Significance
Schizophrenia and bipolar disorder are psychiatric syndromes with a significant social and economic burden that share a common symptom, psychosis, attributed to excessive dopamine release. Despite years of intensive research, the causes of these devastating diseases are still unknown. In this work, a mouse line with a selective deletion of the molecular target of antipsychotics, dopamine D2 receptors, from the most affected neuron subtype in patients, parvalbumin interneurons, results in animals with schizophrenia-like phenotypes and resistance to the broadly used antipsychotic aripiprazole. Therefore, this genetic dissection provides clues about the intrinsic molecular mechanism leading to a dysregulated dopamine system and the development of psychiatric conditions like schizophrenia, bringing opportunities to develop diagnostic and treatment approaches.
Keywords: parvalbumin, DRD2, psychosis, mouse, deletion
Abstract
Excessive dopamine neurotransmission underlies psychotic episodes as observed in patients with some types of bipolar disorder and schizophrenia. The dopaminergic hypothesis was postulated after the finding that antipsychotics were effective to halt increased dopamine tone. However, there is little evidence for dysfunction within the dopaminergic system itself. Alternatively, it has been proposed that excessive afferent activity onto ventral tegmental area dopaminergic neurons, particularly from the ventral hippocampus, increase dopamine neurotransmission, leading to psychosis. Here, we show that selective dopamine D2 receptor deletion from parvalbumin interneurons in mouse causes an impaired inhibitory activity in the ventral hippocampus and a dysregulated dopaminergic system. Conditional mutant animals show adult onset of schizophrenia-like behaviors and molecular, cellular, and physiological endophenotypes as previously described from postmortem brain studies of patients with schizophrenia. Our findings show that dopamine D2 receptor expression on parvalbumin interneurons is required to modulate and limit pyramidal neuron activity, which may prevent the dysregulation of the dopaminergic system.
The dopaminergic hypothesis of psychosis was postulated after the finding that antipsychotics are antagonists of dopamine receptors, particularly D2 receptors (DRD2) (1). It stated that psychotic symptoms are caused by a “hyperdopaminergic state.” This hypothesis evolved and now postulates a dopamine imbalance between different brain regions. Increased subcortical dopaminergic neurotransmission may be responsible of the psychotic aspects, whereas reduced prefrontal dopaminergic neurotransmission may underlie negative and cognitive aspects (2, 3). Although the dopaminergic hypothesis is the most perdurable in psychiatry, there is limited direct evidence of impairment within the dopaminergic system itself. Alternatively, it has been proposed that impaired afferent circuits onto ventral tegmental area (VTA) dopaminergic neurons lead to a dysregulated dopamine release (4). The ventral hippocampus (vHipp) is highly relevant in this respect, since it has been shown that increased pyramidal neuron activity from this region indirectly promotes an increase in the number of tonic firing dopamine neurons in the VTA to release dopamine (5, 6). These observations are supported by human PET studies, showing an increased activity of the hippocampus in patients (7). Therefore, an aberrant dopamine neuron modulation from the vHipp may be crucial in the etiology of psychiatric diseases.
On the other hand, the consistent observation of reduced mRNA and protein levels of GAD67, one of the enzymes that synthesize GABA, from postmortem brain studies of patients with schizophrenia and bipolar disorder, led to the proposal of a different hypothesis for these psychiatric conditions, the GABAergic (8, 9). Interestingly, GAD67 levels are reduced in a particular subset of cortical interneurons, those expressing the calcium-binding protein parvalbumin (PV), and accordingly, levels of PV mRNA are also reduced (10). Increasing evidence suggests that disrupted PV interneuron (PVI) function might be central in the pathophysiology of schizophrenia (11, 12) and bipolar disorder (9). PVI are also required to drive cortical gamma oscillations (13), essential for working memory performance, a functional activity reportedly impaired in patients (14).
A recurrent observation in some psychiatric conditions is a disrupted balance between excitation and inhibition (E/I) that leads to cognitive and behavioral defects (15). PVI play a central role in the establishment of this balance (16). The inhibitory activity of this subtype of interneuron is promoted by dopamine stimulation onto DRD2 (17, 18). Interestingly, this response begins at the end of adolescence (19) and depends on β-Arrestin 2 signaling (20). Therefore, a developmental switch to an active DRD2 signaling within PVI may be required along the transition from adolescence to adulthood to set a delicate physiological E/I ratio and attain a balanced neuronal network.
As accumulating evidence posits PVI defects playing a central role in the development of psychiatric diseases, and DRD2, the target of most antipsychotics, are developmentally expressed in this subtype of interneurons (19, 21), we generated a mouse line with a selective deletion of DRD2 exclusively from PVI. We found that conditional mutant animals show adult onset of schizophrenia-like behaviors and a range of endophenotypes as those described for bipolar disorder and schizophrenia. Mutant DRD2 animals also showed limited or even blunt pharmacological response to aripiprazole, a widely used antipsychotic prescribed for both conditions. Therefore, conditional DRD2 mutant mouse line may be a useful tool to deepen our understanding of the pathological mechanisms that lead to psychiatric syndromes with overlapped dimensions.
Results
We generated a mouse line with a selective deletion of DRD2 from PVI by breeding a PV-Cre line (22) with a DRD2 floxed allele mouse line (23) (Fig. S1). By postnatal day 20 (P20), a conditional GFP reporter line showed that Cre recombinase is only expressed in PVI (Fig. S2), an evidence of reliable expression from the PV driver. Electrophysiological recordings from PVI showed no substantial differences in membrane properties between genotypes (Table S1). At P60, Cre recombinase activity faithfully recapitulated PV expression (Fig. S3 A–F). Combined in situ hybridization and immunohistochemistry showed that DRD2 mRNA was absent from PVI in conditional mutants (Fig. S3 G–I). Functional evidence of selective DRD2 loss of function was obtained by patch-clamp recordings of action potentials in the absence or presence of the DRD2 selective agonist quinpirole (19) (Fig. S3 J–L). When quinpirole was present, control PVI increased their firing rate (19). In contrast, conditional mutant interneurons were refractory to the DRD2 agonist. Therefore, general fast-spiking properties were not changed despite the lack of a functional DRD2 expression on PVI.
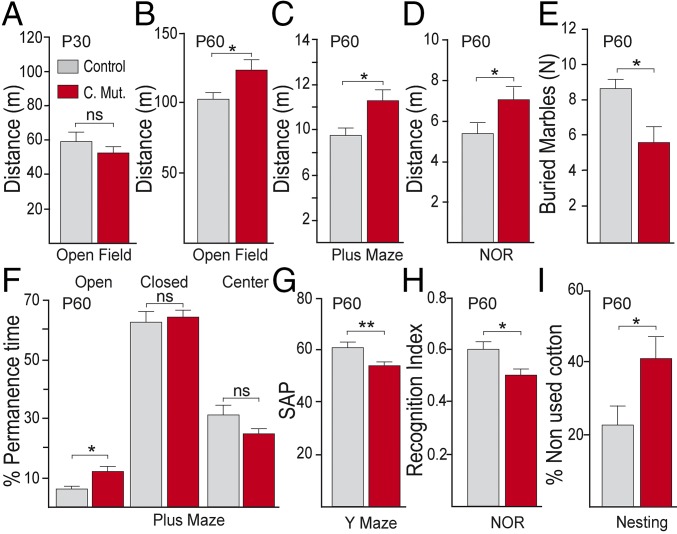
To evaluate the effect of DRD2 deletion, we performed a broad behavioral analysis with young (P30) and adult (P60) control and conditional mutant animals. Total distance traveled by young control or conditional DRD2 mutant animals was not significantly different in the open field (OF) (Fig. 1A), plus maze (PM), or novel object recognition (NOR) test (Fig. S4 A and B). In contrast, adult conditional mutants showed a significant increase in locomotor activity, regardless of the context in which they were analyzed (Fig. 1 B–D). Anxiety or risk-taking behavior was assessed by the PM experiment. Adult conditional DRD2 mutants showed a significant increase in the proportion of time spent in the open arms of the maze (Fig. 1F), an index of increased risk-taking behavior, whereas young animals of both genotypes spent similar proportion of time in each arm (Fig. S4C). Adult conditional DRD2 mutants buried significantly less marbles than controls in the marble-burying test (Fig. 1E and Fig. S4D), which is evidence of abnormal anxiety. Alternation between arms of a Y maze engages working memory that is modulated by dopamine. Adult, but not young, conditional mutants, alternated significantly less between the arms of the maze, reaching chance levels (Fig. 1G and Fig. S4E), suggesting an impaired working memory performance. The NOR test showed that adult control animals spent significantly more time exploring the novel object, whereas conditional DRD2 mutant animals spent an equal amount of time between novel and familiar objects (Fig. 1H and Fig. S4F), as if both objects were novel. Finally, we studied the motivation of animals to build a nest by unthreading a provided pressed cotton piece and found that adult conditional DRD2 mutant animals unthreaded significantly less cotton to build a nest and built nests were disorganized compared with control animals (Fig. 1I and Fig. S4G), an index of reduced motivation. In summary, our results show that the selective deletion of the DRD2 from PVI causes an adult onset of schizophrenia-like behaviors.
Fig. 1.
Adult onset of schizophrenia-like phenotypes in conditional DRD2 mutant animals. (A and B) Total distance in an open field, (C) plus maze, and (D) novel object recognition (NOR) test from (A) young (P30) animals or (B–D) adult (P60) animals. Anxiety level in adult animals was evaluated by the marble-burying test (E) and the plus maze (F). (E) Number of buried marbles in the marble-burying test. (F) Percentage of permanence time in open, closed, or center areas of the plus maze from adult animals. (G) Working memory performance was evaluated by the spontaneous alternation percentage (SAP) in the Y maze. (H) Recognition memory was assessed by the recognition index in the NOR test. (I) Percentage of nonused cotton in the nesting test. *P < 0.05, **P < 0.01; ns, not significant. Data are presented as mean ± SEM. n = 10–20 animals per group. Unpaired t test (A–H); Wilcoxon–Mann–Whitney (I).
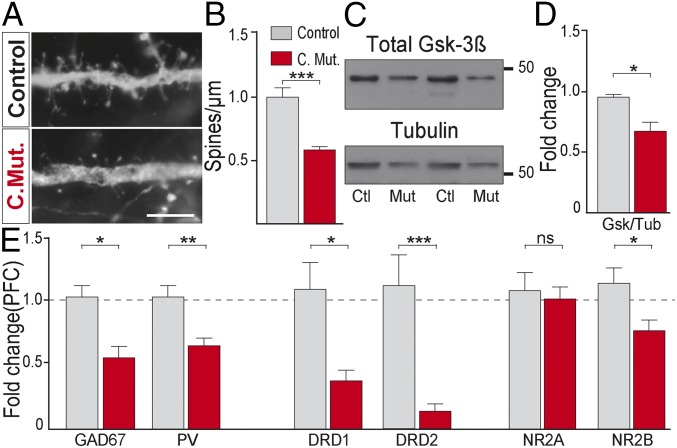
Impaired cognition, working memory deficits, and poor functional outcome are reported to arise from an impaired prefrontal cortex (PFC) (24). We therefore explored whether selective DRD2 deletion adversely impacts PFC physiology. As it has been shown that dopamine signaling through DRD2 regulates spine morphogenesis of pyramidal neurons (25) and reduced spine density has been observed in postmortem studies from patients, we measured dendritic spine density and found that it was significantly reduced in conditional DRD2 adult mutants compared with control animals (Fig. 2 A and B). It has been shown that DRD2 signaling depends on β-arrestin2 expression (26) and that the mood stabilizer lithium disrupts this signaling complex, inhibiting glycogen synthase kinase 3β (Gsk-3β) activity (27). Expression of Gsk3β have been found to be reduced in schizophrenia (28). Therefore, we determined the level of Gsk-3β in PFC from both genotypes and found that it was significantly decreased in conditional DRD2 mutants compared with controls (Fig. 2 C and D). As reduced GAD67 and PV protein and mRNA expression is a hallmark of schizophrenia, we quantified the relative mRNA expression of both genes by qRT-PCR in PFC (prelimbic and infralimbic region) and found that they were significantly reduced in conditional mutants (Fig. 2E) without a significant change in PVI density (Fig. S5 D–F). DRD1 and DRD2 mRNA levels were also significantly reduced in PFC (Fig. 2E) but in the striatum only DRD1 levels were reduced (Fig. S6). Extensive evidence shows that a reduced neurotransmission through the N-methyl d-aspartate (NMDA) receptor (NMDAR) recapitulates dimensions of psychiatric conditions like schizophrenia (29). We therefore explored the expression levels of two NMDAR subunits in the PFC: NR2A and NR2B. Interestingly, NR2B mRNA, but not NR2A, was significantly reduced in conditional DRD2 mutants (Fig. 2E). As PVIs are central in the generation of oscillatory activity within brain networks essential for working memory (30), we performed simultaneous local field potential (LFP) recordings from PFC and hippocampus (CA1 region) and found that oscillations in the alpha band (8–12 Hz) modulated the amplitude of the gamma band (30–70 Hz), and this modulation was significantly increased in the PFC of conditional DRD2 mutant mice (Fig. S7), as reported in patients (31). In summary, the selective deletion of DRD2 from PVI causes PFC endophenotypes highly reminiscent of psychiatric conditions at molecular, cellular, and physiological levels.
Fig. 2.
PFC endophenotypes in conditional DRD2 mutants. (A) Micrographs of dendritic spines from pyramidal neurons of control and conditional DRD2 mutant animals. (Scale bar: 5 µm.) (B) Quantification of dendritic spine density (n = 7–9 dendrites from three animals from each genotype). (C) Immunoblots from PFC homogenates in control and conditional DRD2 mutant mice of total Gsk3β and Tubulin and (D) their respective quantification (n = 5–6 animals each genotype). (E) mRNA fold change (n = 5–8 animals from each genotype) for GAD67, PV, DRD1, DRD2, NR2A, and NR2B in PFC. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant. Data are presented as mean ± SEM. Unpaired t test (B, D, and E).
We then quantified levels of dopamine (DA) and its metabolites (DOPAC and HVA) by HPLC in striatum and PFC of adult animals and found a significant increase of DOPAC level in the striatum and a significant reduction of DA and DOPAC in the PFC of conditional mutants (Fig. 3 A and B and Fig. S8), in line with the cortico-striatal imbalance hypothesis (2).
Fig. 3.
Dysregulated dopaminergic system in conditional mutants. (A) HPLC measurement of dopamine (DA) and (B) the metabolite DOPAC in prefrontal cortex (PFC) and striatum from control and conditional mutants. (C) Total distance traveled in an open field for 60 min after the administration of a single dose of saline or 5 mg/kg amphetamine. *P < 0.05, **P < 0.01; ns, not significant. Data are presented as mean ± SEM. Unpaired t test (A–C).
Chronic amphetamine administration to rodents is a well-established pharmacological model that resembles the psychotic symptoms of the disease, inducing a persistent sensitization that exaggerates the hyperactivity produced by a single amphetamine challenge (32, 33). We injected adult animals of both genotypes with an acute dose of amphetamine and then measured total locomotion in an open field and found that conditional DRD2 mutants were hypersensitive to the drug, as they exhibited a significant increase in total locomotion compared with controls (Fig. 3C and Fig. S9), suggesting a previous endogenous mechanism that sensitized the dopaminergic system.
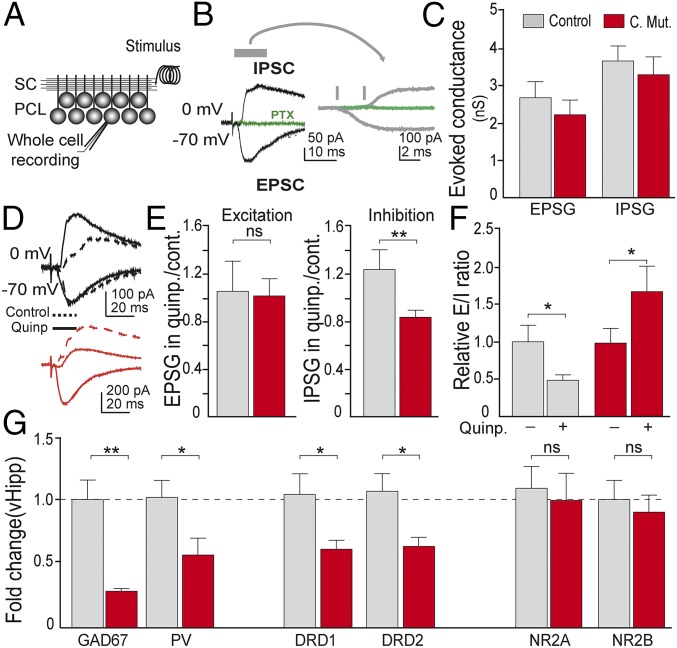
Afferent circuits to VTA dopaminergic neurons modulate dopamine output, particularly from the ventral hippocampus (4–6). To explore whether this region was affected after the selective deletion of DRD2 from PVI, we evaluated the responses of vHipp CA1 pyramidal neurons to afferent activity and performed electrophysiological recordings in acute slices from control and conditional DRD2 adult (P60) mutant animals. We carried out whole-cell voltage-clamp recordings to measure excitatory and inhibitory currents [excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs)] evoked in response to stimulation of the Schaffer collateral (SC) pathway, the main afferent pathway to CA1 (Fig. 4A). IPSCs were completely blocked with picrotoxin, a GABAA receptor antagonist (Fig. 4B). As expected, stimulation of SC produced monosynaptic excitation followed by disynaptic feedforward inhibition (Fig. 4B). In control conditions, the amplitude of the evoked EPCSs and IPSCs did not differ between genotypes (Fig. 4C). However, in the presence of the selective DRD2 agonist quinpirole, the responses to afferent stimulation were different. While excitation did not change in the presence of quinpirole, inhibition significantly increased in recordings from control slices but decreased in neurons from conditional mutant animals (Fig. 4 D and E). This differential effect on inhibition generated a change in the E/I balance in response to afferent activation (Fig. 4F). In control animals, the presence of DRD2 agonist quinpirole decreased the E/I ratio, indicating that activation of DRD2 may normally reduce afferent responses. In contrast, the increase in the E/I ratio observed in neurons from conditional mutant animals may produce an increased response of pyramidal neuron population when afferent activity arrives.
Fig. 4.
Disinhibited hippocampus in conditional mutants. (A) Scheme of experimental recording configuration. (B) Representative traces of EPSCs and IPSCs in response to stimulation of SCs. Right traces are an amplified timescale to show the delay between monosynaptic excitation and disynaptic feedforward inhibition. (C) Amplitude of the evoked conductance measured in control or conditional DRD2 mutant animals in control conditions. (D) Representative traces of IPSCs and EPSCs measured in response to SC stimulation in control conditions (dashed lines) or in presence of the selective DRD2 agonist quinpirole (1 μM) (solid lines). (E) Amplitude of conductance in control or conditional DRD2 mutant animals in response to afferent stimulation. Amplitudes in presence of quinpirole are normalized by the amplitude obtained in control conditions. (F) Effect of quinpirole on E/I ratio relative to control conditions. (G) Abnormal gene expression in ventral hippocampus. mRNA fold change (n = 5–8 animals from each genotype). *P < 0.05, **P < 0.01; ns, not significant. Data are presented as mean ± SEM. Unpaired t test (C and G); n = 6–10 cells from five animals each genotype. Paired t test (E and F).
As vHipp showed impaired inhibition following DRD2 selective deletion from PVI, we asked whether inhibitory markers were also affected and quantified the relative expression of GAD67 mRNA. We found that both GAD67 and PV mRNA were significantly reduced in conditional mutants (Fig. 4G), although there were no differences in vHipp PVI density between genotypes (Fig. S5 A–C). We also explored DRD1 and DRD2 mRNA levels and found a significant reduction of both receptors in conditional mutants (Fig. 4G), but the expression levels of NR2A and NR2B were not different between control and conditional mutant animals (Fig. 4G). In summary, our results show that the selective deletion of DRD2 from PVI causes an impaired inhibition of vHipp pyramidal neurons, abnormal expression of genes involved in the modulation of the E/I ratio, hypersensitivity to amphetamine, and unbalanced prefrontal–striatal dopamine neurotransmission.
As DRD2 conditional mutants exhibit an array of schizophrenia-like phenotypes and DRD2 is the target of most antipsychotics, we explored to what extent the functional activity of DRD2 from PVI was essential to mediate antipsychotic action. We thus treated both control and conditional DRD2 adult mutant animals with two different psychotomimetics: amphetamine or MK-801 (an NMDAR antagonist). These two compounds produced an increase in total locomotion in mice (Fig. 5), an effect that resembles psychotic symptoms (32). Each psychotomimetic was used alone or in combination with an antipsychotic: risperidone, a DRD2 antagonist, or aripiprazole, a partial DRD2 agonist. When risperidone was administered in combination with either MK-801 or amphetamine, total locomotion was reduced to basal levels in both genotypes (Fig. 5 A and B and Fig. S9), suggesting that risperidone antagonism onto DRD2 might have prevented the effect of massively released dopamine and that DRD2 expression in PVI was not required by risperidone to reduce locomotor activity. Control animals treated with aripiprazole in combination with amphetamine significantly reduced total locomotion (Fig. 5C and Fig. S9), but conditional DRD2 mutants showed only a partial reduction in total locomotion (Fig. 5C). Moreover, when animals were treated with aripiprazole in combination with MK-801, control mice significantly reduced total locomotion but conditional DRD2 mutants were completely refractory to aripiprazole treatment (Fig. 5D and Fig. S9). These results indicate that expression of DRD2 on PVI is required by aripiprazol to recover from the induced hyperlocomotor phenotype. As this compound provides a partial agonism onto DRD2, it may promote vHipp inhibitory activity of PVI over pyramidal neurons and thus reduce dopamine release. Our results highlight the relevance of this subtype of dopamine receptors and PVI in the mechanism of action of aripiprazole.
Fig. 5.
Impaired response to aripiprazole in conditional DRD2 mutant animals. (A and C) Total locomotion in an open field for 30 min after the administration of amphetamine (5 mg/kg) i.p. or (B and D) MK-801 (0.2 mg/kg) and the effect of (A and B) risperidone (0.025 mg/kg) or (C and D) aripiprazole (0.6 mg/kg). (A) Combined effect of risperidone and amphetamine (B) risperidone and MK-801, (C) aripiprazole and amphetamine, and (D) aripiprazole and MK-801 on control and conditional mutant adult mice. *P ≤ 0.05, **P ≤ 0.01, ***P < 0.001; ns, not significant; data are presented as mean ± SEM. One-way ANOVA with Bonferroni test as post hoc; n = 8–14 animals per group (A–D).
Discussion
Here, we generated a mouse line with a selective deletion of DRD2, the target of most antipsychotic drugs, from PVI, the most prominent GABAergic interneuron subtype affected in schizophrenia pathophysiology, and evaluated the consequences at molecular, cellular, physiological, and behavioral levels.
Schizophrenia is generally diagnosed between the end of adolescence to the beginning of adulthood by the presence of visible signs and symptoms, fundamentally psychotic episodes (34). Accordingly, young control and conditional DRD2 mutants did not exhibit any behavioral deficit. In contrast, adult DRD2 mutant animals showed increased locomotor activity, increased risk-taking behavior, abnormal anxiety levels, cognitive deficits, and reduced motivation, a set of behaviors reminiscent of schizophrenia, suggesting that the selective deletion of DRD2 from PVI recapitulates the late developmental onset of schizophrenia-like behaviors.
The PFC is central for cognitive performance and executive functions. Excessive or reduced dopamine neurotransmission in the PFC has a strong impact on working memory and functional outcome (24). Postmortem studies of PFC from affected patients consistently show phenotypes that includes reduced expression of GABAergic markers and reduced synaptic connections, among others. The observation of schizophrenia-like behaviors in adult DRD2 conditional mutant animals prompted us to search for reported endophenotypes associated to this psychiatric disease. We performed a series of experiments in PFC and found the following: (i) reduced GAD67 and PV mRNA levels (35); (ii) reduced DRD1 (36) and DRD2 mRNA levels; (iii) reduced NR2B mRNA levels (37); (iv) reduced Gsk-3β protein levels (28); (v) reduced pyramidal neuron dendritic spine density (38); (vi) excessive cross-frequency coupling between alpha and gamma bands (31); and (vii) reduced prefrontal dopamine and DOPAC content with a concomitant increase in striatal DOPAC (2). Unbalanced levels of dopamine and its metabolites between cortical and subcortical regions agree with a hyperactive mesostriatal and a hypoactive mesocortical systems (3). The observation of a reduced NR2B mRNA expression is highly relevant, as it may support a reduced neurotransmission through NMDAR, in line with the proposed hypoglutamatergic hypothesis of the schizophrenia. Reduced GAD67 and PV mRNA levels may cause an attenuated inhibitory function, as the expression of both genes is activity dependent (39). Taken together, these results show that the selective deletion of DRD2 from PVI not only exhibits adult onset of schizophrenia-like behaviors but also recapitulates prevalent endophenotypes recurrently described in patient studies.
A well-established signaling pathway from DRD2 is the down-regulation of cAMP production and PKA activity. However, β-Arrestin2–dependent signaling pathway downstream of DRD2 inhibits Akt and activates Gsk3β, a protein involved in dopamine-dependent behaviors (26). β-Arrestin2 is required in PVI to increase the firing frequency upon DRD2 activation (20) and thus inhibit pyramidal neurons. Our work supports this finding, as specific DRD2 deletion from PVI results in reduced inhibitory activity, pyramidal disinhibition, aripiprazole refractoriness, and schizophrenia-like behaviors. Therefore, promoting a biased DRD2/β-Arrestin2 signaling selectively in PVIs may be a promising strategy to stimulate inhibitory activity and restore a physiological E/I balance.
Different experimental approaches have shown that vHipp interventions result in adult emergence of schizophrenia-like phenotypes affecting, with particular emphasis, the PVI population (40, 41). Previous reports also suggested that PVI impairment is central in the etiology of schizophrenia. Genetic models with specific receptor deletion from PVI show phenotypes resembling different aspects of schizophrenia pathophysiology (11, 12). Moreover, an interneuron transplant approach into the vHipp predominantly with PVI precursors reversed psychosis-like phenotypes in an animal model of hippocampal disinhibition (42). Our work reinforces the notion that PVI plays an essential role in the development of psychiatric diseases.
Conditional DRD2 animals show a dysregulated dopamine system, unveiled after the acute amphetamine treatment. Our results suggest that, in adult DRD2 conditional mutants, there might have been a mechanism that sensitized the dopaminergic system, as a single dose of amphetamine produced a hypersensitive behavior, an expected phenotype in an animal model of schizophrenia (43). This mechanism may involve an excessive dopamine release. Exacerbated vHipp pyramidal neuron activity has been proposed to underlie this behavior in amphetamine-sensitized mice (33) and in an animal model of schizophrenia (44). In line with these results, we found that dopamine-mediated inhibitory activity of vHipp PVI is impaired, leading to an increased E/I ratio. Therefore, our results suggest that, in control animals, DRD2-mediated PVI activation may promote the inhibition of vHipp pyramidal neurons. In contrast, in conditional mutants, DRD2-dependent PVI inhibitory activity is impaired and pyramidal neurons may become hyperactive. A disinhibited vHipp may in turn promote an aberrant modulation of dopamine release from midbrain dopaminergic neurons after adverse environmental stimulus (5, 6). As DRD2 also promotes the activity of PFC pyramidal neurons (45), PVI disinhibition and heightened dopamine tone may have compensated and reduced DRD1 and DRD2 mRNA expression. Therefore, DRD2 from vHipp PVI may function as a feedback mechanism to finely modulate dopamine release. As a consequence, in DRD2 mutants, an aberrant homeostatic adaptation may take place during the transition from adolescence to adulthood to adapt the affected circuits to an increased dopamine neurotransmission and an impaired E/I balance. Once the animal reaches adulthood, the involved neural circuit may be established in a pathological conformation leading to the observed pathological phenotypes (25, 46–48).
Antipsychotics must exhibit antagonism on DRD2 to successfully manage psychosis. Aripiprazole is an exception, as it is a partial agonist of this receptor. Our pharmacological experiments show that risperidone does not depend on DRD2 expression on PVI to recover animals from an induced hyperlocomotor phenotype, but aripiprazole requires a functional DRD2 expression in this subtype of GABAergic interneurons to accomplish this recovery. Therefore, promoting the inhibitory activity of PVI by an agonist of DRD2 like aripiprazole may reduce the excessive activity of pyramidal neurons, confining the population activity of midbrain dopaminergic neurons to limit dopamine release.
Altogether, the absence of a functional DRD2 signaling promoting the inhibitory activity of PVI to modulate and limit pyramidal neuron excitation may drive an excessive dopaminergic neurotransmission. This excessive dopaminergic neurotransmission may promote an abnormal neuronal circuit conformation leading to schizophrenia-like phenotypes in adulthood. DRD2 conditional mouse line may be a useful tool to study unknown aspects of dopamine system in relation to schizophrenia, bipolar, and other psychiatric conditions. Further research on DRD2 downstream signaling in this particular subtype of interneurons may lead to the identification of target molecules to finely modulate and promote its inhibitory activity.
Materials and Methods
Animals.
A mouse colony was established by mating DRD2flox/flox with PV+/Cre::DRD2flox/flox. Only male mice were used for experiments. Animals were maintained in a controlled environment (20–22 °C, 12-h light/dark cycle) and were group housed in ventilated cages with food and water ad libitum. Genomic DNA was obtained from ear punch biopsies to identify each genotype. Experiments were performed in accordance with the Principles of Laboratory Animal Care and under approved guidelines of the Institutional Animal Care and Use Committee, Instituto de Biología y Medicina Experimental, Consejo Nacional de Investigaciones Cientificas y Técnicas.
Recordings of Pyramidal Cells.
Pyramidal neurons in CA1 area were recorded with infrared differential interference contrast (DIC) video microscopy. Whole-cell recordings were performed using microelectrodes (4–8 MΩ), amplified, digitized, and acquired at 20 kHz using the pClamp10 software. Membrane capacitance and input resistance were obtained from current traces evoked by a hyperpolarizing step of 10 mV. Evoked monosynaptic EPSCs and IPSCs were recorded after SC stimulation. EPSCs were isolated by voltage clamping pyramidal neurons at the reversal potential of the IPSC (∼−70 mV). IPSCs were recorded at the reversal potential of the EPSC (∼0 mV).
qRT-PCR.
PFC and vHipp were dissected from control and conditional mutant mice and were lysed in Tripure Isolation Reagent (Roche). The mRNA was retrotranscribed with Transcriptor First Strand cDNA Synthesis Kit (Roche). For qRT-PCR, FastStart Universal SYBR Green Master Kit (Roche) was used.
Determination of Dopamine and Its Metabolites.
The neurochemical detection of DA and DOPAC was performed by HPLC. Tissues were weighed, homogenized, and deproteinized. Homogenates were centrifuged and supernatants (20 µL) were injected onto a reverse-phase column. Endogenous concentrations of DA, DOPAC, and HVA were determined using a Shimadzu LC-1OAS bomb and an electrochemical detector (Bioanalytical Systems). Peak heights were measured and quantified based on standard curves.
Western Blots.
PFC tissue was dissected and homogenized in lysis buffer. Samples were run on 12% SDS/PAGE gels. Gels were transferred onto PVDF membranes and probed with primary antibodies overnight. Subsequently, immune complexes were incubated with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) along with ECL Western blotting detection reagents (Thermo Scientific). Signals were acquired in G-Box (Syngene) and quantified with ImageJ.
Behavior and Pharmacology.
Behavioral experiments were conducted during the early dark phase of the light/dark cycle and performed blinded to genotype. Performed tests were as follows: open field, elevated plus maze, marble burying test, Y-maze, novel object recognition test (NOR), and nesting test. All tests were videotaped and analyzed using ANY-maze Video Tracking System (Stoelting). For drug experiments, mice were injected with vehicle or drug, placed into the test apparatus, and monitored. Drugs used were as follows: MK-801, aripiprazole (Maprimed), risperidone (Maprimed), and amphetamine (Sigma).
Statistical Analysis.
The data are presented as mean ± SEM. Statistical analysis was carried out in GraphPad Prism 5. Data were evaluated by t test, ANOVA followed by post hoc tests or Mann–Whitney–Wilcoxon test for nonparametric data. P < 0.05 was considered significant.
For full detailed materials and methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Fundación Rene Baron, Fundación Williams, Fundación Bunge y Born, Maprimed, Valeria Poggi, Sonia Espindola, Dr. Rubinstein, Dra. Avale, and Dr. Tomas Falzone. This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT) [PICT 2012-2024 (to D.M.G.), PICT 2013-0182 (to A.M.-B.), and PICT 2015-0364 (to A.M.-B.)], FOCEM-MERCOSUR (COF 03711) (A.M.-B.), Fundacion Roemmers (D.M.G.), and Fundación Florencio Fiorini (D.M.G.). The work of E.T., L.B., C.M., M.B.O., and M.N.D.G. was supported by doctoral fellowships from Consejo Nacional de Investigaciones Cientificas y Técnicas, ANPCYT, or Fundación Bunge y Born.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719897115/-/DCSupplemental.
References
- 1.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: A review. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 3.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 7.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- 9.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlen M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartley AF, et al. Interneuron transcriptional dysregulation causes frequency-dependent alterations in the balance of inhibition and excitation in hippocampus. J Neurosci. 2015;35:15276–15290. doi: 10.1523/JNEUROSCI.1834-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng KY, et al. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng K-Y, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urs NM, et al. Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci USA. 2016;113:E8178–E8186. doi: 10.1073/pnas.1614347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello EP, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floresco SB. Prefrontal dopamine and behavioral flexibility: Shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia J-M, Zhao J, Hu Z, Lindberg D, Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nat Neurosci. 2013;16:1627–1636. doi: 10.1038/nn.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu J-M, et al. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu J-M, et al. A β-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167:98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haenschel C, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popov T, Popova P. Same clock, different time read-out: Spontaneous brain oscillations and their relationship to deficient coding of cognitive content. Neuroimage. 2015;119:316–324. doi: 10.1016/j.neuroimage.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 32.van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: Pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: A mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 35.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 36.Okubo Y, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 37.Laruelle M. Schizophrenia: From dopaminergic to glutamatergic interventions. Curr Opin Pharmacol. 2014;14:97–102. doi: 10.1016/j.coph.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 41.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilani AI, et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci USA. 2014;111:7450–7455. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson SE, Sohal VS. Dopamine D2 receptors modulate pyramidal neurons in mouse medial prefrontal cortex through a stimulatory G-protein pathway. J Neurosci. 2017;37:10063–10073. doi: 10.1523/JNEUROSCI.1893-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cordon I, et al. Coupling in the cortico-basal ganglia circuit is aberrant in the ketamine model of schizophrenia. Eur Neuropsychopharmacol. 2015;25:1375–1387. doi: 10.1016/j.euroneuro.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Chen P, Gu Z, Liu W, Yan Z. Glycogen synthase kinase 3 regulates N-methyl-d-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol. 2007;72:40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- 48.Li Y-C, Xi D, Roman J, Huang Y-Q, Gao W-J. Activation of glycogen synthase kinase-3β is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci. 2009;29:15551–15563. doi: 10.1523/JNEUROSCI.3336-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- 50.Franklin K, Paxinos G. 2008. The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego), Compact 3rd Ed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.