Abstract
Foundation species define the ecosystems they live in, but ecologists have often characterized dominant plants as foundational without supporting evidence. Giant kelp has long been considered a marine foundation species due to its complex structure and high productivity; however, there is little quantitative evidence to evaluate this. Here, we apply structural equation modelling to a 15-year time series of reef community data to evaluate how giant kelp affects the reef community. Although species richness was positively associated with giant kelp biomass, most direct paths did not involve giant kelp. Instead, the foundational qualities of giant kelp were driven mostly by indirect effects attributed to its dominant physical structure and associated engineering influence on the ecosystem, rather than by its use as food by invertebrates and fishes. Giant kelp structure has indirect effects because it shades out understorey algae that compete with sessile invertebrates. When released from competition, sessile species in turn increase the diversity of mobile predators. Sea urchin grazing effects could have been misinterpreted as kelp effects, because sea urchins can overgraze giant kelp, understorey algae and sessile invertebrates alike. Our results confirm the high diversity and biomass associated with kelp forests, but highlight how species interactions and habitat attributes can be misconstrued as direct consequences of a foundation species like giant kelp.
Keywords: ecosystem engineering, foundation species, competition, facilitation, kelp forest, subtidal
1. Background
While in the Galapagos, bent over the Beagle's rail, Charles Darwin noted that ‘The number of living creatures of all Orders, whose existence intimately depends on the kelp, is wonderful … Amidst the leaves of this plant numerous species of fish live, which nowhere else could find food or shelter; with their destruction the many cormorants and other fishing birds, and otters, seals, and porpoises, would soon perish also …’ (p. 252 of [1]). Darwin considered the giant kelp, Macrocystis pyrifera, what ecologists now call a foundation species, or ecosystem engineer [2]—a single species that increases species richness and food-web complexity [3] by creating spatial structure and influencing physical conditions and ecosystem processes [4–6]. As such, removing foundation species should impact many associated species [7,8], and restoring foundation species aids ecosystem restoration [9–11]. Foundation species are expected to decline with future climate change [12,13], resulting in cascading evolutionary, ecological and environmental changes [7,8]. Despite their importance, our understanding of how foundation species influence community structure and function is lacking [5,8,14].
Since Darwin, marine ecologists have obsessed over how giant kelp affects reef communities [15], reef food webs [16,17], sandy beach [18,19] and deep-water ecosystems [20,21], hydrodynamics [22,23], biogeochemistry [24,25], and even early human migrations [26,27]. The stark differences inside and outside giant kelp forests are as obvious to today's scuba divers and anglers as they were to Darwin. Occurring in temperate waters across the world's oceans, giant kelp grows to the surface where its massive floating canopy creates habitat used by many species [15]. Beneath the canopy, shading can reduce macroalgal production [28], thereby favouring sessile invertebrates [29,30]. Water flow slows through the forest [22,23,31], and kelp facilitates turbulent mixing [32], potentially altering deposition of sediment, detritus, phytoplankton and larval settlement [33]. Most notably, kelp forests are higher in biodiversity and different in community composition than adjacent ‘barren’ reef areas, where sea urchins have over-grazed kelp [16,34,35]. Yet there are many factors that could drive differences between urchin barrens and kelp forests, not the least of which is urchins themselves, which can consume many if not most sessile reef species [36–38]. In other words, although kelp forest communities are diverse, this could be due to a direct kelp effect, as assumed by the foundation species concept, or a variety of alternatives, including a generalized macroalgal effect, indirect effects of kelp, or simply a joint dependence on hard substrate by giant kelp and many other species. Removal experiments are perhaps the gold standard for measuring foundational effects, but are necessarily small scale. Some kelp-removal experiments have documented that kelp can shade out understorey algae [39,40], but effects on other species, particularly mobile species, are often weak or undetectable [41,42]. Thus, Darwin's broad claims about giant kelp, as for many claims about foundation species, remain largely unquantified.
Here, we evaluate the paradigm that giant kelp is a foundation species that directly shapes the rocky reef community and thereby promotes biodiversity. We first examine the extent to which giant kelp correlates with species richness overall. We then consider whether giant kelp increases biodiversity and abundance of particular functional groups on rocky reefs through both direct or indirect effects, rather than simply being associated with overall biodiversity. We also evaluate how sea urchins, the often-cast antagonist in the system, affect kelp and biodiversity. To do so, we analyse a 15-year time series of kelp forest community data to quantify the relationship between giant kelp, reef structural complexity and biodiversity.
2. Material and methods
We hypothesized that if giant kelp is a foundation species, its biomass should be associated with higher species richness through provision of habitat and resources [6]. We also considered the following alternative hypotheses for what determines reef diversity and biomass. (i) Rock habitat drives positive associations between kelp and other reef species. (ii) Sea urchins determine community composition by consuming algae and sessile invertebrates. (iii) Bottom-up effects drive community composition (i.e. increased basal diversity and biomass increase their consumers' diversity and biomass, and prey diversity and biomass increases predator diversity and biomass). (iv) Biomass within a functional group drives biodiversity of that group. We rejected, a priori, a potential logical path from sea urchin predators (sheephead wrasse Semicossyphus pulcher, spiny lobster Panulirus interruptus and sunflower star Pycnopodia helianthoides) to sea urchins in a preliminary model, because these sea urchin predators were not abundant at the sampled sites—the first two species are depressed in abundance and body size because of fishing and the third because of biogeographic patterns (such associations exist at other sites where predators are protected from fishing [34,43]).
(a). Field surveys
Annual kelp forest community surveys were conducted in late July–early August from 2001 to 2016 by the Santa Barbara Coastal Long Term Ecological Research program (http://sbc.lternet.edu). Divers sampled 39 fixed 40 m × 2 m plots distributed among eleven kelp forest sites in the Santa Barbara Channel, USA. In each plot, divers measured density and size of giant kelp, understorey kelps, large mobile invertebrates and reef-associated fishes. Smaller benthic mobile invertebrates and understorey algae were counted and sized within six 1 m2 quadrats placed at 8 m intervals along the 40 m axis of each plot. For sessile invertebrates and understorey algae that are impractical to count as individuals, percentage cover was calculated based on sampling 80 uniformly spaced points within each plot. Substrate type was assessed for the same points and has been simplified in this analysis to percentage cover rock (including loose cobble and boulders).
(b). Community structure metrics
Species sizes and abundances were converted into biomass using species-specific relationships developed for kelp, understorey algae, invertebrates and fish in the region as per Reed et al. [44]. Diversity was calculated as diversity of order 1, which corresponds to the exponential of the Shannon entropy index (H) [45]:
 |
where s is the total number of species and pi is the proportional biomass of the ith species. Diversity of order 1 represents the effective number of equally common species, weighting each species according to its proportional biomass, without favouring rare or common species [45]. Proportional biomass was calculated based on decalcified dry biomass. Species richness was measured as the unique number of species observed each year at a given transect, and corresponds to diversity of order 0 [45]. We first analysed the reef community as a whole, and then split the community into four functional groups based on mobility and trophic position [17]: (i) understorey macroalgae, (ii) sessile invertebrates (suspension feeders), (iii) mobile grazers (herbivores, omnivores and detritivores) and (iv) mobile predators (electronic supplementary material, table S1).
(c). Statistical analyses
We used linear mixed-effects models to test for relationships between giant kelp biomass and species richness (as a whole and by functional group), with kelp biomass (fixed), year (fixed) and site (random) effects. We used richness in these analyses rather than diversity because the species varied greatly in size and biomass and thus the abundance of a few large species would have dominated overall diversity metrics. We further evaluated these relationships for ‘giant kelp-associated’ species, as classified by Graham [16] based on their frequency at sites in the Channel Islands National Park, which were categorized as kelp forests or urchin barrens [34], and for the biomass of each group.
Because overall species richness associations might not reflect the complex networks of interactions among species, we used piecewise structural equation modelling (SEM) to investigate multiple direct and indirect relationships among giant kelp, sea urchins and community biomass and diversity. Classical SEM assumes that all observations are independent, whereas piecewise SEM can account for hierarchically structured observations through a mixed modelling framework [46]. Sampling location was treated as a random factor to account for location-specific environmental variation. However, piecewise SEM cannot disentangle cyclic or reciprocal relationships in the same model, so we used the literature and our knowledge of natural history to remove unlikely directional paths to avoid the potential for cyclic relationships (table 1).
Table 1.
Hypothesized paths and their signs considered in SEM models. Column headings indicate path origins; row headings are destinations; column abbreviations reflect row labels. Path directions were based on ecological relationships in kelp forests as summarized by Schiel & Foster [15]. Grey + and − symbols indicate possible path directions that were not explicitly tested in our model.
| path origin |
||||||
|---|---|---|---|---|---|---|
| kelp | sea urchins | understorey macroalgae | sessile invertebrates | mobile grazers | mobile predators | |
| kelp | −a | − | − | |||
| sea urchins | + | + | + | − | ||
| understorey macroalgae | −a | −a | − | − | ||
| sessile invertebrates | + | −a | −a | − | − | |
| mobile grazers | + | + | + | − | ||
| mobile predators | +a | + | + | +a | +a | |
aPaths that were significant in the model.
We performed all statistical analyses in R v. 3.3.0. We fitted mixed models using the R package lme4 version 1.1–12 [47] and piecewise SEM analyses using the package piecewiseSEM [46]. We used the d-separation test [48] to evaluate whether any non-hypothesized independent relationships were significant and whether including a missing path could improve a model. We reported conditional (R2c, all factors) coefficients of determination for each generalized linear mixed effect model included in our final piecewise SEM. We tested whether accounting for temporal autocorrelation using a continuous first-order autoregressive autocorrelation structure [49] would increase model fit. However, the SEMs accounting for temporal autocorrelation resulted in higher Akaike information criterion scores, suggesting that modelling temporal autocorrelation did not increase our ability to understand this system. For this reason, we reported only models without temporal autocorrelation. We standardized all quantitative predictors to mean of 0 and variance of 1.
3. Results
(a). Univariate relationships
Overall species richness was weakly associated with giant kelp biomass. Although the relationship explained little variation (marginal R2 = 0.092; table 2; electronic supplementary material, figure S1), the slope showed 4.9 more species with each kg m−2 increase in kelp biomass (table 2), resulting in approximately 10–15 fewer species in an unforested reef than in one with maximum kelp biomass (electronic supplementary material, figure S1A). Confining the analysis to the ‘kelp-associated’ species as defined by Graham [16] weakened the fit of the relationship (marginal R2 = 0.036; table 2; electronic supplementary material, figure S1B), and the effect of kelp biomass on ‘kelp-associated’ species richness was smaller in magnitude than the effect on overall richness. This pattern was apparent across the functional groups, and less than 6% of variation in species richness was explained by kelp biomass in any group (table 2). No relationships were observed between giant kelp and the species richness of mobile grazers, either in total or kelp-associated. In general, the biomass of the functional groups was unrelated to giant kelp biomass, and associations that were present were weak (electronic supplementary material, table S2). Biomass of kelp-associated species was positively associated with kelp biomass (marginal R2 = 0.03; electronic supplementary material, table S2), and the slope showed an increase of 0.05 kg dry mass m−2 with each kg m−2 increase in kelp biomass.
Table 2.
The intercept, slope, marginal coefficient of determination (R2), and p-values of linear mixed-effect models testing the effect of giant kelp biomass (kg m−2 dry mass) on species richness of the entire community and four functional groups. Year (fixed) and site (random) factors were also included in the model; coefficients represent the effect of kelp only. We also considered these relationships including only the subset of giant kelp-associated species (GKA) as defined by Graham [16] for each group. Here n is the number of species in each group. Significant relationships are bolded.
| n | intercept | slope | R2 | p | |
|---|---|---|---|---|---|
| total community | 205 | −618.01 | 4.91 | 0.037 | <0.001 |
| GKA community | 51 | −374.37 | 3.43 | 0.095 | <0.001 |
| macroalgae | 58 | −402.43 | 0.82 | 0.051 | 0.047 |
| GKA macroalgae | 18 | −317.18 | 1.48 | 0.106 | <0.001 |
| sessile invertebrates | 68 | −138.35 | 3.33 | 0.044 | <0.001 |
| GKA sessile invertebrates | 18 | −19.32 | 1.35 | 0.066 | <0.001 |
| mobile grazers | 16 | 36.98 | −0.23 | 0.004 | 0.083 |
| GKA mobile grazers | 4 | −5.46 | 0.02 | 0.002 | 0.544 |
| mobile predators | 60 | −126.48 | 1.49 | 0.023 | <0.001 |
| GKA mobile predators | 11 | −31.34 | 0.57 | 0.018 | <0.001 |
(b). Piecewise structural equation modelling analyses
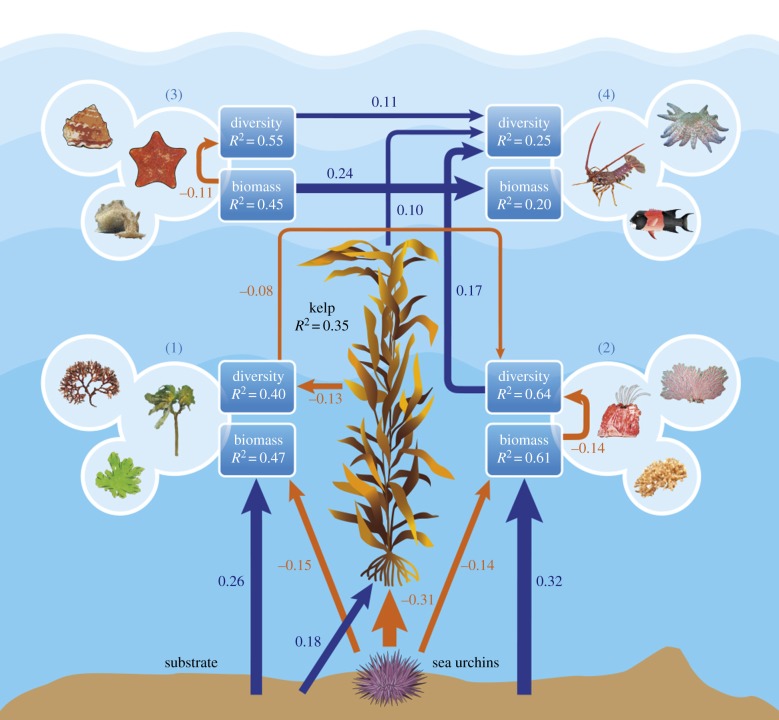
Results from SEM show that hard substrate was strongly associated with sessile species biomass, including giant kelp biomass, and that giant kelp biomass was negatively associated with sea urchin biomass (figure 1). Giant kelp had only two significant direct paths: a direct positive association with mobile predator diversity and a direct negative association with macroalgal diversity (figure 1). Understorey macroalgal diversity had a direct negative association with sessile invertebrate diversity. This suggests that kelp decreased macroalgal diversity, which increased sessile invertebrate diversity, which enhanced predator diversity (figure 1). Surprisingly, kelp was not associated with non-urchin mobile grazer diversity or biomass.
Figure 1.
Piecewise SEM model of the effect of giant kelp biomass and sea urchin biomass on biomass and diversity order 1 (see Material and methods) of four taxonomic functional groups, represented by the blue boxes: (1) understorey macroalgae, (2) sessile invertebrates, (3) mobile grazers and (4) mobile predators. Effects of substrate on the biomass of sessile groups are also shown. Arrows represent unidirectional relationships among variables. Blue arrows denote positive relationships, and orange arrows negative relationships. Arrows for non-significant paths (p ≥ 0.05) are not shown. The thicknesses of the significant paths reflect the magnitude of the standardized regression coefficients given alongside. R2-values inside boxes are conditional R2.
Most direct paths among species did not involve giant kelp. Mobile grazer diversity and biomass had positive paths to the diversity of mobile predators that feed on them (figure 1). In addition to their negative associations with kelp, sea urchins likewise had negative associations with understorey macroalgae and sessile invertebrates. We did not find evidence that biomass increased diversity. In fact, for sessile invertebrates and mobile grazers, biomass was negatively associated with diversity (figure 1). Biomass and diversity were unrelated for macroalgae and predators. Partial correlation plots of the significant relationships in the SEM model are shown in the electronic supplementary material, figure S2.
4. Discussion
From a predator's perspective, giant kelp is clearly a foundational species. This is consistent with the view that giant kelp supports fish habitat, and that the physical structure and refuge from predation provided by the giant kelp canopy helps explain high fish abundance and diversity in the kelp forest [50–55]. In addition, kelp harbours many small invertebrates (not measured in our study), including crustaceans and gastropods that consume kelp and are prey for higher trophic levels [56,57]. Juvenile fishes, such as rockfishes, recruit to the canopy and can also be a food source for larger fish [53]. Giant kelp is thus both a refuge from predation and a habitat for prey.
In addition to directly providing habitat for predators, giant kelp indirectly promotes sessile invertebrates [29,30]. The kelp canopy suppresses understorey macroalgae [28,58], including juvenile giant kelp [40,59] that would otherwise outcompete slow-growing sessile invertebrates that thrive in shaded habitats [60,61]. For instance, after removing giant kelp at one of our sites (Mohawk Reef), understorey algal abundance and richness increased and sessile invertebrate abundance and richness decreased [30,62]. The results presented here differ in that we observed a negative path from macroalgal to sessile invertebrate diversity, but this path was not evident for biomass. This could be due to unaccounted for environmental variables affecting both groups. Although it is global in distribution, Macrocystis varies in morphology, size and population dynamics, and in regions where giant kelp forests are less extensive and more dynamic than in southern California [63], its structural effect may be less pronounced. For example, in southern Chile, removing the Macrocystis canopy has only subtle effects on understorey algal assemblages [58].
Our study suggests that, at least in southern California, giant kelp influences diversity mainly through structure and shading rather than as food, an effect that has been termed a habitat cascade [64]. We saw no evidence for a positive path between giant kelp and non-urchin grazer diversity or biomass, as would be expected if kelp was a dominant food source for these species. Although many kelp forest grazers eat kelp, most also eat other macroalgae and sessile invertebrates, suggesting that giant kelp is a substitutable resource in the kelp forest food web. Likewise, no direct path was evident from giant kelp to suspension feeding sessile invertebrates. Kelps are often hypothesized to contribute significantly to the nutritional requirements of suspension feeding invertebrates through detrital pathways [65]. Our results align with previous studies using stable isotopes and direct measurements of detrital production by giant kelp that suggest reef suspension feeders are dependent on phytoplankton, not kelp detritus, for trophic support [30,66–68]. A kelp effect was only weakly evident in simple relationships between biodiversity, but SEM helped identify indirect pathways that connected giant kelp to diversity.
Plot size can affect results of ecological studies, particularly those involving mobile species. The transects used in this study were 40 × 2 m, certainly smaller than the home range of many of the mobile organisms, such as fishes. The fact that we saw a positive response of mobile predators to kelp abundance at the transect scale suggests that mobile species do respond to kelp on small scales. It is possible that these behavioural associations are easier to observe for species that use kelp for structural habitat (and must remain near it to gain the benefit) than for species that use kelp for food. Senescing kelp falls to the seafloor and can then drift away from its source. This drift can decouple small-scale correlations between live kelp abundance and grazer biomass and diversity. However, our transects were large enough to reveal positive associations between standing giant kelp biomass and detrital kelp biomass from five of our transects over 9 years (n = 152, p = 0.0003, R2 = 0.1) [69], suggesting we should have detected strong links between kelp and mobile grazers if they existed.
Giant kelp could be a significant resource for many species that are unaccounted for in the survey data, because they are small, live in cryptic habitats or both. The two main cryptic habitats created by giant kelp are the holdfast, an intricate mass of root-like haptera that holds the kelp plant to the seafloor, and the dense frond canopy. The canopy harbours larger species than the holdfast and is not covered by our surveys. Canopy specialists, such as the kelp perch (Brachyistius frenatus), and other fishes that tend to be more abundant in the canopy than near the bottom, such as giant kelpfish (Heterostichus rostratus) [52], senorita (Oxyjulis californica), kelp rockfish (Sebastes atrovirens) and juvenile kelp bass (Paralabrax clathratus) [50,52], might have stronger relationships with kelp than our data indicate. For instance, in central California, seven ‘midwater’ fish species were most affected by a large-scale kelp-removal experiment (1 ha) [51]. By contrast, relatively few large mobile invertebrate species live in the canopy; in our area, these include the kelp crab (Pugettia producta) and Norris's topsnail (Norrisia norrisii).
Giant kelp is also likely to affect small organisms that are uncounted in the surveys. An exhaustive study of invertebrate fauna associated with kelp fronds found 114 species, mostly small crustaceans, with abundance and diversity highest near the bottom and decreasing into the canopy [70]. Amphipod and shrimp abundance averaged over 8000 individuals per kg (wet mass) of kelp fronds; mass of kelp fronds averages approximately 2.5–5 kg wet mass per m2 at our study sites [71] and can get much higher [72]. On the bottom, average densities of these macrofaunal organisms have been measured at greater than 30 000 per m2 [73], suggesting that the kelp canopy can be a significant habitat for such reef macrofauna. Indeed, kelp canopy mesograzers contribute a significant portion of carbon to the tissues of predatory fishes [57]. Kelp holdfasts harbour diverse and abundant invertebrate communities [15], although similar communities form in understorey macroalgae and artificial substrates [74].
The trophic groups in our SEM analysis, particularly the grazers and predators, combine species that differ in diet. For example, some predators feed mainly on sessile invertebrates while others feed on mobile prey, including other predators [75]. Because detailed diet information was not available, and diet can be size-dependent, we used broad trophic groups, rather than food-web analysis. Future work on kelp forest food webs could shed more light on species interactions.
Sea urchin predators can influence kelp abundance via trophic cascades [76], especially where fishing is not permitted [77]. Although our analysis did not test for top-down effects, our results suggest that reducing sea urchin biomass will increase reef biodiversity directly and indirectly. At present, fishing has reduced the size and in some cases population abundance of key sea urchin predators [43,78]. Sea otters, voracious urchin predators, have only sporadically recolonized the region since the fur trade in the eighteenth century [79]. However, as a new marine protected area network matures [80], and if sea otters re-establish in the region, then we might expect sea urchins to decline due to increased predation, favouring kelp and its dependent species.
5. Conclusion
It is remarkable that Darwin recognized the ecological importance of giant kelp standing on a ship. Although our study was done in southern California rather than South America, our results largely agree with Darwin's observation that kelp forests contain high species richness and biomass. This association, however, is largely driven by the structural attributes of kelp and its shared affinity for hard substrate with other reef species. Our analysis points to sea urchin grazing, giant kelp abundance and substrate type as important drivers. Hard substrate supports many species, driving most of the positive relationships among them. Sea urchins, however, can exclude sessile animals and macroalgae, leading to barren patches. Where sea urchins are not too abundant, understorey algae can outcompete sessile invertebrates for space or giant kelp can outcompete understorey algae for light, indirectly facilitating sessile invertebrate diversity and providing shelter for the predators that feed on them. In regions or sites where giant kelp does not grow in dense stands, its influence on the community may be weaker [58]. Most rocky reefs are community mosaics defined by these processes, and the heterogeneity that they produce undoubtedly facilitates reef diversity [81].
Supplementary Material
Acknowledgements
We thank C. Nelson, S. Harrer, and the many UCSB graduate and undergraduate students who helped with collection of data in the field, and D. Morton, who provided comments on the manuscript. Any use of trade, product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US government.
Ethics
All data were collected under Californian legal requirements. Any collections were made under California Fish and Wildlife Department permit SC-7193 to SBC LTER.
Data accessibility
The datasets supporting this article are available on the website of the Santa Barbara Coastal Long Term Ecological Research program http://sbc.lternet.edu//data/dataCollectionsPortal.html
Authors' Contributions
R.J.M. designed the study and drafted the manuscript; R.J.M., L.K. and T.L. carried out the statistical analyses; D.C.R. and A.R. collected field data; K.D.L., T.L., L.K., D.C.R. and A.R. gave essential input to study design and writing. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The research was supported by the US National Science Foundation's Long Term Ecological Research program (OCE 9982105, 0620276 and 1232779), by the NASA Biodiversity and Ecological Forecasting program (NNX14AR62A), the Bureau of Ocean and Energy Management Ecosystem Studies program (MC15AC00006) and NOAA in support of the Santa Barbara Channel Biodiversity Observation Network.
References
- 1.Darwin C. 1909. The voyage of the Beagle. New York, NY: PF Collier & Son. [Google Scholar]
- 2.Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. ( 10.1890/0012-9658(1997)078%5B1946:PANEOO%5D2.0.CO;2) [DOI] [Google Scholar]
- 3.Baiser B, Whitaker N, Ellison A. 2013. Modeling foundation species in food webs. Ecosphere 4, art146 ( 10.1890/ES13-00265.1) [DOI] [Google Scholar]
- 4.Dayton PK. 1972. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Colloquium on conservation problems in Antarctica (ed. Parker B.), pp. 81–96 Lawrence, KS: Allen Press. [Google Scholar]
- 5.Ellison A, et al. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 3, 479–486. ( 10.1890/1540-9295(2005)003%5B0479:LOFSCF%5D2.0.CO;2) [DOI] [Google Scholar]
- 6.van der Zee E, et al. 2016. How habitat-modifying organisms structure the food web of two coastal ecosystems. Proc. R. Soc. B 283, 20152326 ( 10.1098/rspb.2015.2326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith M, Knapp A. 2003. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 6, 509–517. ( 10.1046/j.1461-0248.2003.00454.x) [DOI] [Google Scholar]
- 8.Ellison A, Barker-Plotkin A, Foster D, Orwig D. 2010. Experimentally testing the role of foundation species in forests: the Harvard Forest Hemlock Removal Experiment. Methods Ecol. Evol. 1, 168–179. ( 10.1111/j.2041-210X.2010.00025.x) [DOI] [Google Scholar]
- 9.Parrotta J, Turnbull J, Jones N. 1997. Introduction—Catalyzing native forest regeneration on degraded tropical lands. Forest Ecol. Manag. 99, 1–7. ( 10.1016/S0378-1127(97)00190-4) [DOI] [Google Scholar]
- 10.Padilla F, Pugnaire F. 2006. The role of nurse plants in the restoration of degraded environments. Front. Ecol. Environ. 4, 196–202. ( 10.1890/1540-9295(2006)004%5B0196:TRONPI%5D2.0.CO;2) [DOI] [Google Scholar]
- 11.Halpern B, Silliman B, Olden J, Bruno J, Bertness M. 2007. Incorporating positive interactions in aquatic restoration and conservation. Front. Ecol. Environ. 5, 153–160. ( 10.1890/1540-9295(2007)5%5B153:IPIIAR%5D2.0.CO;2) [DOI] [Google Scholar]
- 12.Gaston K, Fuller R. 2007. Biodiversity and extinction: losing the common and the widespread. Progress Phys. Geogr. 31, 213–225. ( 10.1177/0309133307076488) [DOI] [Google Scholar]
- 13.Berggren A, Bjorkman C, Bylund H, Ayres M. 2009. The distribution and abundance of animal populations in a climate of uncertainty. Oikos 118, 1121–1126. ( 10.1111/j.1600-0706.2009.17558.x) [DOI] [Google Scholar]
- 14.Orwig D, Plotkin A, Davidson E, Lux H, Savage K, Ellison A. 2013. Foundation species loss affects vegetation structure more than ecosystem function in a northeastern USA forest. Peerj 1, e41 ( 10.7717/peerj.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiel DR, Foster MS. 2015. The biology and ecology of giant kelp forests. Oakland, CA: University of California Press. [Google Scholar]
- 16.Graham MH. 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7, 341–357. ( 10.1007/s10021-003-0245-6) [DOI] [Google Scholar]
- 17.Byrnes J, Reed D, Cardinale B, Cavanaugh K, Holbrook S, Schmitt R. 2011. Climate-driven increases in storm frequency simplify kelp forest food webs. Glob. Change Biol. 17, 2513–2524. ( 10.1111/j.1365-2486.2011.02409.x) [DOI] [Google Scholar]
- 18.Dugan J, Hubbard D, McCrary M, Pierson M. 2003. The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beaches of southern California. Estuarine Coast. Shelf Sci. 58, 25–40. ( 10.1016/S0272-7714(03)00045-3) [DOI] [Google Scholar]
- 19.Lastra M, Page H, Dugan J, Hubbard D, Rodil I. 2008. Processing of allochthonous macrophyte subsidies by sandy beach consumers: estimates of feeding rates and impacts on food resources. Mar. Biol. 154, 163–174. ( 10.1007/s00227-008-0913-3) [DOI] [Google Scholar]
- 20.Harrold C, Light K, Lisin S. 1998. Organic enrichment of submarine-canyon and continental-shelf benthic communities by macroalgal drift imported from nearshore kelp forests. Limnol. Oceanogr. 43, 669–678. ( 10.4319/lo.1998.43.4.0669) [DOI] [Google Scholar]
- 21.Vetter EW, Dayton PK. 1999. Organic enrichment by macrophyte detritus, and abundance patterns of megafaunal populations in submarine canyons. Mar. Ecol. Prog. Ser. 186, 137–148. ( 10.3354/meps186137) [DOI] [Google Scholar]
- 22.Jackson G. 1997. Currents in the high drag environment of a coastal kelp stand off California. Cont. Shelf Res. 17, 1913–1928. ( 10.1016/S0278-4343(97)00054-X) [DOI] [Google Scholar]
- 23.Gaylord B, et al. 2007. Spatial patterns of flow and their modification within and around a giant kelp forest. Limnol. Oceanogr. 52, 1838–1852. ( 10.4319/lo.2007.52.5.1838) [DOI] [Google Scholar]
- 24.Fram J, Stewart H, Brzezinski M, Gaylord B, Reed D, Williams S, MacIntyre S. 2008. Physical pathways and utilization of nitrate supply to the giant kelp, Macrocystis pyrifera. Limnol. Oceanogr. 53, 1589–1603. ( 10.4319/lo.2008.53.4.1589) [DOI] [Google Scholar]
- 25.Delille B, Borges A, Delille D. 2009. Influence of giant kelp beds (Macrocystis pyrifera) on diel cycles of pCO2 and DIC in the Sub-Antarctic coastal area. Estuarine Coast. Shelf Sci. 81, 114–122. ( 10.1016/j.ecss.2008.10.004) [DOI] [Google Scholar]
- 26.Erlandson J, Rick T. 2010. Archaeology meets marine ecology: the antiquity of maritime cultures and human impacts on marine fisheries and ecosystems. Ann. Rev. Mar. Sci. 2, 231–251. ( 10.1146/annurev.marine.010908.163749) [DOI] [PubMed] [Google Scholar]
- 27.Erlandson J, Braje T, Gill K, Graham M. 2015. Ecology of the kelp highway: did marine resources facilitate human dispersal from northeast Asia to the Americas? J. Island Coast. Archaeol. 10, 392–411. ( 10.1080/15564894.2014.1001923) [DOI] [Google Scholar]
- 28.Miller R, Reed D, Brzezinski M. 2011. Partitioning of primary production among giant kelp (Macrocystis pyrifera), understory macroalgae, and phytoplankton on a temperate reef. Limnol. Oceanogr. 56, 119–132. ( 10.4319/lo.2011.56.1.0119) [DOI] [Google Scholar]
- 29.Arkema K, Reed D, Schroeter S. 2009. Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90, 3126–3137. ( 10.1890/08-1213.1) [DOI] [PubMed] [Google Scholar]
- 30.Miller R, Page H, Reed D. 2015. Trophic versus structural effects of a marine foundation species, giant kelp (Macrocystis pyrifera). Oecologia 179, 1199–1209. ( 10.1007/s00442-015-3441-0) [DOI] [PubMed] [Google Scholar]
- 31.Jackson G, Winant C. 1983. Effect of a kelp forest on coastal currents. Cont. Shelf Res. 2, 75–80. ( 10.1016/0278-4343(83)90023-7) [DOI] [Google Scholar]
- 32.Rosman J, Denny M, Zeller R, Monismith S, Koseff J. 2013. Interaction of waves and currents with kelp forests (Macrocystis pyrifera): Insights from a dynamically scaled laboratory model. Limnol. Oceanogr. 58, 790–802. ( 10.4319/lo.2013.58.3.0790) [DOI] [Google Scholar]
- 33.Morton D, Anderson T. 2013. Spatial patterns of invertebrate settlement in giant kelp forests. Mar. Ecol. Prog. Ser. 485, U75–U102. ( 10.3354/meps10329) [DOI] [Google Scholar]
- 34.Behrens M, Lafferty K. 2004. Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar. Ecol. Prog. Ser. 279, 129–139. ( 10.3354/meps279129) [DOI] [Google Scholar]
- 35.Ling S, Johnson C. 2009. Population dynamics of an ecologically important range-extender: kelp beds versus sea urchin barrens. Mar. Ecol. Prog. Ser. 374, 113–125. ( 10.3354/meps07729) [DOI] [Google Scholar]
- 36.Harrold C, Reed D. 1985. Food availability, sea-urchin grazing, and kelp forest community structure. Ecology 66, 1160–1169. ( 10.2307/1939168) [DOI] [Google Scholar]
- 37.Andrew N. 1993. Spatial heterogeneity, sea-urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74, 292–302. ( 10.2307/1939293) [DOI] [Google Scholar]
- 38.Byrnes J, Cardinale B, Reed D. 2013. Interactions between sea urchin grazing and prey diversity on temperate rocky reef communities. Ecology 94, 1636–1646. ( 10.1890/11-2310.1) [DOI] [PubMed] [Google Scholar]
- 39.Dayton P. 1975. Experimental studies of algal canopy interactions in a sea otter dominated kelp community at Amchitka Island, Alaska. Fish. Bull. 73, 230–237. [Google Scholar]
- 40.Reed D, Foster M. 1984. The effects of canopy shading on algal recruitment and growth in a giant kelp forest. Ecology 65, 937–948. ( 10.2307/1938066) [DOI] [Google Scholar]
- 41.Syms C, Jones G. 1999. Scale of disturbance and the structure of a temperate fish guild. Ecology 80, 921–940. ( 10.1890/0012-9658(1999)080%5B0921:SODATS%5D2.0.CO;2) [DOI] [Google Scholar]
- 42.O'Connor K, Anderson T. 2010. Consequences of habitat disturbance and recovery to recruitment and the abundance of kelp forest fishes. J. Exp. Mar. Biol. Ecol. 386, 1–10. ( 10.1016/j.jembe.2010.01.016) [DOI] [Google Scholar]
- 43.Hamilton S, Caselle J. 2015. Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. R. Soc. B 282, 20141817 ( 10.1098/rspb.2014.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed D, Nelson J, Harrer S, Miller R. 2016. Estimating biomass of benthic kelp forest invertebrates from body size and percent cover data. Mar. Biol. 163, 101 ( 10.1007/s00227-016-2879-x) [DOI] [Google Scholar]
- 45.Jost L. 2006. Entropy and diversity. Oikos 113, 363–375. ( 10.1111/j.2006.0030-1299.14714.x) [DOI] [Google Scholar]
- 46.Lefcheck J. 2016. PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 47.Bates D, Machler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 48.Shipley B. 2013. The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 94, 560–564. ( 10.1890/12-0976.1) [DOI] [PubMed] [Google Scholar]
- 49.Pinheiro M, Rua A, Dias F. 2013. Dynamic factor models with jagged edge panel data: taking on board the dynamics of the idiosyncratic components. Oxford Bull. Econ. Stat. 75, 80–102. ( 10.1111/obes.12006) [DOI] [Google Scholar]
- 50.Ebeling AW, Larson RJ, Alevizon WS. 1980. Habitat groups and island-mainland distribution of kelp-bed fishes off Santa Barbara, California. In Multidisciplinary symposium on the California Islands (ed. Power DM.), pp. 403–431. Santa Barbara, CA: Santa Barbara Museum of Natural History. [Google Scholar]
- 51.Bodkin J. 1988. Effects of kelp forest removal on associated fish assemblages in central California. J. Exp. Mar. Biol. Ecol. 117, 227–238. ( 10.1016/0022-0981(88)90059-7) [DOI] [Google Scholar]
- 52.Holbrook S, Carr M, Schmitt R, Coyer J. 1990. Effect of giant kelp on local abundance of reef fishes: the importance of ontogenic resource requirements. Bull. Mar. Sci. 47, 104–114. [Google Scholar]
- 53.Carr M. 1991. Habitat selection and recruitment of an assemblage of temperate zone reef fishes. J. Exp. Mar. Biol. Ecol. 146, 113–137. ( 10.1016/0022-0981(91)90257-W) [DOI] [Google Scholar]
- 54.Angel A, Ojeda F. 2001. Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: the effect of habitat complexity. Mar. Ecol. Prog. Ser. 217, 81–91. ( 10.3354/meps217081) [DOI] [Google Scholar]
- 55.Lowe C, Topping D, Cartamil D, Papastamatiou Y. 2003. Movement patterns, home range, and habitat utilization of adult kelp bass Paralabrax clathratus in a temperate no-take marine reserve. Mar. Ecol. Prog. Ser. 256, 205–216. ( 10.3354/meps256205) [DOI] [Google Scholar]
- 56.Davenport A, Anderson T. 2007. Positive indirect effects of reef fishes on kelp performance: the importance of mesograzers. Ecology 88, 1548–1561. ( 10.1890/06-0880) [DOI] [PubMed] [Google Scholar]
- 57.Koenigs C, Miller R, Page H. 2015. Top predators rely on carbon derived from giant kelp Macrocystis pyrifera. Mar. Ecol. Prog. Ser. 537, 1–8. ( 10.3354/meps11467) [DOI] [Google Scholar]
- 58.Santelices B, Ojeda F. 1984. Effects of canopy removal on the understory algal community structure of coastal forests of Macrocystis pyrifera from southern South America. Mar. Ecol. Prog. Ser. 14, 165–173. ( 10.3354/meps014165) [DOI] [Google Scholar]
- 59.Pearse J, Hines A. 1979. Expansion of a central california kelp forest following the mass mortality of sea urchins. Mar. Biol. 51, 83–91. ( 10.1007/BF00389034) [DOI] [Google Scholar]
- 60.Miller R, Etter R. 2008. Shading facilitates sessile invertebrate dominance in the rocky subtidal Gulf of Maine. Ecology 89, 452–462. ( 10.1890/06-1099.1) [DOI] [PubMed] [Google Scholar]
- 61.Miller R, Etter R. 2011. Rock walls: small-scale diversity hotspots in the subtidal Gulf of Maine. Mar. Ecol. Prog. Ser. 425, 153–165. ( 10.3354/meps09025) [DOI] [Google Scholar]
- 62.Reed D, Rassweiler A, Miller R, Page H, Holbrook S. 2016. The value of a broad temporal and spatial perspective in understanding dynamics of kelp forest ecosystems. Mar. Freshwater Res. 67, 14–24. ( 10.1071/MF14158) [DOI] [Google Scholar]
- 63.Graham M, Vasquez J, Buschmann A, Gibson R, Atkinson R, Gordon J. 2007. Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr. Mar. Biol. 45, 39–88. [Google Scholar]
- 64.Thomsen M, Wernberg T, Altieri A, Tuya F, Gulbransen D, McGlathery K, Holmer M, Silliman B. 2010. Habitat cascades: the conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 50, 158–175. ( 10.1093/icb/icq042) [DOI] [PubMed] [Google Scholar]
- 65.Miller R, Page H. 2012. Kelp as a trophic resource for marine suspension feeders: a review of isotope-based evidence. Mar. Biol. 159, 1391–1402. ( 10.1007/s00227-012-1929-2) [DOI] [Google Scholar]
- 66.Page HM, Reed DC, Brzezinski MA, Melack JM, Dugan JE. 2008. Assessing the importance of land and marine sources of organic matter to kelp forest food webs. Mar. Ecol. Prog. Ser. 360, 47–62. ( 10.3354/meps07382) [DOI] [Google Scholar]
- 67.Yorke C, Miller R, Page H, Reed D. 2013. Importance of kelp detritus as a component of suspended particulate organic matter in giant kelp Macrocystis pyrifera forests. Mar. Ecol. Prog. Ser. 493, 113–125. ( 10.3354/meps10502) [DOI] [Google Scholar]
- 68.Miller R, Page H, Brzezinski M. 2013. δ13C and δ15N of particulate organic matter in the Santa Barbara Channel: drivers and implications for trophic inference. Mar. Ecol. Prog. Ser. 474, 53–66. ( 10.3354/meps10098) [DOI] [Google Scholar]
- 69.Reed D. 2017. SBC LTER: Reef: Long-term experiment: Kelp removal: Detritus biomass. Environmental Data Initiative. See https://portal.lternet.edu/nis/mapbrowse?packageid=knb-lter-sbc.25.18 ( 10.6073/pasta/490d9479fe3dfffe42140650246b870a) [DOI] [Google Scholar]
- 70.Coyer J. 1984. The invertebrate assemblage associated with the giant kelp, Macrocystis pyrifera, at Santa Catalina Island, California—a general description with emphasis on amphipods, copepods, mysids, and shrimps. Fish. Bull. 82, 55–66. [Google Scholar]
- 71.Reed D, Washburn L, Rassweiler A, Miller R, Bell T, Harrer S. 2016. Extreme warming challenges sentinel status of kelp forests as indicators of climate change. Nat. Commun. 7, 13757 ( 10.1038/ncomms13757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed D, Rassweiler A, Arkema K. 2009. Density derived estimates of standing crop and net primary production in the giant kelp Macrocystis pyrifera. Mar. Biol. 156, 2077–2083. ( 10.1007/s00227-009-1238-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holbrook S, Schmitt R. 1992. Causes and consequences of dietary specialization in surfperches: patch choice and intraspecific competition. Ecology 73, 402–412. ( 10.2307/1940748) [DOI] [Google Scholar]
- 74.Dearn SL. 1987. The fauna of subtidal articulated coralline mats: composition, dynamics, and effects of spatial heterogeneity. Stanislaus, CA: California State University. [Google Scholar]
- 75.Hobson E, Chess J. 2001. Influence of trophic relations on form and behavior among fishes and benthic invertebrates in some California marine communities. Environ. Biol. Fishes 60, 411–457. ( 10.1023/A:1011027312001) [DOI] [Google Scholar]
- 76.Estes J, Duggins D. 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr. 65, 75–100. ( 10.2307/2937159) [DOI] [Google Scholar]
- 77.Lafferty K. 2004. Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 14, 1566–1573. ( 10.1890/03-5088) [DOI] [Google Scholar]
- 78.Kay M, Lenihan H, Kotchen M, Miller C. 2012. Effects of marine reserves on California spiny lobster are robust and modified by fine-scale habitat features and distance from reserve borders. Mar. Ecol. Prog. Ser. 451, 137–150. ( 10.3354/meps09592) [DOI] [Google Scholar]
- 79.Lafferty K, Tinker M. 2014. Sea otters are recolonizing southern California in fits and starts. Ecosphere 5, art50 ( 10.1890/ES13-00394.1) [DOI] [Google Scholar]
- 80.Saarman E, Carr M. 2013. The California Marine Life Protection Act: A balance of top down and bottom up governance in MPA planning. Mar. Policy 41, 41–49. ( 10.1016/j.marpol.2013.01.004) [DOI] [Google Scholar]
- 81.Parnell P. 2015. The effects of seascape pattern on algal patch structure, sea urchin barrens, and ecological processes. J. Exp. Mar. Biol. Ecol. 465, 64–76. ( 10.1016/j.jembe.2015.01.010) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available on the website of the Santa Barbara Coastal Long Term Ecological Research program http://sbc.lternet.edu//data/dataCollectionsPortal.html