Abstract
Because phenotypic plasticity can operate both within and between generations, phenotypic outcomes are often shaped by a complex history of environmental signals. For example, parental and embryonic experiences with predation risk can both independently and interactively influence prey offspring traits early in their life. Parental and embryonic risk experiences can also independently shape offspring phenotypes throughout an offspring's ontogeny, but the persistence of their interactive effects throughout offspring ontogeny is unknown. We examined the effects of parental and embryonic experiences with predation risk on the response of 1-year-old prey (the carnivorous snail, Nucella lapillus) offspring to current predation risk. We found that parental and embryonic risk experiences had largely independent effects on offspring performance and that these effects were context dependent. Parental experience with risk had strong impacts on multiple offspring traits in the presence of current risk that generally improved offspring performance under risk, but embryonic risk experience had relatively weaker effects and only operated in the absence of current risk to reduce offspring growth. These results illustrate that past environmental experiences can dynamically shape organism phenotypes across ontogeny and that attention to these effects is key to a better understanding of predator/prey dynamics in natural systems.
Keywords: parental effects, embryonic effects, developmental plasticity, transgenerational plasticity, life history, Nucella lapillus
1. Introduction
Organisms frequently respond to changes in their environment via phenotypic plasticity, and such modifications have clear implications for individual fitness [1–3] and community and ecosystem dynamics [4,5]. Phenotypic expression can also be influenced by an individual's previous or historical experiences that persist to affect future performance (e.g. carryover effects [6]). One particularly influential period is embryonic development [7]. Both theoretical and empirical work indicates that the environmental conditions experienced as an embryo can affect an organism's phenotype at emergence [8,9], but also have outsized impacts on its lifetime fitness trajectory [10–12]. Moreover, embryonic experience can persist through major life-history shifts such as metamorphosis [13] and because of its influence on adult traits, have lasting impacts on multiple generations [14].
Organisms can also exhibit phenotypic plasticity in response to their parents' environmental experience via parental effects (i.e. transgenerational phenotypic plasticity [15–17]). Parental effects may be adaptive for offspring (and therefore parents) if they appropriately anticipate future conditions and hence mitigate uncertainty in heterogeneous environments [18,19]. Adaptive parental effects are, therefore, likely to be more prevalent in systems where a parent's environment is a strong predictor of its offspring's environment and when environmental conditions vary over ecologically relevant time scales [18,20]. Such parental effects operate in a variety of systems and in response to a diverse array of environmental cues, even in species without parental care (e.g. predation risk [21], environmental quality [22] and climate change [23]). Parental effects can also have remarkable longevity throughout the lifetime of offspring [24,25] and extend beyond one generation (grandparental effects [26]). Hence, the influence of parental experience on offspring is probably a pervasive feature of natural systems that has important effects on phenotypic expression.
While parental and embryonic effects each have independent and lasting impacts on individual fitness, they may also interact to influence individual performance. Reinforcement between parental and embryonic environments, for example, may more reliably indicate the conditions that an individual is likely to encounter than either signal alone. Parental and embryonic effects are more likely to interact in systems where offspring have low dispersal potential and thus develop in conditions similar to their parents or where species have short life cycles relative to the rate of environmental change [12]. Despite the potential for parental and embryonic experiences to interact, our understanding of how and when this interaction operates in offspring and whether it changes across offspring ontogeny is quite limited. Theory predicts that more recent environmental signals should have a stronger influence on organismal traits because the reliability of information obtained from a changing environment declines over time [27]. Hence, closer temporal proximity between the parental and embryonic environments and those experienced immediately after emergence may increase the likelihood that parental and embryonic effects have greater impacts early in offspring ontogeny. There is evidence for ontogenetic shifts in the independent influence of parental (e.g. [28,29]) and embryonic (e.g. [30]) effects. For example, bryozoans whose mothers experienced high loads of copper pollutant performed better as larvae when exposed to copper themselves, but these effects changed over time such that maternal effects diminished offspring performance at later life stages, particularly in stressful conditions [31]. Thus, the nature and strength of any interactive effects between parental and embryonic experience may also change over an individual's lifetime.
Predation risk, where predators scare rather than consume their prey, can be a strong driver of prey behaviour and performance in many systems. In the presence of predation risk, prey often seek refuge in safe habitats to reduce their risk of being consumed [32,33], but by doing so their foraging and performance can suffer [34,35]. These effects can cascade throughout the community and ecosystem with important consequences for population dynamics [36], resource abundance [37] and nutrient cycling [38]. Furthermore, both parental and early life experiences with predation risk can independently affect offspring traits at emergence [9,39] and later in life [40,41]. Donelan & Trussell [42] also found that parental experience with predation risk impacted the size of prey offspring at emergence in a rocky intertidal snail, but only if the offspring were also exposed to risk as embryos. Whether this interaction between parental and embryonic experience with predation risk persists throughout offspring ontogeny, however, remains unknown.
The snail Nucella lapillus, an important intermediate consumer on rocky shores, increases its use of refuge habitats [43], reduces its foraging behaviour [44], produces less tissue [45] and grows less efficiently [46] in the presence of predation risk from the green crab (Carcinus maenas). Parental experience with green crab predation risk, however, can reduce these fitness consequences in adult Nucella offspring [47] and, when combined with embryonic risk exposure, reduce the physiological costs of predation risk in offspring as embryos, thereby allowing them to achieve a larger size at emergence [42]. To explore whether parental and embryonic exposures to predation risk interact to influence the response (behaviour and performance) of offspring to predation risk later in life history, we conducted a laboratory experiment where we manipulated parental experience with predation risk, embryonic experience with risk and exposure to current risk in 1-year-old, subadult Nucella. Our results suggest that the strength and nature of parental and embryonic effects can change during offspring ontogeny and that organisms can dynamically integrate past environmental experiences into current phenotypic outcomes. Parental effects, however, appear to have lasting and substantial impacts on prey performance and probably play an important role in predator/prey dynamics in natural systems.
2. Material and methods
We examined the effects of parental experience (presence/absence) and embryonic experience (presence/absence) with predation risk from the green crab C. maenas on the response of 1-year-old N. lapillus (a carnivorous snail, hereafter Nucella) offspring to current green crab predation risk (presence/absence). Offspring were born and raised in the flow-through seawater facilities at the Marine Science Center in Nahant, MA, USA. Parent Nucella (males and females > 20 mm shell length [48]) were collected from an exposed rocky intertidal shore in Nahant, MA, USA, in early February 2015, returned to the Marine Science Center, and held separately by sex until late spring to allow females to expel any stored sperm (Nucella can store sperm for up to three months [49]) prior to experimental mating. Importantly, we collected snails of a similar age class (i.e. size) and from a relatively small area (approx. 20 m2) at the same site. Nucella are direct developers and are not highly mobile [50] so individuals in a small area have probably experienced similar environmental conditions throughout their lives.
In mid-May, male and female Nucella were randomly paired to create 50 mating pairs. Our experimental design independently manipulated parental and embryonic risk environments, so it was necessary to prevent females from depositing egg capsules in the presence of risk. We, therefore, created a week-long mating cycle consisting of two stages: (i) the risk manipulation stage, where parent snails were placed in the presence or absence of predation risk for 3 days, but kept separately and thus not allowed to mate, followed by (ii) the mating stage, where the male and female in a given pair were placed together in the same chamber in the absence of risk for 4 days to mate and deposit egg capsules.
In the risk manipulation stage, each male and female in a given pair was placed in its own perforated jar (8 × 10 cm, dia. × h) with six blue mussels (Mytilus edulis, 13.8 ± 1.4, mean shell length ± s.d.) for food. Jars containing the male and female in a given pair were placed together in a larger plastic bucket (24 × 24 cm, dia. × h) that was independently supplied with flowing seawater and also contained a perforated ‘risk manipulation’ chamber (11.5 × 10 cm, dia. × h). This risk manipulation chamber housed either one male green crab (73.7 ± 2.2 mm, mean carapace width ± s.d.) with two Nucella for food (risk present) or two Nucella alone (risk absent). Importantly, Nucella are highly sensitive to the presence of Carcinus even without the presence of food snails (e.g. [46]) and do not respond to the presence of other large intertidal crab species [51]. Food mussels and food snails were replenished each week. After 3 days in the risk manipulation stage, the male and female in each parent pair were moved to a separate mating stage bucket (24 × 24 cm, dia. × h) where they were placed together in the same perforated mating chamber (11.5 × 10 cm, dia. × h) that received an independent supply of flowing water. Parents remained in the mating stage for 4 days to mate and deposit egg capsules, hence all egg capsule deposition occurred in the absence of risk. After 4 days, each pair was re-separated and placed in their original risk manipulation bucket, as before. This cycle continued for 12 weeks. Because there were 50 parent pairs, there were 50 independent buckets in the risk manipulation stage and 50 independent buckets in the mating stage.
Mating chambers were inspected each week for newly deposited egg capsules. If we found newly deposited egg capsules, this new clutch was removed and divided approximately in half; each half was then placed into its own mesh-lined tea infuser (Upton Tea Imports). One tea infuser from each clutch was placed into its own bucket (17 × 14 cm, dia. × h) that also contained a perforated chamber (10 × 10 × 7 cm, l × w × h) that housed one adult male green crab (presence of embryonic risk). The other tea infuser from that clutch was placed into its own bucket which contained an empty perforated container (absence of embryonic risk). Each new clutch was similarly divided so that each embryonic risk manipulation bucket contained only one tea infuser; there were a total of 75 independent embryonic risk manipulation buckets (n = 39 for presence of embryonic risk, n = 36 for absence of embryonic risk). All egg capsules remained in the presence or absence of embryonic risk for one week. After one week, egg capsules were moved to a new tea infuser that was placed in its own plastic jar (8 × 10 cm, dia. × h) that received risk-free, flowing seawater.
Six weeks after egg capsule deposition, we began to inspect tea infusers every 2–3 days for the emergence of Nucella offspring. Newly emerged offspring (1.2 ± 0.1 mm, mean shell length ± s.d.) were given approximately 200 juvenile blue mussels (1.1 ± 0.2 mm, mean shell length ± s.d.) for food immediately after emergence and each week thereafter until they were large enough to switch to larger mussels (4.6 ± 1.9 mm, mean shell length ± s.d.). Before the onset of winter, offspring from each tea infuser were transferred to perforated plastic jars (8 × 10 cm, dia. × h) that were placed in a larger plastic bucket that, owing to logistical constraints, also contained five other jars that held offspring from the same parental experience×embryonic experience treatment combination. Each bucket received its own supply of seawater and ‘winter bucket’ had no effect on initial shell or tissue mass of offspring (p > 0.1). Nucella offspring were fed an ad libitum supply of blue mussels and held in these risk-free conditions until the following summer. The offspring used in this experiment are siblings of those used in our earlier work [42].
The following July, approximately 1 year after their emergence from egg capsules, we exposed Nucella offspring to the presence and absence of current predation risk in a laboratory mesocosm experiment at the Marine Science Center. Nucella offspring were 14–19 mm in shell length and, therefore, were considered subadults [45] that were approaching sexual maturity [48]. Mesocosms (27 × 15 × 5 cm, l × w × h) consisted of two chambers separated by a perforated wall: an upstream chamber with a perforated roof for the manipulation of predation risk and a downstream chamber that housed experimental Nucella offspring and their food. We manipulated exposure to current predation risk by placing one male green crab (75.2 ± 3.7 mm, mean carapace width ± s.d.) with two Nucella food snails (risk present) or two Nucella food snails alone (risk absent) in the upstream chamber. The downstream chamber held four experimental Nucella offspring (16.3 ± 1.6 mm, mean shell length ± s.d.) from the same treatment combination (parental experience × embryonic experience) and 60 blue mussels (13.0 ± 2.0 mm, mean shell length ± s.d.) for food. Mussels were placed on top of a granite tile (15 × 15 × 1 cm, l × w × h) that was elevated on 1 cm PVC spacers to create a narrow space under the tile to provide a refuge for Nucella offspring [44]. Each mesocosm was placed in a larger plastic box (33 × 19 × 12 cm, l × w × h) that received an independent supply of flowing seawater. There were eight replicates for each treatment combination (n = 64), and the experiment ran for 25 days. Offspring from 23 parent pairs (10 pairs in the presence of parental risk, 13 in the absence of parental risk) were distributed evenly among the mesocosms as appropriate, and 6–12 (mean = 8.4) families were represented in each treatment combination.
Every 3–4 days, we monitored Nucella offspring refuge use in each mesocosm by recording the location of each snail. Offspring were considered in refuge if they were found underneath the tile, while all other locations were considered risky habitat. We calculated the proportion of Nucella offspring in refuge in each mesocosm by counting the number of snails found in refuge during a given observation and dividing by the total number of snails in that mesocosm (n = 4). We made a total of seven behavioural observations. We measured Nucella offspring tissue growth (final − initial tissue, g) by marking each snail with a numbered bee tag and weighing them using a non-destructive buoyant weighing technique [52] at the beginning and end of the experiment. We converted tissue growth to its energetic equivalent (Joules, J) using empirically derived equations (electronic supplementary material, appendix S1) that convert measured tissue growth into dry tissue mass (milligrams, mg [53]) and dry tissue mass into its energetic equivalent (J, [50]). We determined Nucella foraging activity by counting the number of mussels consumed (indicated by a drill hole on remaining shell) and used the maximum shell length (millimetres, mm) of each consumed mussel to calculate its dry tissue weight (mg [54]) and tissue energetic value (19.5 J mg−1 [55]). Per capita Nucella offspring foraging activity was then determined by dividing the total amount of energy (Joules) consumed by all offspring in a given replicate by the number of offspring in that replicate (n = 4).
We calculated Nucella offspring growth efficiency by dividing individual tissue growth (Joules) by the average per capita foraging activity (Joules) in that replicate. Growth efficiency is a measure of an individual's ability to convert ingested energy into body mass and while it integrates changes in growth and foraging, it can also be directly reduced by predation risk [46]. We focused on tissue growth because tissue is more energetically expensive to produce than shell in Nucella [56] and is, therefore, a better indicator of an individual's total energetic requirements.
We analysed Nucella offspring refuge use and foraging activity using separate type III ANOVAs that considered parental experience with predation risk, embryonic experience with risk and current exposure to risk as fixed effects. Refuge use and foraging activity analyses were done on replicate averages (n = 64); for refuge use, we used the average proportion of offspring in refuge during the seven observations. Because we cannot account for individual offspring foraging rates, we calculated the per capita foraging rate for each replicate and applied it to all offspring in that replicate.
We analysed individual Nucella offspring tissue growth (Joules) and growth efficiency using separate split-plot type III ANOVAs that considered parental experience with predation risk, embryonic experience with risk and current risk exposure as fixed effects and included parent pair (i.e. family) nested within parental experience with risk as a random effect to account for potential differences in the response of parent pairs to predation risk. Because there were multiple Nucella in each replicate, replicate was considered a random effect nested within the parental, embryonic and current risk treatments. Finally, embryonic bucket ID was included as a random block effect.
We conducted the analyses in JMP 11 using REML variance estimates and explored any significant interactions using least-square (LS) contrasts to compare group means. Two replicates (−parental risk/−embryonic risk/+current risk and +parental risk/+embryonic risk/+current risk) were excluded because half of the offspring died mid-way through the experiment. We explored the significance of the random effects using likelihood ratio tests ([57]; see the electronic supplementary material). Data are available in the Dryad Digital Repository [58], and ANOVA and likelihood ratio test results are provided in the electronic supplementary material.
3. Results
In the presence of current risk, Nucella offspring used refuge more often (current risk: F1,54 = 55.7, p < 0.0001; figure 1), but the offspring of risk-experienced parents used refuges 29% less than offspring of risk-naive parents (parental experience × current risk: F1,54 = 7.9, p = 0.007, LS contrast: p = 0.001; figure 1). Refuge use was not affected by embryonic risk experience (F1,54 = 1.1, p = 0.3).
Figure 1.
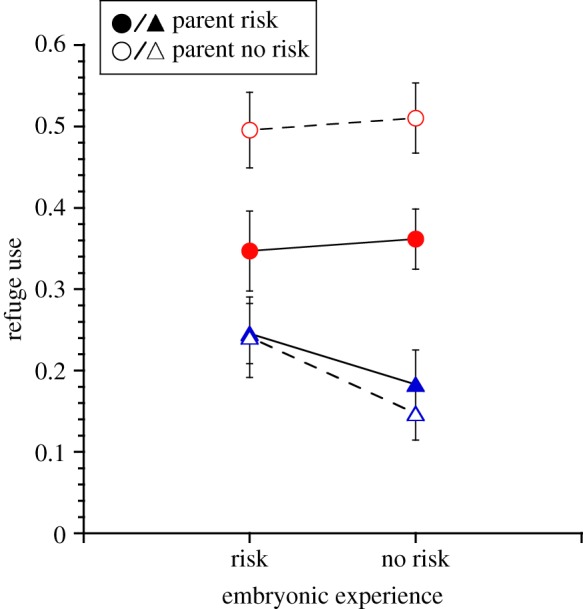
Mean (±s.e.) refuge use of offspring Nucella lapillus in the presence (circles) and absence (triangles) of current predation risk from the green crab Carcinus maenas. Offspring experienced the presence and absence of green crab predation risk as embryos and were produced by parents that experienced the presence (filled symbols) and absence (open symbols) of green crab predation risk. The three-way interaction is not significant here (parental × embryonic × current: p = 0.8), but is shown for ease of comparison to figure 2. n = 8 for all treatment combinations except: −parental risk/−embryonic risk/+current risk and +parental risk/+embryonic risk/+current risk. (Online version in colour.)
Despite differences in refuge use, there were no effects of parental or embryonic experiences with risk on offspring foraging activity (all p > 0.14), but offspring did forage less in the presence of current risk (current risk: F1,54 = 466.7, p < 0.0001; electronic supplementary material, figure S1). By contrast, parental, embryonic and current risk experiences interactively influenced offspring tissue growth (parental experience × embryonic experience × current risk: F1,53.3 = 4.3, p = 0.043; figure 2). In the presence of current risk, offspring of risk-experienced parents grew 128% more tissue than offspring of risk-naive parents (LS contrast: p = 0.05; figure 2), but there was no effect of parental risk experience in the absence of current risk (LS contrast, p = 0.8). In the absence of current risk, embryonic risk experience influenced tissue growth for offspring of risk-naive parents (LS contrast: p = 0.01) but not for offspring of risk-experienced parents (LS contrast p = 0.6). Offspring of risk-naive parents that did not experience risk as embryos grew 18% more tissue than offspring that did experience risk as embryos. There was no effect of embryonic risk experience on offspring tissue growth in the presence of current risk (LS contrast: p = 0.5).
Figure 2.
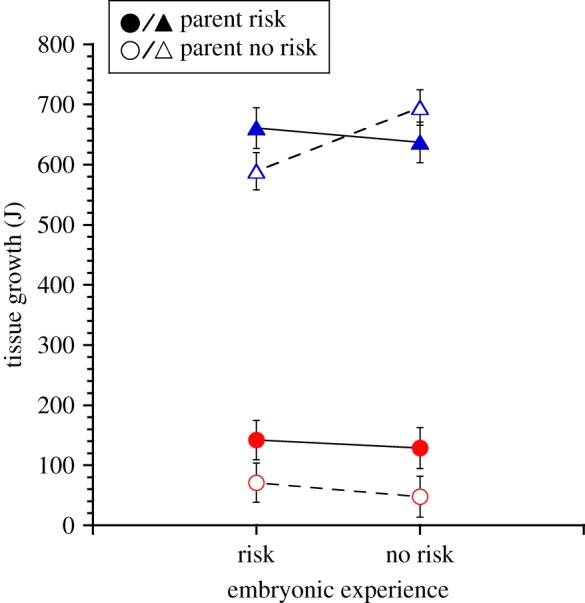
Mean (±s.e.) tissue growth (Joules, J) of offspring Nucella lapillus in the presence (circles) and absence (triangles) of current predation risk from the green crab Carcinus maenas. Offspring experienced the presence and absence of green crab predation risk as embryos and were produced by parents that experienced the presence (filled symbols) and absence (open symbols) of green crab predation risk. n = 8 for all treatment combinations except: −parental risk/−embryonic risk/+current risk and +parental risk/+embryonic risk/+current risk. (Online version in colour.)
Offspring also grew less efficiently in the presence of current risk (F1,53.2 = 312.9, p < 0.0001; figure 3), but offspring of risk-experienced parents were 88% more efficient than the offspring of risk-naive parents (parental experience × current risk: F1,53.2 = 8.8, p = 0.004, LS contrast: p = 0.03; figure 3) in the presence of current risk. There was no effect of parental risk experience on growth efficiency in the absence of current risk (LS contrast: p = 0.7). There was also an interaction between embryonic risk experience and current risk (F1,53.0 = 4.2, p = 0.046), but post hoc tests revealed no differences between means (LS contrasts, presence of embryonic risk: p = 0.2, absence of embryonic risk: p = 0.3) Growth efficiency was not affected by embryonic risk experience (F1,1.3 = 0.05, p = 0.8). Finally, none of the random effects, including parent pair, affected offspring growth or growth efficiency (electronic supplementary material, table S3).
Figure 3.
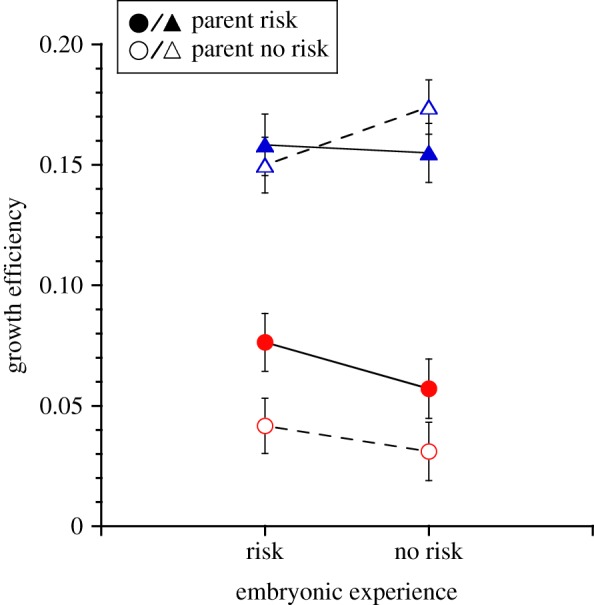
Mean (±s.e.) growth efficiency of offspring Nucella lapillus in the presence (circles) and absence (triangles) of current predation risk from the green crab Carcinus maenas. Offspring experienced the presence and absence of green crab predation risk as embryos and were produced by parents that experienced the presence (filled symbols) and absence (open symbols) of green crab predation risk. The three-way interaction is not significant here (parental × embryonic × current: p = 0.5), but is shown for ease of comparison to figure 2. n = 8 for all treatment combinations except: −parental risk/−embryonic risk/+current risk and +parental risk/+embryonic risk/+current risk. (Online version in colour.)
4. Discussion
In the presence of current green crab predation risk, Nucella offspring used refuges more and had lower foraging activity, growth and growth efficiency than those in the absence of current risk (figures 1–3). These results are consistent with previous work where Nucella increase their antipredator behaviour and exhibit reduced performance in the presence of risk (e.g. [45]). Parental experience with predation risk, however, largely reversed these effects—offspring of risk-experienced parents spent less time in refuge, grew more and had higher growth efficiency in the presence of current risk than offspring of risk-naive parents. These results support our previous work that parental experience with green crab predation risk improves the performance of 1-year-old Nucella offspring [47]. Importantly, while our risk manipulation treatment exposed offspring to constant predation risk throughout the experiment, Nucella that are intermittently (e.g. 25% of the time) exposed to risk from Carcinus experience similar fitness consequences as those in constant risk [59].
In the presence of current risk, offspring of risk-experienced parents spent less time in refuge habitats than offspring of risk-naive parents (figure 1), suggesting that parental effects impact how offspring manage risk. Parental risk experience may increase the reliability of information communicated by predator cues to offspring and thus enhance the ability of offspring to validate the potential persistence of predation risk in their environment. Temporal variation in risk can be a strong driver of prey behaviour; both theoretical and empirical work suggest that prey which are consistently exposed to high risk display weaker antipredator behaviour than those exposed to shorter, more variable risk (risk allocation hypothesis [53,60]). Parental experience with risk may also influence how offspring perceive variability in their risk environment by decreasing uncertainty about future risk conditions. Hence, offspring may be emboldened to leave a refuge even after short risk exposure because they judge the costs of prolonged antipredator behaviour (e.g. reduced foraging) to be higher than its benefits. By contrast, the offspring of risk-naive parents may have greater uncertainty about present and future risk conditions and, therefore, remain in refuge longer even as the costs of doing so escalate. Prey will only confront such decisions when the costs of antipredator behaviour are sufficiently great, which is predicted to occur only after prolonged periods of risk exposure such as those in our experiment (25 days [33,61]). Therefore, while our results differ from studies in other systems showing that offspring of risk-experienced parents display greater antipredator behaviour during very brief risk exposures [62,63], the costs of such behaviours probably manifest only after longer periods of risk exposure.
Interestingly, parental experience with risk did not impact offspring foraging activity in either the presence or absence of current risk (electronic supplementary material, figure S1) despite differences in refuge use. This similarity in foraging may have emerged because the offspring of risk-experienced and risk-naive parents employed different foraging strategies. For example, offspring of risk-naive parents may move back and forth between the risky and refuge habitats, whereas offspring of risk-experienced parents remain in the risky habitat. However, more frequent behavioural observations would be necessary to explore this hypothesis. While other experiments in this system have detected a clear trade-off between foraging and hiding (e.g. [44,64]), the current study indicates that this issue can be quite nuanced. Despite this observed similarity in foraging, the offspring of risk-experienced parents grew more tissue than offspring of risk-naive parents (figure 2) because they had higher growth efficiency in the presence of current risk (figure 3). These results suggest that parental effects operated through physiological changes in offspring in response to current predation risk. Exposure to predation risk often has negative effects on prey growth efficiency [46,65] because prey allocate energy away from growth to support increased respiration and other physiological pathways that mitigate the impacts of stress [66,67]. Elsewhere [42] we have shown that parental risk experience probably contributes to reduced respiration rates in Nucella embryos that are exposed to green crab risk cues, suggesting that offspring of risk-experienced parents exhibit a weaker stress response or require less energy to do so.
Parental effects often operate through epigenetic modifications in offspring that make genes more or less accessible to transcription [68,69], which may impact the energy required for gene expression. We do not know if parental effects act through epigenetic modifications in this system and this intriguing hypothesis awaits future work. Moreover, despite apparent improvements in offspring growth and physiology, we have yet to establish whether these changes reflect adaptive parental effects because we have not observed their impacts on the reproductive output of parents or the survival of offspring. The reduction in antipredator behaviour among offspring of risk-experienced parents could improve offspring survival under extended periods of risk by reducing the risk of starvation (see above), which may outweigh the risk of being eaten. In any case, the positive relationship between individual size and fecundity observed in many systems (e.g. [70]) suggests that parental experience with risk may increase the reproductive output of subadult offspring Nucella in the presence of current risk.
Importantly, parental effects only operated when offspring were exposed to current risk, and such context-dependency is common in the expression of parental effects [17,31,71,72]. Parental effects may be more beneficial to offspring when parental and offspring environments are similar [73–75] and when offspring face adverse conditions [76] such as those under predation risk. Our results did not reveal costs of parental risk experience for offspring in the absence of current risk, which is surprising given previous work showing that parental or early life effects can be maladaptive when they do not accurately predict future offspring environmental conditions [73–75]. However, the adaptive value of parental effects may depend upon the specific mechanism through which they impact offspring fitness. If parental effects act to reduce the physiological costs of predation risk in their offspring as suggested by our results, such changes would be unnecessary in benign, risk-free conditions. It is also possible that despite our monitoring of offspring traits across multiple stages of life history (this study and [42]), we have yet to isolate the trait or stage of ontogeny where such costs are evident. Finally, the costs of parental effects in this system may only appear in the parent generation [19], which was not monitored here.
Although embryonic experience with predation risk did not significantly affect offspring refuge use, foraging activity or growth efficiency in either the presence or absence of current risk, it did affect offspring tissue growth in the absence of current risk—offspring that were exposed to predation risk as embryos produced less tissue than offspring that were not exposed to risk as embryos (figure 2). These reductions in offspring tissue growth in response to embryonic risk experience were not driven by changes in growth efficiency as they were for offspring based on parental risk experience (figure 3). Interestingly, however, these size patterns correspond with those that we found for offspring at emergence in our earlier work—offspring of risk-naive parents emerged smaller from development if they were exposed to risk as embryos [42]. We hypothesize that a silver spoon effect [77,78] may be operating: offspring that experienced relatively benign, risk-free conditions as embryos were relatively better off and, therefore, grew more as 1-year-old adults than those that experienced stressful, risky conditions as embryos. Hence, low-stress conditions early in life may have lasting and positive effects on offspring performance later in life. It is possible that these changes in offspring growth based on embryonic risk experiences impact the willingness of offspring to forage. While only correlative, our results suggest that in the absence of current risk, offspring that experienced risk as embryos consumed on average approximately 125 fewer Joules per capita and produced on average approximately 106 J less tissue than offspring that did not experience risk as embryos. Foraging can be inherently risky regardless of habitat [79]; for example, consuming a mussel can leave Nucella vulnerable for extended periods of time [80]. Offspring that experienced risk as embryos may be more averse to such risk, regardless of their current risk conditions, which may have driven the slight reductions in tissue growth.
We found that embryonic risk experience had no effect on Nucella offspring performance in the presence of current risk, possibly because the impact of embryonic risk experience on offspring growth was relatively small compared to the effect of current risk exposure. Indeed, the overall magnitude of the embryonic effect was much smaller than the effects of either parental or current risk exposure: when operating, parental and current risk experiences suppressed offspring growth by 100% and 85%, respectively, whereas embryonic experience with risk-reduced growth by only 17%. Hence, even if embryonic effects were operating on offspring fitness in the presence of current risk, they may not have been substantial enough for us to detect.
The parents of the offspring used in this experiment were collected directly from the field, which may introduce heterogeneity in parental risk experiences. We attempted to minimize these potential effects by collecting parents from a relatively small spatial area (see Material and methods) and including ‘family’ in our statistical models. However, we recognize that all organisms are probably influenced by past experiences, either directly or indirectly (e.g. parental or grandparental) and that the response of parents to risk may further depend on experiences not manipulated in our experiment. Nevertheless, our results show a strong effect of parental experience despite these potential differences, suggesting that parental experience with risk prior to mating can have important impacts on offspring performance.
Our results reveal that the effects of parental and embryonic experiences are largely independent later in ontogeny. By contrast, our previous work [42] (on siblings of the offspring studied here) found that these effects early in offspring ontogeny operated synergistically to impact offspring size at emergence. Embryonic effects may be more influential during early life history because embryonic cues are deemed more reliable during early stages of ontogeny. Theory predicts that the use of environmental information should decline over time as organisms obtain more recent, and therefore relevant, information [27]. One might expect this rationale to apply to parental effects, but our results suggest that parental effects continued to have strong impacts on offspring throughout ontogeny. Hence, it appears that offspring may ‘trust’ their parents more than themselves when evaluating risk. Parental experience is the earliest possible source of information for offspring and, therefore, may inherently have more profound effects on phenotypic outcomes [12,27,81]. Furthermore, the strength and persistence of parental effects may be driven by their influence on offspring physiology, which may have more significant consequences for offspring than behavioural changes alone.
In summary, our results suggest that parental and embryonic experiences can affect offspring performance, but that their relative importance and tendency to interact may change over time based on environmental context. The persistence of parental and embryonic effects may also depend on the mechanisms through which they operate, so an exploration of the pathways that drive such effects is probably important when examining the role of parental and embryonic effects across an individual's lifetime. We suggest that changes in physiological performance may be more influential on offspring lifetime fitness than changes in foraging rates. Our results demonstrate that attention to the complexity of offspring responses based on parental and embryonic experiences will be essential to robustly predict how natural populations and communities will respond to changing environments.
Supplementary Material
Acknowledgements
We thank Erin Bucci, Rachel Dowley, Erin Sayre and Sydney Stenquist for their enormous help with all aspects of experimental set-up and maintenance, Kyle Pepperman and the Downeast Institute for supplying juvenile mussels, Jessica Torossian for mussel transport, Eric Sanford for experimental advice, Catherine Matassa for intellectual feedback and three anonymous reviewers for their helpful comments. This is part of the PhD dissertation of S.C.D. It is contribution no. 364 from the Marine Science Center.
Ethics
This work was conducted in accordance with the guidelines of the Association for the Study of Animal Behavior and the animal care guidelines of Northeastern University's Institutional Animal Care and Use Committee (IACUC).
Data accessibility
Data are available at the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.ks622 [58].
Authors' contributions
Both authors conceived of the study, S.C.D. designed and coordinated the study, carried out the statistical analyses and drafted the manuscript. Both authors contributed equally to manuscript revisions and give final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was generously supported by National Science Foundation grants to G.C.T. (IOS-1557901, OCE-0963010, Academic Research Infrastructure Recovery and Reinvestment Program, and OCE-1458150).
References
- 1.West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Ann. Rev. Ecol. Syst. 20, 249–278. ( 10.1146/annurev.es.20.110189.001341) [DOI] [Google Scholar]
- 2.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 3.Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH. 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217. ( 10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- 4.Schmitz OJ. 2003. Top predator control of plant biodiversity and productivity in an old field ecosystem. Ecol. Lett. 6, 156–163. ( 10.1046/j.1461-0248.2003.00412.x) [DOI] [Google Scholar]
- 5.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 6.O'Connor CM, Norris DR, Crossin GT, Cooke SJ. 2014. Biological carryover effects: linking common concepts and mechanisms in ecology and evolution. Ecosphere 5, 1–11. ( 10.1890/ES13-00388.1) [DOI] [Google Scholar]
- 7.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Williams TD. 1994. Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol. Rev. 69, 35–59. ( 10.1111/j.1469-185X.1994.tb01485.x) [DOI] [PubMed] [Google Scholar]
- 9.Warkentin KM. 1995. Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc. Natl Acad. Sci. USA 92, 3507–3510. ( 10.1073/pnas.92.8.3507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 11.De Block M, Stoks R. 2005. Fitness effects from egg to reproduction: bridging the life history transition. Ecology 86, 185–197. ( 10.1890/04-0116) [DOI] [Google Scholar]
- 12.English S, Fawcett TW, Higginson AD, Trimmer PC, Uller T, Gaillard J-M, Bronstein JL. 2016. Adaptive use of information during growth can explain long-term effects of early life experiences. Am. Nat. 187, 620–632. ( 10.1086/685644) [DOI] [PubMed] [Google Scholar]
- 13.Pechenik JA. 2006. Larval experience and latent effects: metamorphosis is not a new beginning. Integr. Comp. Biol. 46, 323–333. ( 10.1093/icb/icj028) [DOI] [PubMed] [Google Scholar]
- 14.Naguib M, Gil D. 2005. Transgenerational body size effects caused by early developmental stress in zebra finches. Biol. Lett. 1, 95–97. ( 10.1098/rsbl.2004.0277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardo J. 1996. Maternal effects in animal ecology. Am. Zool. 36, 83–105. ( 10.1093/icb/36.2.83) [DOI] [Google Scholar]
- 16.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Räsänen K, Kruuk L. 2007. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421. ( 10.1111/j.1365-2435.2007.01246.x) [DOI] [Google Scholar]
- 18.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 19.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 20.Burgess SC, Marshall DJ. 2014. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123, 769–776. ( 10.1111/oik.01235) [DOI] [Google Scholar]
- 21.Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63. ( 10.1038/43425) [DOI] [Google Scholar]
- 22.Fox CW, Thakar MS, Mousseau TA. 1997. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am. Nat. 149, 149–163. ( 10.1086/285983) [DOI] [PubMed] [Google Scholar]
- 23.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861. ( 10.1038/nclimate1599) [DOI] [Google Scholar]
- 24.LaMontagne JM, McCauley E. 2001. Maternal effects in Daphnia: what mothers are telling their offspring and do they listen? Ecol. Lett. 4, 64–71. ( 10.1046/j.1461-0248.2001.00197.x) [DOI] [Google Scholar]
- 25.Naguib M, Nemitz A, Gil D. 2006. Maternal developmental stress reduces reproductive success of female offspring in zebra finches. Proc. R. Soc. B 273, 1901–1905. ( 10.1098/rspb.2006.3526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C. 2012. Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 52, 77–88. ( 10.1093/icb/ics04) [DOI] [PubMed] [Google Scholar]
- 27.Dufty AM, Clobert J, Møller AP. 2002. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190–196. ( 10.1016/S0169-5347(02)02498-9) [DOI] [Google Scholar]
- 28.Lindholm AK, Hunt J, Brooks R. 2006. Where do all the maternal effects go? Variation in offspring body size through ontogeny in the live-bearing fish Poecilia parae. Biol. Lett. 2, 586–589. ( 10.1098/rsbl.2006.0546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andree SR, Feiner ZS, Bledsoe JW, Cragun AM, Höök TO. 2015. Ontogenetic variability of maternal effects in an iteroparous fish. Ecol. Freshw. Fish 24, 384–396. ( 10.1111/eff.12153) [DOI] [Google Scholar]
- 30.Pahkala M, Laurila A, Merilä J. 2001. Carry-over effects of ultraviolet–B radiation on larval fitness in Rana temporaria. Proc R. Soc. B 268, 1699–1706. ( 10.1098/rspb.2001.1725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall DJ. 2008. Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89, 418–427. ( 10.1890/07-0449.1) [DOI] [PubMed] [Google Scholar]
- 32.Sih A, Petranka JW, Kats LB. 1988. The dynamics of prey refuge use: a model and tests with sunfish and salamander larvae. Am. Nat. 132, 463–483. ( 10.1086/284865) [DOI] [Google Scholar]
- 33.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 34.Kats LB, Dill LM. 1998. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5, 361–394. ( 10.1080/11956860.1998.11682468) [DOI] [Google Scholar]
- 35.McCauley SJ, Rowe L, Fortin M-J. 2011. The deadly effects of ‘nonlethal’ predators. Ecology 92, 2043–2048. ( 10.1890/11-0455.1) [DOI] [PubMed] [Google Scholar]
- 36.Sheriff MJ, Krebs CJ, Boonstra R. 2010. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology 91, 2983–2994. ( 10.1890/09-1108.1) [DOI] [PubMed] [Google Scholar]
- 37.Matassa CM, Trussell GC. 2011. Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92, 2258–2266. ( 10.1890/11-0424.1) [DOI] [PubMed] [Google Scholar]
- 38.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209. ( 10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 39.Coslovsky M, Richner H. 2011. Predation risk affects offspring growth via maternal effects. Funct. Ecol. 25, 878–888. ( 10.1111/j.1365-2435.2011.01834.x) [DOI] [Google Scholar]
- 40.Peckarsky BL, Taylor BW, McIntosh AR, McPeek MA, Lytle DA. 2001. Variation in mayfly size at metamorphosis as a developmental response to risk of predation. Ecology 82, 740–757. ( 10.1890/0012-9658(2001)082%5B0740:VIMSAM%5D2.0.CO;2) [DOI] [Google Scholar]
- 41.McGhee KE, Pintor LM, Suhr EL, Bell AM. 2012. Maternal exposure to predation risk decreases offspring antipredator behaviour and survival in threespined stickleback. Funct. Ecol. 26, 932–940. ( 10.1111/j.1365-2435.2012.02008.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donelan SC, Trussell GC. 2018. Synergistic effects of parental and embryonic exposure to predation risk on prey offspring size at emergence. Ecology 99, 68–78. ( 10.1002/ecy.2067) [DOI] [PubMed] [Google Scholar]
- 43.Trussell GC, Ewanchuk PJ, Matassa CM. 2006. Habitat effects on the relative importance of trait- and density-mediated indirect interactions. Ecol. Lett. 9, 1245–1252. ( 10.1111/j.1461-0248.2006.00981.x) [DOI] [PubMed] [Google Scholar]
- 44.Donelan SC, Grabowski JH, Trussell GC. 2017. Refuge quality impacts the strength of nonconsumptive effects on prey. Ecology 98, 403–411. ( 10.1002/ecy.1647) [DOI] [PubMed] [Google Scholar]
- 45.Matassa CM, Donelan SC, Luttbeg B, Trussell GC. 2016. Resource levels and prey state influence antipredator behavior and the strength of nonconsumptive predator effects. Oikos 125, 1478–1488. ( 10.1111/oik.03165) [DOI] [Google Scholar]
- 46.Trussell GC, Ewanchuk PJ, Matassa CM. 2006. The fear of being eaten reduces energy transfer in a simple food chain. Ecology 87, 2979–2984. ( 10.1890/0012-9658(2006)87%5B2979:TFOBER%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 47.Donelan SC, Trussell GC. 2015. Parental effects enhance risk tolerance and performance in offspring. Ecology 96, 2049–2055. ( 10.1890/14-1773.1) [DOI] [PubMed] [Google Scholar]
- 48.Etter RJ. 1989. Life history variation in the intertidal snail Nucella lapillus across a wave-exposure gradient. Ecology 70, 1857–1876. ( 10.2307/1938118) [DOI] [Google Scholar]
- 49.Crothers J. 1985. Dog-whelks: an introduction to the biology of Nucella lapillus (L.). Field Stud. 6, 291–360. [Google Scholar]
- 50.Hughes RN. 1972. Annual production of two Nova Scotian populations of Nucella lapillus (L.). Oecologia 8, 356–370. ( 10.1007/BF00367538) [DOI] [PubMed] [Google Scholar]
- 51.Large SI, Smee DL. 2010. Type and nature of cues used by Nucella lapillus to evaluate predation risk. J. Exp. Mar. Biol. Ecol. 396, 10–17. ( 10.1016/j.jembe.2010.10.005) [DOI] [Google Scholar]
- 52.Palmer AR. 1982. Growth in marine gastropods: a non-destructive technique for independently measuring shell and body weight. Malacologia 23, 63–74. [Google Scholar]
- 53.Matassa CM, Trussell GC. 2014. Prey state shapes the effects of temporal variation in predation risk. Proc. R. Soc. B 281, 20141952 ( 10.1098/rspb.2014.1952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burrows MT, Hughes RN. 1990. Variation in growth and consumption among individuals and populations of dogwhelks, Nucella lapillus: a link between foraging behaviour and fitness. J. Anim. Ecol. 59, 723–742. ( 10.2307/4891) [DOI] [Google Scholar]
- 55.Elner RW, Hughes RN. 1978. Energy maximization in the diet of the shore crab, Carcinus maenas. J. Anim. Ecol. 47, 103–116. ( 10.2307/3925) [DOI] [Google Scholar]
- 56.Palmer AR. 1992. Calcification in marine molluscs: how costly is it? Proc. Natl Acad. Sci. USA 89, 1379–1382. ( 10.1073/pnas.89.4.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 58.Donelan SC, Trussell GC.2018. Data from: Parental and embryonic experiences with predation risk affect prey offspring behavior and performance. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
- 59.Trussell GC, Matassa CM, Luttbeg B. 2011. The effects of variable predation risk on foraging and growth: less risk is not necessarily better. Ecology 92, 1799–1806. ( 10.1890/10-2222.1) [DOI] [PubMed] [Google Scholar]
- 60.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 61.Werner EE, Gilliam JF, Hall DJ, Mittelbach GG. 1983. An experimental test of the effects of predation risk on habitat use in fish. Ecology 64, 1540–1548. ( 10.2307/1937508) [DOI] [Google Scholar]
- 62.Giesing ER, Suski CD, Warner RE, Bell AM. 2010. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc. R. Soc. B 278, 1753–1759. ( 10.1098/rspb.2010.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storm JJ, Lima Steven L. 2010. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am. Nat. 175, 382–390. ( 10.1086/650443) [DOI] [PubMed] [Google Scholar]
- 64.Trussell GC, Ewanchuk PJ, Matassa CM. 2008. Resource identity modifies the influence of predation risk on ecosystem function. Ecology 89, 2798–2807. ( 10.1890/08-0250.1) [DOI] [PubMed] [Google Scholar]
- 65.McPeek MA, Grace M., Richardson JM. 2001. Physiological and behavioral responses to predators shape the growth/predation risk trade-off in damselflies. Ecology 82, 1535–1545. ( 10.1890/0012-9658(2001)082%5B1535:PABRTP%5D2.0.CO;2) [DOI] [Google Scholar]
- 66.Slos S, Stoks R. 2008. Predation risk induces stress proteins and reduces antioxidant defense. Funct. Ecol. 22, 637–642. ( 10.1111/j.1365-2435.2008.01424.x) [DOI] [Google Scholar]
- 67.Hawlena D, Schmitz OJ. 2010. Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat. 176, 537–556. ( 10.1086/656495) [DOI] [PubMed] [Google Scholar]
- 68.Jablonka E, Lamb MJ. 1998. Epigenetic inheritance in evolution. J. Evol. Biol. 11, 159–183. ( 10.1046/j.1420-9101.1998.11020159.x) [DOI] [Google Scholar]
- 69.Holeski LM, Jander G, Agrawal AA. 2012. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27, 618–626. ( 10.1016/j.tree.2012.07.011) [DOI] [PubMed] [Google Scholar]
- 70.Hone˘k A. 1993. Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66, 483–492. ( 10.2307/3544943) [DOI] [Google Scholar]
- 71.Badyaev AV, Uller T. 2009. Parental effects in ecology and evolution: mechanisms, processes and implications. Phil. Trans. R. Soc. B 364, 1169–1177. ( 10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plaistow S, Benton T. 2009. The influence of context-dependent maternal effects on population dynamics: an experimental test. Phil. Trans. R. Soc. B 364, 1049–1058. ( 10.1098/rstb.2008.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Love OP, Williams TD. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149. ( 10.1086/590959) [DOI] [PubMed] [Google Scholar]
- 74.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheriff M, Love O. 2013. Determining the adaptive potential of maternal stress. Ecol. Lett. 16, 271–280. ( 10.1111/ele.12042) [DOI] [PubMed] [Google Scholar]
- 76.Räsänen K, Laurila A, Merilä J. 2005. Maternal investment in egg size: environment and population-specific effects on offspring performance. Oecologia 142, 546–553. ( 10.1007/s00442-004-1762-5) [DOI] [PubMed] [Google Scholar]
- 77.Madsen T, Shine R. 2000. Silver spoons and snake body sizes: prey availability early in life influences long-term growth rates of free-ranging pythons. J. Anim. Ecol. 69, 952–958. ( 10.1111/j.1365-2656.2000.00477.x) [DOI] [Google Scholar]
- 78.Grafen A. 1988. On the uses of data on lifetime reproductive success. In Reproductive success studies of individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), pp. 454–471. Chicago, IL: University of Chicago. [Google Scholar]
- 79.Brown JS. 1999. Vigilance, patch use and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1, 49–71. (doi:10.1.1.489.6835) [Google Scholar]
- 80.Miller LP. 2013. The effect of water temperature on drilling and ingestion rates of the dogwhelk Nucella lapillus feeding on Mytilus edulis mussels in the laboratory. Mar. Biol. 160, 1489–1496. ( 10.1007/s00227-013-2202-z) [DOI] [Google Scholar]
- 81.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12, S3 ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Donelan SC, Trussell GC.2018. Data from: Parental and embryonic experiences with predation risk affect prey offspring behavior and performance. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available at the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.ks622 [58].


