Significance
Cell cycle progression is tightly controlled in healthy organisms and often perturbed in human diseases, including, most prominently, many forms of cancers. Cyclin-dependent protein kinases and their inhibitors, such as p19INK4d, regulate the different stages of the cell cycle. Here, we demonstrate how sequential phosphorylation of p19INK4d at two sites first destabilizes and then unfolds the N-terminal half of the protein, which dissociates its cyclin-dependent protein kinase-inhibitory complex and primes p19INK4d for cellular degradation. Our results define a structural mechanism by which phosphorylation-induced protein unfolding controls a key step in cell cycle progression.
Keywords: cell cycle, p19INK4d, protein unfolding, protein phosphorylation, NMR spectroscopy
Abstract
Cell cycle progression is tightly regulated by cyclin-dependent kinases (CDKs). The ankyrin-repeat protein p19INK4d functions as a key regulator of G1/S transition; however, its molecular mode of action is unknown. Here, we combine cell and structural biology methods to unravel the mechanism by which p19INK4d controls cell cycle progression. We delineate how the stepwise phosphorylation of p19INK4d Ser66 and Ser76 by cell cycle-independent (p38) and -dependent protein kinases (CDK1), respectively, leads to local unfolding of the three N-terminal ankyrin repeats of p19INK4d. This dissociates the CDK6–p19INK4d inhibitory complex and, thereby, activates CDK6. CDK6 triggers entry into S-phase, whereas p19INK4d is ubiquitinated and degraded. Our findings reveal how signaling-dependent p19INK4d unfolding contributes to the irreversibility of G1/S transition.
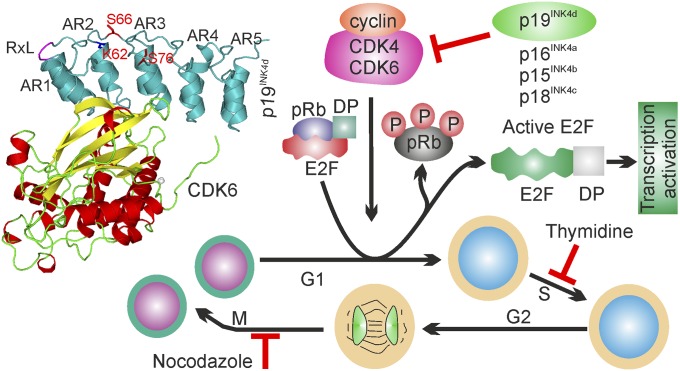
Cell cycle progression from the G1 to S-phase is tightly coupled to the transcriptional control of genes involved in growth and DNA replication (1). In mammalian cells, G1/S transition is primarily mediated by the E2F family of transcription factors (2). E2F proteins E2F1 to E2F8 form heterodimeric E2 promoter-binding–protein-dimerization partner complexes (E2F–DP) with members of the distantly related DP family of proteins (3). Most E2F proteins harbor dedicated protein binding domains that interact with E2F targets upon phosphorylation (4) to form inactive, ternary E2F–DP complexes (5) that stall cells in G1. Such cascades also prevent cells from replicating damaged DNA (6). To successfully enter S-phase, cyclin-dependent protein kinase (CDK)–cyclin complexes (7) hyperphosphorylate (5) and disrupt the E2F–DP assemblies (8) and, in turn, activate E2F-dependent gene expression necessary for G1/S transition (Fig. 1). Therefore, CDKs, such as CDK4/6, play major roles in cell cycle progression. CDK4/6 are controlled via their regulatory subunits including D-type cyclins and specific cyclin-dependent kinase inhibitors (CKIs), of which two families are known. Specifically, CKIs belonging to the INK4 family of proteins (p16INK4a, p15INK4b, p18INK4c, and p19INK4d) (Fig. 1) inhibit CDK4 and CDK6, whereas CKIs of the Cip/Kip family (p21Cip1,WAF–1, p27Kip1, and p57Kip2) act on CDK2 and other CDKs (7, 9, 10). p19INK4d has distinct features compared with the other four members of the INK4 family; mRNA and protein levels accumulate during S-phase and sharply decline at the onset of G2. High amounts of p19INK4d inhibit CDK4/6-cyclin D activity and determine the length of G1, also ensuring the directionality of cell cycle progression (7). In exponentially growing U2OS cells, p19INK4d is found to be phosphorylated at two sites, tentatively assigned to Ser66 and Ser76 (11). Additional regulation by ubiquitination appears to be restricted to p19INK4d, thought to involve Lys62 and depend on CDK4 binding. Given that cellular concentrations of p19INK4d oscillate during the cell cycle, it has been proposed that regulated modification events at these sites determine the fate of intracellular p19INK4d (11).
Fig. 1.
Schematic showing the function of p19INK4d. (Left) Structure of the CDK6–p19INK4d complex (PDB ID code 1BLX). ARs of p19INK4d are presented as AR1–AR5, regulatory sites Ser66, Ser76, and Lys62 are shown red and the RxL motif in magenta. (Right) The signaling pathways regulated by the INK family members. Red blocks (┴) highlight the cell cycle arrest points used in this study.
In-cell NMR allows the study of proteins and their posttranslational modifications in cellular environments (12–14). Alternatively, purified isotope-labeled proteins of interest may be added directly to unlabeled, native cell lysates to study how endogenous cellular proteins, such as kinases, phosphatases, or proteases act on them (15, 16). Here, we adopted the latter approach to gain mechanistic insights into the phosphorylation behavior of full-length human p19INK4d and to interrogate structural and functional consequences of such modifications. We show that p19INK4d Ser66 and Ser76 are phosphorylated by cellular p38 and CDK1 in a strictly stepwise and cell cycle-dependent manner. In response, p19INK4d locally unfolds and dissociates from CDK6, which triggers Lys62 ubiquitination and, ultimately, leads to cellular p19INK4d degradation. Hence, we demonstrate how the combined use of cell biology and NMR spectroscopy enables insights into the molecular mechanisms that regulate p19INK4d activity during cell cycle progression.
Results
p19INK4d Phosphorylation at Ser66.
p19INK4d is an ankyrin-repeat (AR) protein that harbors five evenly spaced helix-turn-helix motifs: AR1 amino acids 9–29, AR2 amino acids 54–62, AR3 amino acids 77–95, AR4 amino acids 110–128, and AR5 amino acids 142–159 (Fig. 1). AR1 and AR2 mediate inhibitory CDK4/6 binding (17). Phosphorylation of Ser66 and Ser76 within the linker region connecting AR2 and AR3 is thought to regulate p19INK4d activity, but kinases that modify these sites have not been identified. We expressed and purified full-length human p19INK4d (residues 1–166) from Escherichia coli and added the recombinant protein to lysates that we prepared from various cultured cell lines (e.g., HeLa, U2OS, HEK-293 grown asynchronously, and from Drosophila melanogaster embryos). 32P-incorporation and autoradiography exposure confirmed phosphorylation by endogenous enzymes (Fig. S1A). To identify respective p19INK4d phosphorylation sites, we similarly prepared 15N-isotope–labeled protein that we added to corresponding lysates for 3 h, before we recorded 2D 1H–15N HSQC (heteronuclear single-quantum coherence) and 1D 31P NMR spectra on the resulting lysate mixtures. The NMR resonance assignment of p19INK4d has previously been reported (18) (Fig. S2F). Because NMR chemical shifts are sensitive indicators of residue-specific chemical environments, phosphorylation-induced chemical-shift changes conveniently identify the respective modification sites. One added benefit of detecting protein phosphorylation by NMR is that conformational rearrangements occurring in response to such modifications result in additional chemical-shift changes that are readily interpretable in structural terms as well. Incubation of p19INK4d in each of the four lysates resulted in the phosphorylation of p19INK4d, manifested by pronounced chemical-shift changes of Ser66 resonance signals (Fig. 2A, shown for HeLa cells lysate, and Fig. S1 B–D). Although residues close to Ser66 also displayed slight alterations in their cross-peak positions (red in Fig. 2A, Lower), other p19INK4d serines, including Ser76, were unaffected. These results indicated that all five ARs remained structurally intact upon Ser66 phosphorylation. In the 1D 31P NMR spectrum, phospho-Ser66 gave rise to a characteristic new resonance at −1 ppm, which was clearly offset from the bulk phosphate-buffer signal at 2 ppm (Fig. 2A, Inset). We confirmed the presence of a single protein-phosphate moiety by MALDI-TOF mass spectrometry (Fig. S2 A and B). To verify the identity of Ser66 as the primary phosphorylation site, we repeated these experiments with alanine-substituted, mutant p19INK4d (e.g., S66A). We detected neither 32P incorporation nor the appearance of the 31P-NMR resonance signal at −1 ppm. These data confirmed that endogenous kinases in lysates prepared from asynchronously growing mammalian cells, or from Drosophila embryos, phosphorylated p19INK4d at Ser66.
Fig. 2.
NMR-detected cell cycle-dependent phosphorylation and local unfolding of p19INK4d. (Upper) Overlays of 2D 1H–15N HSQCs of p19INK4d before (black) and after (red) incubation with (A) asynchronous cell lysate, (B) S-phase HeLa cell lysate, or (C) after incubation of the doubly phosphorylated protein by alkaline phosphatase. (Lower) Spectral changes upon incubation mapped on the p19INK4d structure. The macroscopic helix dipole moments of helices 4 and 6 are depicted by black arrows in A. Mapping of cross-peak on the p19INK4d structure in A and B: red, missing cross-peak; blue, no change; and gray, slight chemical shift or cannot say. Insets show the 1D 31P spectra identifying (A) one (−1 ppm), (B) two (−1 ppm and 4 ppm), and (C) no protein-bound phosphate. The sharp signal at 2 ppm originates from the phosphate buffer (asterisk).
Stepwise Phosphorylation of Ser66 and Ser76 Induces Local Unfolding.
Having preformed the previous set of experiments in lysates of asynchronously growing cells, we were unable to delineate cell cycle-specific contributions to the observed phosphorylation reaction. To our surprise, we also did not detect endogenous p19INK4d in our lysates (Fig. S2C, lane 1). Because the G1/S transition is tightly controlled by CDK4/6 and INK4, we speculated that the kinase targeting p19INK4d might only be active in a fraction of our collected cells, specifically those in S-phase. To test this hypothesis, we subjected HeLa cells to a double thymidine block to arrest them in S-phase (19). We detected abundant amounts of endogenous p19INK4d in these lysates (Fig. S2C, lane 2). Upon thymidine removal and G2 release, p19INK4d levels dropped below the detection limit within 6 h (Fig. S2C, lanes 3 and 4). These findings reaffirmed earlier studies with proliferating cells (20) that cellular p19INK4d concentrations were highest in S-phase and virtually absent in G1, thus consolidating the notion of a genuine oscillatory behavior.
Next, we sought to investigate p19INK4d modifications in lysates of S-phase–arrested cells. 32P incorporation and autoradiography exposure confirmed p19INK4d phosphorylation (Fig. S2D). The 1D 31P NMR experiments revealed the previously observed phospho-resonance at −1 ppm, plus a new signal at 4 ppm, suggesting the presence of a second modified residue under these conditions (Fig. 2B, Inset). MALDI-TOF mass spectrometry indeed confirmed phosphorylation of p19INK4d at two sites (Fig. S2 A and E). To also interrogate possible structural changes of doubly phosphorylated p19INK4d, we recorded 2D 1H–15N HSQC spectra on exogenously added, isotope-labeled protein in lysates of S-phase–arrested HeLa cells (Fig. 2B). Surprisingly, we found all p19INK4d residues of AR1, AR2, and AR3 at new peak positions, narrowly dispersed around 8 ppm along the proton dimension (Fig. 2B and Fig. S2F). Such NMR features are highly characteristic of unfolded protein states. In contrast, residues within AR4 and AR5 exhibited no chemical-shift changes, indicating that these ARs remained folded upon dual p19INK4d phosphorylation. Having established that Ser66 likely constituted one of the modified p19INK4d residues, we set out to identify the second phosphorylation site. To this end, we mutated Ser76 to alanine (S76A) and repeated the S-phase lysate NMR experiment. The 2D 1H–15N HSQC spectra showed that AR1–3 residues, along with those of AR4 and AR5, maintained their original cross-peak positions, hinting toward the structural preservation of all ARs (Fig. 3F and Fig. S2G). Moreover, we clearly detected Ser66 phosphorylation in S76A p19INK4d, suggesting that primary phosphorylation of this site was not affected in the mutant background. Taken together, these results led us to conclude that Ser76 constituted the second p19INK4d modification site in S-phase–arrested cell extracts, that phosphorylation of Ser66 was pursued independent of Ser76 modification, and that double phosphorylation of Ser66 and Ser76 resulted in local unfolding of ankyrin repeats AR1–3.
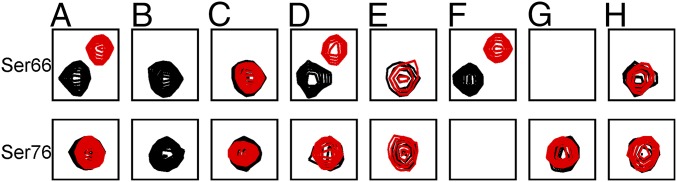
Fig. 3.
Phosphorylation status of Ser66 and Ser76 monitored by NMR spectroscopy. Sections of the overlaid 2D 1H–15N HSQC spectra of p19INK4d before (black) and after (red) incubation with cell lysate are depicted. (A) p19INK4d incubated in asynchronous HeLa cell lysate (Fig. 2A). (B) p19INK4d incubated in S-phase HeLa cell lysate (Fig. 2B). (C) Initially doubly phosphorylated p19INK4d after incubation with alkaline phosphatase (Fig. 2C). (D) Inhibition of Ser76 phosphorylation by the CDK1 inhibitor IV (p19INK4d incubated in S-phase HeLa cell lysate). (E) Inhibition of Ser66 phosphorylation by p38 inhibitor SB203580 (p19INK4d incubated in asynchronous HeLa cell lysate). (F) p19INK4d S76A variant incubated in S-phase HeLa cell lysate. (G) p19INK4d S66A variant incubated in S-phase HeLa cell lysate. (H) p19INK4d incubated with S-phase HeLa cell lysate in the presence of the p38 inhibitor SB203580. The missing cross-peaks in F and G are because of the substitution of Ser with Ala at position 76 and 66, respectively. The entire 2D spectrum for each of the eight experiments is depicted in Fig. 2 and Figs. S2–S4 and S7.
To confirm that the observed conformational changes were indeed due to phosphorylation, and to test whether local AR1–3 unfolding was reversible, we added a nonspecific alkaline phosphatase to Ser66-, Ser76-modified p19INK4d. Dephosphorylation resulted in the disappearance of phospho–protein resonances in 1D 31P NMR spectra (Fig. 2C, Inset) and recovered all p19INK4d resonances at their original cross-peak positions in 2D 1H–15N HSQC spectra (Figs. 2C and 3C). These experiments confirmed that: (i) double phosphorylation caused unfolding and (ii) that unfolding was fully reversible. They further excluded contributions by other posttranslational protein modifications, which may have remained undetected in lysate NMR experiments.
p38 and CDK1 Phosphorylate Ser66 and Ser76, Respectively.
We observed Ser66 phosphorylation in lysates of asynchronously growing cells, as well as in lysates of cells arrested in S-phase, which suggested that this modification is mediated by a kinase that is not stringently cell cycle-regulated. In contrast, we only detected Ser76 phosphorylation in S-phase lysates, arguing for an enzyme with cell cycle-specific activity. To identify the respective kinases that modified Ser66 and Ser76 of p19INK4d, we treated S-phase lysates with different kinase inhibitors. Compounds targeting CDK1, CDK4, or protein kinase A (PKA) did not affect Ser66 phosphorylation (Fig. S3 A–C). In contrast, selective inhibition of the constitutively expressed kinase p38 (21–23) abolished its modification (Fig. 3E and Fig. S3D). We further confirmed p38 selectivity and specificity in reconstituted kinase reactions with isolated wild-type and S66A-mutant p19INK4d (Fig. S3E). Furthermore, in an autoradiography experiment, p19INK4d S66A remained silent (Fig. S3E). p38 is a stress-activated MAP kinase protein family and we found Ser66 to get phosphorylated under various conditions not related to the cell cycle. Therefore, p38 may not be the only kinase that phosphorylates Ser66.
Following a similar approach, we found that different CDK1 inhibitors abolished Ser76 phosphorylation, whereas modification of Ser66 was unperturbed (Fig. 3D and Fig. S4 A and B). In earlier studies, CDK2 was found to phosphorylate Ser76 in response to DNA damage (24). The herein used CDK1 inhibitor IV inhibits CDK2 to some extent at elevated concentrations (25), but the specific CDK1 inhibitor III does not (26). Sole inhibition of CDK2 by inhibitor I (Fig. S4C) and, as expected, treating S-phase cell lysates with CDK4 or PKA inhibitors, showed no effects (Fig. S4 D and E). Together, these results suggested that, predominantly, CDK1 mediated Ser76 phosphorylation. Accordingly, we found that CDK1-cyclin B reconstituted kinase reactions modified wild-type but not S76A-mutant p19INK4d (Fig. S5A). CDK1, in complex with cyclins A, B, D, or E, functions as a key cell cycle regulator (27–29) and p19INK4d exhibits several features of a canonical CDK1 substrate (30). It harbors multiple serine or threonine residues followed by a proline (e.g., minimal Ser/Thr-Pro motifs, including Ser66, Ser76, and Thr141), and it contains a classic Arg–X–Leu CDK docking site, which all remain accessible when p19INK4d is bound to CDK4/6 (Fig. 1, Left). To further substantiate the role of CDK1 in Ser76 phosphorylation, we arrested HeLa cells in M-phase, in which CDK1/cyclin B activity is the highest, and prepared lysates to which we added 15N isotope-labeled p19INK4d. The 2D NMR experiments revealed local unfolding of AR1–3, manifested by spectral characteristic that were indistinguishable from results in S-phase–arrested cell extracts (Fig. S5 B–D). Indeed, NMR spectra of isotope-labeled p19INK4d in reconstituted mixtures of isolated p38α and CDK1/cyclin B showed identical features (Fig. S6). In summary, our combined results strongly suggested that p38 and CDK1 constitute the cellular kinases that phosphorylate Ser66 and Ser76 of p19INK4d, respectively.
p19INK4 Phosphorylation Is a Two Step Process.
Having identified p38 and CDK1 as likely kinase candidates for cellular p19INK4d phosphorylation, we set out to gain mechanistic insights into the Ser66 and Ser76 modification process. Specifically, we asked whether Ser76 phosphorylation required Ser66 modification as a preceding event. We had found that phosphorylation of Ser76 did not occur in the S66A-mutant background (Fig. 3G) or upon p38 inhibition in S-phase cell lysates (Fig. 3H and Fig. S7 A and B), thus raising the possibility that Ser66, Ser76 phosphorylation may constitute a hierarchical, sequential process. Protein phosphorylation sites are generally located in accessible loop and linker regions of folded substrates (30). In p19INK4d, Ser66 is surface-exposed, whereas Ser76 is part of the helical AR3 motif and rather occluded (Fig. 1, Left). As mentioned above, Ser66 phosphorylation led to several NMR chemical-shift changes of residues in its vicinity (Fig. 2A). We hypothesized that these reported on local destabilization of p19INK4d residues surrounding the modification site, probably via repulsive electrostatic interactions of the Ser66 phosphate moiety and the negative charge of the net dipole moments at the C-terminal ends of helices 4 and 6 in AR2 and AR3, respectively (Fig. 2A, and arrows on the corresponding p19INK4d structure). In agreement with this model, residues in these helices displayed the largest chemical-shift changes upon Ser66 phosphorylation (Fig. 2A). To assess the degree of helix destabilization in response to Ser66 modification, we performed proton-deuteron (H-D) backbone amide exchange experiments by NMR. AR3 and AR4 residues in wild-type p19INK4d exhibited protection factors above 105 (Fig. S7C) (31), which reduced significantly upon phosphorylation (red in Fig. S7 D–F). Surprisingly, even though p19INK4d residues within AR1, -4, and -5 did not show chemical-shift changes when Ser66 was phosphorylated, we measured greater solvent-amide proton exchange at these “remote” structural elements, synonymous with lower thermodynamic stabilities in the presence of modified Ser66. Based on these findings, we hypothesized that extended structural destabilization may provide the necessary access for Ser76 phosphorylation by CDK1 (Fig. 1). Local unfolding of AR1–3 may then occur due to additional breaking of hydrogen bonds, such as the one between Ser76 Oγ and V69 O′, for example.
Phosphorylation Dissociates the CDK6–p19INK4d Complex.
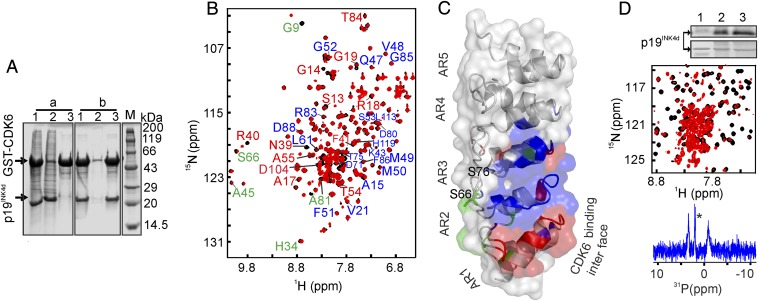
How does p19INK4d phosphorylation correlate with its function as a cell cycle regulator? The crystal structure of the inhibitory CDK6–p19INK4d complex reveals its molecular architecture (17) but it remained unclear how the assembly dissociates and whether p19INK4d modifications affected this process. Therefore, we examined p19INK4d phosphorylation in context of the assembled CDK6–p19INK4d complex. In vitro pull-down assays showed that unmodified p19INK4d specifically bound to GST–CDK6 (31), whereas doubly phosphorylated p19INK4d did not (Fig. 4A), likely because a folded AR1–2 interface is required for CDK6 binding (Fig. 1, Left). In a second experiment, we followed 15N-p19INK4d binding to unlabeled CDK6 by NMR spectroscopy. As we expected from the crystal structure of the complex, p19INK4d AR1 and AR2 residues exhibited pronounced chemical-shift changes upon addition of CDK6 (Fig. 4 B and C). No chemical-shift changes were observed when we added CDK6 to doubly phosphorylated p19INK4d, which confirmed the absence of binding and recapitulated our pull-down results. When we treated assembled CDK6–15N-p19INK4d with S-phase–arrested cell lysates, 2D NMR spectra revealed local unfolding of AR1–3, similar to the isolated protein upon Ser66 and Ser76 phosphorylation (Fig. 4D, Middle). Indeed, 1D 31P NMR spectra and radioisotope incorporation experiments confirmed the presence of both modifications (Fig. 4D, Bottom). We concluded that p19INK4d double phosphorylation and subsequent local unfolding dissociated the p19INK4d–CDK6 complex.
Fig. 4.
Functional implications of p19INK4d phosphorylation. (A) Pull-down assay of p19INK4d using GST–CDK6. The loading in a and b is: lane 1, input (GST–CDK6 beads + p19INK4d); lane 2, supernatant; and lane 3, pellet of pull down. In A, a is doubly phosphorylated p19INK4d, b is p19INK4d as positive control. M, protein molecular mass marker. (B) CDK6–p19INK4d complex formation. Overlaid 2D 1H–15N HSQC spectra of 15N-labeled p19INK4d before (black) and after addition of unlabeled CDK6 (red). Blue labels indicate residues of p19INK4d that interact with CDK6 via their side chains, as revealed by the crystal structure of the complex (17), but which do not influence the backbone chemical shift of p19INK4d. Red labels indicate the backbone chemical-shift changes upon complex formation both monitored by NMR and reported in the crystal structure. Green labels indicate additional chemical-shift changes observed only by NMR and not reported in the crystal structure. These residues are mapped on the structure of p19INK4d as shown in the C. (D, Top) Image showing autoradiography of: lane 1, untreated p19INK4d; lane 2, p19INK4d; and lane 3, CDK6–p19INK4d complex treated with S-phase cell extract. (Top) The autoradiogram and the corresponding Coomassie brilliant blue stained gel below. (Middle) The overlaid 2D 1H–15N HSQC spectra of 15N-labeled p19INK4d after the CDK6–p19INK4d complex incubated with extract of S-phase cells (red) and untreated p19INK4d (black). (Bottom) The respective 31P NMR spectrum. The asterisk indicates the phosphate buffer signal.
Ubiquitination Requires p19INK4d Unfolding.
Many proteins turn into E3 ubiquitin ligase substrates upon phosphorylation, causing them to be degraded by the ATP-dependent ubiquitin/proteasome system (32, 33). Here, we showed that p19INK4d phosphorylation at Ser66 and Ser76 caused local unfolding of AR1–3, which, in turn, released the protein from CDK6. What was the cellular fate of free doubly modified p19INK4d? We reasoned that the locally unfolded protein may become ubiquitinated and eventually degraded, which would explain the rapid disappearance of endogenous p19INK4d in lysates of cells released from S-phase arrest (Fig. S2C). Indeed, this drop of endogenous p19INK4d level upon thymidine removal and G2 release could be prevented by CDK1 inhibition during the S-phase (Fig. 5), confirming the coupling between Ser76 phosphorylation of p19INK4d and its cell cycle-dependent degradation.
Fig. 5.
Phosphorylation–dependent degradation of endogenous p19INK4d. Immunoblot of p19INK4d in HeLa cell extracts: lane 1, S-phase cells; lane 2, asynchronous cells; lane 3, S-phase cells and 6 h after thymidine release; lane 4, S-phase cells at 6 h after thymidine release in the presence of CDK1 inhibitor (Upper). Actin is shown as loading control (Lower).
In line with this model, we expected AR1–3 unfolding to also expose Lys62, a canonical ubiquitination site of p19INK4d. To test this hypothesis, we performed in vitro ubiquitination assays with S-phase–arrested cell lysates and prephosphorylated p19INK4d. We did not detect ubiquitination of unmodified or Ser66-phosphorylated protein. Only Ser66- and Ser76-phosphorylated p19INK4d were evidently modified (Fig. S8). As expected, neither S66A nor S76A-mutant p19INK4d was ubiquitinated.
In summary, these findings (Fig. 6) suggest that cellular ubiquitination of p19INK4d likely depends on its phosphorylation and concomitant structural state. In support of our hypothesis, we conclude that cell cycle-dependent, stepwise phosphorylation of Ser66 and Ser76 induced local unfolding of AR1–3, which, in turn, dissociated p19INK4d from CDK6 and exposed Lys62 for subsequent ubiquitination, which likely served as the signal for intracellular degradation.
Fig. 6.
Summary of the findings of this study, which occur in the S-phase of the cell cycle. Step 1: phosphorylation of p19INK4d by p38. Steps 2 and 3: phosphorylation of p19INK4d by CDK1 followed by unfolding of AR1–AR3 and dissociation of the CDK6–p19INK4d complex. Step 4: polyubiquitination of p19INK4d. Step 5: degradation of p19INK4d.
Discussion
To initiate G1/S transition, CDK4/6 phosphorylates trigger factors, such as the retinoblastoma Rb protein, which, in turn, release E2F transcription factors required for cell cycle progression (Fig. 1). Unmodified p19INK4d binds CDK4/6 and inhibits its function (34). Multisite phosphorylation of INK4 proteins, such as p19INK4d and p18INK4c in asynchronously growing cells, has been observed previously (11, 34) and characterized in vitro by Ser-to-Glu substitutions mimicking phosphorylation (35), but the functional consequences of these modifications remained enigmatic. Here, we established that phosphorylation of p19INK4d Ser66 by p38 kinase broadly destabilizes N-terminal ARs, which appears to constitute a necessary priming event for the stepwise modification of Ser76 by CDK1. Double phosphorylation induces local unfolding of p19INK4d AR1–3, which dissociates the inhibitory CDK4/6–p19INK4d complex. In turn, modified, free, and unfolded p19INK4d undergoes ubiquitination, which predestines the protein for cellular degradation. A schematic summary of this process is depicted in Fig. 6. One key feature of this model is the switch-like function that the cell cycle-dependent protein kinase CDK1 imposes on the network. Clearly, CDK1-mediated phosphorylation of p19INK4d Ser76 appears to determine the functional outcome of the entire regulatory hub. In the absence of CDK1 activity, and Ser76 phosphorylation, p19INK4d blocks cell cycle progression by binding to CDK4/6 and, thereby, inhibits their functions. In this regard, the cell cycle-independent, primary phosphorylation of p19INK4d Ser66 maintains the system in a poised state that is primed for activation by CDK1. Once CDK1 activity is triggered, p19INK4d Ser76 phosphorylation frees CDK4/6 for transcriptional activation, cumulating in cell cycle progression. At the same time, doubly phosphorylated p19INK4d undergoes ubiquitination and eventual degradation, which ensures the irreversibility of the process and, by proxy, the directionality of the cell cycle.
Maybe one of the most striking features of this regulatory network is its structural component. Whereas phosphorylation of the primary p19INK4d substrate site (Ser66) destabilizes structured ARs more globally and thereby “enables” secondary site (Ser76) modification, the actual phosphorylation event “executes” the functional reprogramming step, which is largely driven by a local loss-of-structure mechanism. Unfolding not only disrupts the CDK4/6–p19INK4d complex and, thereby, activates these kinases, it also exposes the previously buried p19INK4d Lys62 residue for ubiquitination, which initiates p19INK4d degradation and clearance. At this level of posttranslational modifications, differences between the four INK4 members are evident, which are not related to the cell cycle-dependent mRNA levels (20). P18INK4c declines much more slowly as cells advance through G1/S phase compared with p19INK4d. The major ubiquitin acceptors Lys46 and Lys112 are buried in p18INK4c ankyrin repeats AR2 and AR4, and this INK4 member lacks any S/T-P motif. Therefore, we speculate that the much shorter cellular half-life of p19INK4d results from the here disclosed mechanism of Lys62 exposure.
Differences in the thermodynamic stabilities of individual ARs in other AR-containing proteins were found to determine the folding pathways (31) of the NF-κB inhibitor IκBα (36), the Notch receptor (37), and tANK (38). However, these examples undergo structural rearrangements in the absence of posttranslational modifications. Phosphorylation-induced unfolding of p19INK4d is uniquely mediated by two sequential modification steps, which jointly dissolve the N-terminal half of this classically folded protein domain. To our knowledge, there exists only one other example of phosphorylation-mediated destruction of a fully structured protein entity, which is the KH1 domain of the K-homology splicing regulator protein KSRP (39). In that case, insertion of a negative charge at a single serine residue (Ser193) disrupts the globular KH1 fold and, in turn, exposes a linear sequence motif that confers nuclear translocalization of KSRP.
The S/T-P motif is conserved in the consensus sequence of ARs, which structurally well align, for example, the INK4 members (p19INK4d, p18INK4c, p16INK4a, p15INK4b) with GABPβ, myotrophin, and 53BP2 (18, 40). Ser66 is not conserved in any of the other homologs and is therefore unique as a priming site for p19INK4d phosphorylation. The transcription factor GABP β-subunit contains four ARs and it has been speculated that MAPK/ERK-mediated phosphorylation involves the S/T-P motifs in AR2 (Ser39-Pro40) and AR3 (Thr73-Pro74) (41), the latter corresponding to Ser76-Pro77 in p19INK4d. Along the same lines, among the four ARs in myotropin, only AR3 harbors such a motif at Thr70-Pro71, and its phosphorylation has been discussed during initiation of cardiac hypertrophy (42).
Within these structural homologs, only p19INK4d contains one further S/T-P motif at position Thr141-Pro142. This threonine was found to be phosphorylated by PKA during DNA damage response following, for example, UV irradiation or cisplatin treatment of cells (24). A putative priming site is Ser130 between AR5 and AR6, at a structural position homologous to Ser66. Destabilization of scaffold repeats AR5 and AR6 (31, 35) might cause the same unfolding of p19INK4d and genotoxic stress-induced CDK4/6 release, a hypothesis that has to be experimentally addressed in the future. These examples document that phosphorylation-induced unfolding of ARs, typically involved in protein–protein interactions, and as delineated in molecular detail here for p19INK4d, might represent an evolutionary conserved principle in cellular signaling.
Materials and Methods
Protein Expression and Purification.
p19INK4d wild-type and mutants were expressed in BL21(DE3) pLysS cells and purified as described previously (31). Additional details are provided in SI Materials and Methods.
Cell Lines and Synchronization.
Cervical carcinoma (HeLa), HEK–293 and human osteosarcoma (U2OS) cells were employed and synchronization was achieved by double incubation with thymidine (S-phase) or Nocodazole (M-phase). All details are described in SI Materials and Methods.
Protein Assays.
The kinase assay was performed by incubating cell lysate (0.5–1 mg/mL total protein concentration) of exponentially growing or synchronized cells in the presence of 32P ATP (5 µCi 32P-γ-ATP) and cAMP (5 µM) with 30 µg of p19INK4d for 3 h at 37 °C. Aliquots of the mixtures were analyzed by SDS/PAGE and labeled polypeptides were detected by autoradiography. Additional details, together with the in vitro kinase assay with isolated p38 or CDK1/cyclin B1, the CDK6-p19INK4d pull-down assay, and the p19INK4d ubiquitination assay are given in SI Materials and Methods.
NMR Spectroscopy Experiments.
The cells were lysed as mentioned above in the presence of kinase assay buffer, protease inhibitors, and phosphatase inhibitors. The lysate was collected by centrifugation. For the NMR spectroscopy experiment, lysate (15–20 mg/mL) was mixed with 30 µM of 15N-labeled p19INK4d or its mutants, 100 µM ATP, and 5 µM cAMP. This reaction mixture was incubated for 3 h at 37 °C. Subsequently, the reaction samples were dialyzed using a 3.5-kDa cut-off membrane in phosphate buffer (20 mM Na2HPO4, 25 mM NaCl, 25 mM KCl, and pH 7.4) for 17 h to remove the unincorporated ATP, small molecules, and to minimize buffer effects to facilitate a proper chemical shift analysis of NMR cross-peaks. These samples were also analyzed by MALDI-TOF mass spectrometry. Details about the acquisition of NMR experiments and the 1H/2H exchange detected by NMR are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Gunter Reuter for Drosophila melanogaster embryos; Ernest D. Laue and Wei Zhang for GST–CDK6; Stefan Gröger for NMR spectroscopy support; and Gary Sawers and René Keil for very helpful discussions. Autoradiography was performed in the Leibniz Institute of Plant Biochemistry Halle. This work has been supported by grants from the Deutsche Forschungsgemeinschaft (GRK 1026, SFB TRR102), the Bundesministeriums für Bildung und Forschung (ProNet–T3), and the European Fund for Regional Development by the European Union.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719774115/-/DCSupplemental.
References
- 1.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 2.Zwicker J, Liu N, Engeland K, Lucibello FC, Müller R. Cell cycle regulation of E2F site occupation in vivo. Science. 1996;271:1595–1597. doi: 10.1126/science.271.5255.1595. [DOI] [PubMed] [Google Scholar]
- 3.Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA. In vivo association of E2F and DP family proteins. Mol Cell Biol. 1995;15:2536–2546. doi: 10.1128/mcb.15.5.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Cho Y. Structural similarity between the pocket region of retinoblastoma tumour suppressor and the cyclin-box. Nat Struct Biol. 1997;4:390–395. doi: 10.1038/nsb0597-390. [DOI] [PubMed] [Google Scholar]
- 5.Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005;15:520–527. doi: 10.1016/j.gde.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Das SK, et al. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim Biophys Acta. 2005;1726:328–335. doi: 10.1016/j.bbagen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 9.Harper JW, Elledge SJ. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 10.Pines J. Cell cycle: Reaching for a role for the Cks proteins. Curr Biol. 1996;6:1399–1402. doi: 10.1016/s0960-9822(96)00741-5. [DOI] [PubMed] [Google Scholar]
- 11.Thullberg M, Bartek J, Lukas J. Ubiquitin/proteasome-mediated degradation of p19INK4d determines its periodic expression during the cell cycle. Oncogene. 2000;19:2870–2876. doi: 10.1038/sj.onc.1203579. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Selenko P. Cellular structural biology. Curr Opin Struct Biol. 2010;20:640–648. doi: 10.1016/j.sbi.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Selenko P, et al. In situ observation of protein phosphorylation by high-resolution NMR spectroscopy. Nat Struct Mol Biol. 2008;15:321–329. doi: 10.1038/nsmb.1395. [DOI] [PubMed] [Google Scholar]
- 14.Theillet FX, et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature. 2016;530:45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- 15.Theillet FX, et al. Site-specific NMR mapping and time-resolved monitoring of serine and threonine phosphorylation in reconstituted kinase reactions and mammalian cell extracts. Nat Protoc. 2013;8:1416–1432. doi: 10.1038/nprot.2013.083. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, et al. N-terminal phosphorylation of parathyroid hormone (PTH) abolishes its receptor activity. ACS Chem Biol. 2014;9:2465–2470. doi: 10.1021/cb5004515. [DOI] [PubMed] [Google Scholar]
- 17.Brotherton DH, et al. Crystal structure of the complex of the cyclin D-dependent kinase Cdk6 bound to the cell-cycle inhibitor p19INK4d. Nature. 1998;395:244–250. doi: 10.1038/26164. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner R, et al. Structure of human cyclin-dependent kinase inhibitor p19INK4d: Comparison to known ankyrin-repeat-containing structures and implications for the dysfunction of tumor suppressor p16INK4a. Structure. 1998;6:1279–1290. doi: 10.1016/s0969-2126(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 19.Whitfield ML, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forget A, et al. Differential post-transcriptional regulation of two Ink4 proteins, p18 Ink4c and p19 Ink4d. Cell Cycle. 2008;7:3737–3746. doi: 10.4161/cc.7.23.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 22.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 24.Marazita MC, et al. CDK2 and PKA mediated-sequential phosphorylation is critical for p19INK4d function in the DNA damage response. PLoS One. 2012;7:e35638. doi: 10.1371/journal.pone.0035638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassilev LT, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brachwitz K, et al. Evaluation of the first cytostatically active 1-aza-9-oxafluorenes as novel selective CDK1 inhibitors with P-glycoprotein modulating properties. J Med Chem. 2003;46:876–879. doi: 10.1021/jm021090g. [DOI] [PubMed] [Google Scholar]
- 27.Hochegger H, et al. An essential role for Cdk1 in S phase control is revealed via chemical genetics in vertebrate cells. J Cell Biol. 2007;178:257–268. doi: 10.1083/jcb.200702034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 29.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 30.Holt LJ, et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löw C, et al. Folding mechanism of an ankyrin repeat protein: Scaffold and active site formation of human CDK inhibitor p19(INK4d) J Mol Biol. 2007;373:219–231. doi: 10.1016/j.jmb.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 32.Kong SK, Chock PB. Protein ubiquitination is regulated by phosphorylation. An in vitro study. J Biol Chem. 1992;267:14189–14192. [PubMed] [Google Scholar]
- 33.Nguyen LK, Kolch W, Kholodenko BN. When ubiquitination meets phosphorylation: A systems biology perspective of EGFR/MAPK signalling. Cell Commun Signal. 2013;11:52. doi: 10.1186/1478-811X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thullberg M, et al. Distinct versus redundant properties among members of the INK4 family of cyclin-dependent kinase inhibitors. FEBS Lett. 2000;470:161–166. doi: 10.1016/s0014-5793(00)01307-7. [DOI] [PubMed] [Google Scholar]
- 35.Löw C, Homeyer N, Weininger U, Sticht H, Balbach J. Conformational switch upon phosphorylation: Human CDK inhibitor p19INK4d between the native and partially folded state. ACS Chem Biol. 2009;4:53–63. doi: 10.1021/cb800219m. [DOI] [PubMed] [Google Scholar]
- 36.Cervantes CF, Handley LD, Sue SC, Dyson HJ, Komives EA. Long-range effects and functional consequences of stabilizing mutations in the ankyrin repeat domain of IκBα. J Mol Biol. 2013;425:902–913. doi: 10.1016/j.jmb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrick D. Biological regulation via ankyrin repeat folding. ACS Chem Biol. 2009;4:19–22. doi: 10.1021/cb900003f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Löw C, et al. Structural insights into an equilibrium folding intermediate of an archaeal ankyrin repeat protein. Proc Natl Acad Sci USA. 2008;105:3779–3784. doi: 10.1073/pnas.0710657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díaz-Moreno I, et al. Phosphorylation-mediated unfolding of a KH domain regulates KSRP localization via 14-3-3 binding. Nat Struct Mol Biol. 2009;16:238–246. doi: 10.1038/nsmb.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkataramani R, Swaminathan K, Marmorstein R. Crystal structure of the CDK4/6 inhibitory protein p18INK4c provides insights into ankyrin-like repeat structure/function and tumor-derived p16INK4 mutations. Nat Struct Biol. 1998;5:74–81. doi: 10.1038/nsb0198-74. [DOI] [PubMed] [Google Scholar]
- 41.Flory E, Hoffmeyer A, Smola U, Rapp UR, Bruder JT. Raf-1 kinase targets GA-binding protein in transcriptional regulation of the human immunodeficiency virus type 1 promoter. J Virol. 1996;70:2260–2268. doi: 10.1128/jvi.70.4.2260-2268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta S, Sen S. Myotrophin-kappaB DNA interaction in the initiation process of cardiac hypertrophy. Biochim Biophys Acta. 2002;1589:247–260. doi: 10.1016/s0167-4889(02)00178-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.