Significance
Argonaute proteins are present in bacteria, archaea, and eukaryotes. They play an important role in a wide range of biological processes, from transcriptional and translational gene expression regulation to defense against viruses and silencing of mobile genetic elements. Here we present mechanistic insights into the interactions of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated Marinitoga piezophila Argonaute (MpAgo) with its guide RNA (gRNA) and RNA substrates. By modifying the 5′-nucleotide of the gRNA, we demonstrate that MpAgo–gRNA complexes (RNPs) are easily programmable, have high affinity to fully complementary RNA substrates, and can discriminate by more than 300-fold between substrates that differ by only a single nucleotide. These MpAgo RNPs should be useful for probing endogenous RNAs in living cells.
Keywords: Argonaute, CRISPR, small noncoding RNA, RNA editing, inosine
Abstract
Argonaute proteins (Agos) are present in all domains of life. Although the physiological function of eukaryotic Agos in regulating gene expression is well documented, the biological roles of many of their prokaryotic counterparts remain enigmatic. In some bacteria, Agos are associated with CRISPR (clustered regularly interspaced short palindromic repeats) loci and use noncanonical 5′-hydroxylated guide RNAs (gRNAs) for nucleic acid targeting. Here we show that using 5-bromo-2′-deoxyuridine (BrdU) as the 5′ nucleotide of gRNAs stabilizes in vitro reconstituted CRISPR-associated Marinitoga piezophila Argonaute–gRNA complexes (MpAgo RNPs) and significantly improves their specificity and affinity for RNA targets. Using reconstituted MpAgo RNPs with 5′-BrdU-modified gRNAs, we mapped the seed region of the gRNA and identified the nucleotides of the gRNA that play the most significant role in targeting specificity. We also show that these MpAgo RNPs can be programmed to distinguish between substrates that differ by a single nucleotide, using permutations at the sixth and seventh positions in the gRNA. Using these specificity features, we employed MpAgo RNPs to detect specific adenosine-to-inosine–edited RNAs in a complex mixture. These findings broaden our mechanistic understanding of the interactions of Argonautes with guide and substrate RNAs, and demonstrate that MpAgo RNPs with 5′-BrdU-modified gRNAs can be used as a highly specific RNA-targeting platform to probe RNA biology.
Argonautes (Agos) are nucleic acid-guided proteins present in organisms from all three domains of life (1). In eukaryotes, Argonautes (eAgos) play central roles in RNA interference (RNAi) and micro-RNA (miRNA) pathways that coordinate a wide range of cellular processes including transcriptional and translational gene regulation (2, 3), silencing of mobile genetic elements (4, 5), and host defense (6). eAgos bind single-stranded RNAs (ssRNA), such as small interfering RNAs (siRNAs), miRNAs, or PIWI (P-element induced wimpy testis)-interacting RNAs (piRNAs), which act as templates for the recognition of complementary ssRNA targets, leading either to Ago cleavage of the targeted RNA (7) or to the recruitment of additional components of the RNA degradation machinery (8–10).
Although prokaryotes lack RNAi pathways (11), prokaryotic Agos (pAgos) are thought to contribute to host defense against foreign DNA (12). Despite their structural similarity to eAgos (Fig. 1A), pAgos are more diverse in their biochemical behavior than their eukaryotic counterparts. For example, some pAgos can use either DNA or RNA guides, and can target either DNA or RNA substrates (12–15). Differing from other Agos, the clustered regularly interspaced short palindromic repeats (CRISPR) locus-associated pAgo subfamily found in Marinitoga piezophila, Thermotoga profunda, and Marinitoga sp. 1155 (16) uses 5′-hydroxylated (5′-OH) guide RNAs (gRNAs) rather than 5′-phosphorylated guides. Furthermore, the M. piezophila Ago (MpAgo) cleaves both ssDNA and ssRNA in vitro in a gRNA-dependent manner (16). However, the origin of endogenous MpAgo guides and the physiological function of MpAgo remain enigmatic.
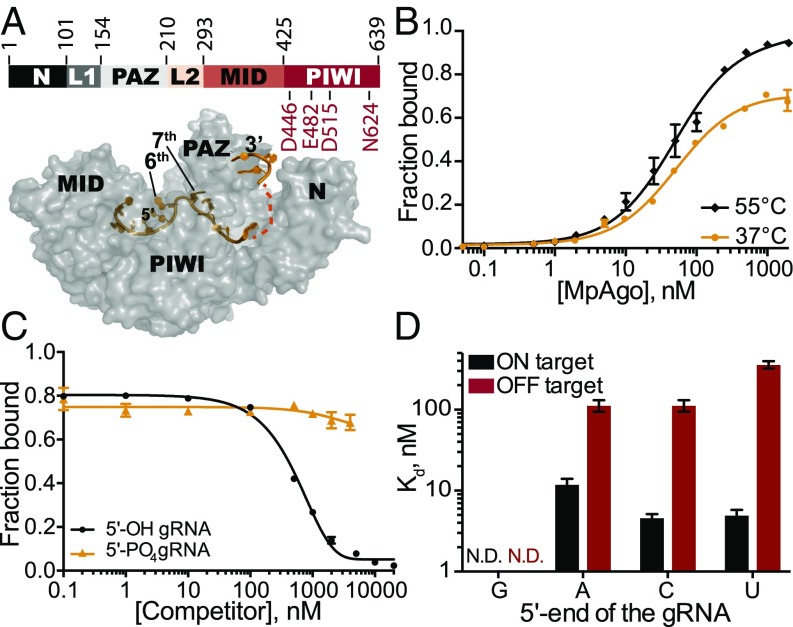
Fig. 1.
Stability and specificity of in vitro reconstituted MpAgo RNPs. (A, Top) Organization of MpAgo’s domains and (A, Bottom) the structure (PDB ID: 5I4A) of MpAgo (gray) bound to its guide RNA (gRNA, orange). The bilobed structure includes a PAZ (PIWI-Argonaute-Zwille) domain that anchors the 3′-end of the gRNA, whereas the second lobe includes a MID and a PIWI domain that binds the 5′ end of the gRNA, and includes the catalytic residues, as marked. The gRNA bound to MpAgo is bent at the sixth and seventh nts. (B) MpAgo binding of gRNA at two temperatures: 37 °C (orange) and 55 °C (black). The fraction of bound gRNA is plotted as a function of MpAgo concentration. The data fit with a standard binding isotherm (solid lines) yield Kd values at 37 °C and 55 °C of 52 ± 4 nM and 49 ± 5 nM, respectively. Data are represented as the mean ± SD from three independent experiments. (C) Competition-binding assay at 37 °C to examine displacement of gRNA from the MpAgo RNP complex, with 5′-OH RNA competitor (black) and 5′-PO4 RNA competitor (orange). The adjusted half-inhibitory concentrations (IC50) are 0.25 ± 0.08 μM and >4.5 μM for the 5′-OH RNA and 5′-PO4 RNA competitors, respectively. Data are represented as the mean ± SD from three independent experiments. (D) Substrate ssRNA filter-binding assays with catalytically inactive MpAgo RNP formed with a 5′-G, 5′-A, 5′-U, or 5′-C gRNA and either fully complementary substrate (ON target, black) or noncomplementary ssRNA substrate (OFF target, red) in the presence of 2 μg/mL heparin. The obtained average Kd values are plotted as the mean ± SD from three independent experiments. N.D., not detected.
Here we present biochemical analyses of guide-dependent RNA targeting by MpAgo. We found that MpAgo is easily programmed with gRNAs to form RNA–protein complexes (RNPs). Reconstituted MpAgo RNPs bound fully complementary RNA substrates with moderate affinity and specificity. However, incorporation of 5-bromo-2′-deoxyuridine (BrdU) at the 5′ end of gRNAs stabilized the MpAgo–gRNA complex (MpAgo RNP) and greatly increased MpAgo RNP affinity and specificity in binding substrate RNAs. Moreover, MpAgo RNPs with 5′-BrdU gRNAs selectively bound substrates that differ by only one nucleotide, which enabled isolation of inosine-edited RNA substrates from a complex mixture.
Results
MpAgo–gRNA RNPs Bind Fully Complementary RNA Substrates with Moderate Affinity and Specificity.
The CRISPR locus-associated MpAgo can be programmed with a short 5′-OH gRNA to cleave single-stranded DNA and RNA substrates in vitro (16). We used catalytically active MpAgo for cleavage assays and catalytically inactive MpAgo for binding assays (16). Because the endogenous guides and targets for M. piezophila are not known, we investigated MpAgo interactions with a previously published 5′-OH 21-nucleotide (nt) gRNA (SI Appendix, Table S1) (16). We determined the equilibrium dissociation constant of MpAgo for its gRNA at 55 °C, using a filter binding assay, as M. piezophila grows at 45–70 °C (17). We observed that MpAgo binds the 5′-OH gRNA with ∼50 nM affinity (Fig. 1B). MpAgo RNP reconstituted at 37 °C, which would be compatible with expression and assembly of MpAgo RNPs in mesophilic (i.e., human) cells, bound the 5′-OH gRNA at 37 °C with a similar affinity as that at 55 °C, although with slightly diminished overall binding (Fig. 1B).
To assess the stability of the reconstituted MpAgo RNP in vitro, preassembled MpAgo RNP complexes were titrated with either excess 5′-OH or 5′-phosphorylated competitor gRNAs of identical sequence. We first measured the off-rate (koff) of 5′-OH gRNAs prebound to MpAgo at 55 °C to be ∼0.03 min−1, with a small population of MpAgo RNPs remaining stable for long times in the presence of excess competitor gRNA (SI Appendix, Fig. S1A). Using incubations of about 45 times the half-life of the MpAgo-gRNA complex, the observed half-inhibitory concentrations (IC50) for 5′-OH and 5′-phosphorylated gRNAs reveal that MpAgo has a very low affinity for 5′-phosphorylated gRNAs (Fig. 1C), in agreement with published data (16). We obtained similar results for the stability and 5′-end specificity of MpAgo RNPs assembled at 37 °C (SI Appendix, Fig. S1A).
We then tested the stability of MpAgo RNPs reconstituted at 37 °C and 55 °C in the presence of RNA targets. MpAgo RNPs immobilized on beads through a biotin tag on the 3′ end of a 33-nt gRNA were incubated with a 10-fold excess of mRNA containing a fully complementary target site (ON-target RNA), or an excess of total RNA purified from HEK293T cells (OFF-target RNA) (SI Appendix, Fig. S2A). Notably, in the presence of the ON-target RNA, we observed more than 50% displacement of MpAgo from MpAgo RNPs assembled at 37 °C, and nearly 30% displacement from MpAgo RNPs reconstituted at 55 °C (SI Appendix, Fig. S2B). However, using 22-nt gRNAs (radiolabeled on the 3′ end), MpAgo RNPs were stable in the presence of ON- and OFF-target RNAs (SI Appendix, Fig. S2C). Collectively, these results indicate that the temperature of the reconstitution reaction does not affect the stability of the MpAgo RNPs. However, binding of fully complementary target RNAs to MpAgo RNPs with gRNAs that have longer 3′ extensions can destabilize the RNPs (SI Appendix, Fig. S2 B and D).
Previously, it was shown that MpAgo does not have a preference for the 5′-terminal nucleotide of the gRNA in targeting ssDNA substrates for cleavage (16). Because the cellular target of MpAgo is not known, and some Agos including MpAgo target ssRNA in vitro (16), we tested whether the 5′-end nucleotide of the gRNA affects the ability of MpAgo RNPs to bind and cleave RNA. Regardless of the gRNA 5′ nucleotide identity (SI Appendix, Fig. S3A), MpAgo cleaved ssRNA substrates with similar rates and efficiencies (SI Appendix, Fig. S3B). We then examined whether the 5′-terminal nucleotide of the gRNA influences ssRNA substrate binding efficiency and specificity in vitro, using an RNA substrate that was either fully complementary (ON target) or noncomplementary (OFF target) to the gRNA, over a range of MpAgo RNP concentrations (Fig. 1D and SI Appendix, Fig. S3 C and F). Whereas ON target binding efficiencies of RNPs with 5′-A, C or U gRNAs are comparable, the specificity is affected by the identity of the 5′-terminal position of the gRNA. RNPs programmed with 5′-U gRNAs are more specific than the RNPs programmed with 5′-A and 5′-C gRNAs, whereas MpAgo RNPs programmed with a 5′-G gRNA bind fully complementary target RNAs inefficiently, even though the cleavage rates are similar when using RNPs programmed with all four gRNAs (SI Appendix, Fig. S3 A and B). This implies that efficient cleavage does not require tight binding of ssRNA substrates. The 5′-nt preference of MpAgo for 5′-U gRNAs is not a result of differences in gRNA binding affinity, as we found the MpAgo affinities for gRNAs to be similar with all four 5′-nucleotides (SI Appendix, Fig. S3G). The fact that MpAgo RNP cleaves only complementary substrates (16) indicates that nonspecific binding of the ssRNA substrates obtained here is likely gRNA independent and could be caused by incomplete assembly of the MpAgo RNP, allowing nonspecific RNA binding to the gRNA binding cleft of free MpAgo (16).
Chemical Modification of the 5′-Terminal Nucleotide of the gRNA Improves MpAgo RNP Specificity and Affinity for ssRNA Substrates.
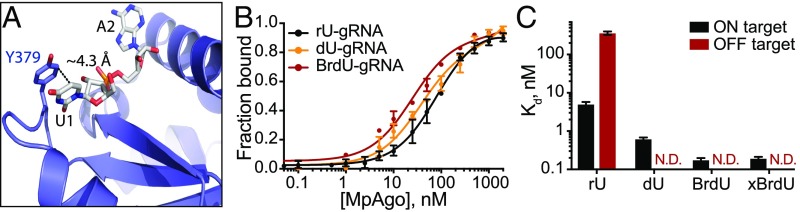
Despite low nanomolar affinity of the MpAgo RNP for ssRNA substrates, the moderate specificity of MpAgo RNPs (Fig. 1D) prompted us to engineer a more specific MpAgo RNP. The previously published structure of MpAgo revealed that the 5′-terminal nucleotide of the bound gRNA is flipped into a pocket formed by MpAgo’s middle (MID) and PIWI domains (Fig. 1A), where it stacks on the aromatic ring of tyrosine 379 (Y379) (Fig. 2A) (16). We tested whether the close proximity of the 5′-terminal nucleotide and the Y379 aromatic ring would favor crosslinking of the gRNA to MpAgo. We used a gRNA containing BrdU at the 5′-end position (SI Appendix, Table S1) and crosslinked it to the protein using 305 nm UV light.
Fig. 2.
MpAgo RNP assembly using 5′-end modified gRNAs and specificity for binding complementary ssRNA substrates. (A) MpAgo (blue) (PDB ID: 5I4A) forms a binding pocket for the 5′-end nucleotide (U1) of the gRNA (gray) that stacks on the aromatic ring of tyrosine residue Y379. (B) MpAgo and gRNA binding curves obtained for 5′-end ribonucleotide (rU, black), 2′-deoxyuridine (dU, orange), and BrdU (red) containing gRNAs at 55 °C. The fraction of bound gRNA is plotted as a function of MpAgo concentration. The data fit with a standard binding isotherm (solid lines) reveal MpAgo binds the rU, dU, and BrdU gRNAs at 55 °C with average Kd values of 81 ± 9 nM, 37 ± 6 nM, and 25 ± 3 nM, respectively. Data are represented as the mean ± SD from three independent experiments. (C) The average Kd values of the MpAgo RNPs for either fully complementary substrate (ON target, black) or noncomplementary ssRNA substrate (OFF target, red) were obtained using MpAgo RNPs formed with a rU, dU, or BrdU gRNA, or with a BrdU gRNA with subsequent 1 h exposure to 305 nm UV light for gRNA crosslinking to MpAgo (xBrdU). The obtained Kd values are plotted as the mean ± SD from three independent experiments. N.D., not detected.
Although UV crosslinking reached ∼22–33% efficiency (SI Appendix, Fig. S4), analysis of noncrosslinked MpAgo RNPs revealed that the 5′-BrdU modification alone was sufficient to improve MpAgo RNP affinity and specificity for substrate RNAs (Fig. 2C and SI Appendix, Fig. S5). Replacing the 5′-end ribonucleotide (rU) of the gRNA with 2′-deoxyuridine (dU) or BrdU slightly increased MpAgo affinity for the gRNA, with each modification making a positive contribution (Fig. 2B). To assess the effect of these 5′-end gRNA modifications on MpAgo RNP stability, we measured the off-rates (koff) of 5′-end dU and BrdU gRNAs prebound to MpAgo at 55 °C as ∼0.024 min−1 and 0.011 min−1, with a small population of MpAgo RNPs remaining stable for long times in the presence of excess competitor gRNA (SI Appendix, Fig. S5A). These data indicate that the BrdU modification stabilizes the MpAgo RNP. Interestingly, in optimized conditions (1:1.5 MpAgo:gRNA ratio and 2 μg/mL of heparin), a dU at the gRNA 5′-end increased MpAgo RNP affinity for a fully complementary ssRNA substrate (21-nt complementarity) by 10 times (SI Appendix, Figs. S3F and S5B), and a 5′-BrdU further increased MpAgo RNP affinity for the ssRNA substrate to better than 200 pM (SI Appendix, Fig. S5C). Notably, these substitutions also decreased OFF-target binding to undetectable levels (Fig. 2C and SI Appendix, Fig. S5 B and C). In the case of 5′-BrdU, these improvements in target binding did not depend on UV crosslinking (Fig. 2C and SI Appendix, Fig. S5 C and D). The following experiments were carried out with UV-crosslinking; however, these results indicate this is unnecessary for 5′-BrdU gRNAs to exert their effects on MpAgo RNPs.
MpAgo Uses a Seed Region on the gRNA with Highest Sensitivity at the Third, Sixth, Seventh, and Ninth Nucleotides.
The seed sequence in the gRNA contributes greatly to the specificity of Ago RNP interactions with target substrates. The canonical seed region of all eAgos and some pAgos is composed of the second to eighth nts of the gRNA and has an A-form helical conformation in the Ago RNP complex (18–23). Furthermore, the second to fourth bases of the seed sequence are exposed to the solvent for interaction with the complementary substrate (22). Notably, in contrast to eAgos and pAgos, the previously determined structure of an MpAgo–gRNA complex revealed that the gRNA and the predicted seed region have a unique conformation: a sharp kink between the sixth and seventh nucleotide of the gRNA that disrupts continuous A-like helical base stacking of the second through eighth nucleotides (16). Interestingly, the tolerance for dinucleotide mismatches across the MpAgo gRNA when targeting ssDNA revealed that the mismatches at the fifth to 15th nts of the gRNA abolish the cleavage of ssDNA substrates the most (24). These structural and biochemical studies suggest that the seed region of the MpAgo may differ from the canonical seed used by previously characterized eAgos and pAgos.
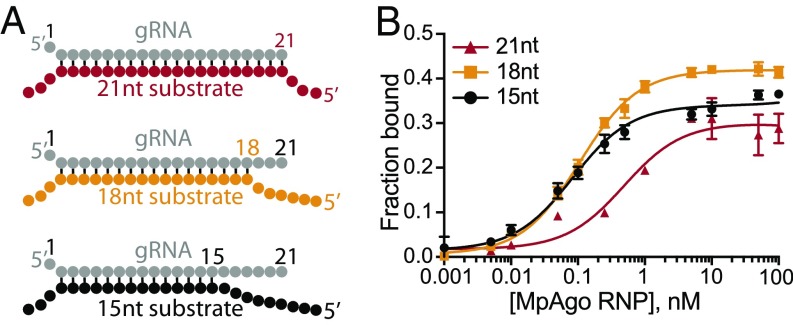
We investigated whether mismatches of three or six nucleotides between ssRNA target and nucleotides at the 3′ end of 5′-BrdU gRNAs affect MpAgo RNP binding to ssRNA substrate (Fig. 3A). Surprisingly, in optimized conditions (1:1.5 MpAgo:gRNA ratio and 10 μg/mL heparin; SI Appendix, Fig. S6 A and B), reducing complementarity between the ssRNA and gRNA to 18 or 15 nts enhanced the affinity of MpAgo RNP substantially, with Kd values of ∼100 pM (Fig. 3B). Because of the high affinity of 15-nt target RNAs (Fig. 3B), we mapped the seed region of MpAgo when targeting ssRNA substrates, using a baseline of 15-nt complementarity to target ssRNAs (Fig. 3A). We introduced single-nucleotide mismatches in the RNA substrate at the positions corresponding to the first to 15th nucleotides in a 21-nucleotide-long gRNA, keeping the sequence of the gRNA constant (SI Appendix, Fig. S7A and Table S1), and determined the equilibrium dissociation constants, using filter binding assays (Fig. 4A and SI Appendix, Fig. S7B). Because of the location of the first gRNA nucleotide in a separate binding pocket (Fig. 2A), there is no effect of a mismatch at the first position. We noticed that the Kd values for the substrates that have mismatches spanning the second to 12th positions (with the exception of the ninth position) are at least five times higher relative to the Kd value for the fully complementary ssRNA substrate (Fig. 4A).
Fig. 3.
MpAgo RNP programmed with 5′-BrdU gRNA binds ssRNA substrates with high affinity. (A) Cartoon of three duplexes of the gRNA and different ssRNA substrates with varying degrees of complementarity to the gRNA used in binding assays: full 21-nt complementarity (Top, red), 18-nt complementarity with three mismatches at the 3′-end of the gRNA (Middle, orange), and 15-nt complementarity with six mismatches at the 3′-end of the gRNA (Bottom, black). The first nt at the 5′-end of the gRNA does not base pair because of MpAgo interactions (Fig. 2A). (B) Filter-binding assays with 21-nt complementarity (red), 18-nt complementarity (orange), or 15-nt complementarity (black) in the presence of 10 μg/mL heparin. The amount of bound ssRNA substrate is plotted as a function of MpAgo RNP concentration. The obtained average Kds for MpAgo RNP and the substrates with 21-, 18-, and 15-nt complementarity are 500 ± 115 pM, 107 ± 6 pM, and 83 ± 8 pM, respectively. Data are represented as the mean ± SD from three independent experiments.
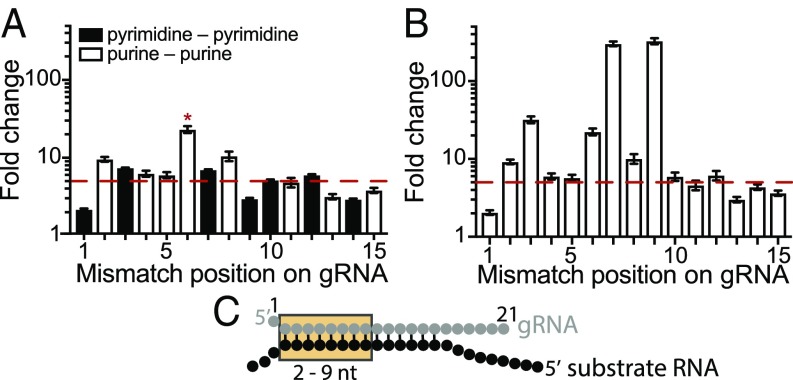
Fig. 4.
Determination of the gRNA seed region of MpAgo RNPs. (A) Substrate ssRNA filter-binding assays with ssRNA substrates that contain a single-nucleotide mismatch along the gRNA from the first to 15th position. The sequence of the gRNA was kept constant, and the ratios of the average Kds of the substrates that create mismatch (Kdmm) to the fully complementary substrate (KdCompl; fold change = Kdmm/KdCompl) were plotted against the position of the mismatch. The red dash line indicates the value equal to ∼5 times the Kd for the fully complementary ssRNA substrate. Asterisk (*), A–G mismatch at the sixth position (guide RNA–substrate RNA). The Kd for a G–A mismatch at the sixth position is 779 ± 71 pM, using a different gRNA (SI Appendix, Table S1). (B) The ratios of the average Kds of the substrates that create purine–purine mismatch (Kdmm) to the fully complementary substrate (KdCompl; fold change = Kdmm/KdCompl) were plotted against the position of the mismatch. The red dashed line indicates a value equal to 5. The purine–purine mismatches at the second through 12th positions decrease the affinity of MpAgo RNP more than fivefold. The third, sixth, seventh, and ninth positions reduce the affinity of MpAgo RNP the most: 32-, 22-, 301-, and 327-fold respectively. (C) The gRNA-substrate RNA duplex with the seed region (orange box).
The type of mismatch also affects the observed Kd value for target binding. In the context of scanning target mismatches to one gRNA, purine–purine mismatches (A–G mismatch in gRNA substrate at the sixth position, and G–A mismatches at the second and eighth positions) in general weakened target binding substantially more than pyrimidine–pyrimidine mismatches (Fig. 4A and SI Appendix, Fig. S5). To check whether the negative effect of the mismatch at the sixth position is the result of the nature of an A–G mismatch, we exchanged the gRNA sequence to obtain a G–A mismatch at the sixth position, which resulted in a Kd very similar to those for substrates that form G–A mismatches at the second and eighth positions (Fig. 4A and SI Appendix, Fig. S9D). We used additional gRNAs and substrates (SI Appendix, Table S1) to probe purine–purine mismatches at the third, seventh, ninth, 10th, 12th, and 14th positions to more rigorously define the seed region for MpAgo RNPs (SI Appendix, Fig. S8). The observed Kd values indicate that the MpAgo RNP seed region spans nucleotides 2–9 and is highly sensitive to mismatches at the third, sixth, seventh, and ninth positions (Fig. 4B). Taken together, our data suggest that MpAgo RNP has an extended seed compared with the canonical seed in eAgos and some pAgos (18–23) when targeting ssRNAs (Fig. 4C).
MpAgo RNPs Can Be Programmed to Distinguish Between Single-Nucleotide Variants.
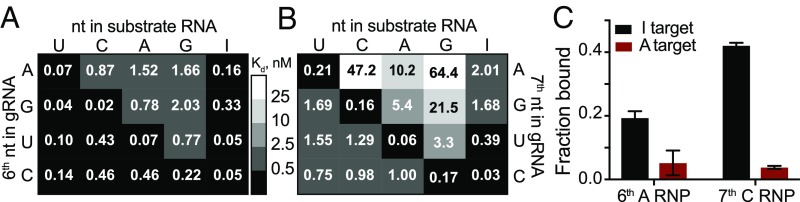
Using the sixth and seventh nucleotides located at the kink of the gRNA (16) (Fig. 1A), we investigated whether MpAgo RNPs could distinguish between RNA substrates that differ by only one nucleotide at the corresponding complementary position. Using MpAgo RNPs programmed with 5′-BrdU gRNAs that have A, G, U, or C either at the sixth or at the seventh position and RNA substrates with all permutations at the corresponding positions (SI Appendix, Figs. S9 and S10), we measured average dissociation constants for the sixth and seventh position (Fig. 5 A and B). Notably, at the sixth position, MpAgo RNP has the highest affinity for the fully complementary substrates (gRNA:substrate base pairs G:C, U:A, A:U, and C:G), substrates that form pyrimidine–pyrimidine mismatches (U–U and C–U), and the substrate that forms a G•U wobble. High-affinity binding of substrates with U–U and C–U mismatches suggests these mismatches are well accommodated in the gRNA–substrate RNA duplex at this position. Interestingly, only the G•C base pair-forming substrate binds more strongly than the substrate that creates a G•U wobble, whereas the binding affinities of the substrates that form U:A, A:U, and C:G Watson–Crick base pairs are two or more times times weaker. Purine–purine mismatches destabilize substrate RNA binding most, with A–A, A–G, G–A, and G–G mismatches increasing the Kd values 20- to 56-fold relative to Watson–Crick pairs. Moreover, MpAgo RNP binding affinity to ssRNA also depends on which nucleotide of the mismatch is on the gRNA strand. The binding affinity of the substrate forming a G•U wobble is 21 times tighter than that of the substrate forming a U•G wobble. Similar trends are observed for other types of mismatches (e.g., G–A vs. A–G, C–A vs. A–C, and C–U vs. U–C; Fig. 5A). Even in the case of perfectly matching gRNA–substrate duplexes, a C:G base pair binds 13 times more tightly than a G:C base pair.
Fig. 5.
5′-BrdU-gRNA MpAgo RNP ssRNA substrate binding specificity at the sixth and seventh positions. Filter-binding assays were performed using 5′-BrdU-gRNA MpAgo RNPs programmed with A, G, U, or C gRNA at the sixth (A) or at the seventh (B) positions and ssRNA substrates containing U, C, A, G, or I at the corresponding position. The average Kds were extracted and are represented in the heat maps. (C) A filter-binding assay to isolate the A-to-I edited substrate (black), but not the nonedited substrate (red), from 500 ng total RNA purified from HEK239T cells, in the presence of 200 ng/μL yeast tRNA. For this assay MpAgo RNP programmed with a 5′-BrdU gRNA containing either A at its sixth or C at its seventh position was used. The fraction of the ssRNA substrate bound to MpAgo RNP was quantified and plotted as the mean ± SD from three independent experiments.
In contrast to the sixth position, at the seventh position, MpAgo RNP has the highest affinity only for fully complementary substrates across the 15 nucleotides (gRNA:substrate base pairs G:C, U:A, A:U and C:G). Even though pyrimidine–pyrimidine mismatches and the G•U and U•G wobble affect the binding affinities the least, they increase the Kd values ∼10- to 20-fold relative to Watson–Crick pairs, suggesting that even these mismatches are not well accommodated in the gRNA–substrate RNA duplex at this position. Purine–purine mismatches (A–A, A–G, G–A, and G–G) and purine–pyrimidine mismatch A–C destabilize substrate RNA binding even more, increasing the Kd values 10- to 100-fold relative to Watson–Crick pairs. Similar to the sixth position, MpAgo RNP binding affinity to ssRNA also depends on which nucleotide of the mismatch is on the gRNA strand. The binding affinity of the substrate forming a C–A mismatch is 47 times tighter than that of the substrate forming an A–C mismatch. Similar trends are observed for other types of mismatches (e.g., G–A vs. A–G, G•U vs. U•G, and C–U vs. U–C; Fig. 5B). Collectively, our analysis of the sixth and seventh positions of the gRNA and corresponding positions of the ssRNA substrate indicate that MpAgo monitors not only the type of mismatch but also the identity of the bases forming the mismatch.
Next we tested whether MpAgo RNPs have high enough specificity to recognize modified nucleotides in RNA substrates. For instance, adenosine deaminases acting on RNA (ADARs) catalyze adenosine to inosine (A-to-I) editing of certain mRNAs (25, 26). Compared with adenosine, inosine has very different base-paring properties (27). We tested whether MpAgo RNPs could be programmed to distinguish between A-to-I edited and nonedited RNA substrates using MpAgo RNPs programmed with gRNAs that have A, G, U, or C at either the sixth or the seventh positions and substrate with inosine at the corresponding complementary positions (Fig. 5 A and B and SI Appendix, Figs. S9 and S10). Although MpAgo RNPs have low picomolar affinity for the inosine-containing substrate, irrespective of which nucleotide is used at the sixth position of the gRNA, the presence of an A at the sixth position of the gRNA leads to ∼10-fold weaker binding to substrates with an A instead of I (Fig. 5A and SI Appendix, Fig. S9). Intriguingly, at the seventh position, MpAgo RNP binds the I-containing substrate weaker than at the sixth position with the exception of the MpAgo RNP that has C at the seventh position (Fig. 5B and SI Appendix, Fig. S10). Moreover, the presence of a C at the seventh position of the gRNA leads to ∼33-fold weaker binding to substrates with an A instead of I (Fig. 5B and SI Appendix, Fig. S10).
We then investigated whether MpAgo RNP programmed with the 5′-BrdU gRNA containing either A at its sixth or C at its seventh position could be used to isolate A-to-I edited RNAs from a complex mixture. We spiked in either a radiolabeled I-containing RNA substrate or a radiolabeled A-containing RNA substrate into HEK293T total RNA and incubated the mixtures with MpAgo RNP programmed with a gRNA containing either A at its sixth or C at its seventh position. We also substituted bulk tRNA for heparin in the binding reactions to make the conditions of binding reactions more physiological. At the sixth position, MpAgo RNP bound an I-containing substrate with ∼19% efficiency, whereas the A-harboring RNA substrate was bound only with 5% efficiency. At the seventh position, MpAgo RNP bound an I-containing substrate with ∼42% efficiency, although the A-harboring RNA substrate was bound only with 4% efficiency (Fig. 5C). These results are consistent with the specificity seen with pure substrates in filter-binding assays (SI Appendix, Figs. S9 and S10) at the concentration of MpAgo RNP used with the complex mixture (500 pM MpAgo RNP). These results demonstrate that MpAgo RNPs can distinguish between the RNA substrates that differ by only one nucleotide with high specificity, and that it can be programmed to isolate A-to-I edited substrates from complex mixtures.
Discussion
As opposed to all other known Agos, the CRISPR-associated Agos in the M. piezophila family bind chemically distinct 5′-hydroxylated gRNAs rather than 5′-phosphorylated guides. This implies that the origin and biological assembly pathway of these Ago RNPs are also likely to be unique. It is possible that MpAgo gRNAs are generated by nucleases associated with the CRISPR operon; namely, Cas6, Csx1, or Csm6 (28–30). All three nucleases produce 5′-hydroxylated short RNAs (31–33) that could serve as MpAgo guides. We speculate that either Csx1 or Csm6, but not Cas6, is responsible for generating gRNAs for MpAgo, as Cas6 produces short RNAs that contain a conserved (8-nt) repeat sequence derived from the CRISPR array at their 5′ end (32). The conserved sequence spans the most important region of the seed sequence (Fig. 4), which would defeat the functional role of this region of the gRNA. The fact that the source of guides is likely unique for these CRISPR-associated Agos suggests that their biological role could differ from other pAgos (8, 13–15), a question that will require future experiments to answer.
In addition to the unknown origin of MpAgo gRNAs, the physiological targets for MpAgo are currently unclear. We showed that MpAgo cleaves ssRNA substrates with a cleavage rate of 0.39–0.64 min−1, similar to rates observed for ssDNA substrate cleavage [kobs ∼ 0.55 min−1 (16)]. This implies that both ssRNA and ssDNA may be physiological targets of MpAgo. Future experiments to identify the preferred in vivo targets of MpAgo will require analysis of the concentration dependence of MpAgo cleavage reactions, as well as estimates of ssDNA and ssRNA concentrations in M. piezophila bacterial cells. We also found that MpAgo, once formed as an RNP, has moderate affinity (∼5 nM Kd) and specificity for target RNAs (Fig. 1D and SI Appendix, Fig. S3 D–F). Remarkably, simple substitution of the 5′-end nucleotide of the gRNA with 5′-dU greatly increases the affinity and specificity of MpAgo RNPs for RNA substrates (Fig. 2C and SI Appendix, Fig. S5B). Although intended for UV crosslinking, use of 5′-BrdU gRNAs without crosslinking further increases MpAgo RNPs affinity and specificity for target RNAs (Fig. 2C and SI Appendix, Fig. S5C). Moreover, MpAgo RNPs programmed with 5′-BrdU gRNAs are twice as stable compared with the RNPs programmed with 5′-rU gRNAs (SI Appendix, Figs. S1 and S5A) and can bind complementary RNA substrates more than 300 times more efficiently than noncomplementary RNAs with a single-nt mismatch (Fig. 4B).
The specificity of the Ago RNP’s interaction with its substrates is determined by the seed sequence in the guide RNA. We have shown the MpAgo RNP seed region spans the second to ninth nucleotides of the gRNA when targeting ssRNA. This is distinct from the extended seed region when MpAgo targets ssDNA substrates (24), but similar to the seed region reported for other Agos, with the third, sixth, seventh, and ninth positions affecting MpAgo RNP’s specificity in binding target RNAs the most. With eAgos, it has been reported that the fourth and fifth nt of the gRNA contribute the most to specificity (34), although other reports suggest central positions of the guide RNA can also confer specificity (35). In MpAgo, the sixth–seventh positions may serve as conformational checkpoints, as the guide RNA is kinked between the sixth and seventh nucleotides (Fig. 1A). We hypothesize that base pairing of the sixth and seventh nucleotides releases the distortion in the gRNA and allows further 5′ to 3′ propagation of the guide–substrate base pairing. The structure of MpAgo RNP bound to a DNA substrate suggests that two protein helices of the L2 domain and a loop of the PIWI domain could enable MpAgo to sense the shape of the minor groove at the sixth and seventh position of the gRNA and ensure that the gRNA–RNA substrate duplex is complementary (SI Appendix, Fig. S11) (24), although MpAgo RNP interactions with DNA and RNA substrates are likely to be distinct (Fig. 4). Analysis of substrate specificity at the sixth and seventh nt of the gRNA demonstrate that MpAgo monitors not only the position and type of the mismatch but also the identity and position of the bases forming the mismatch. This discovery reveals a coding feature of the MpAgo RNP that enables it to discriminate between substrate RNAs differing by only a single nucleotide.
Interestingly, the MpAgo RNP can also be programmed to distinguish between target RNAs that are posttranscriptionally modified (i.e., that contain common base modifications such as inosine). Despite inosine’s ability to base pair with all four canonical bases, it forms the most stable base pair with cytidine. Therefore, it is recognized as guanosine by the translational machinery when it is present in coding mRNA sequences and can cause amino acid substitutions (36). In addition, perturbations of A-to-I editing correlate with several neurological diseases (37). Despite the prevalence of A-to-I editing, the functions of most editing sites remain unknown. The method primarily used to identify editing sites is based on the comparison of cDNA and corresponding genomic DNA sequences (36). However, there is no reliable method to investigate the function, cellular localization, and interaction networks of specific, edited RNA transcripts in vivo. The ability of MpAgo RNPs to isolate A-to-I edited RNAs from a complex mixture should enable deeper exploration of the biological function of inosine modifications in RNAs. In principle, MpAgo RNPs could also be used to isolate mRNAs with C-to-U deaminations (38), using gRNAs with an A at the seventh position (Fig. 5B).
MpAgo has moderate affinity for 5′-hydroxylated gRNAs (Kd ∼ 50–100 nM for all RNA guides that contain ribonucleotide at the 5′-end, and ∼25 nM for 5′-BrdU guides) compared with other Agos [Kd ∼ 1–7 nM for Rhodobacter sphaeroides Ago (39) and human Argonaute 2, respectively (40)]. Notably, the higher affinity of MpAgo RNPs for ssRNA substrates (Kd ∼ 5 nM for RNPs programmed with 5′-ribo gRNAs, and ∼0.02–0.5 nM for RNPs programmed with 5′-BrdU guides) than of MpAgo for gRNAs (Kd 50–104 nM for 5′-ribo gRNAs, and ∼25 nM for 5′-BrdU gRNAs) implies that binding of gRNA to MpAgo is stabilized by binding complementary RNA substrates (Figs. 1 B and D, 2 B and C, and 5 A and B). Thus, MpAgo is relatively easily programmed with defined gRNAs to target diverse RNAs of interest, and furthermore can be programmed at 37 °C, suitable for use in mesophilic cells. MpAgo RNPs with 5′-BrdU gRNAs bind complementary ssRNA substrates with much higher affinities than most RNA binding proteins currently employed for RNA targeting. Whereas the MS2 and PP7 coat proteins, the λN protein, repurposed RCas9, and more recently characterized type II-A Cas9 bind their RNA substrates with 1.6 nM–5 nM affinities, only the CRISPR nuclease Csy4 binds its RNA hairpin substrate with similar affinity to MpAgo, ∼50 pM (41–46). Of these systems, only Streptococcus pyogenes and Staphylococcus aureus Cas9 are easily programmable, and S. pyogenes Cas9 requires a PAMmer DNA oligonucleotide for efficient RNA binding (44, 46–48). Notably, the cellular concentration of mRNAs in mammalian cells is generally in the 10–100 pM range, except for the most abundant transcripts (49, 50), matching the affinity range for MpAgo RNPs programmed with 5′-BrdU gRNAs. Finally, these MpAgo RNPs can be used to discriminate single-nucleotide variants and identify A-to-I edited target RNAs. In the future, it should be possible to use 5′-BrdU gRNA-loaded MpAgo RNPs to analyze, image, and manipulate untagged RNAs in live cells with single-nucleotide resolution.
Materials and Methods
A full description of the materials and methods used in this study, including in vitro RNA synthesis and radiolabeling, in vitro reconstitution of MpAgo RNP, MpAgo unloading assays, in vitro binding and cleavage assays, MpAgo-gRNA crosslinking experiments, A-to-I edited substrate isolation assays, and a list of oligonucleotides, is provided in the SI Appendix.
Supplementary Material
Acknowledgments
We thank the members of the J.H.D.C. and the J.A.D. laboratories, especially Steven C. Strutt, Emine Kaya, and Kevin W. Doxzen, for their technical assistance and helpful discussions. A.L. acknowledges support from the Human Frontiers in Science Program (Postdoctoral Fellowship LT 000102/2015-L). This work was supported by National Institutes of Health Grant R01-GM065050 (to J.H.D.C.) and by a Frontiers Science award from the Paul Allen Institute (to J.A.D.). J.A.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: The authors are filing a patent application covering the present work.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717725115/-/DCSupplemental.
References
- 1.Swarts DC, et al. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. 2014;21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe TA, et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 3.Sigova A, Rhind N, Zamore PD. A single Argonaute protein mediates both transcriptional and posttranscriptional silencing in Schizosaccharomyces pombe. Genes Dev. 2004;18:2359–2367. doi: 10.1101/gad.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabara H, et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 5.Houwing S, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins C, et al. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature. 2005;436:1044–1047. doi: 10.1038/nature03957. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 10.Behm-Ansmant I, et al. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shabalina SA, Koonin EV. Origins and evolution of eukaryotic RNA interference. Trends Ecol Evol. 2008;23:578–587. doi: 10.1016/j.tree.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olovnikov I, Chan K, Sachidanandam R, Newman DK, Aravin AA. Bacterial argonaute samples the transcriptome to identify foreign DNA. Mol Cell. 2013;51:594–605. doi: 10.1016/j.molcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swarts DC, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. 2014;507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y-R, et al. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell. 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarts DC, et al. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA. Nucleic Acids Res. 2015;43:5120–5129. doi: 10.1093/nar/gkv415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaya E, et al. A bacterial Argonaute with noncanonical guide RNA specificity. Proc Natl Acad Sci USA. 2016;113:4057–4062. doi: 10.1073/pnas.1524385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alain K, et al. Marinitoga piezophila sp. nov., a rod-shaped, thermo-piezophilic bacterium isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol. 2002;52:1331–1339. doi: 10.1099/00207713-52-4-1331. [DOI] [PubMed] [Google Scholar]
- 18.Elkayam E, et al. The structure of human argonaute-2 in complex with miR-20a. Cell. 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486:368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirle NT, Sheu-Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346:608–613. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008;456:921–926. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008;456:209–213. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doxzen KW, Doudna JA. DNA recognition by an RNA-guided bacterial Argonaute. PLoS One. 2017;12:e0177097. doi: 10.1371/journal.pone.0177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner RW, Smith JE, Cooperman BS, Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc Natl Acad Sci USA. 1989;86:2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- 27.Kawase Y, Iwai S, Inoue H, Miura K, Ohtsuka E. Studies on nucleic acid interactions. I. Stabilities of mini-duplexes (dG2A4XA4G2-dC2T4YT4C2) and self-complementary d(GGGAAXYTTCCC) containing deoxyinosine and other mismatched bases. Nucleic Acids Res. 1986;14:7727–7736. doi: 10.1093/nar/14.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynen M, Snel B, Lathe W, 3rd, Bork P. Predicting protein function by genomic context: quantitative evaluation and qualitative inferences. Genome Res. 2000;10:1204–1210. doi: 10.1101/gr.10.8.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galperin MY, Koonin EV. Who’s your neighbor? New computational approaches for functional genomics. Nat Biotechnol. 2000;18:609–613. doi: 10.1038/76443. [DOI] [PubMed] [Google Scholar]
- 30.Aravind L. Guilt by association: contextual information in genome analysis. Genome Res. 2000;10:1074–1077. doi: 10.1101/gr.10.8.1074. [DOI] [PubMed] [Google Scholar]
- 31.Niewoehner O, Jinek M. Structural basis for the endoribonuclease activity of the type III-A CRISPR-associated protein Csm6. RNA. 2016;22:318–329. doi: 10.1261/rna.054098.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22:3489–3496. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheppard NF, Glover CVC, 3rd, Terns RM, Terns MP. The CRISPR-associated Csx1 protein of Pyrococcus furiosus is an adenosine-specific endoribonuclease. RNA. 2016;22:216–224. doi: 10.1261/rna.039842.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wee LM, Flores-Jasso CF, Salomon WE, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151:1055–1067. doi: 10.1016/j.cell.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du Q, Thonberg H, Wang J, Wahlestedt C, Liang Z. A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res. 2005;33:1671–1677. doi: 10.1093/nar/gki312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns CM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 37.Maas S, Kawahara Y, Tamburro KM, Nishikura K. A-to-I RNA editing and human disease. RNA Biol. 2006;3:1–9. doi: 10.4161/rna.3.1.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng B, Burant CF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;260:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi T, Ito K, Murakami R, Uchiumi T. Structural basis for the recognition of guide RNA and target DNA heteroduplex by Argonaute. Nat Commun. 2016;7:11846. doi: 10.1038/ncomms11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deerberg A, Willkomm S, Restle T. Minimal mechanistic model of siRNA-dependent target RNA slicing by recombinant human Argonaute 2 protein. Proc Natl Acad Sci USA. 2013;110:17850–17855. doi: 10.1073/pnas.1217838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternberg SH, Haurwitz RE, Doudna JA. Mechanism of substrate selection by a highly specific CRISPR endoribonuclease. RNA. 2012;18:661–672. doi: 10.1261/rna.030882.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeCuyer KA, Behlen LS, Uhlenbeck OC. Mutants of the bacteriophage MS2 coat protein that alter its cooperative binding to RNA. Biochemistry. 1995;34:10600–10606. doi: 10.1021/bi00033a035. [DOI] [PubMed] [Google Scholar]
- 43.Cilley CD, Williamson JR. Analysis of bacteriophage N protein and peptide binding to boxB RNA using polyacrylamide gel coelectrophoresis (PACE) RNA. 1997;3:57–67. [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connell MR, et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature. 2014;516:263–266. doi: 10.1038/nature13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao JA, Patskovsky Y, Almo SC, Singer RH. Structural basis for the coevolution of a viral RNA-protein complex. Nat Struct Mol Biol. 2008;15:103–105. doi: 10.1038/nsmb1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strutt SC, Torrez RM, Kaya E, Negrete OA, Doudna JA. RNA-dependent RNA targeting by CRISPR-Cas9. eLife. 2018;7:e32724. doi: 10.7554/eLife.32724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelles DA, et al. Programmable RNA tracking in live cells with CRISPR/Cas9. Cell. 2016;165:488–496. doi: 10.1016/j.cell.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batra R, et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell. 2017;170:899–912.e10. doi: 10.1016/j.cell.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 50.Albayrak C, et al. Digital quantification of proteins and mRNA in single mammalian cells. Mol Cell. 2016;61:914–924. doi: 10.1016/j.molcel.2016.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.