Significance
Mevalonate is a building block of archaeal lipids. Three enzymes are involved in its biosynthesis: acetoacetyl-CoA thiolase (thiolase), 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase (HMGCS), and HMG-CoA reductase. The thiolase reaction is highly endergonic, which means that archaea have to find a way to overcome this low-flux bottleneck. Our work revealed the presence of a thiolase/HMGCS complex, which directly couples the endergonic thiolase reaction to the exergonic HMGCS reaction. An unexpected third protein spatially connects the thiolase and HMGCS. Strikingly, these two enzymes share the same substrate-binding site. Genomic information indicated that the presence of a thiolase/HMGCS complex is common in most of archaea and many bacteria. Such a natural intermediate-channeling system could lead to new strategies to improve biotechnological mevalonate synthesis.
Keywords: isoprenoid synthesis, enzymatic channeling, mevalonate production, archaea, X-ray crystallography
Abstract
Many reactions within a cell are thermodynamically unfavorable. To efficiently run some of those endergonic reactions, nature evolved intermediate-channeling enzyme complexes, in which the products of the first endergonic reactions are immediately consumed by the second exergonic reactions. Based on this concept, we studied how archaea overcome the unfavorable first reaction of isoprenoid biosynthesis—the condensation of two molecules of acetyl-CoA to acetoacetyl-CoA catalyzed by acetoacetyl-CoA thiolases (thiolases). We natively isolated an enzyme complex comprising the thiolase and 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase (HMGCS) from a fast-growing methanogenic archaeon, Methanothermococcus thermolithotrophicus. HMGCS catalyzes the second reaction in the mevalonate pathway—the exergonic condensation of acetoacetyl-CoA and acetyl-CoA to HMG-CoA. The 380-kDa crystal structure revealed that both enzymes are held together by a third protein (DUF35) with so-far-unknown function. The active-site clefts of thiolase and HMGCS form a fused CoA-binding site, which allows for efficient coupling of the endergonic thiolase reaction with the exergonic HMGCS reaction. The tripartite complex is found in almost all archaeal genomes and in some bacterial ones. In addition, the DUF35 proteins are also important for polyhydroxyalkanoate (PHA) biosynthesis, most probably by functioning as a scaffold protein that connects thiolase with 3-ketoacyl-CoA reductase. This natural and highly conserved enzyme complex offers great potential to improve isoprenoid and PHA biosynthesis in biotechnologically relevant organisms.
Biological systems have evolved to efficiently catalyze a myriad of enzymatic reactions in metabolism. Among these reactions, some exhibit extremely unfavorable thermodynamics. One prominent example, found in the tricarboxylic acid (TCA) cycle, is the oxidation of malate to oxaloacetate that is catalyzed by malate dehydrogenase (MDH). Under physiological conditions, the equilibrium constant for this reaction in the forward direction is Keq = (2.86 ± 0.12) × 10−5 (1); estimated ΔG°′ = +30.3 kJ/mol using eQuilibrator (2). If considered as an isolated reaction alone, this unfavorable step would drastically reduce the flux through the TCA cycle. However, recent in vivo chemical cross-linking experiments have shown that MDH forms a weakly associated complex with the next enzyme in the TCA cycle, citrate synthase (CS) (3, 4). The oxaloacetate produced by MDH might be electrostatically guided from the active site of the MDH to the active site of the CS without freely diffusing into the bulk solvent (5, 6). The direct substrate channeling from one active site to the other could lead to an increased consumption of oxaloacetate by CS in a low-concentration microenvironment and allows high flux through the MDH/CS complex despite unfavorable thermodynamics of the first reaction (5). This coupling mechanism balances the first endergonic reaction with the second and drives the high rate of citrate formation required (4, 5).
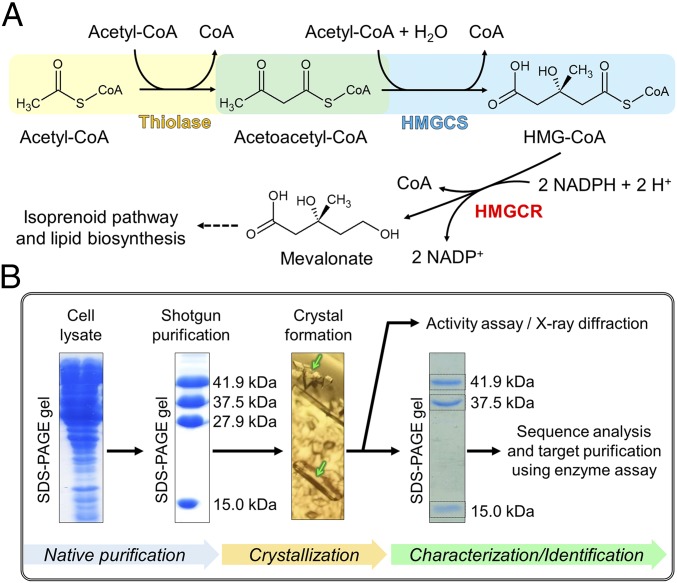
Another example of a reaction with unfavorable thermodynamics in central carbon metabolism is the nondecarboxylative Claisen condensation of two acetyl-CoA molecules into acetoacetyl-CoA catalyzed by acetoacetyl-CoA thiolases (thiolases); Keq = (1.1 ± 0.2) × 10−5 (7); estimated ΔG°′ = +26.1 kJ/mol using eQuilibrator (2). Thiolases operate in all domains of life and are a part of several important metabolic pathways. There are two types of thiolases (8). Degradative thiolases are involved in the β-oxidation of fatty acids, where they catalyze the thermodynamically favorable reaction of thiolytic cleavage of a wide range of 3-ketoacyl-CoAs into shorter acyl-CoAs and acetyl-CoA. Biosynthetic thiolases operate in the polyhydroxyalkanoate (PHA) and mevalonate biosynthesis pathways, where they catalyze the thermodynamically unfavorable condensation of two molecules of acetyl-CoA to acetoacetyl-CoA. Both types of thiolases have significant sequence identity and show a similar protein fold (8). In the mevalonate pathway, acetoacetyl-CoA is condensed with a third acetyl-CoA molecule to form 3-hydroxy-3-methylglutaryl (HMG)-CoA by HMG-CoA synthase (HMGCS) and then reduced to mevalonate by HMG-CoA reductase (HMGCR) using NADPH as reductant (Fig. 1A). The exergonic HMGCS and HMGCR reactions make the total mevalonate biosynthetic reaction thermodynamically favorable.
Fig. 1.
Mevalonate pathway and purification strategy. (A) Reaction scheme of the three enzymes involved in the mevalonate pathway. (B) Workflow applied in this work to identify and analyze the thiolase/HMGCS complex. Proteins in the cell extract were isolated by shotgun purification (SI Appendix). Two different crystalline forms appeared (green arrows) from a fraction. Crystals were washed and dissolved. The protein bands on SDS/PAGE of the dissolved crystals were used for mass spectrometric identification. Based on the protein-sequence analysis, the function of the crystallized proteins was predicted and the thiolase/HMGCS activity was measured by the HMGCR-coupled assay. The thiolase/HMGCS complex was purified again from the cell extract using the enzyme assay (target purification), and the purified enzyme complex was enzymologically characterized.
The mevalonate pathway is found in eukaryotes, archaea, as well as some gram-positive bacteria, where it forms the starting compound of isoprenoid biosynthesis (9). Isoprenoids are one of the largest groups of natural products and display a wide variety of biological functions. They serve as hormones, photosynthetic pigments, quinones, plant defense compounds, and membrane components (10). Particularly, archaea require a lot of isoprenoids as building blocks of the membrane lipid bilayer. This raises the question of how archaea realize high flux through the mevalonate pathway despite the unfavorable thermodynamics of the thiolase reaction.
Here, we show that the thiolase and HMGCS of the methanogenic archaeon Methanothermococcus thermolithotrophicus form a 380-kDa enzyme complex in combination with a small scaffold protein (Pfam family: DUF35), which connects the two enzymes. The crystal structure of the native complex revealed a unique, shared CoA-binding site formed by both the thiolase and HMGCS subunits. Structural and biochemical data indicate that the complex uses this shared binding site to couple the endergonic nondecarboxylative Claisen condensation to the exergonic HMG-CoA–forming reaction. This complex is conserved in most of archaea and can be found in many bacteria.
Results
A Tripartite Complex Found by Native Purification.
We initially identified the presence of the thiolase/HMGCS complex from M. thermolithotrophicus, which is a fast-growing methanogen with a doubling time of about 30 min in a minimum mineral medium at 65 °C (11, 12), by the shotgun purification of its proteome (Fig. 1B and SI Appendix). After identification of the multicomponent enzyme, we purified the thiolase/HMGCS complex from the cell lysate by following mevalonate-producing activity using a coupled-enzyme assay containing HMGCR. In the purification steps, the total activity increased 4.4-fold after the first two purification steps, which indicated that there are some inhibitory or regulatory components for the reactions in the lysate that were lost during these purification steps. At the end of the five-step purification, the specific activity increased 1,000-fold (Table 1). Four protein bands were visible on SDS/PAGE in the final fraction (Fig. 1B and SI Appendix, Fig. S1).
Table 1.
Target purification of the thiolase/HMGCS complex
| Purification step | Protein, mg | Total activity, U | Specific activity, U/mg | Purification, -fold |
| Soluble fraction | 4,000 | 31 | 0.008 | 1 |
| DEAE Sepharose | 2,300 | 99 | 0.043 | 5.4 |
| Q Sepharose | 1,100 | 130 | 0.12 | 15 |
| Hydroxyapatite | 310 | 59 | 0.19 | 24 |
| Source 15 Phe | 4.3 | 43 | 10 | 1,300 |
The activity was determined using HMGCR-coupled assay. The assays contained 2 mM acetyl-CoA, 2 µM HMGCR, and 450 µM NADPH in 100 mM Hepes-NaOH pH 7.5.
Multiple crystalline forms were observed in one crystallization drop. We dissolved single crystals and tested the enzyme activity (Fig. 1B). A type of crystals exhibited the HMG-CoA–forming enzyme activity from acetyl-CoA and contained three proteins as shown by SDS/PAGE; the ratio of the band intensities was ∼1:1:1. By mass spectrometric sequencing, these proteins were annotated as the 41.9-kDa thiolase, the 37.5-kDa HMGCS, and a 15-kDa DUF35-family protein. The function of the DUF35-family protein was, at that point, unknown (13). Complexing of the DUF35 protein with thiolase and HMGCS, however, was in line with the observation that DUF35 proteins are typically encoded within the same transcriptional unit. Other crystals in the crystallization drop, which did not exhibit the HMG-CoA–forming activity, contained a protein with a molecular mass of 27.9 kDa present in the SDS/PAGE. This protein was identified by mass spectrometric sequencing as thiazole synthase, which is not functionally related to the mevalonate pathway.
Architecture of the Thiolase/HMGCS Complex.
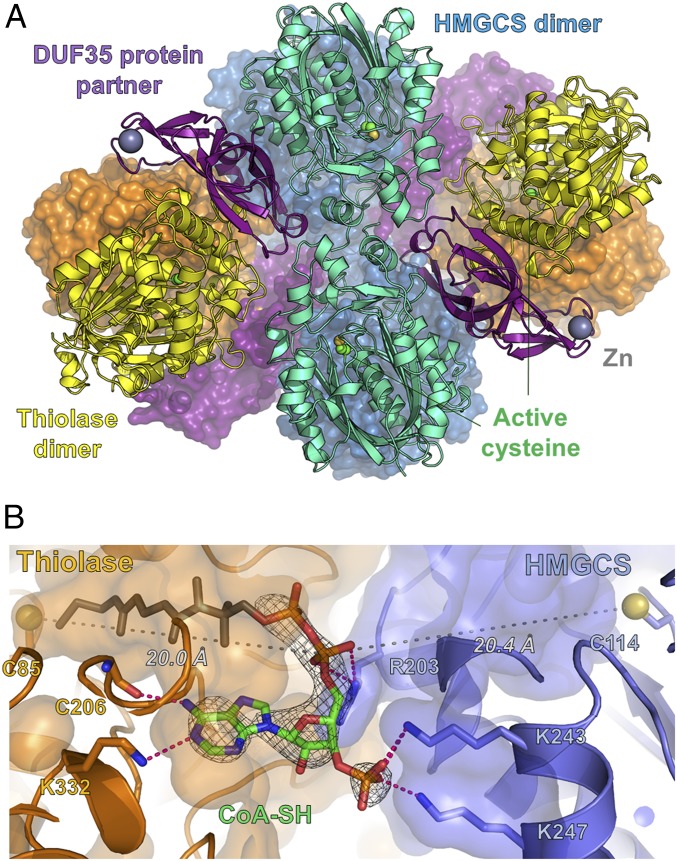
The crystals containing thiolase, HMGCS, and the DUF35 protein were used for X-ray diffraction experiments. We solved the structure of this enzyme complex using the newly developed terbium-containing phasing reagent, Tb-Xo4 (14). Diffraction data of the Tb-Xo4–soaked crystals were collected at the terbium LIII absorption edge, and the 16 terbium sites with high occupancy were used to determine the structure by means of anomalous phasing (Materials and Methods and SI Appendix, Fig. S2). The complex contained two dimers of thiolase, two dimers of HMGCS, and four monomers of the DUF35-family protein (Fig. 2A). The structure of the thiolase dimer in the complex showed the highest similarity to the lone-standing Scp2 type-II thiolases from eukaryotic parasites [i.e., Leishmania mexicana, PDB ID code 3ZBG, root mean square deviation (rmsd) of 1.0 Å], which are folded as a five-layered αβαβα catalytic domain (SI Appendix, Fig. S3A) (15–17). The HMGCS dimer from M. thermolithotrophicus has a fold very similar to the HMGCSs from eukaryotes (i.e., Homo sapiens, PDB ID code 2P8U, rmsd 1.8 Å) and gram-positive bacteria, which adopt a five-layered αβαβα catalytic fold (SI Appendix, Fig. S3B) (18, 19). However, the HMGCS from M. thermolithotrophicus is reduced in size and lacks a C-terminal extension of about 50 to 100 amino acids compared with HMGCSs from eukaryotes and gram-positive bacteria.
Fig. 2.
Thiolase and HMGCS are assembled in an active complex via the DUF35 protein, which provides a bridging platform for the organization of a shared CoA-binding site. (A) Quaternary representation of the thiolase/HMGCS complex in cartoon and surface models. Zn(II) ion and reactive cysteines are shown in ball-and-stick models. Color code highlights the different proteins: yellow/orange for thiolase dimers, cyan/dark blue for the HMGCS dimers, and purple for the DUF35 protein. (B) Binding site of acyl-CoA made at the interface of both enzymes. CoA (adenosine moiety in green) and CoA-binding residues are shown in the stick model. The active-site cysteines are shown as yellow balls and sticks. The 2Fo − Fc electron density from the omit map for CoA-SH is contoured at 1.0 σ.
The third component of the complex, the DUF35 protein, has high similarity with a homolog from Sulfolobus solfataricus, whose structure has been solved by a structural genomics initiative (PDB ID code 3IRB, rmsd 2.9 Å) (SI Appendix, Fig. S3 C and D) (13). A role in lipid and polyketide antibiotic biosynthesis was proposed for this homolog (13). The DUF35-encoding genes are also found in operons containing sterol-carrier protein X–related thiolases (20–22). In the crystal structure of the S. solfataricus DUF35 homolog, the N-terminal helix is bound to the hydrophobic core of the protein and also bound to another DUF35 monomer (13). This helix was speculated to serve as a scaffold for binding other proteins. In contrast, in the DUF35 protein from the thiolase/HMGCS complex, the hydrophobic core of the DUF35 protein binds to the thiolase dimer, and its N-terminal segment interacts with one thiolase subunit (SI Appendix, Fig. S3C). A Zn(II) ion is coordinated in its rubredoxin domain, and the rubredoxin binds to the second thiolase subunit. Furthermore, the core of the DUF35 protein displays multiple contacts with the thiolase and the HMGCS dimers with a total interface of 2,019 Å2 and 1,640 Å2, respectively (SI Appendix, Fig. S4). Compared with the DUF35 interaction, thiolase and HMGCS dimers perform only direct contacts, with an area of interaction below 300 Å2. The interface area between thiolase-DUF35-HMGCS is governed by electrostatic ringlike interactions in the periphery and some hydrophobic contacts close to the center, which are largely conserved throughout methanogens (SI Appendix, Fig. S5). Thus, the small DUF35 protein functions as a scaffold to connect the thiolase and HMGCS dimers.
Thiolase and HMGCS Share a Single CoA-Binding Site.
We investigated the substrate-binding sites of the thiolase/HMGCS complex by soaking with CoA, acetyl-CoA, and HMG-CoA. We obtained one dataset at 2.95 Å resolution (SI Appendix, Table S1) from a soak with 100 mM acetyl-CoA for 1.5 min at 18 °C (see Materials and Methods). In this structure, an extra electron density appeared at the interface of the two enzyme subunits and was modeled as an acetyl-CoA molecule (SI Appendix, Fig. S6). The adenine moiety of the acetyl-CoA molecule was bound to the thiolase part, and the phosphate groups were fixed by three salt bridges to the HMGCS part. The adenine- and phosphate-binding sites are located at the equivalent positions of the acetyl-CoA binding sites of the lone-standing thiolase and HMGCS, respectively (SI Appendix, Fig. S7). This finding indicated that thiolase and HMGCS in the enzyme complex have only one common acyl-CoA binding site, which is shared by both enzymes (Fig. 2B). While the adenine interaction is well resolved in our structure, there is only partial electron density visible for the pantetheinyl arm reaching into the thiolase active site. This is probably due to the flexibility of the pantetheinyl arm that can swing between the thiolase and HMGCS active sites. By using noncrystallographic symmetry on the omit map, we were able to detect peaks of electron density close to the two known catalytic cysteines in the thiolase (Cys85) and HMGCS (Cys114) (SI Appendix, Fig. S8). Since these two residues could be temporally acetylated in the catalytic reactions (15), we interpreted these densities as partially acetylated cysteine sulfurs.
The fused acetyl-CoA binding site suggested that the two enzymatic reactions are directly coupled via the common CoA-binding site. In this hypothetical mechanism, the intermediate acetoacetyl-CoA swings from the thiolase active site to the HMGCS active site, where the intermediate is directly converted to HMG-CoA without releasing it into the bulk solvent.
Kinetic Assays Indicating Channeling.
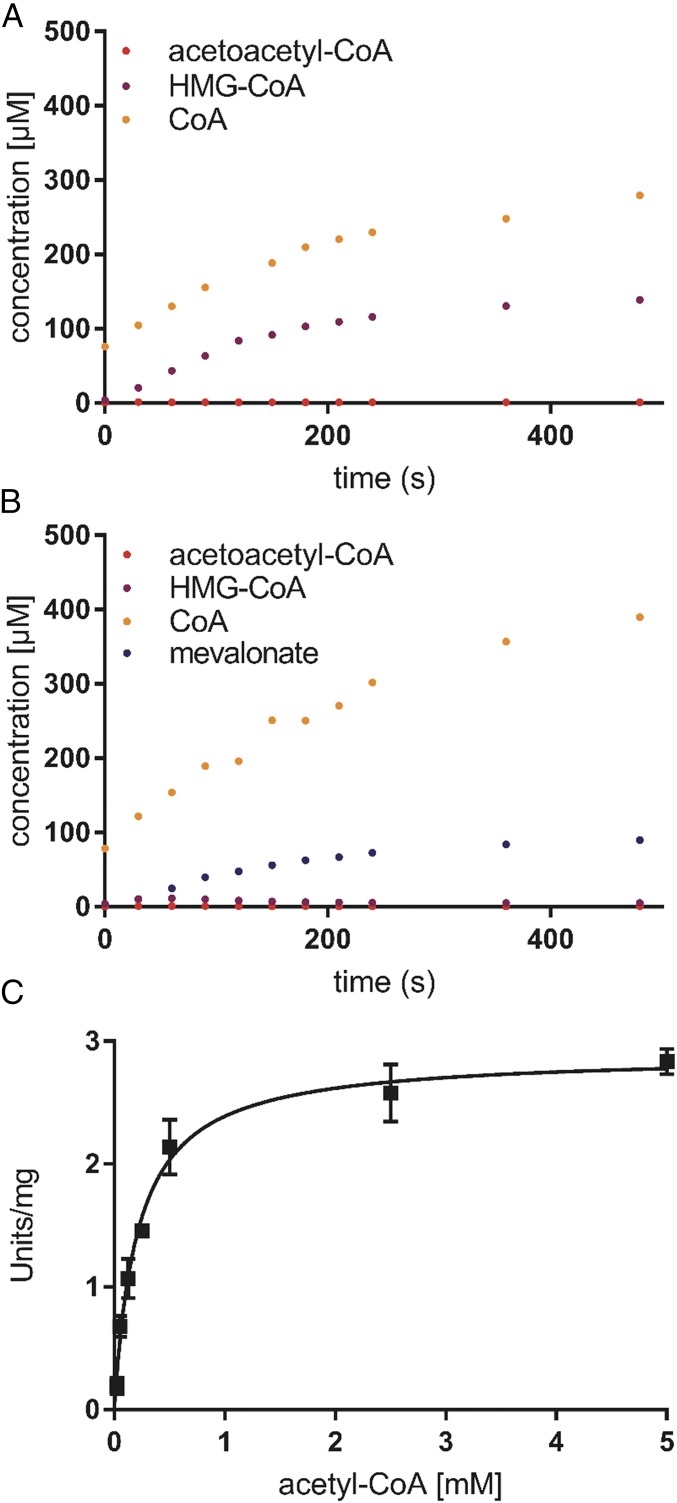
The enzyme assay using HPLC-MS showed that the thiolase/HMGCS complex alone is able to convert acetyl-CoA to HMG-CoA (Fig. 3A). Addition of an excess amount of HMGCR to the assay increased neither the reaction rate nor the stoichiometry of the final products (Fig. 3B). These findings indicated that the coupled reactions of thiolase and HMGCS are thermodynamically favorable, which allows the production of HMG-CoA from acetyl-CoA. To determine the kinetic parameters of the thiolase/HMGCS complex, we used a photometrical assay with an excess amount of HMGCR. In this assay, oxidation of NADPH was followed. The thiolase/HMGCS complex showed a specific activity of 2.9 ± 0.2 U/mg, considering the purity of the complex (60% judged by SDS/PAGE), and a Km,app for acetyl-CoA of 210 μM at 65 °C and at pH of 7.5 (Fig. 3C).
Fig. 3.
Activity assay of the thiolase/HMGCS complex. (A) Production of acetoacetyl-CoA, HMG-CoA, and CoA catalyzed by the thiolase/HMGCS complex at 60 °C determined by the LC-MS analysis. The assays contained 100 mM Hepes-KOH pH 7.5, 0.1 mg/mL thiolase/HMGCS complex, and 2 mM acetyl-CoA. (B) Production of acetoacetyl-CoA, HMG-CoA, CoA, and mevalonate catalyzed by the thiolase/HMGCS complex in the presence of HMGCR at 60 °C determined by the LC-MS analysis. The assay contained 100 mM Hepes-KOH pH 7.5, 0.1 mg/mL thiolase/HMGCS complex, 200 μM NADPH, and 1.35 μM HMGCR. (C) Effect of substrate (acetyl-CoA) concentration on the reaction rate catalyzed by the thiolase/HMGCS complex. The activity was measured with the HMGCR-coupled assay by following the oxidation of NADPH at 340 nm. Standard errors of triplicate samples are shown.
In the next step, we tested whether acetoacetyl-CoA is released into the bulk solvent during the catalysis. We quenched assays during the steady state of the reactions that either contained only the thiolase/HMGCS complex or the complex and HMGCR. Using HPLC-MS analysis, we could not detect any acetoacetyl-CoA above the detection limit of 100 nM in any of the assays, indicating that acetoacetyl-CoA concentrations were below 100 nM during steady-state catalysis of the thiolase/HMGCS complex. It is unlikely that such low concentrations of acetoacetyl-CoA in solution can account for the measured turnover rates of 2.9 U/mg (kcat,app = 4.6 s−1) of the complex if we compare it to the kinetic parameters of the archaeal HMGCS of Haloferax volcanii [kcat = 4.6 s−1, Km,acetoacetyl-CoA = 1.4 µM (23)]. The data, therefore, indicate that acetoacetyl-CoA was consumed directly by the HMGCS without release of the intermediate from the active site of the thiolase into the bulk solvent.
We next investigated whether external acetoacetyl-CoA can enter the active site of the HMGCS by adding external acetoacetyl-CoA to the thiolase/HMGCS complex. The kinetic data show that addition of external acetoacetyl-CoA slows down the overall turnover of the reaction, which suggests that external acetoacetyl-CoA is able to diffuse into the active site and acts as a competitive inhibitor by binding to the acetyl-CoA binding site (SI Appendix, Fig. S9). Such an inhibitory effect of acetoacetyl-CoA was shown for several lone-standing HMGCSs (24, 25). Furthermore, we tested the utilization of acetoacetyl-CoA by a stable isotope-labeling experiment using the HMGCR-coupled assay. We started the reaction of the complex in the presence of 1 mM [1,2-13C2] acetyl-CoA and 1 mM unlabeled acetoacetyl-CoA (SI Appendix, Fig. S10). Mass spectrometric analysis of the HMGCR-coupled reaction products showed that the thiolase/HMGCS complex preferentially used the unlabeled acetoacetyl-CoA (SI Appendix, Table S2). This finding confirmed that external acetoacetyl-CoA was diffusible into the active site, which suggested that the acetoacetyl-CoA intermediate formed from acetyl-CoA is not trapped in an internal compartment.
To assess the effect of complex formation on activity, we heterologously expressed the three proteins individually in Escherichia coli. Unfortunately, we were unable to purify the thiolase, as it was always found in insoluble inclusion bodies. The purified HMGCS did not show any activity toward acetoacetyl-CoA (concentrations of up to 10 mM acetoacetyl-CoA were tested) in coupled assays with HMGCR, suggesting that the contribution of the thiolase to the binding site is required for activity.
DUF35 Protein Is a Universal Scaffold in Archaea for Lipid and PHA Biosynthesis.
We found that almost all archaea encode the three proteins of the thiolase/HMGCS complex (thiolase, HMGCS, and DUF35) (SI Appendix, Fig. S11 and Tables S3 and S4), which indicated that the tripartite complex is essential in archaea for lipid biosynthesis. In some archaea, the DUF35 protein is directly fused to the C terminus of HMGCS (i.e., in Halobacterium). The only exception is the recently discovered group of Lokiarchaea, which neither encodes a lone-standing DUF35 protein nor a DUF35-HMGCS fusion protein. However, the HMGCS of Lokiarchaea contains a C-terminal extension with a similar size as the DUF35 protein but with a unique sequence. This C-terminal extension might fulfill a similar role as the DUF35 protein in the thiolase/HMGCS complex.
The DUF35 proteins are also found in the two PHA biosynthetic gene clusters of Haloferax mediterranei that are known to produce branched and unbranched polyalkanoates. Hou et al. (26) showed that the DUF35 proteins are essential for PHA biosynthesis. A double knockout of both DUF35 proteins completely abolished PHA production in H. mediterranei. This suggests that in PHA biosynthesis, the DUF35 protein also acts as a scaffold that brings together the thiolase with a 3-ketoacyl-CoA reductase, which couples the endergonic thiolase reaction to the exergonic reduction of acetoacetyl-CoA to 3-hydroxybutyryl-CoA.
Notably, the DUF35 scaffolding proteins are also found throughout the genomes of many bacteria (SI Appendix, Table S4). The bacterial DUF35 homologs are colocated with a thiolase and, in many cases, either a HMGCS or a 3-ketoacyl-CoA reductase homolog, which suggests that these DUF35 proteins also organize tripartite mevalonate and/or PHA biosynthetic complexes in bacteria, although no such enzyme complexes are reported so far.
In eukaryotes that also synthesize isoprenoids via the mevalonate pathway, no DUF35 homologs are found. Interestingly, however, eukaryotic and bacterial lone-standing HMGCSs contain a C-terminal hairpin [e.g., residues 487 to 508 for the mitochondrial human HMGCS, PDB ID code 2WYA (19)] that superimposes well with the C-terminal part of the DUF35 scaffolding protein (i.e., residues 100 to 129 of the DUF35) from the archaeal tripartite complex (SI Appendix, Fig. S12). These eukaryotic HMGCSs have been characterized to act as lone-standing enzymes; however, the structural homology of the C terminus suggests that they might complex with other proteins in the cells.
Discussion
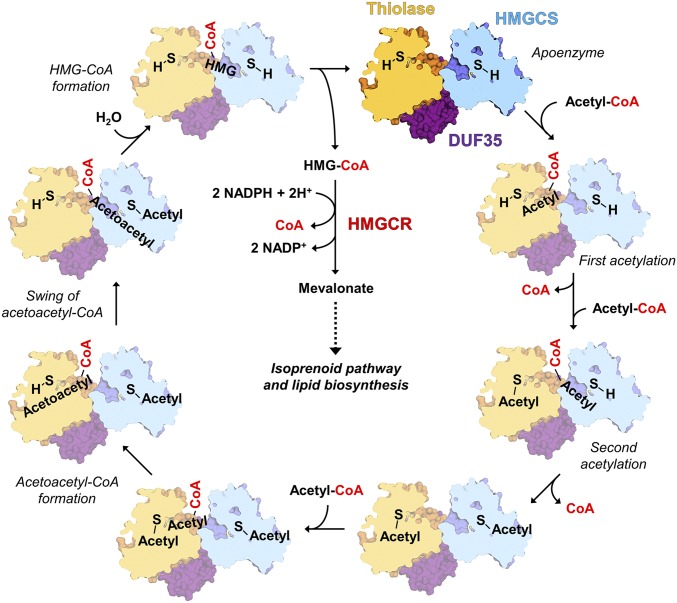
The identification of this natural channeling complex highlights the importance of native purification as a method to discover physiological enzyme complexes. The isolation of the native thiolase/HMGCS complex enabled us to find the third partner of these two enzymes, the DUF35-family protein, whose functions have been enigmatic. The DUF35 protein organizes the spatial colocalization of the thiolase and HMGCS dimers to form the thiolase/HMGCS complex. A shared acyl-CoA binding site is formed between the thiolase and the HMGCS. This shared binding site could enable acetoacetyl-CoA produced by the thiolase to swing directly from the thiolase into the HMGCS active site. This couples the endergonic first reaction with the exergonic second one to increase overall biosynthetic flux. The proposed reaction scheme is shown in Fig. 4. Both active-site cysteines have to be acetylated first to ensure formation of acetoacetyl-CoA and HMG-CoA. Desynchronized reaction order will lead to unreactive enzyme substrate/intermediate complexes, as seen in the inhibitory effect of added bulk-solvent acetoacetyl-CoA (see Kinetic Assays Indicating Channeling). To perform such a sequential-ordered reaction, the thiolase and HMGCS subunits have to communicate with each other through conformational changes.
Fig. 4.
Proposed reaction sequence of the thiolase/HMGCS complex. The active-site Cys85 of thiolase (in yellow) and Cys114 of HMGCS (in blue) are shown as S-(H). In the initial two steps, both specific cysteine residues of thiolase and the HMGCS are acetylated. In the next step, thiolase forms acetoacetyl-CoA using the third molecule of acetyl-CoA. The acetoacetyl-pantetheinyl arm of acetoacetyl-CoA swings to the acetylated HMGCS active site and is converted to the final product HMG-CoA. The released HMG-CoA is further processed by HMGCR to form mevalonate through the mevalonate pathway.
The high total kinetic rate of the thiolase/HMGCS complex (2.9 ± 0.2 U/mg) indicated that the highly endergonic thiolase reaction was not only thermodynamically overcome but also kinetically accelerated by the channeling system in this enzyme complex, most probably by means of the shared CoA-binding site. A similar shared CoA-binding site has been reported for the bacterial multienzyme complex that catalyzes the last three sequential reactions in the fatty acid β-oxidation cycle (27). In this complex, 2-enoyl-CoA hydratase, 3-ketoacyl-CoA thiolase, and 3-hydroxyacyl-CoA dehydrogenase share the same CoA-binding site and use the long-spanning pantetheinyl arm to swing the intermediate from one active site to the next ones.
This mode of action of the thiolase/HMGCS complex resembles that of polyketide and fatty acid synthases that also channel catalytic intermediates from one active site to the other (28). However, in contrast to the latter complexes, the intermediates of the thiolase/HMGCS are not covalently bound. It should be noted that polyketide synthases and the fatty acid synthases use decarboxylative Claisen condensation for the C–C bond formation. Instead of condensing two molecules of acetyl-CoA, they condense acetyl-CoA with malonyl-CoA, releasing CO2 during the reaction. This release of CO2 serves as the driving force to catalyze the condensation reaction. The malonyl-CoA is synthesized from acetyl-CoA via ATP-dependent carboxylation by acetyl-CoA carboxylase. The condensation of two molecules of acetyl-CoA by polyketide synthases and fatty acid synthases, therefore, uses one molecule of ATP. The archaeal tripartite complex, on the other hand, saves one molecule of ATP by coupling the endergonic condensation of two molecules of acetyl-CoA directly to the exergonic formation of HMG-CoA.
Although the presented tripartite complex is universal to most archaea, it does not appear to be essential for acetyl-CoA condensation reactions in some bacteria and, in particular, eukaryotes, which completely lack a DUF35 scaffolding protein. However, in bacteria and eukaryotes, the demand for isoprenoids is generally lower compared with archaea, which use isoprenoids as a major component of membrane lipids. It remains to be seen in bacteria and eukaryotes whether lone-standing enzymes are sufficient to sustain isoprenoid production, or whether other mechanisms might be operating that help to overcome the unfavorable condensation reaction (e.g., high acetyl-CoA pools and compartmentalization).
Dueber et al. (29) found that channeling by artificial connection of E. coli thiolase AtoB with the HMGCS and HMGCR from Saccharomyces cerevisiae increased the production of mevalonate over lone-standing enzymes in E. coli about 77-fold. Our discovery of a naturally evolved channeling strategy in archaea harbors great potential to further improve the heterologous production of mevalonate in biotechnologically relevant strains.
Materials and Methods
Cultivation and Native Purification of Proteins.
M. thermolithotrophicus (DSM 2095) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen and was grown at 65 °C as described in ref. 30 and SI Appendix. The methods for purification of the thiolase/HMGCS complex are provided in SI Appendix, SI Materials and Methods.
Enzymatic Activity Assay of the Thiolase/HMGCS Complex.
The assay coupled with HMGCR was carried out with a Cary-4000 UV-visible spectrometer (Agilent) at 60 °C by following NADPH consumption at 340 nm using 10-mm quartz cuvettes (Hellma). To measure the activity of the fractions obtained in the purification steps, the assay solution contained 100 mM Hepes-KOH pH 7.5, 2 mM acetyl-CoA, 450 µM NADPH, and 2 µM HMGCR. Kinetic parameters of the purified thiolase/HMGCS complex were determined at 60 °C using the assay solution containing 500 mM potassium phosphate buffer pH 7.5, 400 µM NADPH, 2 µM HMGCR, 4.5 µg/mL thiolase/HMGCS complex, and varying concentrations of acetyl-CoA. The liquid chromatography (LC)-MS experiments were done with the Agilent 6550 iFunnel Q-TOF LC/MS system equipped with an electrospray ionization source set to positive ionization mode. Compounds were separated on an RP-18 column (50 × 2.1 mm, particle size 1.7 µm, Kinetex XB-C18; Phenomenex) using a mobile phase system composed of 50 mM ammonium formate pH 8.1 and methanol. Assays contained 100 mM Hepes-KOH pH 7.5, 0.1 mg/mL thiolase/HMGCS complex, and 2 mM acetyl-CoA, and, in the coupled assay, additionally contained 200 µM NADPH and 1.35 µM HMGCR. Samples were quenched with 5% formic acid (final concentration) and centrifuged for 5 min at 17,000 × g to remove precipitated proteins, and then the supernatant was analyzed by LC-MS. For this assay, the thiolase/HMGCS complex stored for about 2 wk at −80 °C was used. The activity of the stored preparation was 15% of that of the fresh sample. Compounds in the assay were quantified using external standard curves prepared in the same buffer system (SI Appendix, Fig. S13).
Crystallization of the Thiolase/HMGCS Complex from M. thermolithotrophicus.
Crystallization was done under air at 18 to 20 °C. The best-diffracting crystals were obtained using the sitting drop method (in a 24-well junior clover plate from Jena Bioscience). The crystallization reservoir contained 100 mM Tris⋅HCl pH 8.0, 25 to 28% (vol/vol) pentaerythritol ethoxylate (15/4 EO/OH, average molecular mass ∼797 Da), and 50 mM MgCl2. The crystallization drop contained 1 to 2 μL of the purified complex (50 to 60 mg/mL) and 1 μL of the mother liquor. The best-diffracting crystal was obtained in the presence of 10 mM Tb-Xo4 (14) (SI Appendix, Table S1). For phasing, the crystals obtained in the crystallization solution in the presence of 10 mM Tb-Xo4 were soaked with 100 mM Tb-Xo4. The substrate-soaking experiment was done using crystals, which were obtained in the absence of Tb-Xo4. The best data were obtained when the crystals were soaked for 1.5 min in the mother liquor supplemented with 100 mM acetyl-CoA. The thiolase/HMGCS crystals were also soaked with several concentrations of CoA, acetyl-CoA, and HMG-CoA; however, the majority of the crystals lost diffraction power, which probably came from conformational changes of the enzymes upon substrate binding.
Structural Analysis.
A first, intensive screening using crystals soaked in heavy atoms was performed on the BM30A beamline at the European Synchrotron Radiation Facility (ESRF). Soaking of the crystals in the mother liquor supplemented with 100 mM Tb-Xo4 (14) for 6 min gave the best result. The heavy-atom derivative exhibited a very strong anomalous signal without altering the diffraction quality. After an X-ray fluorescent scan, a dataset was collected at the Tb LIII edge on Proxima 2A beamline (SOLEIL synchrotron). Diffraction frames were integrated using XDS (31), and the integrated intensities were scaled using the CCP4 program SCALA (32). The structure was solved de novo by the single-wavelength anomalous diffraction method. First, we localized the 39 terbium sites using SHELX software (33) and phased with Autosol from the PHENIX package (34). The model building was completed with Buccaneer from the CCP4 package (35). Molecular replacements were done with PHASER using this first model to solve the structure of the native and the acyl-CoA thiolase/HMGCS complex. Models were completed and improved in COOT (36) before refinement with BUSTER (37) or PHENIX (34). These models were then optimized through iterative rounds of refinement and model building. Automated noncrystallography symmetry and translation–liberation–screw-rotation provided in the BUSTER and PHENIX program suites were applied. The final refinement rounds were carried out with the hydrogens in riding position and were omitted for the final deposition. Refinement statistics are summarized in SI Appendix, Table S1.
The figures were generated with PyMOL (Version 1.5; Schrödinger, LLC). The structural homologs search was done with the DALI server (38). Contact areas between the DUF35 protein and the two other enzymes were analyzed with the PISA server (39).
Supplementary Material
Acknowledgments
We thank the Proxima 2A staff at the SOLEIL synchrotron and the staffs from the beamlines ID29 and ID23-1 for their help and advice during data collection measurements. We are also grateful for the beam time on BM30A from the ESRF for intensive diffraction screening. This work was supported by grants from the Max Planck Society, and we acknowledge financial support from the Agence Nationale de la Recherche (Grant ANR-13-BS07-0007-02 Ln23).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.J.B. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (thiolase/HMGCS complex native, PDB ID code 6ET9; and thiolase/HMGCS soaked with acetyl-CoA, PDB ID code 6ESQ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718649115/-/DCSupplemental.
References
- 1.Guynn RW, Gelberg HJ, Veech RL. Equilibrium constants of the malate dehydrogenase, citrate synthase, citrate lyase, and acetyl coenzyme A hydrolysis reactions under physiological conditions. J Biol Chem. 1973;248:6957–6965. [PubMed] [Google Scholar]
- 2.Flamholz A, Noor E, Bar-Even A, Milo R. eQuilibrator—The biochemical thermodynamics calculator. Nucleic Acids Res. 2012;40:D770–D775. doi: 10.1093/nar/gkr874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Minteer S. Krebs cycle metabolon: Structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew Chem Int Ed Engl. 2015;54:1851–1854. doi: 10.1002/anie.201409336. [DOI] [PubMed] [Google Scholar]
- 4.Lindbladh C, et al. Preparation and kinetic characterization of a fusion protein of yeast mitochondrial citrate synthase and malate dehydrogenase. Biochemistry. 1994;33:11692–11698. doi: 10.1021/bi00205a004. [DOI] [PubMed] [Google Scholar]
- 5.Wheeldon I, et al. Substrate channelling as an approach to cascade reactions. Nat Chem. 2016;8:299–309. doi: 10.1038/nchem.2459. [DOI] [PubMed] [Google Scholar]
- 6.Shatalin K, Lebreton S, Rault-Leonardon M, Vélot C, Srere PA. Electrostatic channeling of oxaloacetate in a fusion protein of porcine citrate synthase and porcine mitochondrial malate dehydrogenase. Biochemistry. 1999;38:881–889. doi: 10.1021/bi982195h. [DOI] [PubMed] [Google Scholar]
- 7.Lan EI, Liao JC. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc Natl Acad Sci USA. 2012;109:6018–6023. doi: 10.1073/pnas.1200074109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heath RJ, Rock CO. The Claisen condensation in biology. Nat Prod Rep. 2002;19:581–596. doi: 10.1039/b110221b. [DOI] [PubMed] [Google Scholar]
- 9.Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA. 2000;97:13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner T, Wegner CE, Kahnt J, Ermler U, Shima S. Phylogenetic and structural comparisons of the three types of methyl-coenzyme M reductase from Methanococcales and Methanobacteriales. J Bacteriol. 2017;199:e00197-17. doi: 10.1128/JB.00197-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber H, Thomm M, König H, Thies G, Stetter KO. Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch Microbiol. 1982;132:47–50. [Google Scholar]
- 13.Krishna SS, et al. The structure of SSO2064, the first representative of Pfam family PF01796, reveals a novel two-domain zinc-ribbon OB-fold architecture with a potential acyl-CoA-binding role. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1160–1166. doi: 10.1107/S1744309110002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engilberge S, et al. Crystallophore: A versatile lanthanide complex for protein crystallography combining nucleating effects, phasing properties, and luminescence. Chem Sci. 2017;8:5909–5917. doi: 10.1039/c7sc00758b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modis Y, Wierenga RK. A biosynthetic thiolase in complex with a reaction intermediate: The crystal structure provides new insights into the catalytic mechanism. Structure. 1999;7:1279–1290. doi: 10.1016/s0969-2126(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 16.Harijan RK, et al. Crystal structures of SCP2-thiolases of Trypanosomatidae, human pathogens causing widespread tropical diseases: The importance for catalysis of the cysteine of the unique HDCF loop. Biochem J. 2013;455:119–130. doi: 10.1042/BJ20130669. [DOI] [PubMed] [Google Scholar]
- 17.Harijan RK, et al. The SCP2-thiolase-like protein (SLP) of Trypanosoma brucei is an enzyme involved in lipid metabolism. Proteins. 2016;84:1075–1096. doi: 10.1002/prot.25054. [DOI] [PubMed] [Google Scholar]
- 18.Campobasso N, et al. Staphylococcus aureus 3-hydroxy-3-methylglutaryl-CoA synthase: Crystal structure and mechanism. J Biol Chem. 2004;279:44883–44888. doi: 10.1074/jbc.M407882200. [DOI] [PubMed] [Google Scholar]
- 19.Shafqat N, Turnbull A, Zschocke J, Oppermann U, Yue WW. Crystal structures of human HMG-CoA synthase isoforms provide insights into inherited ketogenesis disorders and inhibitor design. J Mol Biol. 2010;398:497–506. doi: 10.1016/j.jmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Kühner S, et al. Substrate-dependent regulation of anaerobic degradation pathways for toluene and ethylbenzene in a denitrifying bacterium, strain EbN1. J Bacteriol. 2005;187:1493–1503. doi: 10.1128/JB.187.4.1493-1503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuthner B, Heider J. Anaerobic toluene catabolism of Thauera aromatica: The bbs operon codes for enzymes of beta oxidation of the intermediate benzylsuccinate. J Bacteriol. 2000;182:272–277. doi: 10.1128/jb.182.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murdoch RW, Hay AG. Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology. 2013;159:621–632. doi: 10.1099/mic.0.062273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanNice JC, Skaff DA, Wyckoff GJ, Miziorko HM. Expression in Haloferax volcanii of 3-hydroxy-3-methylglutaryl coenzyme A synthase facilitates isolation and characterization of the active form of a key enzyme required for polyisoprenoid cell membrane biosynthesis in halophilic archaea. J Bacteriol. 2013;195:3854–3862. doi: 10.1128/JB.00485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steussy CN, et al. X-ray crystal structures of HMG-CoA synthase from Enterococcus faecalis and a complex with its second substrate/inhibitor acetoacetyl-CoA. Biochemistry. 2005;44:14256–14267. doi: 10.1021/bi051487x. [DOI] [PubMed] [Google Scholar]
- 25.Lowe DM, Tubbs PK. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985;227:591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou J, et al. Haloarchaeal-type β-ketothiolases involved in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthesis in Haloferax mediterranei. Appl Environ Microbiol. 2013;79:5104–5111. doi: 10.1128/AEM.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa M, Tsuchiya D, Oyama T, Tsunaka Y, Morikawa K. Structural basis for channelling mechanism of a fatty acid β-oxidation multienzyme complex. EMBO J. 2004;23:2745–2754. doi: 10.1038/sj.emboj.7600298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S, Tsai SC. The type I fatty acid and polyketide synthases: A tale of two megasynthases. Nat Prod Rep. 2007;24:1041–1072. doi: 10.1039/b603600g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dueber JE, et al. Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol. 2009;27:753–759. doi: 10.1038/nbt.1557. [DOI] [PubMed] [Google Scholar]
- 30.Wagner T, Koch J, Ermler U, Shima S. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science. 2017;357:699–703. doi: 10.1126/science.aan0425. [DOI] [PubMed] [Google Scholar]
- 31.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheldrick GM. Experimental phasing with SHELXC/D/E: Combining chain tracing with density modification. Acta Crystallogr D Biol Crystallogr. 2010;66:479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afonine PV, et al. phenix.model_vs_data: A high-level tool for the calculation of crystallographic model and data statistics. J Appl Crystallogr. 2010;43:669–676. doi: 10.1107/S0021889810015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 36.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bricogne G, et al. 2016 BUSTER (Global Phasing Ltd., Cambridge, UK), Version 2.10.3. [Google Scholar]
- 38.Holm L, Laakso LM. Dali server update. Nucleic Acids Res. 2016;44:W351–W355. doi: 10.1093/nar/gkw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.