Abstract
Background
Growth hormone deficiency (GHD) is a potential consequence of traumatic brain injury (TBI), including sport-related concussion (SRC). GH stimulation testing is required for definitive diagnosis; however, this is resource intensive and can be associated with adverse symptoms or risks. Measurement of serum IGF-1 is more practical and accessible, and pituitary tumour patients with hypopituitarism and low serum IGF-1 have been shown to have a high probability of GHD. We aimed to evaluate IGF-1 measurement for diagnosing GHD in our local TBI population.
Methods
We conducted a retrospective chart review of patients evaluated for GHD at the TBI clinic and referred for GH stimulation testing with insulin tolerance test (ITT) or glucagon stimulation test (GST) since December 2013. We obtained demographics, TBI severity, IGF-1, data pertaining to pituitary function, and GH stimulation results. IGF-1 values were used to calculate z-scores per age and gender specific reference ranges. Receiver operator curve analysis was performed to evaluate diagnostic threshold of IGF-1 z-score for determining GHD by GST or ITT.
Results
Sixty four patient charts were reviewed. 48 patients had mild, six had moderate, eight had severe TBI, and two had non-traumatic brain injuries. 47 patients underwent ITT or GST. 27 were confirmed to have GHD (peak hGH < 5 μg/L). IGF-1 level was within the age and gender specific reference range for all patients with confirmed GHD following GH stimulation testing. Only one patient had a baseline IGF-1 level below the age and gender specific reference range; this patient had a normal response to GH stimulation testing. ROC analysis showed IGF-1 z-score AUC f, confirming lack of diagnostic utility.
Conclusion
Baseline IGF-1 is not a useful predictor of GHD in our local TBI population, and therefore has no value as a screening tool. TBI patients undergoing pituitary evaluation will require a dynamic test of GH reserve.
Background
Growth hormone deficiency (GHD) is an increasingly recognized potential consequence following traumatic brain injury (TBI) [1, 2] Patients with GHD may present with impaired concentration, memory loss, low energy, depression, anxiety, social isolation, and poor quality of life [1, 3]. Due to the subtle and non-specific nature of these symptoms, as well as the potential overlap with neurologic and psychiatric sequelae of TBI, biochemical testing is crucial in making the diagnosis of GHD [2, 4]. GH stimulation testing is required for definitive diagnosis of GHD; the insulin tolerance test (ITT) is considered the reference standard and the glucagon stimulation test (GST) is an acceptable alternative, however, this testing is time consuming, resource intensive, and can be associated with adverse side effects [5]. Measurement of serum IGF-1, as a potential marker of GH activity, is comparatively more practical and accessible, and hypopituitary patients with low serum IGF-1 values have been shown to have a high probability of GHD [6]. Previous authors showed that in a population comprised of patients with moderate and severe TBIs, an IGF-1 greater than 175 ng/mL had high negative predictive value for true GHD according to a stimulation test with peak GH response of < 3 μg/L, and was therefore helpful in deciding which patients should undergo dynamic testing [7]. However, the IGF-1 method changed from a manual to an automated method during this study, and these findings have not been validated in a population that includes patients with mild, moderate, and severe traumatic brain injuries or sports-related concussion (SRC). The latter point is especially relevant considering patients with mild TBI also have significant risk of neuroendocrine dysfunction [8]. Our primary objective was to evaluate the performance of IGF-1 as a screening tool for GHD in a patient population that includes mild (including SRC), moderate, and severe TBI.
Methods
The procedures followed in this study were in accordance with the ethical standards of the Conjoint Health Research Ethics Board at the University of Calgary, Calgary, Alberta, Canada. We conducted a retrospective review of the electronic medical record of patients referred to endocrinology from the Calgary Brain Injury Program from December 2013–2016 for evaluation of hypopituitarism/GHD with either an ITT or GST. From each patient chart, we collected demographic information including age, sex, weight, and the number of months from injury to endocrinology assessment. We also collected data pertaining to the nature of the TBI including severity (mild, moderate, or severe) and whether the TBI had been classified as a SRC. Severity of injury was classified based on the Mayo Clinic Classification which includes initial GCS, length of loss of consciousness or length of post-traumatic amnesia to retrospectively determine TBI severity [9]. Baseline IGF-1 values were recorded, as well as all biochemical data pertaining to pituitary function. IGF-1 values were used to calculate a z-score for each patient per age and gender specific reference ranges. ITT and GST were performed in a dedicated endocrine testing unit with trained endocrine nurses according previously published standard protocols [10]. The choice of ITT or GST was made according to the availability of sufficient nursing staff as two nurses are required to perform the ITT. A successful insulin hypoglycemic test was determined by the achievement of a nadir blood glucose of < 2.5 mmol/L and all tests achieved this standard. Peak GH response following dynamic GH testing with ITT or GST was used to define the presence or absence of GHD where a peak hGH < 5.0 μg/L was considered abnormal and peak < 3.0 μg/L considered severe GHD. Using the dynamic test as the reference standard, the sensitivity and specificity of IGF-1 levels and IGF-1 Z-score to detect GHD was determined.
Assays
Insulin-like growth factor-1 (IGF-1) method
Serum IGF-1 was measured by a one-step immunometric (sandwich) chemiluminescent immunoassay using the Diasorin Liaison XL platform (DiaSorin, Stillwater, MN, USA). IGF-1 is separated from binding proteins and is captured by a murine monoclonal antibody coated onto solid-phase magnetic particles and a secondary murine monoclonal antibody conjugated with an isoluminol derivative to form an immune complex. After incubation, the unbound material is removed with a wash cycle and starter reagents are added to induce a flash chemiluminescence reaction which is measured by a photomultiplier and is directly proportional to the IGF-1 concentration (ug/L) in the serum. During the period of the study, the assay exhibited imprecision levels of < 8.4%. Age- and gender-specific reference intervals (95th percentile) were adapted according to the manufacturer’s instructions for use. This assay is referenced to the 1st WHO International Standard for Insulin-like Growth Factor-I NIBSC code: 02/254.
Growth hormone method
Growth hormone was measured by a one-step immunometric chemiluminescent immunoassay using the Siemens Immulite 2000 XPi platform (Siemens Healthcare Diagnostics, Tarrytown, NY, USA). Growth hormone is captured by a murine monoclonal antibody coated onto the solid phase bead a rabbit polyclonal antibody conjugated with alkaline phosphatase. After incubation, unbound material is removed by a centrifugal wash and chemiluminescent substrate is added to produce a signal which is directly proportional to the growth hormone concentration (ug/L). During the period of the study, the assay exhibited imprecision levels of < 3.9%. Reference intervals (95th percentile) were adapted according to the manufacturer’s instructions for use. This assay is referenced to WHO NIBSC 2nd International Standard 98/574.
Statistics
Standard descriptive statistics were used to define demographic variables. Group medians were compared using the Mann-Whitney U test. Statistical significance was set at p = 0.05. Receiver operator curve analysis was performed to evaluate diagnostic threshold of IGF-1 z-score for determining GHD by GST or ITT. All statistical calculations were performed using SPSS software version 24 [11].
Results
Sixty four patient charts were retrieved. Baseline demographics according to diagnostic GH status are presented in Table 1. There were 48 mild, 6 moderate, and 8 severe TBIs. Two were excluded from analysis due to having brain injuries of non-traumatic etiology. One patient passed away during the interval between assessment and dynamic testing. 14 patients did not receive GH stimulation testing after endocrinology assessment. Of these patients, six were not felt to have symptoms in keeping with GHD at the time of endocrinology assessment so were not referred on for testing. Five patients declined testing due to improvement in their symptoms at the time of endocrinology assessment, or unwillingness to undergo testing procedures. Three agreed to testing but were lost to follow-up before testing was performed. Of the 47 patients that underwent GH stimulation testing with ITT (n = 24, 51%) or GST (n = 23, 49%), 27 (57%) were confirmed to have GHD (based on a peak GH level of 5 μg/L or less), 18 (38%) had normal results to stimulation testing. Two patients (4%) patients had borderline results with a peak GH levels of 5.2 and 5.5 μg/L following GST and were classified as GHD at the discretion of the treating clinician, as therapy was offered to these patients due to symptomatology in keeping with growth hormone deficiency. Of the patients with confirmed GHD, 20 (43%) had severe GHD with peak GH < 3.0 μg/L or less.
Table 1.
Baseline Demographics
| All patients n = 62 | GH deficient n = 29 | Non-GH deficient n = 18 | |
|---|---|---|---|
| Age (median, IQR) | 43 (21.8) | 46 (17) | 39 (18) p = 0.216 |
| Gender | M: n = 29 | M: n = 15 | M: n = 7 |
| F: n = 33 | F: n = 14 | F: n = 11 | |
| Weight (median, IQR) | 78.1 kg (32.3) | 83 kg (34.0) | 74.2 kg (23.6) p = 0.220 |
| #months to endo referral (median, IQR) | 20 (16) | 24 (13) | 15 (20) p = 0.317 |
| TBI Severity | Mild n = 48 | Mild n = 22 | Mild n = 17 |
| Mod n = 6 | Mod n = 1 | Mod n = 1 | |
| Severe n = 8 | Severe n = 6 | ||
| Documented Sport Concussion | n = 15 | n = 7 | n = 5 |
| Hockey n = 4 | Hockey n = 3 | Basketball n = 1 | |
| Cycling n = 2 | Cycling n = 2 | Dirt biking n = 1 | |
| Basketball n = 2 | Basketball n = 1 | Football n = 1 | |
| Soccer n = 2 | Football n = 1 | Equestrian n = 1 | |
| Football n = 2 | Soccer n = 1 | ||
| Dirt biking n = 1 | |||
| Wrestling n = 1 | |||
| Equestrian n = 1 | |||
| Documented pituitary deficits | n = 4 | n = 2 | n = 1 |
| n = 1 secondary hypothyroidism | n = 1 secondary hypothyroidism | n = 1 secondary hypogonadism and hypothyroidism | |
| n = 2 secondary hypogonadism | n = 1 secondary hypogonadism | ||
| n = 1 secondary hypothyroidism and secondary hypogonadism | |||
| IGF-1 Z score (median, IQR) | −0.193 (2.07) | −0.214 (1.52) | −0.060 (1.24) p = 0.979 |
| Peak GH during stim test (median, IQR) | 3.8 μg/L (6.9) | 1.3 μg/L (2.6) | 9.7 μg/L (4.3) p = < 0.001 |
There was a total of 15 TBIs classified as SRC; seven of which were ultimately proved to be GH deficient. Four patients had documented evidence of other pituitary deficits, which were either central hypothyroidism, central hypogonadism, or both. Of these four patients, one of them had sustained a SRC, though this individual was known to have a history of anabolic steroid use.
For each patient, IGF-1 status was correlated with GH status after stimulation testing (Table 2). Patients were labelled as having “low” IGF-1 if the value fell below the assay reference range for matched age and gender, and “normal” IGF-1 if value was within this reference range. Baseline IGF-1 level was not available for four patients in GH deficient group and two patients in the non- GH deficient group. All but one patient had normal IGF-1 levels, and the single patient with low IGF-1 level tested negative for growth hormone deficiency with a peak GH of 50.2 μg/L. Mean IGF-1 z-score was lower for patients in the non-GHD group, but this was non-significant (p = 0.979). IGF-1 Z-scores’ ability to diagnose GHD were examined by ROC curve analysis, shown in Fig. 1. ROC analysis showed AUC of 0.495, p = 0.959, confirming lack of diagnostic utility.
Table 2.
IGF-1 and GH status
| GH deficient | Non-GH deficient | |
|---|---|---|
| IGF-1 low | 0 | 1 |
| IGF-1 normal | 25 | 15 |
| Median z-score | −0.214 | − 0.060 p = 0.979 |
Fig. 1.
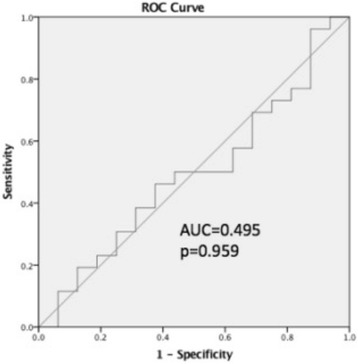
Receiver operating characteristic curve of serum IGF-1 level for diagnosis of growth hormone deficiency by dynamic testing
Discussion
Our results demonstrate that baseline serum IGF-1 level had no value in predicting GH deficiency, emphasizing the need for dynamic testing in this population. These findings contradict those of Zgaljardic et al. [7] There are several differences between our respective studies that may account for this. Our study includes an older patient population, and the majority of our subjects (48/62) sustained mild TBI, while the previous paper only included patients with moderate and severe TBI. Because almost all of our patients had normal IGF-1 levels, we cannot exclude the possibility that a truly low IGF-1 level, if present, may have a high specificity for true GHD as has been seen in other hypopituitary states. However, in our data, the single patient with overtly low IGF-1 was not GH deficient and as a group, the non-GHD patients tended to have lower IGF-1 values than the GHD patients and so it may be that in TBI patients, IGF-1 measurements of any kind have very poor sensitivity or specificity for true GHD. Importantly, the IGF-1 assay used at our centre differs from that used by Zgaljardic et al.; significant variability amongst different commercial IGF-1 immunoassays has been highlighted previously [12]. Furthermore, the different reference interval for each assay is a source of further complexity regarding the comparison of results between studies [13]. While reference intervals need to be method-specific, there is considerable variability in how reference ranges are derived for IGF-1 and may lead to different interpretations for the same patient [14]. Given this variability, it may require multicenter studies to validate robust reference ranges for specific IGF-1 methods to allow for better correlation between studies [12, 15]. Another factor can be due to the regulation of IGF-1 and its association with binding proteins, particularly with IGF binding proteins, whereby IGF-1 immunoassays have relied on displacement of IGF-1 from IGF binding proteins for proper measurement. Further advances in IGF-1 measurement by tandem mass spectrometry may be more specific and ensures the displacement of binding proteins by assay design. However, tandem mass spectrometry methods will similarly require proper validation of reference ranges as well as standardization [16]. In addition, the tandem mass spectrometry method may miss certain IGF-1 protein variants which have unknown functionality and will be unable to distinguish from wild type individuals [17]. Nevertheless, a repeat study that employs the use of a well-developed tandem mass spectrometry method for IGF-1 is warranted and will address the role of assay specificity in addition to variability in reference ranges. Taken together, the limitations in IGF-1 assay standardization and the results presented herein suggest the use of more specific dynamic testing of GH reserve.
Our findings add to a growing body of literature documenting pituitary dysfunction secondary to sport-related concussion. This can present with isolated or multiple pituitary hormone deficits, with GHD being the most common isolated deficit [18, 19]. While previous literature has implicated contact sports involving repetitive head trauma [4], our study includes two patients who developed growth hormone deficiency following an isolated concussion sustained while cycling. Therefore, even individuals who sustain a single concussion from non-contact sports appear to be susceptible to developing some degree of pituitary dysfunction.
Our study has several methodological limitations. Our results may not be generalizable to other institutions that use different IGF-1 assays, as we have outlined above. The referral process was not standardized; decisions about which patients are referred for endocrinology assessment reflect local practice patterns which may differ at other centres. For each patient, only a single dynamic test of GH reserve was performed, however, additional testing is not currently feasible due to cost and resource limitations. In the absence of other pituitary hormone deficits or obvious structural abnormalities in the pituitary, expert opinion suggests that two dynamic tests of GH reserve should be performed in order to confirm isolated GHD [20]. However, an FDA or Health Canada-approved GHRH method is not available in North America for GHRH stimulation testing, while insulin hypoglycemic testing may not be widely available at many centres and thus multi-modality testing may not be possible. In the absence of a “gold standard” diagnostic reference, it is also unknown as to whether the combination of dynamic GH stimulation tests truly improves diagnosis. In theory, diagnostic specificity may be improved but at an unknown loss of diagnostic sensitivity; this requires further study. We are unable to obtain BMI data from our EMR, which may have diagnostic implications as BMI can influence peak GH response, though the evidence on this matter is conflicting [7].
Conclusion
Our study has demonstrated the need for dynamic testing of GH reserve for patients with TBI who are suspected to have GHD at our centre. Individual measurement of IGF-1 levels and interpretation of the results according to method-specific reference ranges is ineffective to screen for GH deficiency in patients with TBI. Sport-related concussion, not just repetitive SRC of any nature appears to be a risk factor for subsequent GHD.
Acknowledgements
None
Funding
There is no funding source to disclose.
Availability of data and materials
The clinical dataset generated and analyzed during the current study are not publicly available as the minimum raw dataset needed to interpret, replicate, and build upon contains indirect patient identifiers (age, gender, and mechanism of head injury) that could put patient confidentiality at risk if seen in the context of the entire dataset. For this reason, we have decided not to publically share our data. However, the dataset can be made available from the corresponding author on reasonable request.
Abbreviations
- GH
Growth hormone
- GHD
Growth hormone deficiency
- GST
glucagon stimulation test
- hGH
human growth hormone
- ITT
insulin tolerance test
- SRC
sport-related concussion
- TBI
traumatic brain injury
Authors’ contributions
KL, CD, and GK were all responsible for study planning and design. KL was responsible for data collection, data analysis, and drafting the manuscript. AC assisted in data analysis and revised the lab methodology section of the manuscript. CD assisted with data analysis and statistics, reviewed all versions of the manuscript and provided minor revisions and editorial feedback. GK assisted with data analysis and statistics, reviewed all versions of the manuscript and provided major revisions and editorial feedback. All authors have given final approval of for work to be published, take responsibility for appropriate portions of the content, and agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
The authors declare that the procedures followed in this study were in accordance with the ethical approval s of the Conjoint Health Research Ethics Board at the University of Calgary, Calgary, Alberta, Canada (REB16–0355). Our study was granted waiver of consent by the Conjoint Health Research Ethics Board on the grounds that contacting all patients was thought to be unfeasible (due to the study’s retrospective nature, we did not have up to date contact information for all participants) as well as the minimal risk of harm to participants given our study did not involve any direct interaction or procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirstie Lithgow, Email: kclithgo@ucalgary.ca.
Alex Chin, Email: alex.chin@cls.ab.ca.
Chantel T. Debert, Email: chantel.debert@ahs.ca
Gregory A. Kline, Email: Gregory.kline@ahs.ca
References
- 1.Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79(7):753–759. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- 2.Brod M, Pohlman B, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Impact of adult growth hormone deficiency on daily functioning and well-being. BMC Res Notes. 2014;7:813. doi: 10.1186/1756-0500-7-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ, Board HA, Group USHS Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab. 2002;87(2):477–485. doi: 10.1210/jcem.87.2.8216. [DOI] [PubMed] [Google Scholar]
- 4.Dubourg J, Messerer M. Sports-related chronic repetitive head trauma as a cause of pituitary dysfunction. Neurosurg Focus. 2011;31(5):E2. doi: 10.3171/2011.8.FOCUS11182. [DOI] [PubMed] [Google Scholar]
- 5.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Society E. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 6.Lissett CA, Jönsson P, Monson JP, Shalet SM, Board KI. Determinants of IGF-I status in a large cohort of growth hormone-deficient (GHD) subjects: the role of timing of onset of GHD. Clin Endocrinol. 2003;59(6):773–778. doi: 10.1046/j.1365-2265.2003.01884.x. [DOI] [PubMed] [Google Scholar]
- 7.Zgaljardic DJ, Guttikonda S, Grady JJ, Gilkison CR, Mossberg KA, High WM, Masel BE, Urban RJ. Serum IGF-1 concentrations in a sample of patients with traumatic brain injury as a diagnostic marker of growth hormone secretory response to glucagon stimulation testing. Clin Endocrinol. 2011;74(3):365–369. doi: 10.1111/j.1365-2265.2010.03935.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanriverdi F, Kelestimur F. Pituitary dysfunction following traumatic brain injury: clinical perspectives. Neuropsychiatr Dis Treat. 2015;11:1835–1843. doi: 10.2147/NDT.S65814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK. The mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24(9):1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- 10.Berg C, Meinel T, Lahner H, Yuece A, Mann K, Petersenn S. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol. 2010;162(3):477–482. doi: 10.1530/EJE-09-0824. [DOI] [PubMed] [Google Scholar]
- 11.IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, N.Y., USA.
- 12.Chanson P, Arnoux A, Mavromati M, Brailly-Tabard S, Massart C, Young J, Piketty ML, Souberbielle JC, Investigators V. Reference values for IGF-I serum concentrations: comparison of six immunoassays. J Clin Endocrinol Metab. 2016;101(9):3450–3458. doi: 10.1210/jc.2016-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranke MB, Osterziel KJ, Schweizer R, Schuett B, Weber K, Röbbel P, Vornwald A, Blumenstock G, Elmlinger MW. Reference levels of insulin-like growth factor I in the serum of healthy adults: comparison of four immunoassays. Clin Chem Lab Med. 2003;41(10):1329–1334. doi: 10.1515/CCLM.2003.203. [DOI] [PubMed] [Google Scholar]
- 14.Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol. 2007;67(1):65–70. doi: 10.1111/j.1365-2265.2007.02836.x. [DOI] [PubMed] [Google Scholar]
- 15.Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, Körner A, Obermayer-Pietsch B, Hübener C, Dahlgren J, et al. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712–1721. doi: 10.1210/jc.2013-3059. [DOI] [PubMed] [Google Scholar]
- 16.Cox HD, Lopes F, Woldemariam GA, Becker JO, Parkin MC, Thomas A, Butch AW, Cowan DA, Thevis M, Bowers LD, et al. Interlaboratory agreement of insulin-like growth factor 1 concentrations measured by mass spectrometry. Clin Chem. 2014;60(3):541–548. doi: 10.1373/clinchem.2013.208538. [DOI] [PubMed] [Google Scholar]
- 17.Hines J, Milosevic D, Ketha H, Taylor R, Algeciras-Schimnich A, Grebe SK, Singh RJ. Detection of IGF-1 protein variants by use of LC-MS with high-resolution accurate mass in routine clinical analysis. Clin Chem. 2015;61(7):990–991. doi: 10.1373/clinchem.2014.234799. [DOI] [PubMed] [Google Scholar]
- 18.Popovic V. GH deficiency as the most common pituitary defect after TBI: clinical implications. Pituitary. 2005;8(3–4):239–243. doi: 10.1007/s11102-006-6047-z. [DOI] [PubMed] [Google Scholar]
- 19.Langelier DM, Kline GA, Debert CT. Neuroendocrine dysfunction in a young athlete with concussion: a case report. Clin J Sport Med. 2017; [DOI] [PubMed]
- 20.Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012;2012:972617. doi: 10.1155/2012/972617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical dataset generated and analyzed during the current study are not publicly available as the minimum raw dataset needed to interpret, replicate, and build upon contains indirect patient identifiers (age, gender, and mechanism of head injury) that could put patient confidentiality at risk if seen in the context of the entire dataset. For this reason, we have decided not to publically share our data. However, the dataset can be made available from the corresponding author on reasonable request.


