Abstract
Background
Repeated nontreponemal serologic test for syphilis titers is recommended to evaluate treatment response. However, it is unknown whether serum rapid plasma reagin (RPR) titer can serve as a surrogate for determining the efficacy of treatment in general paresis (GP) remains unknown.
Methods
We retrospectively reviewed data from 105 GP patients, who were divided into two groups (62 CSF RPR+ patients and 43 CSF RPR- patients) according to reactive RPR test status in CSF. Clinical assessment included the Mini-Mental State Examination (MMSE) scores, CSF examinations (WBC count, protein concentration and RPR titer), and serum tests (RPR titer and TPPA). Among the 105 GP patients, 13 CSF RPR+ patients and 6 CSF RPR- patients had a 12 months follow-up of CSF, serum measures and MMSE.
Results
The median serum RPR titer was significantly higher in CSF RPR+ patients than that in CSF RPR- GP patients, 1:8 [IQR 1:4–1:32] vs. 1:4 [IQR 1:4–1:8] (P < 0.001). The number of CSF RPR+ patients with serum RPR titer≥1:32 was significantly higher when compared with CSF RPR- patients (P = 0.001). For CSF RPR+ patients, the MMSE scores improved or remained constantly after penicillin treatment. For CSF RPR+ patients, the CSF RPR titer declined four-fold in 85% (11/13) of the patients, whereas the serum RPR titer declined four-fold in only 46% (6/13) of the patients, the odds ratio is 6.4 (95% confidence interval 1.0–41.2).
Conclusions
A four-fold decline in CSF RPR titer is a good predictor for treatment efficacy in CSF RPR+ GP patients within 12 months after the completion of therapy.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3062-4) contains supplementary material, which is available to authorized users.
Keywords: Neurosyphilis, General paresis, Rapid plasma regain, HIV-negative
Background
Syphilis, which is caused by Treponema pallidum, is a major health problem worldwide. Cases of syphilitic dementia (general paresis, GP), which is characterized by rapidly progressive cognitive decline with or without psychiatric features, continue to be reported in the modern era [1].
Serum treponemal and non-treponemal tests are the most widely used methods for syphilis diagnosis. Reactive serum treponemal test (TT, including T. pallidum Haemagglutination test (TPHA) and T. pallidum Particle Agglutination test (TPPA)), symptoms and signs of neurosyphilis and reactive venereal disease research laboratory (VDRL) in cerebrospinal fluid (CSF) or CSF white blood cells (WBCs) > 5/μl or CSF protein > 45 mg/dL are used in the diagnosis of symptomatic neurosyphilis [2]. A reactive rapid plasma reagin (RPR) test in CSF is considered to be the diagnostic of neurosyphilis [2, 3], but this test may be nonreactive in patients with GP depending on the criteria used to define neurosyphilis.
Repeated titers of nontreponemal (i.e., RPR, VDRL) serologic test for syphilis has been recommended to evaluate treatment response, with a four-fold decrease from baseline and/or seroreversion in 12–24 months after treatment representing an appropriate response to therapy/serologic cure [4]. For those neurosyphilis with symptoms or signs, resolution of clinical abnormalities is also considered to determine the efficacy of treatment [5].
The goal of this study is to explore whether serum RPR titer could serve as a surrogate for determining the efficacy of treatment in GP.
Methods
Patients
We retrospectively reviewed 105 GP patients (91 males and 14 females). These GP patients were late syphilis and fulfilled all of the following criteria: (1) TPPA and RPR titer were tested in serum and CSF, and were positive in serum TPPA; (2) serologic test was based on RPR positivity in CSF, or WBC count of CSF>5 cells/μL or protein of CSF>45 mg/dL with TPPA positivity in CSF [2]; (3) patient has slowly progressive deterioration in memory, personality, and habits, and with disorientation; (4) exclusion of other diagnosis. These patients were divided into two groups (62 CSF RPR+ patients and 43 CSF RPR- patients) according to reactive RPR status in CSF. Furthermore, since some GP patients were uncooperative, certain information was obtained from their relatives at admission. All patients involved in this study were diagnosed with GP for the first time and they have not received the recommended penicillin therapy [penicillin 18–24 million units intra-venous (IV) daily for 10–14 days] before the diagnosis of GP. Cognitive assessment, the Chinese version of the Mini-Mental State Examination (MMSE), was tested in all cases. It covers the following five areas of cognition: orientation, memory, attention and calculation, recall and language [6]. WBC count, protein concentration and RPR titer in CSF, RPR titer and TPPA in serum were assessed. Human immunodeficiency virus (HIV) infection was excluded in all patients using serological testing.
Thirteen CSF RPR+ GP patients returned for follow-up visits with lumbar puncture, MMSE scores and blood samples in 12 months after penicillin treatment (penicillin G, 24 million units per day for 14 days) (see Additional file 1: Table S1). Fourteen CSF RPR- GP patients returned for follow-up visits with MMSE scores and serological tests in 12 months after penicillin treatment, while 6 of them had a follow-up evaluation of CSF (see Additional file 2: Table S2). An appropriate response to the therapy in the follow-up visit was defined as a four-fold decline in RPR titer which means four-fold decrease or returning to non-reactive in 12 months after treatment.
Statistical analysis
All statistical analyses were performed by the Statistical Program for Social Sciences (SPSS) software (version 22.0, Chicago, IL, USA). Associations between categorical variables were assessed using the χ2 test or Fisher’s exact test. Associations between continuous variables and categorical variables were assessed using the Mann-Whitney U test. P < 0.05 was considered to be statistically significant.
Ethics
The study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Medical Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. The patients or the guardians of patients with severe cognitive impairment provided written informed consent. Lumbar puncture was also performed with informed consent.
Results
Patients
One hundred and five GP patients were divided into two groups (62 CSF RPR+ patients and 43 CSF RPR- patients) according to reactive RPR test status in CSF. The characteristics and CSF abnormalities of GP patients with CSF RPR+ and CSF RPR- are shown in Tables 1 and 2. The serum RPR titers of CSF RPR+ and CSF RPR- patients are shown in Fig. 1. The median serum RPR titer was significantly higher in CSF RPR+ patients than that in CSF RPR- GP patients, 1:8 [IQR 1:4–1:32] vs. 1:4 [IQR 1:4–1:8] (P < 0.001, see Table 1). The number of CSF RPR+ patients with serum RPR titer≥1:32 was significantly higher when compared with CSF RPR- patients (P = 0.001). The number of CSF RPR+ GP patients who had no abnormalities or only one abnormality on initial lumbar puncture was significantly different when compared with CSF RPR- patients (see Table 2). Furthermore, no significant correlation was found between the 1/CSF RPR titer and 1/serum RPR titer in CSF RPR+ GP patients. (r = 0.149, P = 0.249).
Table 1.
Characteristics, CSF and serum measures in CSF RPR+ and CSF RPR- patients
| Characteristic | All Patients (n = 105) | CSF RPR+ Patients (n = 62) | CSF RPR- Patients (n = 43) | P a |
|---|---|---|---|---|
| Male sex | 91/105(87%) | 54/62(87%) | 37/43(86%) | 0.876 |
| Age, median years (IQR) | 52 (47–58) | 53(47–58) | 52 (47–56) | 0.432 |
| MMSE (IQR) | 14(12–17) | 14(12–17) | 14(11–17) | 0.982 |
| CSF WBC count | ||||
| > 5 cells/μl | 26/89(29%) | 14/46(30%)b | 12/43(28%) | 0.793 |
| Median cells/ml (IQR) | 3(1–6) | 3(0.75–6) | 2(1–6) | 0.878 |
| CSF protein concentration | ||||
| > 45 mg/dl | 73/89(82%) | 34/46(74%)b | 39/43(91%) | 0.039 |
| Median mg/dl (IQR) | 62(48–75) | 62.5(45–75.25) | 61(50–75) | 0.577 |
| Reactive CSF RPR | 62/105 (59%) | 62/62(100%) | 0/43(0%) | |
| Median CSF RPR titer (IQR) | – | 1:2 (1:2–1:4) | – | |
| Serum RPR titer | ||||
| ≥ 1: 32 | 21/108(19%) | 19/62(31%) | 2/43(4.7%) | 0.001 |
| 1: 16 | 16/108(15%) | 8/62(13%) | 8/43(18.6%) | 0.424 |
| 1: 1–1: 8 | 68/108(63%) | 35/62(56%) | 33/43(76.7%) | 0.032 |
| Median serum RPR titer (IQR) | 1:8(1:4–1:16) | 1:8(1:4–1:32) | 1:4(1:4–1:8) | < 0.001 |
| Normalized by final follow-up visit | 14/27(52%) | 6/13(46%)c | 8/14(57%)d | 0.568 |
Data are no. (%) of patients, unless otherwise indicated. IQR interquartile range, RPR rapid plasma regain, MMSE mini-mental state examination
aAssociations between categorical variables were assessed using the ×2 test or Fisher’s exact test. Associations between continuous variables and categorical variables were assessed using the Mann-Whitney U test
bData are for 46 patients
cData are for 13 patients
dData are for 14 patients
Table 2.
Cerebrospinal fluid abnormalities in CSF RPR+ and CSF RPR- patients
| Characteristic | All Patients (n = 89) |
CSF RPR+ Patients (n = 46) |
CSF RPR- Patients (n = 43) |
P |
|---|---|---|---|---|
| No abnormalities | 8/89(9%) | 8/46(17%) | 0/43(0) | 0.004 |
| One abnormality | 63/89(71%) | 28/46(61%) | 35/43(81%) | 0.033 |
| Two abnormalities | 18/89(20%) | 10/46(22%) | 8/43(19%) | 0.713 |
No abnormalities, no changes of CSF WBC count (> 5 cells/μl) and CSF protein concentration (> 45 mg/dl); One abnormality, change in CSF WBC count (> 5 cells/μl) or CSF protein concentration (> 45 mg/dl); Two abnormalities, changes in CSF WBC count (> 5 cells/μl) and CSF protein concentration (> 45 mg/dl); RPR, rapid plasma regain
Fig. 1.
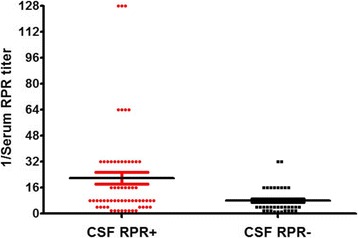
The serum RPR titers for CSF RPR+ and CSF RPR- patients in a scatter plot
Changes of MMSE and RPR titer in serum and CSF
As shown in Tables 3, 13 CSF RPR+ GP patients (including 12 males and 1 female) who received IV penicillin treatment (penicillin G, 24 million units per day for 14 days) had a follow-up evaluation including MMSE and RPR tests in both serum and CSF. The median MMSE change (the changes in MMSE score between last follow-up evaluation in 12 months after penicillin treatment and initial admission) was 4 (IQR 2–11). None of the patients who received the follow-up evaluation showed obvious cognitive deterioration on clinical examination. MMSE scores were higher at post-treatment as compared to pre-treatment (P < 0.001, see Table 3). For CSF RPR+ patients, the CSF RPR titer declined four-fold in 85% (11/13) of the patients, whereas the serum RPR titer declined four-fold in only 46% (6/13) of the patients. The odds ratio is 6.4 (95% confidence interval 1.0–41.2). The changes of CSF WBC and protein measures in 12 patients by follow-up are shown in Table 3.
Table 3.
MMSE and changes of CSF measures and serum RPR titer in CSF RPR+ patients by follow-up
| Variable | pre-treatment | 12 months after treatment | P |
|---|---|---|---|
| Increased CSF WBC counta | 5/12(42%) | 0/12(0%) | 0.012 |
| Increased CSF protein concentrationa | 7/12(58%) | 2/12(17%) | 0.035 |
| Four-fold decline in CSF RPR | – | 11/13(85%) | – |
| Four-fold decline in serum RPR | – | 6/13(46%) | – |
| Median MMSE (IQR) | 13(9.5–15) | 19(16.5–20.5) | < 0.001 |
Increased CSF WBC count means CSF WBC count > 5 cells/μl; Increased CSF protein concentration means CSF protein concentration > 45 mg/dl; RPR, rapid plasma regain; MMSE, mini-mental state examination; aData are for 12 patients
Fourteen CSF RPR- GP patients (including 13 males and 1 female) had a follow-up evaluation of serum RPR titer (see Table 1), while 6 of them had a follow-up evaluation of both CSF and serum RPR titer. The changes of MMSE scores and serum and CSF measures in these 6 patients by follow-up are shown in Table 4.
Table 4.
MMSE and changes of CSF measures and serum RPR titer in CSF RPR- patients by follow-up
| Variable | pre-treatment | 12 months after treatment | P |
|---|---|---|---|
| Increased CSF WBC count | 2/6(33%) | 1/6(17%) | 0.505 |
| Increased CSF protein concentration | 6/6(100%) | 1/6(17%) | 0.003 |
| Four-fold decline in CSF RPR | – | – | – |
| Four-fold decline in serum RPR | – | 3/6(50%) | – |
| Median MMSE (IQR) | 14.5(11–18.25) | 17(13–19.25) | 0.048 |
Increased CSF WBC count means CSF WBC count > 5 cells/μl; Increased CSF protein concentration means CSF protein concentration > 45 mg/dl; RPR, rapid plasma regain; MMSE, mini-mental state examination
Discussion
In this study, we explored the correlation between the RPR titer and GP. Serum RPR titer ≥1:32 has been described as a risk factor for neurosyphilis with HIV infection regardless of the stage of syphilis [7–9]. In this study, we also found that the number of CSF RPR+ patients with serum RPR titer ≥1:32 was significantly higher when compared with CSF RPR- patients.
When patients have symptomatic neurosyphilis, the success of therapy is assessed by normalization of CSF abnormalities, including pleocytosis, elevated protein concentration, or a reactive CSF VDRL test, and by resolution of symptoms and signs [5]. Repeated determination of serum RPR titer is recommended to evaluate treatment response, with a four-fold decrease from baseline and/or seroreversion in 12–24 months after treatment representing an appropriate response to therapy/serologic cure [4]. Marra et al. found that a four-fold decline in serologic RPR titers correlated with resolution of CSF parameters in persons with neurosyphilis, and normalization of serum RPR titer correctly predicted treatment success of neurosyphilis [10]. However, we found a four-fold decline in CSF RPR titer is more likely to occur than four-fold decline in serum RPR titer among these CSF RPR+ GP patients after penicillin treatment. Reactive non-treponemal test in non-HIV-infected late (latent) syphilis patients often remains stable after adequate therapy [2]. Many studies have found the serological response of nontreponemal tests (ie, VDRL, RPR) to treatment was influenced by syphilis stage but not by HIV infection or reinfection [11–14]. Compared to primary syphilis, later stages of syphilis showed a significantly slower treatment response [11, 13–17]. In this study, all the GP patients were late syphilis, which probably explains the 4-fold decline in serum RPR titers might not adequately predict treatment efficacy in GP patients. The majority of patients were early neurosyphilis in the study by Marra et al. [10], which could partially explain why a four-fold decline in CSF RPR titer instead of the serum RPR normalization found by Marra is a good predictor of treatment efficacy in HIV-negative CSF RPR+ GP patients in our finding. According to these results, it should be emphasized that the disease stage of syphilis might lead to different treatment response. Different recommendations may be needed for the follow-up of neurosyphilis depending on the stage of the disease.
During the follow-up at 12 months after penicillin treatment, no significant difference was found in the four-fold decline in serum RPR titer between CSF RPR+ and CSF RPR- patients. According to our data and our analyses, for CSF RPR- patients, normalization of CSF measures, not serum RPR titer value, is a sensible way of assessing treatment efficacy. A cutoff of greater than or equal to 5 cells/μL has been the standard for determining normal CSF values. For CSF RPR- GP patients, CSF WBC count was often normal before treatment, so it is difficult to evaluate the treatment efficacy by assessing the recovery of CSF WBC. CSF protein concentration in neurosyphilis is slow to normalize and may remain elevated even after the normalization of other CSF and clinical abnormalities [10, 18]. Thus, the decision to re-treat should not be based solely on failure of CSF protein concentration to normalize [1]. Importantly, our study found that the number of CSF RPR- patients (91%) with CSF protein concentration > 45 mg/dl was significantly higher when compared with CSF RPR+ patients (74%) (P = 0.028). The normalization of CSF protein concentration occurred in most of CSF RPR- GP patients by follow-up, which suggested that it may be a good indicator of the success of therapy in GP, although the number of this patient group by follow-up is relatively small. Further studies should be conducted to provide more evidence on this question.
Some limitations of our study should be considered. Specifically, a) the study is a retrospective study, in which case the association may be confounded or modified by patient characteristics; b) the large percentage of loss of follow-up leads to small number of the patients with follow-up, which has limited the statistical power; c) further studies are required to assess an association between RPR titers and the clinical features of these cases, and to identify an ideal indicator of the success of therapy for CSF-RPR- GP patients.
Conclusions
The practical implications of our findings are that a four-fold decline in CSF RPR titer is a good predictor for treatment efficacy in CSF RPR+ GP patients within 12 months after completion of therapy. The sole use of serum RPR titer might not adequately predict treatment efficacy of GP. Considering the limitations of this study, larger samples and more follow-up data are needed for future investigation. Furthermore, the association between RPR titers and the clinical features of these cases need to systematically quantified, and more indicators of treatment efficacy need to be investigated for CSF RPR- GP patients.
Additional files
Table S1. Follow-up of MMSE, CSF and serum measures in CSF-RPR+ patients. Thirteen CSF RPR+ GP patients returned for follow-up visits with lumbar puncture, MMSE scores and blood samples in 12 months after penicillin treatment. (DOCX 19 kb)
Table S2. Follow-up of MMSE, CSF and serum measures in CSF-RPR- patients. Fourteen CSF RPR- GP patients returned for follow-up visits with MMSE scores and serological tests in 12 months after penicillin treatment, while 6 of them had a follow-up evaluation of CSF. (DOCX 19 kb)
Acknowledgements
The authors thank Dr. Christina Marra from University of Washington for helpful comments on this manuscript.
Funding
This work was supported by grants (to X Chen) from the Israel Science Foundation (ISF)-the National Natural Science Foundation of China (NSFC) joint program (813111290) and by grant (to Y Jiang) from the Natural Science Foundation of Guangdong Province (2016A030313228) and the National Natural Science Foundation of China (81671182). Yong Chen and Yulun Liu’s research were supported in part by National Institutes of Health under award numbers 1R01LM012607 and 1R01AI130460. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSF
cerebrospinal fluid
- GP
general paresis
- MMSE
Mini-Mental State Examination
- RPR
rapid plasma regain
- TPPA
T. pallidum Particle Agglutination test
- VDRL
Venereal disease research laboratory
- WBC
white blood cells
Authors’ contributions
YJ and XC contributed to the conception and design of the study. YJ, RW, YZ, RF, YL, ZC, FP and XC collected and organized the data. YJ, YL and YC analyzed the data. YJ and XC drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Medical Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. The patients or the guardians of patients with severe cognitive impairment provided written informed consent. Lumbar puncture was also performed with informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3062-4) contains supplementary material, which is available to authorized users.
Contributor Information
Ying Jiang, Email: jiangying722@163.com.
Xiaohong Chen, Phone: +86-20-85513761, Email: xiaohongchenzssy@aliyun.com.
References
- 1.Marra CM. Neurosyphilis. Continuum (Minneap Minn). 2015;21(6):1714–28. [DOI] [PubMed]
- 2.French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. IUST. IUSTI: 2008 European guidelines on the Management of Syphilis. Int J STD AIDS. 2009;20(5):300–309. doi: 10.1258/ijsa.2008.008510. [DOI] [PubMed] [Google Scholar]
- 3.Castro R, Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: an evaluation of the venereal disease research laboratory (VDRL) and the rapid plasma Reagin (RPR) tests. J Clin Lab Anal. 2008;22(4):257–261. doi: 10.1002/jcla.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Chahine LM, Khoriaty RN, Tomford WJ, Hussain MS. The changing face of neurosyphilis. Int J Stroke. 2011;6(2):136–143. doi: 10.1111/j.1747-4949.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- 6.Su X, Shang L, Xu Q, Li N, Chen J, Zhang L, Zhang L, Hua Q. Prevalence and predictors of mild cognitive impairment in Xi'an: a community-based study among the elders. PLoS One. 2014;9:e83217. doi: 10.1371/journal.pone.0083217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, Stoner BP, Augenbraun M, Barker DE, Corbett JJ, Zajackowski M, Raines C, Nerad J, Kee R, Barnett SH. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189(3):369–376. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 8.Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, Sanchez P, Negredo E, Vandenbruaene M, Gatell JM, Clumeck N. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis. 2007;34(3):141–144. doi: 10.1097/01.olq.0000230481.28936.e5. [DOI] [PubMed] [Google Scholar]
- 9.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. AIDS. 2008;22(10):1145–1151. doi: 10.1097/QAD.0b013e32830184df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra CM, Maxwell CL, Tantalo LC, Sahi SK, Lukehart SA. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clin Infect Dis. 2008;47(7):893–899. doi: 10.1086/591534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaute DF, Graf N, Lautenschlager S, Weber R, Bosshard PP. Serological response to treatment of syphilis according to disease stage and HIV status. Clin Infect Dis. 2012;55(12):1615–1622. doi: 10.1093/cid/cis757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson CM, Hook E, 3rd, Shepherd M, Verley J, Rompalo AM. Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann Intern Med. 1994;121:94–100. doi: 10.7326/0003-4819-121-2-199407150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gourevitch MN, Selwyn PA, Davenny K, Buono D, Schoenbaum EE, Klein RS, Friedland GH. Effects of HIV infection on the serologic manifestations and response to treatment of syphilis in intravenous drug users. Ann Intern Med. 1993;118(5):350. doi: 10.7326/0003-4819-118-5-199303010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 15.Romanowski B, Sutherland R, Fick GH, Mooney D, Love EJ. Serologic response to treatment of infectious syphilis. Ann Intern Med. 1991;114:1005–1009. doi: 10.7326/0003-4819-114-12-1005. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem KG, Erbelding EJ, Wiener ZS, Rompalo AM. Serological response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007;83:97–101. doi: 10.1136/sti.2006.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Lopez JJ, Guerrero ML, Lujan R, Tostado SF, de Gorgolas M, Requena L. Factors determining serologic response to treatment in patients with syphilis. Clin Infect Dis. 2009;49:1505–1511. doi: 10.1086/644618. [DOI] [PubMed] [Google Scholar]
- 18.Marra CM, Maxwell CL, Tantalo L, Eaton M, Rompalo AM, Raines C, Stoner BP, Corbett JJ, Augenbraun M, Zajackowski M, Kee R, Lukehart SA. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: does HIV status matter? Clin Infect Dis. 2004;38(7):1001–1006. doi: 10.1086/382532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Follow-up of MMSE, CSF and serum measures in CSF-RPR+ patients. Thirteen CSF RPR+ GP patients returned for follow-up visits with lumbar puncture, MMSE scores and blood samples in 12 months after penicillin treatment. (DOCX 19 kb)
Table S2. Follow-up of MMSE, CSF and serum measures in CSF-RPR- patients. Fourteen CSF RPR- GP patients returned for follow-up visits with MMSE scores and serological tests in 12 months after penicillin treatment, while 6 of them had a follow-up evaluation of CSF. (DOCX 19 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


