Significance
Ketamine is an NMDA receptor (NMDAR) antagonist that alleviates depressive symptoms in patients with treatment-resistant depression within hours of administering a single dose. However, psychoactive side effects limit its use. Understanding the mechanism underlying ketamine’s antidepressant effect is crucial in identifying targets for novel therapeutics with fewer side effects. Here, we report ketamine enhances excitability of pyramidal cells indirectly by reducing synaptic GABAergic inhibition, thus causing disinhibition. Additionally, we investigated whether other NMDAR antagonists and the muscarinic acetylcholine receptor antagonist scopolamine shared this disinhibition mechanism. Our results show that only those antagonists with antidepressant efficacy in humans disinhibit pyramidal cells at a clinically relevant concentration, supporting the concept that disinhibition is likely involved in the antidepressant effect of these antagonists.
Keywords: excitation/inhibition balance, major depression, disinhibition, GABAergic transmission, hippocampal networks
Abstract
Low-dose ketamine, an open-channel N-methyl d-aspartate receptor (NMDAR) antagonist, mediates rapid antidepressant effects in humans that are mimicked in preclinical rodent models. Disinhibition of pyramidal cells via decreased output of fast-spiking GABAergic interneurons has been proposed as a key mechanism that triggers the antidepressant response. Unfortunately, to date, disinhibition has not been directly demonstrated. Furthermore, whether disinhibition is a common mechanism shared among other antagonists with rapid antidepressant properties in humans has not been investigated. Using in vitro electrophysiology in acute slices of dorsal hippocampus from adult male Sprague–Dawley rats, we examined the immediate effects of a clinically relevant concentration of ketamine to directly test the disinhibition hypothesis. As a mechanistic comparison, we also tested the effects of the glycine site NMDAR partial agonist/antagonist GLYX-13 (rapastinel), the GluN2B subunit-selective NMDAR antagonist Ro 25-6981, and the muscarinic acetylcholine receptor (mAChR) antagonist scopolamine. Low-dose ketamine, GLYX-13, and scopolamine reduced inhibitory input onto pyramidal cells and increased synaptically driven pyramidal cell excitability measured at the single-cell and population levels. Conversely, Ro 25-6981 increased the strength of inhibitory transmission and did not change pyramidal cell excitability. These results show a decrease in the inhibition/excitation balance that supports disinhibition as a common mechanism shared among those antagonists with rapid antidepressant properties. These data suggest that pyramidal cell disinhibition downstream of NMDAR antagonism could serve as a possible biomarker for the efficacy of rapid antidepressant therapy.
Ketamine, an open-channel N-methyl d-aspartate receptor (NMDAR) antagonist, has provided a new outlook for treatment-resistant major depressive disorder (MDD) due to its rapid beneficial effects occurring within 2–3 h following i.v. administration (1, 2). Unwanted psychoactive side effects and the abuse potential of ketamine have prompted numerous studies focused on identifying key cellular mechanisms in hippocampus and cortex that are responsible for ketamine’s antidepressant effects and could serve as alternate therapeutic targets.
The “disinhibition hypothesis” posits that rapid NMDAR antagonism on fast-spiking GABAergic inhibitory interneurons, particularly the parvalbumin basket cells (PV BCs), reduces inhibitory input onto pyramidal cells, indirectly increasing their excitability (3–6). This pyramidal cell disinhibition is thought to trigger signaling pathways that help reestablish synaptic communication in brain regions negatively impacted in depression, such as hippocampus and prefrontal cortex (PFC). Published studies from rodent models report that increased phospho-mTOR, BDNF, excitatory transmission mediated by glutamatergic AMPA receptors (AMPARs), and dendritic spine density occur hours after ketamine is metabolized (3–9). The disinhibition hypothesis receives support from early studies showing increased action potential (AP) firing of presumed pyramidal cells recorded in PFC in vivo within 10 min of i.p. ketamine administration concurrent with elevated extracellular glutamate (10, 11). However, other published reports challenge this view (12–15), and implicate tonically active NMDARs, such as those containing the GluN2B subunit, on pyramidal cells and decreased eEF2 signaling, all measured within a time frame well after ketamine’s disappearance from brain (13, 16–18). More recent studies suggest that active ketamine metabolites, specifically (2R, 6R)-hydroxynorketamine (HNK), help mediate the antidepressant effects of ketamine via an AMPAR- or NMDAR-dependent mechanism (19, 20). These metabolites may be responsible for the longer lasting antidepressant response of ketamine compared with other investigational antidepressants, such as the NMDAR partial agonist/antagonist rapastinel (GLYX-13) (21). Although these cellular mechanisms are not agreed upon, studies are in consensus that increased BDNF and AMPAR transmission are important for the antidepressant effects of ketamine (9, 16–18, 22). The possibility remains that all of these identified mechanisms work in concert to mediate the antidepressant effects of ketamine.
The disinhibition hypothesis has yet to be rigorously tested to provide additional support either for or against this concept. If disinhibition is a key mechanism, then it should be shared by other NMDAR antagonists with rapid antidepressant efficacy, as well by the muscarinic actelycholine receptor (mAChR) scopolamine, which has rapid antidepressant responses in both humans and rodents (23–29). Here, using clinically relevant concentrations and a seconds-to-minutes time frame consistent with the rapid effects occurring in vivo when i.v. ketamine reaches brain, we tested whether ketamine and other antagonists rapidly shift the inhibition/excitation (I/E) balance toward excitation through disinhibition of CA1 pyramidal cells. We report that antagonists with significant antidepressant efficacy in humans share the ability to reduce inhibitory input onto pyramidal cells and disinhibit them within minutes.
Results
Ketamine Reduces Inhibitory and Excitatory Input and Disinhibits CA1 Pyramidal Cells.
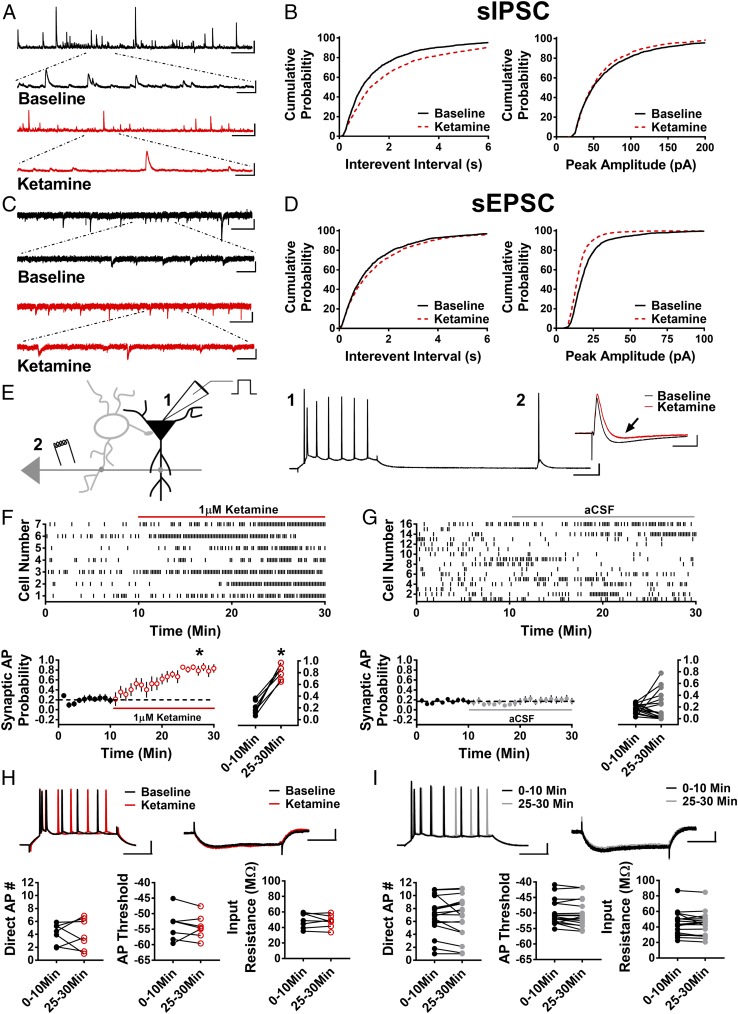
If NMDARs on spontaneously active GABAergic interneurons, such as PV BCs, are an initial target of low-dose ketamine, then a decrease in spontaneous inhibitory postsynaptic current (sIPSC) frequency onto pyramidal cells should be observed during bath application of ketamine. Bath application was used in an attempt to mimic the rapid distribution through brain during in vivo i.v. infusion as in humans, and we chose 1 μM ketamine as this is the estimated concentration reached in human brain during low-dose i.v. administration (30). We performed these experiments during pharmacological blockade of AMPARs to isolate sIPSCs originating from spontaneously active interneurons. Consistent with GABAergic interneurons being a possible target of ketamine, we observed a significant increase in the sIPSC interevent interval (IEI) [Fig. 1 A and B; n = 10 cells, Kolmogorov–Smirnov (KS) maximum vertical deviation (D value) = 0.13, P < 0.0001], reflecting a decrease in frequency, and a near-significant decrease in sIPSC amplitude (Fig. 1 A and B; n = 10 cells, KS D value = 0.05, P = 0.051). The decrease in sIPSC amplitude is likely a consequence of a bias toward removing larger amplitude sIPSCs generated by spontaneous APs in interneurons, such as PV BCs. To determine if this effect of ketamine is selective for inhibitory input, we next examined its effect on spontaneous excitatory postsynaptic currents (sEPSCs). Surprisingly, we found an increase in the sEPSC IEI (decreased frequency) (Fig. 1 C and D; n = 8 cells, KS D value = 0.07, P < 0.01) and a decrease in sEPSC amplitude (Fig. 1 C and D; n = 8 cells, KS D value = 0.2141, P < 0.0001) when cells were held at V = −50 mV [approximate chloride reversal potential (ECl−)] to isolate EPSCs (which reverse at V = 0 mV) from IPSCs, and in no synaptic blockers. However, because we are estimating ECl− at −50 mV, the true ECl− could be more depolarized in some cells, so sIPSCs would be recorded as inward currents. In this case, ketamine could be removing inward currents that are really GABAergic sIPSCs rather than glutamatergic sEPSCs, making it appear as if ketamine is decreasing sEPSC frequency. To test if ketamine actually decreases sEPSC frequency, we recorded sEPSCs in the presence of 100 μM picrotoxin to pharmacologically isolate them, and found that ketamine had no effect on sEPSC IEI (Fig. S1A; n = 5 cells, KS D value = 0.06, P = 0.64). These data indicate a selective effect of ketamine in decreasing the frequency of sIPSCs, with no effect on sEPSC frequency. Furthermore, this finding explains the significant increase in sEPSC IEI (decreased frequency; Fig. 1 C and D) measured in the absence of picrotoxin. However, in contrast to the effect on IEI, ketamine still elicits a significant decrease in sEPSC amplitude in the presence of picrotoxin (Fig. S1B; n = 5 cells, KS D value = 0.14, P < 0.01). This result is likely due to a fraction of the postsynaptic NMDARs being open at the holding potential of −50 mV, allowing for ketamine block, which would not occur when cells are at their typical resting membrane potential (Vrest ∼ −60 to −70 mV). Despite these possible caveats, the net circuit effects of ketamine and other rapid antidepressants can only be revealed when the circuit is kept intact.
Fig. 1.
Ketamine reduces sIPSCs and sEPSCs and disinhibits pyramidal cells. (A) Representative traces of sIPSCs in baseline conditions and in the presence of ketamine. (Scale bars: 100 pA and 5 s; Inset, 100 pA and 500 ms.) (B) Cumulative probability of the IEI showing a significant increase in ketamine (P < 0.0001, n = 10, 1,880 baseline events, 1,250 ketamine events), and there is a trend toward decreased peak amplitude of sIPSCs in the presence of ketamine (P = 0.051, n = 10, 1,880 baseline events, 1,250 ketamine events). (C) Representative traces of sEPSCs in baseline and ketamine conditions. (Scale bars: 20 pA and 3 s; Inset, 20 pA and 500 ms.) (D) Cumulative probability of increased IEI (P < 0.01, n = 8, 1,460 baseline events, 1,440 ketamine events) and decreased peak amplitude (P < 0.001, n = 8, 1,460 baseline events, 1,440 ketamine events) of sEPSCs in the presence of ketamine. (E) Schematic of whole-cell recordings of CA1 pyramidal cells where APs were elicited with a depolarizing current step (1) and electrical stimulation of Schaffer collaterals (2). (Scale bars: 20 mV and 100 ms.) (Inset) Subthreshold EPSP-IPSP sequence from cell 1. The baseline EPSP-IPSP (black) shows a decrease in the IPSP amplitude in the presence of ketamine (red, black arrow). (Scale bars: 2 mV and 50 ms.) (F) Raster plot (Top) and summary plots (Bottom) showing a significant increase in synaptic AP probability with bath application of ketamine (*P < 0.001, n = 7). (G) Raster plot (Top) and summary plots (Bottom) demonstrating no significant change in synaptic AP probability in control cells recorded over 30 min (n = 16). (H) No change in intrinsic properties (AP number generated via direct current injection, AP threshold, or input resistance) in the presence of ketamine. (Scale bars: 20 mV and 100 ms, 2 mV and 100 ms.) (I) No change in intrinsic properties in control recordings. (Scale bars: 20 mV and 100 ms, 2 mV and 100 ms.) All values are mean ± SEM.
The above findings led us to investigate whether the reduction in inhibitory input was significant enough to cause disinhibition of pyramidal cells. We used whole-cell current-clamp recordings of CA1 pyramidal cells and assessed the effect of ketamine on intrinsically driven and synaptically driven excitability (Fig. 1E). Using this approach, we are able to investigate whether ketamine has a direct effect on the membrane properties of CA1 pyramidal cells that could increase excitability and/or an indirect effect on pyramidal cell excitability through modulation of synaptic input onto these cells. A sequence of hyperpolarizing somatic current injections was used to assess possible effects of ketamine on input resistance. This was alternated with a depolarizing somatic current injection, which elicited APs to measure intrinsically driven excitability. Electrical stimulation via an electrode placed in CA1 stratum radiatum was used to activate synaptic drive onto CA1 pyramidal cells, and stimulation strength was set to elicit a subthreshold excitatory postsynaptic potential-inhibitory postsynaptic potential (EPSP-IPSP) sequence in >60% of trials. If ketamine causes disinhibition that is due to a decrease in the strength of inhibitory input onto pyramidal cells rather than to a direct effect on the membrane properties of pyramidal cells, then the same electrical synaptic stimulus strength should increase the probability that synaptic drive generates APs without changing the AP number elicited by the depolarizing somatic current injection. In essence, synaptically generated subthreshold EPSPs should be converted to suprathreshold EPSPs (i.e., APs) if synaptic inhibition is decreased by ketamine. Following a stable baseline of recording, 1 μM ketamine was bath-applied. Consistent with a disinhibition mechanism, ketamine significantly increased the synaptic AP probability (Fig. 1F; 0–10 min: 0.20 ± 0.04, 25–30 min: 0.82 ± 0.05; n = 7 cells; paired t test, P < 0.0001) in the absence of an increase in the number of APs evoked by direct current injection (Fig. 1H; 0–10 min: 4.12 ± 0.60, 25–30 min: 4.04 ± 0.92; n = 7 cells; paired t test, P = 0.91), AP threshold (Fig. 1H; 0–10 min: −53.78 ± 1.82 mV, 25–30 min: −54.21 ± 1.45 mV; n = 7 cells; paired t test, P = 0.67), or decrease in input resistance (Fig. 1H; 0–10 min: 49.05 ± 3.67 MΩ, 25–30 min: 47.74 ± 3.12 MΩ; n = 7 cells; paired t test, P = 0.53). Importantly, the ketamine-induced increase in synaptic AP probability was not observed in the presence of 100 μM picrotoxin (Fig. S2; n = 7 cells; paired t test, P = 0.44), indicating that the effect of ketamine depends on GABAergic transmission. Furthermore, in control experiments, synaptic AP probability remained stable over the 30-min recording period (Fig. 1G; 0–10 min: 0.17 ± 0.02, 25–30 min: 0.21 ± 0.06; n = 17 cells; paired t test, P = 0.55), and there were no changes in AP number generated by direct depolarizing current injection (Fig. 1I; 0–10 min: 6.86 ± 0.71, 25–30 min: 6.67 ± 0.78; n = 17 cells; paired t test, P = 0.51), AP threshold (Fig. 1I; 0–10 min: −49.9 ± 0.98 mV, 25–30 min: −50.26 ± 1.06 mV; n = 17 cells; paired t test, P = 0.20), or input resistance (Fig. 1I; 0–10 min: 45.06 ± 3.81 MΩ, 25–30 min: 43.52 ± 3.76 MΩ; n = 17 cells; paired t test, P = 0.20). These results show that the most immediate effect of ketamine, at a concentration that mimics what occurs in humans treated with i.v. ketamine, is to enhance pyramidal cell excitability by reducing synaptic inhibitory input. This allows excitatory synaptic input to drive pyramidal cells to fire APs independent of a change in intrinsic membrane properties.
GLYX-13 Mimics the Effect of Ketamine and Causes Disinhibition.
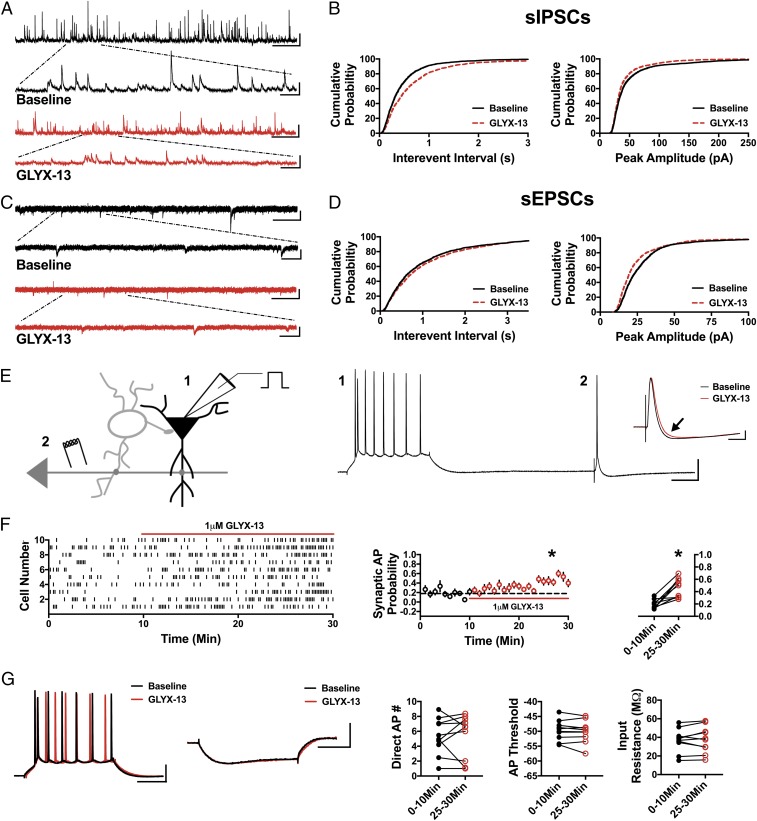
We reasoned that if disinhibition is a key mechanism in triggering downstream signaling pathways required for the antidepressant effects of ketamine, then other NMDAR antagonists known to mediate an antidepressant response should also cause disinhibition. Thus, we next tested the partial NMDAR agonist/antagonist GLYX-13, which blocks NMDAR-mediated current at 1 μM (31). In every measure, GLYX-13 (1 μM) mimicked the effect of ketamine. In the presence of GLYX-13, the sIPSC IEI was increased (Fig. 2 A and B; n = 6 cells, KS D value = 0.15, P < 0.0001) and the sIPSC amplitude was decreased (Fig. 2 A and B; n = 6 cells, KS D value = 0.10, P < 0.0001). Similarly, the sEPSC IEI increased (Fig. 2 C and D; n = 8 cells, KS D value = 0.06, P < 0.01) and sEPSC amplitude decreased (Fig. 2 C and D; n = 8 cells, KS D value = 0.13, P < 0.0001). Most importantly, GLYX-13 (1 μM) significantly increased synaptic AP probability (Fig. 2 E and F; 0–10 min: 0.19 ± 0.02, 25–30 min: 0.47 ± 0.05; n = 10 cells; paired t test, P < 0.001), while the AP number produced by direct somatic current injection was not altered (Fig. 2G; 0–10 min: 5.36 ± 0.77, 25–30 min: 5.45 ± 0.92; n = 10 cells; paired t test, P = 0.90), and neither was the AP threshold (Fig. 2G; 0–10 min: −49.7 ± 1.07 mV, 25–30 min: −50.05 ± 0.92 mV; n = 10 cells; paired t test, P = 0.36) or input resistance (Fig. 2G; 0–10 min: 36.82 ± 3.92 MΩ, 25–30 min: 37.76 ± 4.46 MΩ; n = 10 cells; paired t test, P = 0.53). These data indicate that even though GLYX-13 is a mechanistically different NMDAR antagonist from ketamine, it shares the same circuit effects as ketamine with a net effect of increased probability that excitatory synaptic input will drive pyramidal cells to fire APs through a disinhibition mechanism. Together, these data are consistent with the concept that disinhibition might be the initial trigger in the rapid antidepressant effect of NMDAR antagonists.
Fig. 2.
GLYX-13 mimics ketamine by decreasing sIPSCs and sEPSCs and disinhibiting pyramidal cells. (A) Representative traces demonstrating a decrease in sIPSC frequency and amplitude in the presence of GLYX-13. (Scale bars: 100 pA and 5 s; Inset, 100 pA and 500 ms.) (B) Cumulative probability shows a significant increase in sIPSC IEI in GLYX-13 (P < 0.0001, n = 6, 2,350 baseline events, 2,175 GLYX-13 events) and a decrease in peak amplitude with bath application of GLYX-13 (P < 0.0001, n = 6, 2,350 baseline events, 2,175 GLYX-13 events). (C) Representative traces of sEPSCs in baseline conditions and in the presence of GLYX-13. (Scale bars: 25 pA and 3 s; Inset, 25 pA and 500 ms.) (D) Cumulative probability of the IEI increasing (P < 0.01, n = 8, 1,900 baseline events, 1,700 GLYX-13 events) and peak amplitude of sEPSCs decreasing (P < 0.0001, n = 8, 1,900 baseline events, 1,700 GLYX-13 events) in the presence of GLYX-13. (E) Schematic of whole-cell recordings of CA1 pyramidal cells where APs were elicited with a depolarizing current step (1) and an electrical stimulation of CA3 synapses (2). (Scale bars: 20 mV and 100 ms.) (Inset) Subthreshold EPSP-IPSP sequence from cell 2. The baseline EPSP-IPSP (black) shows a decrease in the IPSP amplitude with bath application of GLYX-13 (red, black arrow). (Scale bars: 2 mV and 50 ms.) (F) Raster plot (Left) and summary plots (Right) showing a significant increase in synaptic AP probability with bath application of GLYX-13 (*P < 0.001, n = 10). (G) No significant change is observed in the AP number generated via direct current injection, AP threshold, or input resistance measured in from the depolarizing and hyperpolarizing current steps (n = 10). (Scale bars: 20 mV and 100 ms, 2 mV and 100 ms.) All values are mean ± SEM.
Ro 25-6981 Elicits the Opposite Effect of Ketamine and GLYX-13 on sIPSCs and No Effect on Excitability.
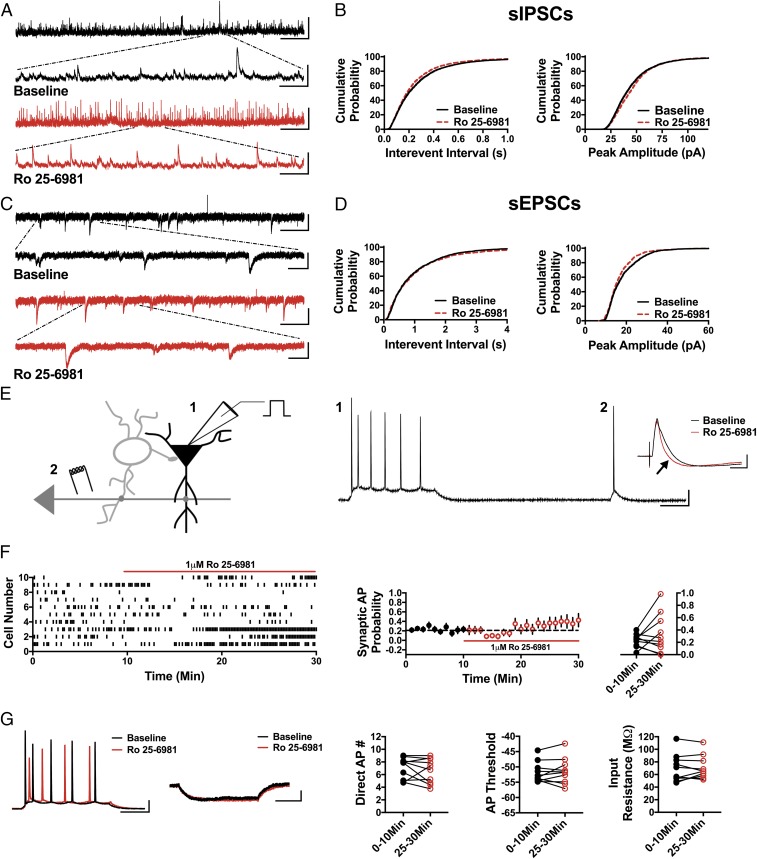
Compared with ketamine, GluN2B subunit-selective NMDAR antagonists have been much less effective at relieving depression symptoms in humans and in preclinical models (32). While some clinical trials have demonstrated antidepressant efficacy of GluN2B subunit-selective NMDAR antagonists, others report no significant improvement in depressive symptoms (33, 34). In preclinical studies, a rapid antidepressant-like effect of GluN2B subunit-selective NMDAR antagonists has been observed (8), but this effect is short-lived (17). Thus, if reducing GABAergic input and disinhibiting pyramidal cells is the initial trigger in the rapid antidepressant effects of NMDAR antagonists, then GluN2B subunit-selective antagonists should be less effective at eliciting these effects in accordance with their decreased antidepressant efficacy. We tested this concept using the GluN2B subunit-selective antagonist, Ro 25-6981. Indeed, 1 μM Ro 25-6981 elicited the opposite effect on sIPSC frequency and amplitude. As shown, sIPSC IEI was significantly decreased, reflecting an increase in frequency, in the presence of Ro 25-6981 (Fig. 3 A and B; n = 6 cells, KS D value = 0.06, P < 0.0001), and the sIPSC amplitude was significantly increased (Fig. 3 A and B; n = 6 cells, KS D value = 0.07, P < 0.0001). Ro 25-6981 had no significant effect on the sEPSC IEI (Fig. 3 C and D; n = 5 cells, KS D value = 0.04, P = 0.18) but did decrease sEPSC amplitude (Fig. 3 C and D; n = 5 cells, KS D value = 0.09, P < 0.0001). As anticipated, Ro 25-6981 fails to increase the probability of synaptically driven APs (Fig. 3 E and F; 0–10 min: 0.23 ± 0.03, 25–30 min: 0.34 ± 0.10; n = 10 cells; paired t test, P = 0.25), and there was no effect on the number of APs elicited by direct somatic depolarizing current injection (Fig. 3G; 0–10 min: 7.07 ± 0.53, 25–30 min: 6.62 ± 0.62; n = 10 cells; paired t test, P = 0.38), AP threshold (Fig. 3G; 0–10 min: −51.79 ± 1.06 mV, 25–30 min: −51.39 ± 1.35 mV; n = 10 cells; paired t test, P = 0.56), or input resistance (Fig. 3G; 0–10 min: 69.71 ± 6.94 MΩ, 25–30 min: 70.99 ± 5.9 MΩ; n = 10 cells; paired t test, P = 0.63). These results demonstrate that a GluN2B subunit-selective NMDAR antagonist does not mimic the circuit effects of either ketamine or GLYX-13.
Fig. 3.
Ro 25-6981 increases sIPSC frequency and amplitude, decreases sEPSC amplitude, and does not significantly alter synaptic AP probability. (A) Representative traces showing an increase in sIPSC frequency in the presence of Ro 25-6981. (Scale bars: 100 pA and 5 s; Inset, 100 pA and 500 ms.) (B) Cumulative probability shows a decrease in sIPSC IEI and increase in sIPSC amplitude during bath application of Ro 25-6981 (IEI: P < 0.0001, peak amplitude: P < 0.0001, n = 6, 3,900 baseline events, 4,400 Ro 25-6981 events). (C) Representative traces showing sEPSCs in baseline conditions and in the presence of Ro 25-6981. (Scale bars: 20 pA and 3 s; Inset, 20 pA and 500 ms.) (D) Cumulative probability shows no change in sEPSC IEI (P = 0.18, n = 5, 1,500 baseline events, 1,300 Ro 25-6981 events) but a significant decrease in sEPSC peak amplitude (P < 0.0001, n = 5, 1,500 baseline events, 1,300 Ro 25-6981 events). (E) Schematic of whole-cell recordings of CA1 pyramidal cells where APs were elicited with a depolarizing current step (1) and an electrical stimulation of CA3 synapses (2). (Scale bars: 20 mV and 100 ms.) (Inset) Representative traces in baseline (black) and Ro 25-6981 (red) from cell 5 in a raster plot demonstrating enhanced inhibition in the EPSP-IPSP sequence (black arrow). (Scale bars: 2 mV and 50 ms.) (F) Raster plot and summary plots displaying no significant change in synaptic AP probability in the presence of Ro 25-6981 (n = 10). (G). No change in the measured intrinsic proprieties (AP number generated via direct current injection, AP threshold, and input resistance) is observed (n = 10). (Scale bars: 20 mV and 100 ms, 2 mV and 100 ms.) All values are mean ± SEM.
Scopolamine Reduces sIPSCs, Increases sEPSC Amplitude, and Disinhibits Pyramidal Cells.
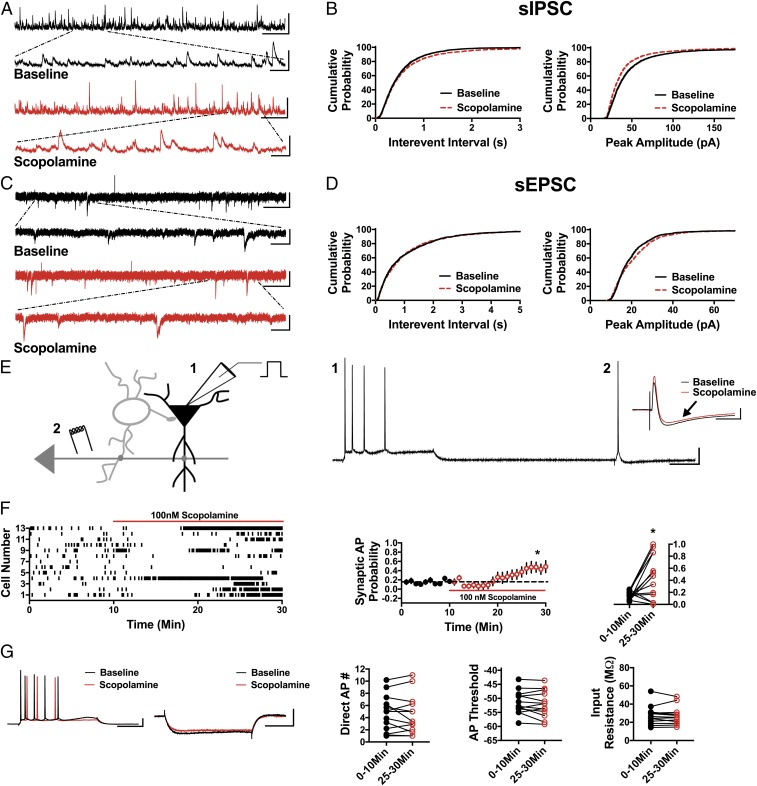
The mAChR antagonist scopolamine elicits a rapid antidepressant response in humans and in preclinical models similar to ketamine (23–29). Therefore, we next asked if scopolamine shares with ketamine the ability to reduce inhibitory input and elicit disinhibition of CA1 pyramidal cells. If so, this would strengthen the concept that disinhibition could be the initial trigger that stimulates the downstream signaling pathways required for the antidepressant behavioral response. Importantly, similar to ketamine, the sIPSC IEI was significantly increased in the presence of 100 nM scopolamine (Fig. 4 A and B; n = 7 cells, KS D value = 0.04, P < 0.05), and sIPSC amplitude was significantly decreased (Fig. 4 A and B; n = 7 cells, KS D value = 0.12, P < 0.0001). Scopolamine had no effect on sEPSC IEI (Fig. 4 C and D; n = 5 cells, KS D value = 0.04, P = 0.29) and increased sEPSC amplitude (Fig. 4 C and D; n = 5 cells, KS D value = 0.09, P < 0.0001). The increase in sEPSC amplitude in the presence of scopolamine potentially results from reducing the inhibitory shunt in CA1 dendrites that results from decreased GABAA receptor (GABAAR) activation. Because scopolamine does not target NMDARs like ketamine and GLYX-13, this indirect effect on sEPSC amplitude can be measured. In accord with the decrease in sIPSC frequency and amplitude, 100 nM scopolamine significantly increased the synaptically driven AP probability (Fig. 4 E and F; 0–10 min: 0.15 ± 0.02, 25–30 min: 0.43 ± 0.12; n = 13 cells; paired t test, P < 0.05) and, similar to ketamine, does this in the absence of a change in APs elicited by direct somatic depolarizing current injection (Fig. 4G; 0–10 min: 4.59 ± 0.78, 25–30 min: 4.45 ± 0.86; n = 13 cells; paired t test, P = 0.65) or a change in AP threshold or input resistance (Fig. 4G; threshold at 0–10 min: −49.05 ± 1.46 mV, 25–30 min: −49.5 ± 1.55 mV; n = 13 cells; paired t test, P = 0.25 and input resistance at 0–10 min: 31.51 ± 2.76 MΩ, 25–30 min: 30.06 ± 2.49 MΩ; n = 13 cells; paired t test, P = 0.18). Ketamine and scopolamine target completely different neurotransmitter receptors yet share the ability to decrease inhibition, increase synaptically driven excitability, and elicit a rapid antidepressant behavioral response. This provides support for the idea that disinhibition could be the initial mechanism that leads to activation of subsequent signaling pathways required for the antidepressant response.
Fig. 4.
Scopolamine reduces sIPSC frequency and amplitude, increases sEPSC amplitude, and disinhibits CA1 pyramidal cells. (A) Representative traces showing scopolamine decreases sIPSC frequency and peak amplitude compared with baseline. (Scale bars: 100 pA and 5 s; Inset, 100 pA and 500 ms.) (B) Cumulative probability showing a significant increase in the sIPSC IEI in the presence of scopolamine (P < 0.05, n = 7, 2,700 baseline events, 2,500 scopolamine events) and a decrease in sIPSC peak amplitude (P < 0.0001, n = 7, 2,700 baseline events, 2,500 scopolamine events). (C) Representative traces from baseline conditions and in the presence of scopolamine. (Scale bars: 20 pA and 3 s; Inset, 20 pA and 500 ms.) (D) Cumulative probability showing no change in sEPSC IEI (P = 0.29, n = 5, 1,300 baseline events, 1,300 scopolamine events) and increased peak amplitude with bath application of scopolamine (P < 0.0001, n = 5, 1,300 baseline events, 1,300 scopolamine events). (E) Schematic of whole-cell recordings of CA1 pyramidal cells showing the effects of scopolamine on intrinsically (1) and synaptically (2) driven APs. (Scale bars: 20 mV and 100 ms.) (Inset) Representative subthreshold EPSP-IPSP traces from cell 12 in the raster plot; compared with the baseline trace (black), scopolamine decreases the IPSP magnitude (red trace, black arrow). (Scale bars: 2 mV and 50 ms.) (F) Raster plot and summary plots showing a significant increase in synaptic AP probability in the presence of scopolamine (*P < 0.05, n = 13). (G) No change in the measured intrinsic properties (Direct AP number, AP threshold, and input resistance) is observed (n = 13). (Scale bars: 20 mV and 100 ms, 4 mV and 100 ms.) All values are mean ± SEM.
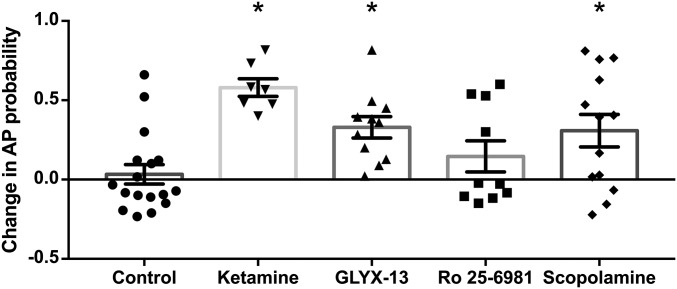
At the Population Level, Ketamine and Scopolamine, but Not GLYX-13 or Ro 25-6981, Cause Rapid Excitation of CA1 Pyramidal Cells.
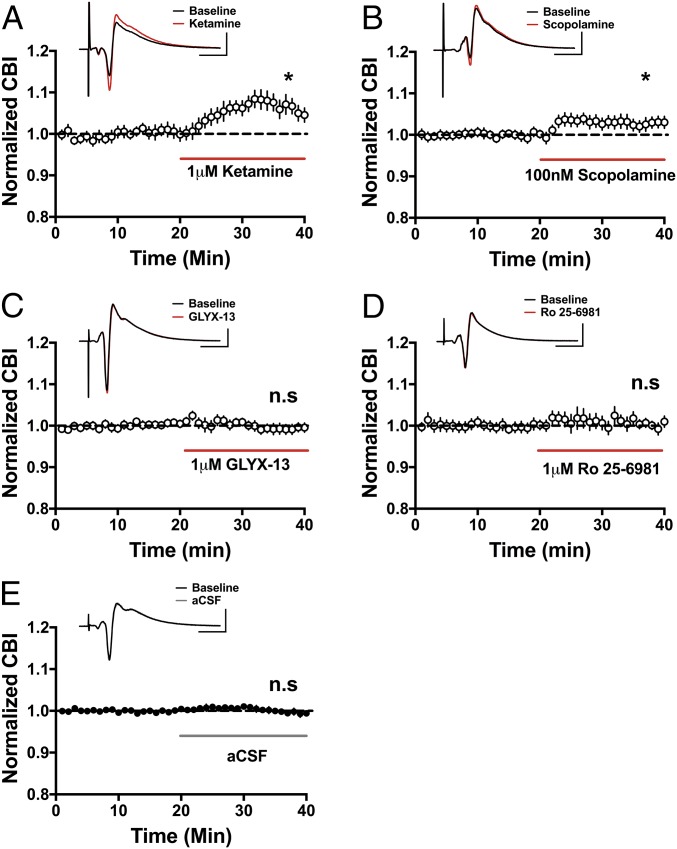
The data above indicate that ketamine, GLYX-13, and scopolamine disinhibit pyramidal cells through their effects on hippocampal circuits as each inhibitor elicited a significant increase in synaptic AP probability compared with the baseline response before each drug application (summarized in Fig. 5). To assess whether the same increase in synaptic driven excitability can be observed at the level of the population of pyramidal cells, we recorded extracellular population spikes (PSs) in CA1 stratum pyramidale evoked via Schaffer collateral stimulation as above. We chose to measure extracellular PSs to enable the assessment of AP activity in a population of neurons, as pyramidal neurons function in vivo in synchronized groups (35, 36). Consistent with the disinhibition hypothesis, ketamine enhanced the excitability of the CA1 pyramidal population measured using coastline burst index (CBI) (Fig. 6A; baseline: 0.99 ± 0.001, ketamine: 1.06 ± 0.014; n = 6 animals; paired t test, P < 0.05). The same was true for scopolamine (Fig. 6B; baseline: 1.00 ± 0.002, 100 nM scopolamine: 1.03 ± 0.01; n = 10 animals; paired t test, P < 0.05). This effect was dose-dependent as a lower dose (30 nM) increased synaptically driven AP probability measured in single cells (Fig. S3B), but this did not translate to the population (Fig. S3D). Similarly, even though GLYX-13 significantly increased synaptically driven AP probability in single-cell recordings (Fig. 2F), this increase in excitability did not reach statistical significance in the population (Fig. 6C; baseline: 1.00 ± 0.005, GLYX-13: 0.99 ± 0.006; n = 12 animals; paired t test, P = 0.25). Additionally, consistent with our findings at the single-cell level (Fig. 3F), Ro 25-6981 had no effect on excitability at the population level (Fig. 6D; baseline: 1.00 ± 0.005, Ro 25-6981: 1.00 ± 0.004; n = 8 animals; paired t test, P = 0.07). Importantly, in control experiments, no changes in excitability were observed when switching between two artificial cerebrospinal fluid (aCSF) solutions (Fig. 6E; first control solution: 1.00 ± 0.002, second control solution: 1.00 ± 0.006; n = 37 animals; paired t test, P = 0.57).
Fig. 5.
Summary of the effects of rapid antidepressants on synaptically driven APs. Ketamine, GLYX-13, and scopolamine significantly increase the synaptic AP probability compared with baseline (ketamine and GLYX-13, P < 0.001 and scopolamine, P < 0.05), indicating pyramidal cells are disinhibited. Conversely, the GluN2B subunit-selective NMDAR antagonist Ro 25-6981 does not alter synaptically driven pyramidal cell excitability. Replotted from Figs. 1–4. Asterisks indicate a significant difference from baseline and drug for each inhibitor. All values are mean ± SEM.
Fig. 6.
Ketamine and scopolamine, but not GLYX-13 or Ro 25-6981, increase excitability in a neural population. In extracellular recordings, bath application of ketamine (A; *P < 0.05, n = 6) and scopolamine (B; *P < 0.05, n = 10) significantly increases pyramidal cell excitability, while there is no effect with GLYX-13 (C, n = 12) or Ro 25-6981 (D, n = 7). n.s., not significant. In control experiments, switching between two containers of aCSF had no effect on excitability (E, n = 37). (Scale bars: 1 mV and 10 ms.) All values are mean ± SEM.
Discussion
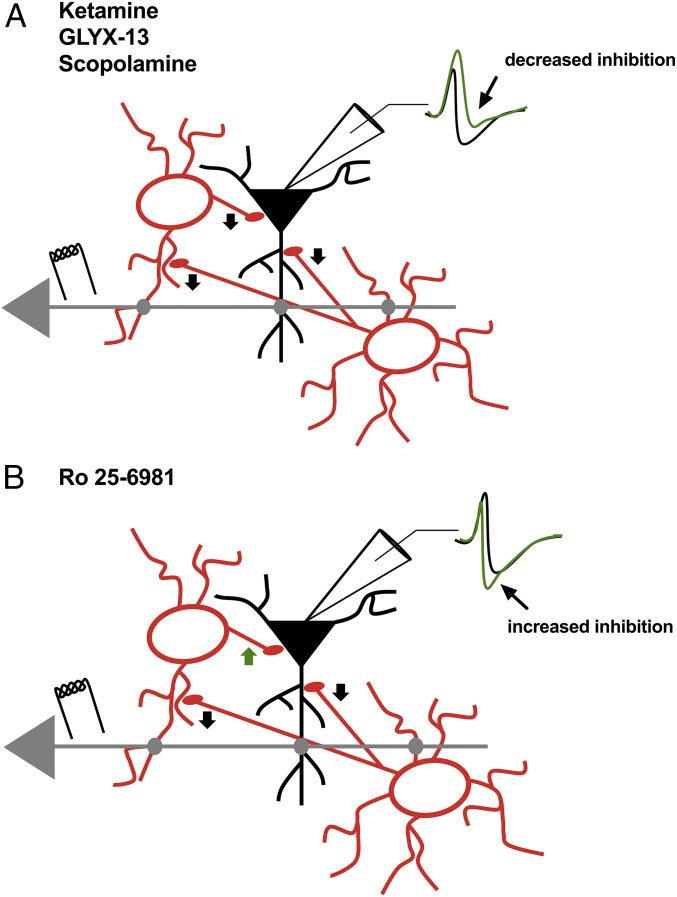
Here, we directly tested the disinhibition hypothesis put forth as a possible mechanism key to ketamine’s antidepressant behavioral response observed hours later (3–6). We find that low-dose ketamine rapidly decreased synaptic inhibition and increased the probability of synaptically driven AP generation, with no effect on intrinsic excitability. Disinhibition was also elicited by the partial NMDAR agonist GLYX-13 and the mAChR antagonist scopolamine (Fig. 7A), both of which have rapid antidepressant effects in humans and rodents (1, 2, 8, 13, 16, 17, 21, 23–28, 37). Given our results, it is tempting to relate the efficacy of pyramidal cell disinhibition to the clinical efficacy of these antagonists in mediating rapid antidepressant responses in both humans and rodent models. We observed variable amounts of disinhibition with these antagonists, with ketamine being the most reliable (summarized in Fig. 5); clinically, there is variable antidepressant efficacy, with ketamine being the most effective (38). Importantly, the GluN2B subunit-selective NMDAR antagonist Ro 25-6981 increases synaptic inhibition and does not disinhibit pyramidal neurons (Fig. 7B), potentially explaining the poor antidepressant efficacy of these subtype-selective NMDAR inhibitors (32–34).
Fig. 7.
Graphical representation of the disinhibition elicited by NMDAR and muscarinic receptor antagonists. (A) Ketamine, GLYX-13, and scopolamine reduce inhibitory input onto pyramidal cells (black arrow heads). This leads to a decrease in the IPSP (arrow, green trace) in the EPSP-IPSP sequence, which shifts the I/E balance toward excitation, leading to a greater probability for synaptic excitatory input to elicit APs. (B) Ro 25-6981 increases inhibitory input onto pyramidal cells, leading to an enhanced IPSP (arrow, green trace) in the EPSP-IPSP sequence and a shift in the I/E balance away from excitation. This is possibly due to only a subset of interneurons in hippocampus having a large number of GluN2B-containing NMDARs. Therefore, Ro 25-6981 may decrease output of a subset of interneurons (black arrow heads), which likely results in disinhibition of other interneurons and more inhibitory drive onto pyramidal cells (green arrowhead).
Given the numerous publications proposing the disinhibition hypothesis of ketamine’s action (3–6), it is striking that a systematic test of whether ketamine elicits disinhibition at low dose is lacking from the literature. A majority of in vitro studies used concentrations much higher (e.g., 20 μM) (16, 18, 39) than what is believed to occur in human brain (30), and electrophysiological measurements were made at time frames too late to capture the most immediate effects on neuronal excitability and synaptic circuits. Thus, this current study has filled this gap. Importantly, we employed 1 μM ketamine, a concentration on the low range of what is estimated in human brain, and made continuous measurements of neuronal and synaptic activity before, during, and after ketamine application in vitro, which revealed the shift in I/E balance and increased synaptically driven excitability. At higher concentrations, ketamine decreases excitatory transmission (40) and has no antidepressant-like effects in rodent models (7), so the concentration used is critical. Likewise, the concentrations of GLYX-13 and Ro 25-6981 used are also critical, as they likely will elicit variable effects on the synaptic network depending upon the magnitude of NMDAR inhibition achieved. The GLYX-13 dose is particularly complicated by its partial antagonist properties, whereby it can potentiate NMDAR currents at very low concentrations (31). Unfortunately, information regarding clinically relevant brain concentrations that elicit antidepressant responses in patients is lacking, limiting our ability to use the most appropriate drug concentrations in preclinical studies. Future studies are needed to determine the dose–response effects of both GLYX-13 and GluN2B subunit-selective NMDAR antagonists, such as Ro 25-6981, on synaptic circuits.
Although ketamine, GLYX-13, and scopolamine, significantly decreased sIPSC frequency, further investigation is necessary to determine if PV BCs are specifically targeted or if other interneurons are involved (3–6). Notably, ketamine’s antidepressant response is preserved in mice with NMDARs genetically removed from PV cells, suggesting other interneurons besides PV cells are potential targets (14). Interneurons expressing M1 and M2 muscarinic receptors have been implicated in the rapid antidepressant response of scopolamine (28), and particularly in somatostatin interneurons that express M1 receptors (26). Regardless of the precise interneuron subtypes targeted by ketamine, GLYX-13, and scopolamine, our results collectively suggest that disinhibition may be the most immediate trigger for the antidepressant response. However, other mechanisms, such as blocking NMDARs on pyramidal cells (13, 15, 16), are also likely involved, since once pyramidal cells are disinhibited, postsynaptic NMDARs will be available for blockade by ketamine and other NMDAR antagonists. This chain of events would lead to the desuppression of eEF2 kinase and increased protein translation (15, 16). In support of the concept that all of these mechanisms are required for the rapid antidepressant effects, memantine, a low-affinity NMDAR antagonist, only reliably causes disinhibition at a concentration (10 μM) above the therapeutic range (0.5–1 μM) (41, 42) and does not deactivate eEF2 kinase or elicit an antidepressant-like response in preclinical rodent models (43). Additionally, memantine has a longer half-life (42, 44) than ketamine, potentially leading to prolonged NMDAR antagonism and circuit effects, possibly explaining its lack of antidepressant efficacy in rodent models (43) and patients (45, 46). While our studies suggest disinhibition may be the initial trigger, deuterated ketamine, a nonhydrolyzable form that cannot be metabolized into HNK, failed to produce antidepressant-like effects in mice (19), indicating that additional mechanisms are clearly important for the antidepressant effect. Another component of the antidepressant effect may involve changes in other brain regions. We investigated the effects of rapid antidepressants on hippocampal synaptic circuits because morphological and functional deficits are observed in hippocampus in humans with MDD (47) and in preclinical models characterized by decreased dendritic complexity, spine density, neuronal excitability, and long-term potentiation (LTP) (48–51). PFC is also implicated in the pathophysiology of MDD (52, 53). Therefore, determining whether disinhibition also occurs in prefrontal cortical circuits will provide a better understanding of how these antagonists work throughout brain.
Our finding that the GluN2B subunit-selective antagonist Ro 25-6981 enhances the strength of inhibition through increasing sIPSC frequency likely provides a mechanistic explanation for the poor performance of this drug class in mediating rapid antidepressant effects in clinical studies and the shorter term effects in rodents (17, 32–34). Only a subset of interneurons has a large proportion of GluN2B-containing NMDARs at glutamatergic synapses in hippocampus and cortex (54), and certain subclasses provide synaptic inhibition onto other interneurons (55). Ro 25-6981 is likely targeting only those interneurons with GluN2B-containing NMDARs (i.e., those derived from the medial ganglionic eminence), which disinhibits other interneurons that lack this NMDAR subtype (i.e., those derived from the caudal ganglionic eminence), leading to the enhanced inhibitory tone onto pyramidal cells that we observe. This suggests that a more global, non–subunit-selective inhibition of NMDARs is necessary to shift the I/E balance toward excitation, which could contribute to the greater therapeutic benefit of ketamine if disinhibition is a key mechanism.
As previously mentioned, the ketamine metabolite HNK can elicit an antidepressant response, which may act through both NMDARs and AMPARs (19, 20). While there are conflicting reports on HNK’s ability to block NMDARs, it seems to enhance glutamatergic transmission mediated by AMPARs, which is required for the antidepressant-like effects in mice (19, 20, 43). Additionally, a new negative allosteric modulator of GABAARs, which reduces inhibition and shifts the I/E balance toward excitation, has a rapid antidepressant-like effect in rodent models (56, 57). Our findings indicate ketamine and GLYX-13 also enhance glutamatergic transmission via CA1 pyramidal cells firing more APs. This mechanism is also shared by scopolamine, but not by the GluN2B subunit-selective NMDAR antagonist Ro 25-6981. Thus, decreasing the I/E balance could potentially be shared by all rapid antidepressants and serve as a biomarker for the efficacy of rapid antidepressant therapy, and prospective therapeutics should be examined for their ability to disinhibit pyramidal cells.
Methods
All experimental procedures were approved by the University of Alabama at Birmingham’s Institutional Animal Care and Use Committee and were performed in accordance with NIH experimental guidelines.
Hippocampal Slice Preparation and Recordings.
Coronal slices (400 μm) were cut from dorsal hippocampus of young adult Sprague–Dawley rats (2–4 mo of age), and whole-cell recordings from pyramidal cells were attained as previously published (58, 59). In all whole-cell experiments, one cell was recorded per slice to avoid possible effects of residual drug. It was necessary to record sEPSCs near the IPSC reversal potential (Vh = −50 mV) and, in the absence of GABAAR antagonists, to keep the inhibitory and excitatory circuits intact. Note that at −50 mV, the voltage-dependent Mg2+ block will likely be removed from a majority of postsynaptic NMDARs, potentially preventing isolation of ketamine’s action solely on fast-spiking interneurons. sIPSCs were recorded (Vh = 0 mV, 10 μM nifedipine) in 10 μM 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX), an AMPAR antagonist, to enrich for sIPSCs from spontaneously active interneurons rather than from interneurons driven by excitatory transmission.
In current-clamp recordings (K-gluconate pipette solution), APs were synaptically evoked via electrical stimulation of Schaffer collaterals (platinum bipolar electrode; FHC) in CA1 stratum radiatum. Stimulus strength was set to elicit a subthreshold EPSP-IPSP sequence in the majority (>60%) of trials, and a possible increase in the probability that subthreshold EPSPs will be converted into synaptically driven APs was calculated before and during drug application. A sequence of depolarizing and hyperpolarizing somatic current injections (300-ms duration) was repeated throughout the recording and occurred 900 ms before electrical stimulation to evoke synaptic responses. First in the sequence, a direct somatic depolarizing current injection was used to assess potential changes in intrinsic excitability. Then, three hyperpolarizing somatic current injections were used to measure input resistance and the integrity of the recording over time.
In extracellular recordings, standard aCSF was used. Schaffer collaterals were stimulated in stratum radiatum, and a glass recording electrode measured the somatic EPSP and PS in CA1 stratum pyramidale. The CBI, which measures the length of the waveform, was used to analyze changes in excitability (60, 61). Baseline stimulus strength was set to 20–40% maximum of PS amplitude to facilitate observing an increase in excitability and to avoid a possible ceiling effect. Drugs were bath-applied following a 20-min stable baseline, and PSs were recorded for an additional 20 min in the continued presence of drug.
Statistics.
Data were acquired using Clampex 10.3 (pClamp; Molecular Devices) and analyzed offline in Clampfit 10.3 (Molecular Devices). For sEPSCs and sIPSCs, the nonparametric KS test was used to test for significance (62). For PS and synaptically driven AP experiments, a two-tailed paired t test was performed to compare changes in excitability between 10 min of baseline and the last 5 min of drug application. In whole-cell recordings, the n number represents a single cell and at least three animals were used per experiment. For extracellular recordings, the n number represents the number of animals; when more than one slice was used in experiments from a single animal, the data were averaged to represent that animal. In all datasets, significance was set to P < 0.05, and statistical analysis was performed using Prism 7 (GraphPad). All graphs were created in Prism 7. Additional details are provided in Supporting Information.
Supplementary Material
Acknowledgments
We thank Dr. Richard Shelton for helpful discussions related to this study. This work was supported by NIH NIMH Grant R56 MH107190 (to L.L.M.) and NIH NIMH Grant 1F31MH110096 (to A.J.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718883115/-/DCSupplemental.
References
- 1.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Duman RS. Pathophysiology of depression and innovative treatments: Remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18:pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Miller OH, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pozzi L, Pollak Dorocic I, Wang X, Carlén M, Meletis K. Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One. 2014;9:e83879, and erratum (2014) 9:e91486. doi: 10.1371/journal.pone.0083879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteggia LM, Gideons E, Kavalali ET. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry. 2013;73:1199–1203. doi: 10.1016/j.biopsych.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Nosyreva E, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanos P, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 21.Preskorn S, et al. GLYX-13 Clinical Study Group Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 22.Garcia LS, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: A randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: A randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarria A, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohleb ES, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voleti B, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witkin JM, et al. M1 and M2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351:448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 29.Martin AE, et al. Further evaluation of mechanisms associated with the antidepressant-like signature of scopolamine in mice. CNS Neurol Disord Drug Targets. 2017;16:492–500. doi: 10.2174/1871527316666170309142646. [DOI] [PubMed] [Google Scholar]
- 30.Hartvig P, et al. Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther. 1995;58:165–173. doi: 10.1016/0009-9236(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55:1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanacora G. What are we learning from early-phase clinical trials with glutamate targeting medications for the treatment of major depressive disorder. JAMA Psychiatry. 2016;73:651–652. doi: 10.1001/jamapsychiatry.2016.0780. [DOI] [PubMed] [Google Scholar]
- 33.Ibrahim L, et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–557. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preskorn SH, et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 35.Kamondi A, Acsády L, Wang XJ, Buzsáki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: Activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Buzsáki G. Hippocampal sharp waves: Their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 37.Burgdorf J, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaso BA, et al. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr Neuropharmacol. 2017;15:57–70. doi: 10.2174/1570159X14666160321123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016;9:ra123. doi: 10.1126/scisignal.aai7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology. 2014;86:273–281. doi: 10.1016/j.neuropharm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Povysheva NV, Johnson JW. Effects of memantine on the excitation-inhibition balance in prefrontal cortex. Neurobiol Dis. 2016;96:75–83. doi: 10.1016/j.nbd.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons CG, Stöffler A, Danysz W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–Too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci USA. 2014;111:8649–8654. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarate CA, Jr, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 46.Smith EG, et al. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: A randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2013;74:966–973. doi: 10.4088/JCP.12m08252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacQueen GM, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CS, Brager DH, Johnston D. Perisomatic changes in h-channels regulate depressive behaviors following chronic unpredictable stress. Mol Psychiatry. April 18, 2017 doi: 10.1038/mp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 50.Bredemann TM, McMahon LL. 17β Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014;42:77–88. doi: 10.1016/j.psyneuen.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 52.Baxter LR, Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 53.Wagner G, et al. Cortical inefficiency in patients with unipolar depression: An event-related FMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 54.Matta JA, et al. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci. 2013;16:1032–1041. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Front Neurosci. 2012;6:165. doi: 10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology. 2015;40:2499–2509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanos P, et al. A negative allosteric modulator for α5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro. 2017;4:ENEURO.0285-16.2017. doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vedder LC, Smith CC, Flannigan AE, McMahon LL. Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus. 2013;23:108–115. doi: 10.1002/hipo.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- 61.Roberson ED, et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36:93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.