Abstract
Comparative analyses of ectotherm susceptibility to climate change often focus on thermal extremes, yet responses to aridity may be equally important. Here we focus on plasticity in desiccation resistance, a key trait shaping distributions of Drosophila species and other small ectotherms. We examined the extent to which 32 Drosophila species, varying in their distribution, could increase their desiccation resistance via phenotypic plasticity involving hardening, linking these responses to environment, phylogeny and basal resistance. We found no evidence to support the seasonality hypothesis; species with higher hardening plasticity did not occupy environments with higher and more seasonal precipitation. As basal resistance increased, the capacity of species to respond via phenotypic plasticity decreased, suggesting plastic responses involving hardening may be constrained by basal resistance. Trade-offs between basal desiccation resistance and plasticity were not universal across the phylogeny and tended to occur within specific clades. Phylogeny, environment and trade-offs all helped to explain variation in plasticity for desiccation resistance but in complex ways. These findings suggest some species have the ability to counter dry periods through plastic responses, whereas others do not; and this ability will depend to some extent on a species' placement within a phylogeny, along with its basal level of resistance.
Keywords: phenotypic plasticity, climate change, desiccation, seasonality, trade-off
1. Introduction
Increasing mean temperatures and extreme events, coupled with substantial changes in precipitation under climate change, threaten the persistence of many organisms [1,2]. This is particularly true for ectotherms, because temperature and precipitation are among the most important drivers of their distributions [3–5]. Most of the research has focused on adaptive responses in thermal traits. But changes in precipitation and water availability, in both cold and dry environments, will impose a significant stress to organisms [6], reduce fitness and influence distributions and survival. Understanding adaptive responses to desiccation stress are thus critical for predicting climate change impacts.
Drosophila species may increase their tolerance to desiccation stress through three main mechanisms: reducing water-loss rates across the cuticle, increasing their tolerance to water-loss and increasing the amount of stored water [7]. All three mechanisms can contribute to variation in desiccation resistance within selected lines of Drosophila melanogaster [8–10], and have been shown to associate with climatic adaptation between Drosophila species [7,11]. Studies have predominantly focused on the genetic mechanisms that underpin evolved shifts in desiccation resistance, but species survival under climate change will in part depend on their capacity to rapidly buffer changes through phenotypic plasticity [12,13]. Rapid adult desiccation hardening has been described in Drosophila species [14,15], where a non-lethal exposure to desiccation stress increases resistance to subsequent exposures. Plastic responses can vary across species [14,15], but whether this variation is predictable across environments, and whether desiccation plasticity may be constrained by basal levels of resistance, is poorly understood.
Two main hypotheses have been developed to explain patterns of variation in plasticity across species, and to identify climatic drivers of plasticity evolution. These hypotheses were developed largely in the context of understanding thermal plasticity [16] but can be extended to other stresses [12]. First, the latitudinal or seasonality hypothesis of Janzen proposes that greater seasonal uniformity of temperature at lower latitudes should select for narrower thermal tolerances and reduced plasticity [16–18]. Theoretical models support this hypothesis and suggest plasticity should be favoured when populations experience spatial and/or temporal environmental heterogeneity [19–24], the environmental cues influencing plasticity are strong and predictable [20,25], and the cost of plasticity is low [26–29]. Based on these theoretical expectations, temperate organisms should evolve broad thermal tolerances and high plasticity to counter large seasonal/daily fluctuations in climate, while tropical organisms should evolve narrow thermal tolerances and reduced plasticity, in response to exposure to a less variable climate. Empirical tests of these hypotheses have mostly focussed on assessing patterns of thermal plasticity and produced equivocal results [18,30–33]. Second, the trade-off hypothesis predicts that plastic responses are linked to physiological constraints such that species with the highest overall tolerance will have the lowest plasticity [34]. Thus, the evolution of plasticity will be dependent on the strength of selection on a trait in each environment. Under the trade-off hypothesis, tropical species that have low climatic stress resistance [5,35,36] might have higher plasticity [37]. Support for the trade-off hypothesis is also mixed [38–40].
Another important, but seldom considered, driver of the evolution of phenotypic plasticity is that of shared evolutionary history. Species may share similar capacities to respond via phenotypic plasticity because they share a more recent common ancestor, frequently termed phylogenetic conservatism (often investigated as the presence of phylogenetic signal in a dataset) [41]. If the capacity to respond plastically in key traits determines persistence under stressful climates and phylogenetic signal is common, then species sensitivity to climatic stress may occur within certain clades. However, the presence of phylogenetic signal can occur for reasons other than those related to constraints. Closely related species may be more likely to occur in similar geographic locations and be exposed to similar environments and similar selection pressures [42]. Under this scenario, the presence of phylogenetic signal may not reflect constraints but instead represent an adaptive process: phylogenetically structured adaptation. Whether phylogenetically structured adaptation frequently explains phylogenetic signal remains to be determined, but current studies demonstrating both processes highlight the importance of dissecting patterns of phylogenetic signal into processes rather than patterns [36,43–45].
Here we quantify plasticity (measured as an induced hardening response) in desiccation resistance across 32 Drosophila species with varying distributions and broad phylogenetic relationships. We focus on desiccation resistance, an important driver of current distributions of Drosophila and other species [5,46]. Further, desiccation resistance is a trait that may become increasingly important in determining species persistence under climate change [46]. Using a design that allows us to explicitly compare species that vary in their basal resistance, we relate plasticity in desiccation resistance to environmental variables. We examine the degree to which plasticity is predictably structured across the phylogeny and whether the relationship between phylogeny and traits is driven by constraints or adaptive processes.
2. Material and methods
(a). Drosophila species maintenance, collection sites and experimental set-up
Species varied in their geographical distribution and sensitivity to desiccation stress and were collected from one of three sources: 21 of the 32 species were collected from the field in Australia between 2010 and 2015, with experiments completed in 2013–2016, seven of the 32 species were obtained from the San Diego stock centre and three species were maintained as long-term laboratory stocks in Denmark (electronic supplementary material, table S1). For further details of maintenance and initiation of experimental flies, see the electronic supplementary material, Material and methods.
(i). Desiccation hardening pre-treatment
One component of plasticity, for desiccation resistance, was characterized as a rapid hardening response [15], where adults are exposed to a short (hours), sub-lethal period of desiccation stress before exposure to a subsequent lethal stress following recovery under benign conditions. For species collected from Australia, hardening responses were assessed on mass-bred populations (see above), while for species obtained from the stock centre assessments were made on a single iso-female line [5,36]. Hardening responses were assessed in each species separately. Female flies were pre-treated (hardened) with a non-lethal desiccation stress of 5–10% relative humidity (RH) by placing them in empty vials (without food) (10 flies per vial) covered with gauze placed into a sealed container with silica gel. Each hardening treatment was replicated 10 times (i.e. 10 vials set-up per treatment, 100 flies per treatment), although this was not always the case (electronic supplementary material, table S1). Control treatments, which assessed un-hardened (basal) desiccation resistance of each species, were set up in a similar manner except that they were maintained on food during the hardening pre-treatment; i.e. they were not exposed to any hardening pre-treatment prior to the lethal desiccation assay.
Following hardening, flies were placed into vials containing food (90–100% humidity) for a 9 h recovery period at 25°C, prior to being assessed for desiccation resistance at 5–10% RH (see below). Hardening treatments for a species were set up in such a way to ensure that they all concluded at the same time, so that the final desiccation assessment could be performed on all treatments at the same time. Desiccation resistance was scored every hour until 50% mortality was observed in each vial, with each vial providing a data point which was analysed as an LT50.
(ii). Climate variables
Global climate data were obtained from the WorldClim dataset from geospatial coordinates from all known collection sites to generate climate information which encompasses the entire distribution of each species (www.worldclim.org) [47]. To test the seasonality hypothesis, we chose to examine the relationship between plasticity and eight temperature and precipitation variables known to be important in the evolution of desiccation stress [5]. For further information on the choice and justification of climate variables, see the electronic supplementary material, Material and methods.
(b). Analyses
(i). Assessing desiccation hardening responses across species: hardening capacity and the hardening response ratio
The effects of hardening pre-treatments on mean desiccation resistance were examined with one-way analyses of variance (ANOVAs) on vial LT50s for each species, with hardening pre-treatment considered a fixed effect. Dunnett's post hoc test was performed to examine differences in hardening pre-treatments on desiccation resistance relative to the control (that is basal) desiccation resistance (electronic supplementary material, table S2). All analyses were performed in R [48]. As each hardening treatment involved a different group of flies, and as species were assessed in different experiments, probabilities were not adjusted for multiple testing.
We quantified hardening responses (the extent of plastic response) across species using two methods (see the electronic supplementary, Material and methods for a justification). Firstly, we calculated hardening capacity (HC-3.5) as the hardened mean after 3.5 h hardening treatment minus the basal mean, and secondly, we calculated the hardening response ratio (HRR) as (maximum hardened mean − basal mean)/hours of maximum pre-treatment [32]. HC-3.5 involves the same amount of physical stress, while HRR provides a measurement of how desiccation resistance changes per hour of pre-treatment, which captures how the timing of the hardening response differs across species (electronic supplementary material, figure S1a,b). A common method to examine whether basal resistance and plasticity are involved in a trade-off is to examine the relationship between HRR and basal resistance. However, as basal resistance is included in the calculation of HRR, this relationship is not independent as basal resistance occurs both in the y and x variables. For this reason, we refrain from making such a comparison [49]. For two species, a 3.5 h desiccation stress-induced mortality, and for these species (electronic supplementary material, figure S1a,b) we estimated HC under a 2 h stress treatment.
(ii). Estimating the relationship between traits and phylogeny: phylogenetic signal and ancestral trait reconstruction
To account for non-independence of data points that may arise due to shared evolutionary history, we used a number of phylogenetic comparative methods to examine the relationship between phylogeny, traits and environment. To incorporate all species examined in this study, we used a composite phylogeny updated from Kellermann et al. [5], which was primarily based on a phylogeny [50] of 22 species. Missing species were incorporated into the phylogeny by standardizing branch lengths from published phylogenies against the phylogeny from van der Linde et al. [49] (electronic supplementary material, table S1). Although a high level of uncertainty around branch lengths and topology of the phylogeny may be expected using this method, currently no single phylogeny incorporating all Drosophila species examined here exists. Furthermore, recent studies have demonstrated that error in branch length and topology is likely to have negligible impacts on estimation of phylogenetic signal [51,52].
To examine whether phylogeny plays a role in shaping basal and hardened desiccation resistance, we estimated phylogenetic signal using two common methods, Pagel's λ and Blomberg's K-statistic, using the phytools package in R [48,53]. These two methods are based on a model of trait evolution via Brownian motion (BM) [54,55]. Pagel's λ was estimated from the residuals, where λ is a scaling parameter which compares the covariances among species to the covariance expected under BM. A λ = 0 indicates no phylogenetic relationship while a λ = 1 means traits are evolving across the phylogeny equal to BM (trait variance is proportional to branch lengths and thus traits show a high level of phylogenetic association/signal). Whether estimates of λ were significantly different from 0 or 1 was assessed by comparing the AIC of the estimated λ to the AIC of λ = 1 or λ = 0. The K-statistic is an estimate of the variance within and between clades and is commonly divided into four scenarios: (i) K not significantly different from 0 indicates no phylogenetic signal, (ii) K = 1 indicates phylogenetic signal and traits are evolving under a BM model, (iii) K greater than 1 suggests traits are more similar than expected under a BM model and (iv) K less than 1 (but greater than 0) suggests traits are less similar than expected under BM, which could be driven by convergent evolution of unrelated species or measurement error [54].
To evaluate the extent to which spatial proximity influenced patterns in desiccation resistance and plasticity, we examined the relationship between traits and space by comparing distance matrices for these two variables with a linear regression analysis [56]. Distances matrices were calculated in R; Euclidean distances were calculated for the traits, while spatial matrices were calculated from the average longitude and latitude data for each species using the fossil package in R [57].
To visualize trait evolution across the phylogeny, we implemented ancestral trait reconstruction using a maximum-likelihood approach for continuous characters, based on a BM model using the phytools program in R [53]. To further explore whether trade-offs between basal desiccation resistance and plasticity were structured across the phylogeny, we divided species into three categories displaying characteristics consistent with or divergent from the trade-off hypothesis (see electronic supplementary, Material and methods for justification of groups). Using these three character states, we used stochastic mapping [58] in the R package Phytools [53] to calculate the proportion of time spent in each state and the posterior probabilities for each internal node; we generated 100 stochastic character maps.
(iii). Association between climate, basal desiccation resistance and adult desiccation hardening
To identify climate variables associated with desiccation plasticity, hardening responses and basal resistance for each species were regressed against them. To account for phylogenetic effects, we used the caper program in R [48,59]. Because collinearity is a common feature of environmental data, the variance inflation factor (VIF) was calculated from the full model including all eight environmental variables. Environmental variables were sequentially removed based on the highest VIF until VIF < 5 (VIF < 5 is standard statistical procedure see [60]). To ensure model selection was not biased by the order in which collinear environmental variables were removed, we initially assessed the contribution of single environmental variables, where single influential environmental variables were removed based on the VIF process we would retain that variable and remove the next influential variable. We then compared AIC to the original model, and this process was performed until we were satisfied that we were not biasing our final model choice. Following this, non-significant environmental variables were sequentially removed from the full model and assessed with AIC; environmental variables were removed until the lowest AIC was found. Where the difference between models was less than 2.5 AIC, we chose the simplest model.
3. Results
We examined the capacity for 32 Drosophila species varying in their distribution and sensitivity to desiccation stress to mount a hardening (plastic) response to a non-lethal desiccation stress. Twenty-one of the 32 species mounted a significantly positive hardening response in at least one treatment (electronic supplementary material, table S2). The treatments that induced a hardening response differed across species (electronic supplementary material, table S2). For those species with high basal resistance, positive hardening responses were generally observed only after the longer pre-treatments, often close to the point at which mortality started to occur (treatments with any mortality were not included in the analyses). For the species that did not show a significant hardening response, the effect of the ‘hardening treatment’ tended to be negative.
(a). Hardening capacity and the hardening response ratio patterns with climate
Hardening capacity at 3.5 h (HC-3.5) was calculated to compare standardized hardening (plastic) responses across species. Of the 32 species examined, eight showed a significant positive hardening response following a 3.5 h desiccation stress pre-treatment. HC-3.5 differed across the species, ranging from negative effects (−2.05 h) to positive effects (2.14 h) (electronic supplementary material, table S2). Across all 32 species, HC-3.5 was related to precipitation and temperature variables: PWARM and TMAX (R2 = 0.345, p < 0.01) (table 1). Of these two variables, PWARM explained the greatest amount of variation, with HC-3.5 increasing with precipitation (table 1 and figure 1c).
Table 1.
Phylogenetic generalized linear model (PGLS) examining the relationship between environment, basal desiccation resistance, two measures of desiccation hardening responses HC-35 and ARR and phylogeny. Estimates of λ = 0 indicate no relationship between traits, environment and phylogeny while a λ = 1 suggests a strong relationship. Two measures of phylogenetic signal within each trait were calculated: K-statistic and λ. K = 0 suggests no relationship between phylogeny and traits, K < 1, K = 1, K > 1. λ interpreted above with the associated AIC value for the current λ versus λ = 0 and λ = 1, with the best fit model highlighted in italics. *p < 0.05, **p < 0.01, ***p < 0.001.
| (a) PGLS |
(b) phylogenetic signal |
|||||
|---|---|---|---|---|---|---|
| slope | R2 | λ | K | λ | AIC | |
| basal∼ | ||||||
| PANN | −0.011 ± 0.002*** | 0.606*** | 0 | 0.818** | 1 | 202.665 |
| TMAX | 0.091 ± 0.035* | (0–0.900) | λ0 213.744 | |||
| λ1 202.665 | ||||||
| basal∼ | ||||||
| PWARM | −0.012 ± 0.005* | 0.598*** | 0 | |||
| TMAX | 0.149 ± 0.050** | (0–0.865) | ||||
| TMIN | −0.054 ± 0.021* | |||||
| PCOLD | −0.018 ± 0.007* | |||||
| HC-3.5∼ | ||||||
| PWARM | 0.004 ± 0.001*** | 0.345** | 0 | 0.595* | 0.695* | 96.479 |
| TMAX | −0.018 ± 0.008* | (0–616) | λ0 100.629 | |||
| λ1 96.479 | ||||||
| HRR∼ | ||||||
| PWARM | 0.0009 ± 0.0003** | 0.256* | 0 | 0.538* | 0.622 | −7.046 |
| PANN | −0.0003 ± 0.0001* | (0–0.599) | λ0 −2.714 | |||
| TMAX | −0.003 ± 0.002 | λ1 −7.046 | ||||
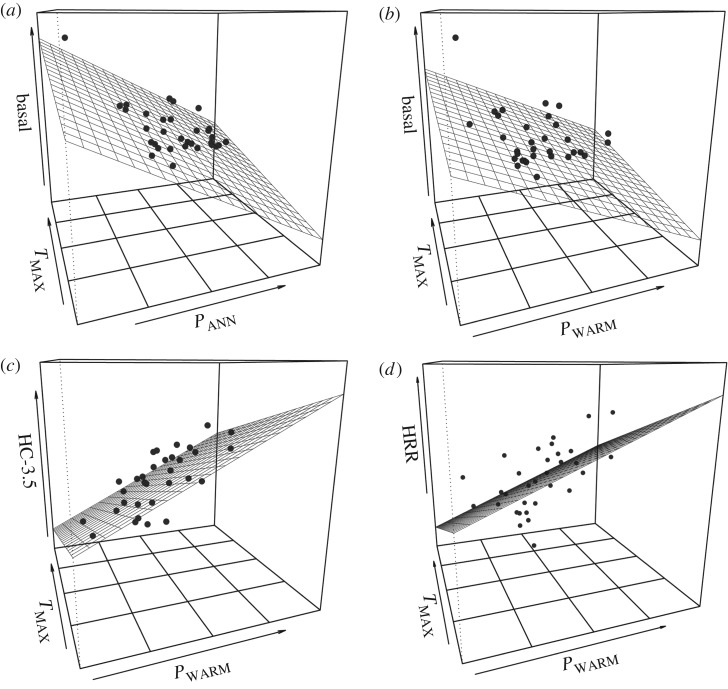
Figure 1.
Relationship between basal resistance, plasticity and environment. General linear model showing the relationship between basal desiccation resistance and desiccation hardening (HC-3.5 and ARR) for the two environmental variables explaining the largest amount of variation in plasticity. (a) Basal desiccation resistance and PANN and TMAX, (b) basal desiccation resistance and PWARM and TMAX, (c) hardening capacity at 3.5 (HC-3.5) and TMAX and PWARM and (d) acclimation response ratio (ARR) and PANN and PWARM.
A further 14 species significantly increased their desiccation resistance compared to the control following desiccation pre-treatments that were greater than 3.5 h (electronic supplementary material, table S2). When we examined the relationship between HRR and environment across all 32 species, we found that two environmental variables, PWARM and PANN, explained approximately 25% of the variation in plasticity (table 1). Similar to results when using HC-3.5, PWARM explained the greatest amount of variation in HRR- plasticity increased with increasing precipitation (higher HRR). However, plasticity also increased with decreasing PANN (table 1 and figure 1d). We found a weak negative relationship between latitude and one measure of plasticity, HC-3.5 (HC-3.5: R2 = 0.117, slope = −0.020 ± 0.009, p = 0.031; HRR: R2 = 0.001, slope = −0.002 ± 0.002, p = 0.389), such that plasticity increased with decreasing latitude.
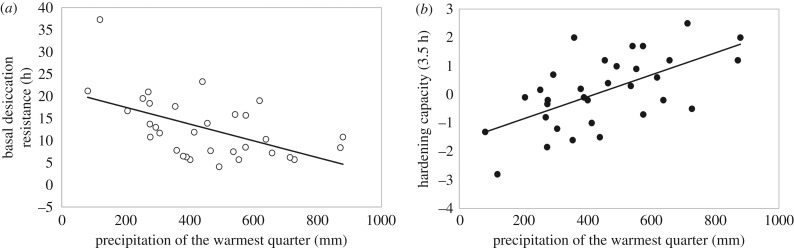
Environmental variables related to temperature and precipitation, PANN and TMAX, explained the greatest amount of variation for basal desiccation resistance (R2 = 0.606, p < 0.001) (table 1 and figure 1a). Although the main environmental drivers of basal desiccation resistance (PANN and TMAX) and plasticity (HC-3.5 and HRR) differed, precipitation was the strongest driver for all traits. Comparing how basal desiccation resistance, HC-3.5 and HRR changed with precipitation, we found an opposing relationship; as precipitation increases, desiccation resistance decreases (figure 2), while plasticity (HC-3.5 and HRR) generally increases (table 1 and figure 2). This result suggests a possible trade-off between basal desiccation resistance and desiccation plasticity; i.e. species with high basal desiccation resistance have a lower capacity to shift their resistance via plasticity.
Figure 2.
Evidence for trade-offs between basal resistance and plasticity. (a) The relationship between mean basal desiccation resistance (white circles) and (b) hardening capacity (HC-3.5) (solid circles) for precipitation of the warmest quarter (PWARM).
(b). Phylogenetic patterns with hardening capacity and basal desiccation resistance
Phylogenetic signal was detected for basal desiccation resistance and both measures of desiccation plasticity (table 1). Stronger phylogenetic signal was detected for basal resistance with λ not significantly different from 1, which is consistent with a BM model of evolution. In addition, a K-statistic (0.818) approaching 1 suggests that basal desiccation resistance is not evolving independently from the phylogeny. The evolution of HC-3.5 and HRR was also associated with phylogeny; values of λ were not significantly different from 1, while K was significantly greater than 0, both consistent with a BM model of plasticity evolution (table 1).
We found a weak association between species distributions and HC-3.5 (HC-3.5: R2 = 0.032, slope = < 0.001 ± <0.001 h km−1, p = 0.001). The small amount of variation that this relationship could explain suggests that the presence of phylogenetic signal in HC-3.5 could mostly be attributed to shared evolutionary history (i.e. phylogenetic signal) rather than phylogenetically structured adaptation related to shared spatial (environmental) associations. Similarly, for both basal desiccation resistance and HRR, we found no evidence for phylogenetically structured adaptation (basal desiccation resistance: R2 = 0.002, slope = < 0.001 ± <0.001 h km−1, p = 0.168; HRR: R2 = 0.003, slope = < 0.001 ± <0.001 h km−1, p = 0.135).
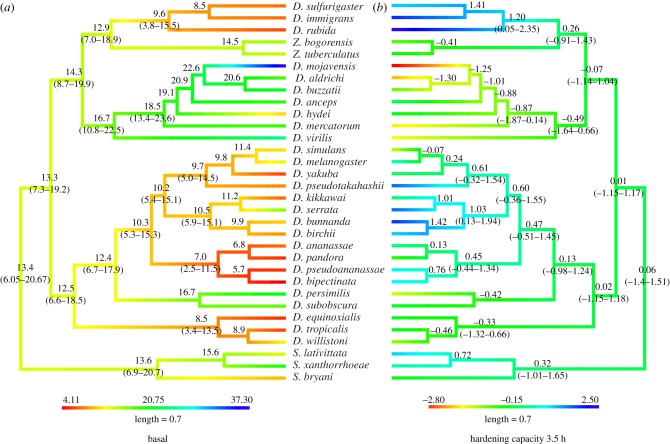
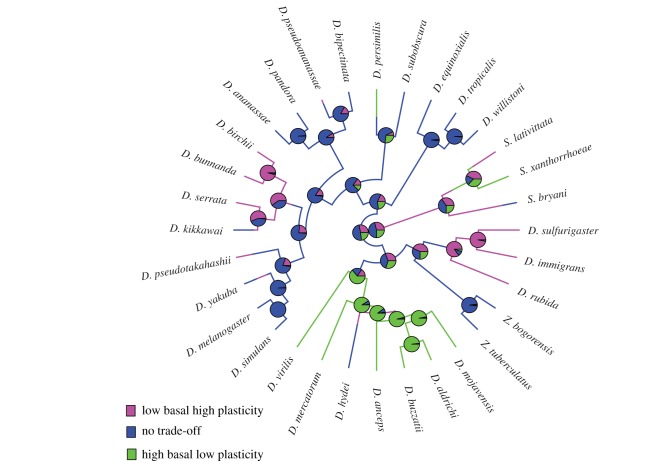
Mapping traits onto the phylogeny via ancestral trait reconstruction indicates the nature of the phylogenetic signal (figure 3). For basal desiccation resistance, clear structuring was evident within clades. For both measures of plasticity, structuring was also evident, although weaker than observed for basal desiccation resistance (figure 3; electronic supplementary material, figure S2). The ancestral state for basal desiccation resistance was an intermediate phenotype (11.9 h) (figure 3), while low plasticity was the ancestral state for both measures of plasticity. There were no instances of high desiccation resistance coupled with high plasticity. Instead, high plasticity appeared to be associated with low basal desiccation resistance, and this pattern was clearer for HC-3.5 than for HRR (figure 3; electronic supplementary material, figure S2). This result is consistent with a possible trade-off between basal desiccation resistance and plasticity, although evidence for a trade-off was not consistent across all species (figure 3). Species displaying characteristics consistent with the trade-off hypothesis, i.e. low basal desiccation resistance and high plasticity and vice versa, tended to occur within certain clades (figure 4; electronic supplementary material, figure S3). Hence, the presence/absence of a trade-off was phylogenetically structured.
Figure 3.
Patterns in basal desiccation resistance and plasticity across the phylogeny. Phylogenetic hypothesis for the 32 Drosophila species examined in this study, branch lengths reflect standardized time. Values next to the branches and the colours represent the ancestral state and the confidence intervals in hours for (a) basal desiccation resistance and (b) hardening capacity.
Figure 4.
Evidence for phylogenetically structured trade-offs in the Drosophila phylogeny. Stochastic character mapping for HC-3.5 for species displaying characteristics in line or divergent with the trade-off hypothesis. Pie graphs represent the posterior probabilities of a character state for each node calculated by re-sampling 100 character histories.
4. Discussion
Climate change poses a threat to the persistence of many organisms [1,2]. Current predictions of climate change risk suggest that tropical and mid-latitude ectothermic species are the most threatened [4,36,61–63]. However, these predictions of risk do not explicitly consider plasticity in key traits and do not consider changes in precipitation, even though precipitation is a key driver of species distribution in small ectotherms like Drosophila [5]. We addressed these gaps in our understanding by investigating whether the capacity of Drosophila species to mount plastic responses under desiccation stress varies predictably with the environment, while accounting for phylogeny. While the physiological mechanisms underlying desiccation resistance have been studied in a number of Drosophila species [15,64–66], the focus of this study was to explicitly test key hypotheses that have been proposed to explain broad patterns in thermal plasticity within the context of desiccation resistance, and to ask whether plasticity in desiccation resistance is structured across the phylogeny.
Environmental variables capturing average/annual shifts in temperature and precipitation, rather than seasonality, were associated with both basal desiccation resistance and induced plasticity for desiccation resistance (HC-3.5 and HRR). In line with previous work [5], PANN explained a large proportion of the variation in basal desiccation resistance. PWARM predominately explained variation in plasticity, with other environmental variables TMAX and PANN also contributing to the variation in HC-3.5 and HRR, respectively. The relationship between environment and HRR was complex, with plasticity increasing with increasing PWARM but also increasing with decreasing PANN. These results suggest that the environment is important in shaping plasticity but that environmental variables may shape the evolution of plasticity in complex ways that depend on the environmental variables and traits being considered.
We found no evidence to support the seasonality hypothesis that tropical species display lower levels of phenotypic plasticity [16,17]. Instead, we found species with low basal desiccation resistance (which were often tropical species) tended to have a greater capacity to increase their desiccation resistance via plasticity compared to species with higher basal resistance (widespread species). This suggests that the evolution of plasticity for desiccation resistance may be constrained by a trade-off between basal desiccation resistance and the capacity to harden. Ancestral trait reconstruction further supported a trade-off (figure 3), however, trade-offs were not universal across the phylogeny; rather they tended to occur within species groups (figure 4). That is, some species showed responses indicative of a trade-off between basal desiccation resistance and desiccation plasticity and others did not, and this pattern tended to be phylogenetically structured. The species-specific nature of possible trade-offs revealed here may in part explain the inconsistent evidence for trade-offs between basal trait values and plasticity reported more broadly in the literature [30,38,40,67–69]. Evidence for a trade-off between basal resistance and plasticity reported here is consistent with theory predicting that the evolution of plasticity must be constrained by costs [70,71]. Although trade-offs were not apparent for all species, we never observed species with high basal resistance and high plasticity, which one would expect if the evolution of plasticity was free from any constraint. Instead, species with low to moderate basal desiccation resistance had no capacity to increase their resistance through plasticity, suggesting that factors other than trade-offs may be limiting the evolution of plasticity across the Drosophila phylogeny.
The strong phylogenetic structuring of plasticity relative to a species basal response (trade-off) could indicate divergent mechanisms underpinning desiccation resistance and plasticity across the phylogeny. Of the three mechanisms that contribute to desiccation resistance (water-loss rates, storage size and tolerance), changes in water-loss rates have been implicated in hardening responses (D. melanogaster) [72], selection responses for increased desiccation resistance [8,10,73], and differences in innate resistance in desert versus mesic species [7]. Changes in water-loss rates, due to rapid changes in cuticular permeability rather than respiratory water-loss, is a likely candidate for plastic responses in D. melanogaster [66,72] but the mechanisms underpinning plasticity may vary across species [14]. Decreasing respiratory water-loss via a reduced metabolic rate has been associated with evolved responses [7,73], but may not contribute to plastic responses, at least in D. melanogaster [66]. Increased water storage and tolerance of water-loss can also contribute to increased desiccation resistance in selected lines of D. melanogaster [8], but do not associate with resistance at the species level [11]. The capability of Drosophila to rapidly change tolerance of water-loss and water storage capacity via rapid hardening has not been studied; but neither of these mechanisms associate with evolved responses in comparative studies [11,74], and increased water storage and tolerance have never evolved in the same selection lines of D. melanogaster (reviewed in [8]), suggesting a limited capacity to evolve these two mechanisms. High desiccation tolerance in desert species was also strongly associated with the phylogeny, indicative of constraints linked to evolutionary history [11]. Whether the phylogenetically structured trade-off between plasticity and innate desiccation resistance is driven by these potential constraints needs to be determined.
We detected strong to moderate phylogenetic signal for basal desiccation resistance and hardening plasticity, respectively, with strong phylogenetic structuring of basal desiccation resistance consistent with previous work [5]. Spatial associations driving common adaptations across the phylogeny were not apparent in the current dataset, suggesting that the phylogenetic signal for basal desiccation resistance and plasticity was related to phylogenetic inertia rather than adaptation. Thus the capacity to alter desiccation resistance either via evolution or plasticity appears limited in drosophilid species, although more so for basal resistance. Few studies have explored the role of phylogeny in shaping patterns of plasticity in traits linked to species distributions, with mixed evidence for phylogenetic signal; one study [45] found moderate phylogenetic signal for plasticity in heat resistance in marine organisms, while flowering date and breeding time, traits that are likely to be comprised both plastic and genetic components, display significant phylogenetic structure [75,76].
A number of caveats apply to this research. Our focus was on rapid hardening responses, but developmental acclimation (rearing in environments with varying humidity) will also contribute to climate change responses [77]. The extent to which developmental plasticity may vary across species has yet to be studied but is worth quantifying in the future. We have not considered the role of behaviour in regulating species exposure to humid environments. Species with low plasticity may have a greater capacity to seek out humid environments, but very little is known on the capacity of Drosophila species to regulate desiccation stress via behaviour. We have examined only one population of each species, and it is possible that plasticity differs across populations. However, evidence of intra-specific variation for desiccation plasticity is limited [15], and even when present, will be smaller than inter-specific variation [78]. In addition, long-term laboratory iso-female lines were examined for some species and these are likely to suffer from laboratory adaptation and inbreeding. This may not be an issue, as a recent study found no consistent relationship between inbreeding and plasticity in stress traits [79,80], and we found no evidence for a relationship between time in the laboratory and phenotypic plasticity in our data (electronic supplementary material, figure S4). However, it is something that should be tested by ideally comparing lines of the same species with different lengths of time in the laboratory.
There is increasing focus on understanding the role that phenotypic plasticity will play in mediating species responses to climate change [32,33,45,81]. Studies have focussed on plastic responses to changing thermal regimes, and reveal that thermal plasticity will have minimal effects on climate change risk. No study has yet comprehensively quantified plastic responses to desiccation stress, and how desiccation plasticity might be distributed across species and environments. Our analyses confirm that basal desiccation resistance is phylogenetically constrained [5], and show for the first time that there is less phylogenetic inertia for desiccation plasticity. While we find some support for the idea that the evolution of plasticity is constrained by a trade-off between basal resistance and plastic responses, this trade-off was phylogenetically structured, occurring only within certain species groups in such a way that was not relatable to the environment or basal resistance. The complex relationship between desiccation plasticity, phylogeny and environment revealed in this study suggests that while some species have the capacity to respond to increasing desiccation stress via plasticity, this capacity seems to be the result of a complex interplay between evolutionary history and natural selection. More studies performed within a phylogenetic framework are required to better our understanding of the processes that facilitate or constrain the evolution of plasticity.
Supplementary Material
Acknowledgements
We are grateful to Jessica Hammond, Katherine Sutton, Fiona Beasley and Jonas L. Andersen for technical assistance.
Data accessibility
Data have been deposited in the Dryad Digital Repository at: https://dx.doi.org/10.5061/dryad.558pt7j [82].
Authors' contributions
V.K., C.M.S. and A.A.H. designed the experiment, V.K. performed and analysed the experiments, C.M.S., J.O. and V.L. provided equipment for the experiments. All authors contributed to the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
The Danish Research Council for Independent Research (FNU) for funding by a large frame grant to J.O. and V.L. and the Australian Research Council for financial support to V.K., C.M.S. and A.A.H. through their Discovery and Fellowship schemes and the Science and Industry Endowment Fund for support to C.M.S. and A.A.H., Monash University for financial support to V.K. and C.M.S. and L'Oreal for financial support to V.K. through their For Women In Science scheme.
References
- 1.IPCC. 2014. Climate change 2014: mitigation of climate change. Contribution of working group III to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 3.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clusella-Trullas S, Blackburn TM, Chown SL. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751. ( 10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 5.Kellermann V, Loeschcke V, Hoffmann AA, Kristensen TN, Fløjgaard C, David JR, Overgaard J. 2012. Phylogenetic constraints in key functional traits behind species' climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 66, 3377 ( 10.1111/j.1558-5646.2012.01685.x) [DOI] [PubMed] [Google Scholar]
- 6.Chown SL, Sorensen JG, Terblanche JS. 2011. Water loss in insects: an environmental change perspective. J. Insect. Physiol. 57, 1070–1084. ( 10.1016/j.jinsphys.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 7.Gibbs AG, Fukuzato F, Matzkin LM. 2003. Evolution of water conservation mechanisms in Drosophila. J. Exp. Biol. 206, 1183–1192. ( 10.1242/jeb.00233) [DOI] [PubMed] [Google Scholar]
- 8.Telonis-Scott M, Guthridge KM, Hoffmann AA. 2006. A new set of laboratory-selected Drosophila melanogaster lines for the analysis of desiccation resistance: response to selection, physiology and correlated responses. J. Exp. Biol. 209, 1837–1847. ( 10.1242/jeb.02201) [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann AA, Parsons PA. 1989. Selection for increased desiccation resistance in Drosophila melanogaster: additive genetic control and correlated responses for other stresses. Genetics 122, 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs AG, Chippindale AK, Rose MR. 1997. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821–1832. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs AG, Matzkin LM. 2001. Evolution of water balance in the genus Drosophila. J. Exp. Biol. 204, 2331–2338. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann A, Parsons PA.. 1991. Evolutionary genetics and environmental stress. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ Jr, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15. ( 10.3354/cr00879) [DOI] [Google Scholar]
- 14.Kalra B, Parkash R, Aggarwal DD. 2014. Divergent mechanisms for water conservation in Drosophila species. Entomol. Exp. Appl. 151, 43–56. ( 10.1111/eea.12169) [DOI] [Google Scholar]
- 15.Hoffmann AA. 1991. Acclimation for desiccation resistance in Drosophila species and population comparisons. J. Insect. Physiol. 37, 757–762. ( 10.1016/0022-1910(91)90110-L) [DOI] [Google Scholar]
- 16.Janzen DH. 1967. Why mountain passes are higher in tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 17.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 18.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 19.Via S, Lande R. 1985. Genotype environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.1111/j.1558-5646.1985.tb00391.x) [DOI] [PubMed] [Google Scholar]
- 20.Gabriel W, Lynch M. 1992. The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 5, 41–59. ( 10.1046/j.1420-9101.1992.5010041.x) [DOI] [Google Scholar]
- 21.Gabriel W, Luttbeg B, Sih A, Tollrian R. 2005. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 166, 339–353. ( 10.1086/432558) [DOI] [PubMed] [Google Scholar]
- 22.Gomulkiewicz R, Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390–411. ( 10.1111/j.1558-5646.1992.tb02047.x) [DOI] [PubMed] [Google Scholar]
- 23.Gavrilets S, Scheiner SM. 1993. The genetics of phenotypic plasticity 5. Evolution of reaction norm shape. J. Evol. Biol. 6, 31–48. ( 10.1046/j.1420-9101.1993.6010031.x) [DOI] [Google Scholar]
- 24.Gilchrist GW. 1995. Specialists and geeralists in changing environments 1. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270. ( 10.1086/285797) [DOI] [Google Scholar]
- 25.Tufto J. 2000. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am. Nat. 156, 121–130. ( 10.1086/303381) [DOI] [PubMed] [Google Scholar]
- 26.van Tienderen PH. 1991. Evolution of generalists and specialists in spatially hetergeneous environments. Evolution 45, 1317–1331. ( 10.1111/j.1558-5646.1991.tb02638.x) [DOI] [PubMed] [Google Scholar]
- 27.Murren CJ, et al. 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115, 293–301. ( 10.1038/hdy.2015.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran NA. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989. ( 10.1086/285369) [DOI] [Google Scholar]
- 29.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 31.Mitchell KA, Sgro CM, Hoffmann AA. 2011. Phenotypic plasticity in upper thermal limits is weakly related to Drosophila species distributions. Funct. Ecol. 25, 661–670. ( 10.1111/j.1365-2435.2010.01821.x) [DOI] [Google Scholar]
- 32.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorensen JG, Kristensen TN, Overgaard J. 2016. Evolutionary and ecological patterns of thermal acclimation capacity in Drosophila: is it important for keeping up with climate change? Curr. Opin. Insect Sci. 17, 98–104. ( 10.1016/j.cois.2016.08.003) [DOI] [PubMed] [Google Scholar]
- 34.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 35.Kellermann V, van Heerwaarden B, Sgro CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244–1246. ( 10.1126/science.1175443) [DOI] [PubMed] [Google Scholar]
- 36.Kellermann V, Overgaard J, Hoffmann AA, Flojgaard C, Svenning JC, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228–16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson MJO, Hoffmann AA. 1996. Acclimation, cross-generation effects, and the response to selection for increased cold resistance in Drosophila. Evolution 50, 1182–1192. ( 10.1111/j.1558-5646.1996.tb02359.x) [DOI] [PubMed] [Google Scholar]
- 38.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 39.Magozzi S, Calosi P. 2015. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Change Biol. 21, 181–194. ( 10.1111/gcb.12695) [DOI] [PubMed] [Google Scholar]
- 40.Calosi P, Bilton DT, Spicer JI. 2008. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 4, 99–102. ( 10.1098/rsbl.2007.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. ( 10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 42.Freckleton RP, Jetz W. 2009. Space versus phylogeny: disentangling phylogenetic and spatial signals in comparative data. Proc. R. Soc. B 276, 21–30. ( 10.1098/rspb.2008.0905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usui T, Butchart SHM, Phillimore AB. 2017. Temporal shifts and temperature sensitivity of avian spring migratory phenology: a phylogenetic meta-analysis. J. Anim. Ecol. 86, 250–261. ( 10.1111/1365-2656.12612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labra A, Pienaar J, Hansen TF. 2009. Evolution of thermal physiology in Liolaemus lizards: adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 174, 204–220. ( 10.1086/600088) [DOI] [PubMed] [Google Scholar]
- 45.Comte L, Olden JD. 2017. Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob. Change Biol. 23, 728–736. ( 10.1111/gcb.13427) [DOI] [PubMed] [Google Scholar]
- 46.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]
- 47.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 48.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation.
- 49.Kelly C, Price TD. 2005. Correcting for regression to the mean in behavior and ecology. Am. Nat. 166, 700–707. ( 10.1086/497402) [DOI] [PubMed] [Google Scholar]
- 50.van der Linde K, Houle D, Spicer GS, Steppan SJ. 2010. A supermatrix-based molecular phylogeny of the family Drosophilidae. Genet. Res. 92, 25–38. ( 10.1017/S001667231000008X) [DOI] [PubMed] [Google Scholar]
- 51.Munkemuller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, Thuiller W. 2012. How to measure and test phylogenetic signal. Methods Ecol. Evol. 3, 743–756. ( 10.1111/j.2041-210X.2012.00196.x) [DOI] [Google Scholar]
- 52.Diniz JAF, Santos T, Rangel TF, Bini LM. 2012. A comparison of metrics for estimating phylogenetic signal under alternative evolutionary models. Genet. Mol. Biol. 35, 673–679. ( 10.1590/S1415-47572012005000053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Revell LJ. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 54.Blomberg SP, Garland T, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. ( 10.1111/j.0014-3820.2003.tb00285.x) [DOI] [PubMed] [Google Scholar]
- 55.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 56.Legendre P, Lapointe FJ, Casgrain P. 1994. Modeling brain evolution from behavior: a permutational regression approach. Evolution 48, 1487–1499. ( 10.1111/j.1558-5646.1994.tb02191.x) [DOI] [PubMed] [Google Scholar]
- 57.Vavrek MJ. 2011. fossil: Palaeoecological and palaeogeographical analysis tools. Palaeontol. Electron. 14, 1T See http://palaeo-electronica.org/2011_1/238/index.html. [Google Scholar]
- 58.Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158. ( 10.1080/10635150390192780) [DOI] [PubMed] [Google Scholar]
- 59.Orme CDL, Freckleton RP, Thomas GH, Petzoldt T, Fritz SA, Isaac NJB. 2013. caper: Comparative analyses of phylogenetics and evolution in R. See http://CRAN.R-project.org/package=caper.
- 60.Rogerson P.A. 2001. Statistical methods for geography. London, UK: Sage. [Google Scholar]
- 61.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Perez HJA, Garland T Jr. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parkash R, Ramniwas S, Kajla B, Aggarwal DD. 2012. Divergence of desiccation-related traits in two Drosophila species of the Takahashii subgroup from the western Himalayas. J. Exp. Biol. 215, 2181–2191. ( 10.1242/jeb.065730) [DOI] [PubMed] [Google Scholar]
- 65.Parkash R, Aggarwal DD, Ranga P, Singh D. 2012. Divergent strategies for adaptation to desiccation stress in two Drosophila species of immigrans group. J. Comp. Physiol. B 182, 751–769. ( 10.1007/s00360-012-0655-x) [DOI] [PubMed] [Google Scholar]
- 66.Stinziano JR, Sove RJ, Rundle HD, Sinclair BJ. 2015. Rapid desiccation hardening changes the cuticular hydrocarbon profile of Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 180, 38–42. ( 10.1016/j.cbpa.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 67.van Heerwaarden B, Kellermann V, Sgro CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687). [DOI] [Google Scholar]
- 68.Kellermann V, Van Heerwaarden B, Sgro C. 2017. How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proc. R. Soc. B 284, 20170447 ( 10.1098/rspb.2017.0447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stenseng E, Braby CE, Somero GN. 2005. Evolutionary and acclimation-induced variation in the thermal limits of heart function in congeneric marine snails (genus Tegula): implications for vertical zonation. Biol. Bull. 208, 138–144. ( 10.2307/3593122) [DOI] [PubMed] [Google Scholar]
- 70.Van Buskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860. ( 10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 71.Sgro CM, Terblanche JS, Hoffmann AA. 2016. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 61, 433–451. ( 10.1146/annurev-ento-010715-023859) [DOI] [PubMed] [Google Scholar]
- 72.Bazinet AL, Marshall KE, MacMillan HA, Williams CM, Sinclair BJ. 2010. Rapid changes in desiccation resistance in Drosophila melanogaster are facilitated by changes in cuticular permeability. J. Insect. Physiol. 56, 2006–2012. ( 10.1016/j.jinsphys.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann AA, Parsons PA. 1993. Direct and correlated responses to selection for desiccation resistance: a comparison of Drosophila melanogaster and D. simulans. J. Evol. Biol. 6, 643–657. ( 10.1046/j.1420-9101.1993.6050643.x) [DOI] [Google Scholar]
- 74.Gibbs AG. 2002. Water balance in desert Drosophila: lessons from non-charismatic microfauna. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 781–789. ( 10.1016/S1095-6433(02)00208-8) [DOI] [PubMed] [Google Scholar]
- 75.Davis CC, Willis CG, Primack RB, Miller-Rushing AJ. 2010. The importance of phylogeny to the study of phenological response to global climate change. Phil. Trans. R. Soc. B 365, 3201–3213. ( 10.1098/rstb.2010.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. 2008. Phylogenetic patterns of species loss in Thoreau's woods are driven by climate change. Proc. Natl Acad. Sci. USA 105, 17029–17033. ( 10.1073/pnas.0806446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aggarwal DD, Ranga P, Kalra B, Parkash R, Rashkovetsky E, Bantis LE. 2013. Rapid effects of humidity acclimation on stress resistance in Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 166, 81–90. ( 10.1016/j.cbpa.2013.05.012) [DOI] [PubMed] [Google Scholar]
- 78.Hoffmann AA, Shirriffs J, Scott M. 2005. Relative importance of plastic vs genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct. Ecol. 19, 222–227. ( 10.1111/j.1365-2435.2005.00959.x) [DOI] [Google Scholar]
- 79.Schou MF, Kristensen TN, Kellermann V, Schloetterer C, Loeschcke V. 2014. A Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. J. Evol. Biol. 27, 1859–1868. ( 10.1111/jeb.12436) [DOI] [PubMed] [Google Scholar]
- 80.Kristensen TN, Loeschcke V, Bilde T, Hoffmann AA, Sgro C, Noreikiene K, Ondresik M, Bechsgaard JS. 2011. No inbreeding depression for low temperayure developmental acclimation across multiple Drosophila species. Evolution 65, 3195–3201. ( 10.1111/j.1558-5646.2011.01359.x) [DOI] [PubMed] [Google Scholar]
- 81.Gunderson AR, Dillon ME, Stillman JH. 2017. Estimating the benefits of plasticity in ectotherm heat tolerance under natural thermal variability. Funct. Ecol. 31, 1529–1539. ( 10.1111/1365-2435.12874) [DOI] [Google Scholar]
- 82.Kellermann V, Hoffmann AA, Overgaard J, Loeschcke V, Sgrò CM.2018. Data from: Plasticity for desiccation tolerance across Drosophila species is affected by phylogeny and climate in complex ways. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kellermann V, Hoffmann AA, Overgaard J, Loeschcke V, Sgrò CM.2018. Data from: Plasticity for desiccation tolerance across Drosophila species is affected by phylogeny and climate in complex ways. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data have been deposited in the Dryad Digital Repository at: https://dx.doi.org/10.5061/dryad.558pt7j [82].