp dsDNA with T□T and irradiated with blue light (t = 0) anaerobically in Tris buffer (50 mM Tris-HCl, 50 mM KCl, 1 mM EDTA, 10% glycerol, pH 7.5). The background magnetic field was 0.4 G, and the applied field was added to this to give the total field strength of 30 G. The magnetic field angle was varied by applying the magnetic field at either a 0°, 45°, or 90° angle relative to the plane of the electrode surface. Standard error was plotted with n = 4. (Bottom) Monitoring CPD repair with AtCRY1ΔC by HPLC. Duplex DNA containing T□T was incubated under anaerobic conditions in solution for 1 h at ambient temperature with AtCRY1ΔC with and without a 6600 G magnetic field, and in the presence or absence of blue light. Phosphodiesterase I was then used to digest the DNA, and the HPLC peak characteristic of the thymine dimer (inset) was quantified and compared.
Importantly, and consistent with the experiments on photolyase, the angle of the magnetic field relative to the plane of the electrode significantly influences the yield of dimer repair by the cryptochrome (Figure 7). A magnetic field perpendicular to the plane of the electrode exhibits the largest effect. Changing the angle of inclination to 45° diminishes the effect, as does applying a field parallel to the plane of the surface. Thus, a cryptochrome with its C-terminal domain removed can in fact serve as a compass by carrying out the CPD repair reaction. Indeed, binding of the C-terminal domain may prevent DNA from accessing the flavin and provide a regulatory element for the reaction.
Conclusions
These experiments illustrate the design of a biological compass that functions at weak magnetic field strengths. Weak magnetic fields significantly affect the repair of CPD lesions by E. coli photolyase and by A. thaliana cryptochrome with removal of its C-terminal domain. This magnetosensitivity is dependent on the magnetic field strength and direction. What is central to the magnetosensitivity we observe with photolyase and cryptochrome is the CPD repair reaction, a reaction involving a short-lived radical pair that governs a subsequent bond-breaking reaction, the dimer repair. Experiments with photolyase active site mutants and uracil-containing lesions point to radical pair chemistry on the CPD as the source of magnetosensitivity. These experiments offer insight into a plausible way that nature could use radical pair chemistry to sense the angle of magnetic fields as weak as Earth’s and certainly suggest the need to examine more closely whether CPD repair plays any role in vivo in magnetic sensing.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to the NIH (GM061077 to J.K.B. and GM074813 to D.Z.) for their financial support. E.C.M.T. appreciates the Croucher Foundation for a postdoctoral fellowship. T.J.Z. is an NSF fellow (DGE-1144469) and would like to thank them for financial support of this research. We thank Jennifer Buz and Joseph Kirschvink for discussion and aid with SQUID measurements. We thank Mona Shahgholi and the Caltech MultiUser Mass Spectrometry Lab for help conducting mass spectrometry experiments. We dedicate this manuscript to Nicholas J. Turro, whose studies on radical pair reactions in weak fields stimulated this work.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00008.
Materials and methods along with figures illustrating additional experimental details and controls (PDF)
Author Contributions
T.J.Z. and J.K.B. designed the experiments. T.J.Z. performed most of the experiments. E.C.M.T. performed gel shift experiments, some electrochemistry experiments, DNA digestion, HPLC, and MS product analysis. D.Z. produced and purified all proteins as well as provided guidance for proper protein handling. T.J.Z. and J.K.B. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Wiltschko W.; Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol., A 2005, 191, 675–693. 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- Hore P. J.; Mouritsen H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. 10.1146/annurev-biophys-032116-094545. [DOI] [PubMed] [Google Scholar]
- Maeda K.; et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 4774–4779. 10.1073/pnas.1118959109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S. Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochim. Biophys. Acta, Bioenerg. 2005, 1707, 1–23. 10.1016/j.bbabio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003, 103, 2203–2238. 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- DeRosa M. C.; Sancar A.; Barton J. K. Electrically monitoring DNA repair by photolyase. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 10788–10792. 10.1073/pnas.0503527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genereux J. C.; Barton J. K. Mechanisms for DNA charge transport. Chem. Rev. 2010, 110, 1642–1662. 10.1021/cr900228f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henbest K. B.; et al. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 14395–14399. 10.1073/pnas.0803620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinker J. D.; Muren N. B.; Gorodetsky A. A.; Barton J. K. Multiplexed DNA-modified electrodes. J. Am. Chem. Soc. 2010, 132, 2769–2774. 10.1021/ja909915m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S. O.; et al. Orienting DNA helices on gold using applied electric fields. Langmuir 1998, 14, 6781–6784. 10.1021/la980874n. [DOI] [Google Scholar]
- Gould I. R.; Turro N. J.; Zimmt M. B. Magnetic field and magnetic isotope effects on the products of organic reactions. Adv. Phys. Org. Chem. 1984, 20, 1–53. 10.1016/S0065-3160(08)60147-1. [DOI] [Google Scholar]
- Zhong D. Electron transfer mechanisms of DNA repair by photolyase. Annu. Rev. Phys. Chem. 2015, 66, 691–715. 10.1146/annurev-physchem-040513-103631. [DOI] [PubMed] [Google Scholar]
- Tan C.; et al. The molecular origin of high DNA-repair efficiency by photolyase. Nat. Commun. 2015, 6, 7302. 10.1038/ncomms8302. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Wang L.; Zhong D. Dynamics and mechanisms of DNA repair by photolyase. Phys. Chem. Chem. Phys. 2015, 17, 11933–11949. 10.1039/C4CP05286B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mees A.; et al. Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science 2004, 306, 1789–1793. 10.1126/science.1101598. [DOI] [PubMed] [Google Scholar]
- Griesbeck A. G.; Mauder H. M.; Stadtmuller S. Intersystem crossing in triplet 1,4-biradicals: conformational memory effects on the stereoselectivity of photocycloaddition reactions. Acc. Chem. Res. 1994, 27, 70–75. 10.1021/ar00039a002. [DOI] [Google Scholar]
- Huels M. A.; Boudaiffa B.; Cloutier P.; Hunting D.; Sanche L. Single, double, and multiple double strand breaks induced in DNA by 3–100 eV electrons. J. Am. Chem. Soc. 2003, 125, 4467–4477. 10.1021/ja029527x. [DOI] [PubMed] [Google Scholar]
- Zimmt M. B.; Doubleday C. Jr.; Turro N. J. Magnetic field effect on the intersystem crossing rate constants of biradicals measured by nanosecond transient UV absorption. J. Am. Chem. Soc. 1985, 107, 6726. 10.1021/ja00309a060. [DOI] [Google Scholar]
- Ahmad M.; Galland P.; Ritz T.; Wiltschko R.; Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2007, 225, 615–624. 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- Müller M.; Carell T. Structural biology of DNA photolyases and cryptochromes. Curr. Opin. Struct. Biol. 2009, 19, 277–285. 10.1016/j.sbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Ozturk N.; Selby C. P.; Zhong D.; Sancar A. Mechanism of photosignaling by drosophila cryptochrome. J. Biol. Chem. 2014, 289, 4634–4642. 10.1074/jbc.M113.542498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. T.; et al. Flavin reduction activates Drosophila cryptochrome. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 20455–20460. 10.1073/pnas.1313336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.; Liu H.; Klejnot J.; Lin C. The cryptochrome blue light receptors. Arabidopsis Book 2010, 8, e0135. 10.1199/tab.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski B. D.; et al. Structure of full-length Drosophila cryptochrome. Nature 2011, 480, 396–399. 10.1038/nature10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heelis P. F.; Payne G.; Sancar A. Photochemical properties of Escherichia coli DNA photolyase: selective photodecomposition of the second chromophore. Biochemistry 1987, 26, 4634–4640. 10.1021/bi00389a007. [DOI] [PubMed] [Google Scholar]
- Ozturk N.; et al. Purification and characterization of a type III photolyase from Caulobacter crescentus. Biochemistry 2008, 47, 10255–10261. 10.1021/bi801085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney S.; et al. Single amino acid substitution reveals latent photolyase activity in Arabidopsis cry1. Angew. Chem., Int. Ed. 2012, 51, 9356–9360. 10.1002/anie.201203476. [DOI] [PubMed] [Google Scholar]
- Brautigam C. A.; et al. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12142–12147. 10.1073/pnas.0404851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K.; et al. Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapsis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry 1995, 34, 6892–6899. 10.1021/bi00020a037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.