Abstract
Background
Recent studies have shown that some vaccines have beneficial effects that could not be explained solely by the prevention of their respective targeted disease(s).
Methods
We used the MarketScan® United States (US) Commercial Claims Databases from 2005–2014 to assess the risk of hospital admission for non-targeted infectious diseases (NTI) in children from 16 through 24 months according to the last vaccine type (live and/or inactivated). We included children continuously enrolled within a month of birth through 15 months who received at least three doses of Diphtheria-Tetanus-acellular Pertussis vaccine by end of 15 months of age. We used Cox regression to estimate hazard ratios (HRs), stratifying by birthdate to control for age, year and seasonality, and adjusting for sex, chronic diseases, prior hospitalizations, number of outpatient visits, region of residence, urban/rural area of domicile, prematurity, low birth weight, and mother’s age.
Results
311,663 children were included. In adjusted analyses, risk of hospitalization for non-targeted infections from ages 16 through 24 months was reduced for those who received live vaccine alone compared with inactivated alone or concurrent live and inactivated vaccines (HR 0.50, 95% CI 0.43, 0.57 and HR 0.78, 95% CI 0.67, 0.91, respectively), and for those who received live and inactivated vaccines concurrently compared with inactivated only (HR 0.64, 95% CI 0.58, 0.70).
Conclusions
We found lower risk of non-targeted infectious disease hospitalizations from 16 through 24 months among US children whose last vaccine received was live compared with inactivated vaccine, as well as concurrent receipt compared with inactivated vaccine.
Introduction
Childhood vaccinations have been one of the greatest public health achievements in the past century. Vaccines are rigorously evaluated pre-licensure in clinical trials establishing their safety and efficacy against their targeted diseases. However, recent studies have identified possible nonspecific vaccine effects in children beyond their targeted infections. Studies in West Africa found that the measles vaccine1 and BCG vaccine2 had beneficial nonspecific effects that could not be explained solely by the prevention of measles and tuberculosis, respectively. Sorup et al in Denmark reported the first study in a high-income country that found receiving the live Measles-Mumps-Rubella (MMR) vaccine as the most recent vaccine was associated with a lower rate of admissions for infections compared with having the most recent the inactivated Diphtheria-Tetanus-acellular Pertussis-inactivated Polio virus-Haemophilus influenzae type b (DTaP-IPV-Hib) vaccine by age 15 months.3
In the United States (US), the current childhood vaccination schedule recommends routine vaccination with live vaccines at age intervals that are similar to those for recommended doses of inactivated vaccines. For example, the recommended age for two of the live vaccines, MMR and varicella (12 to 15 months) overlaps the recommended ages for doses of the following inactivated vaccines: Haemophilus influenzae type b (Hib) [12 to 15 months], pneumococcal conjugate vaccine [12 to 15 months], Hepatitis B vaccine (HBV) [6 to 18 months], inactivated poliovirus vaccine (IPV) [6 to 18 months], hepatitis A (HAV) [12 to 23 months], and DTaP (15 to 18 months).
We assessed hospital admissions due to non-targeted infections (NTI) in a US population of children according to the type of the last vaccine received. We attempted to replicate the study by Sorup and colleagues3 by using similar methods to the extent possible. We also conducted secondary analyses to assess different types of NTIs.
Methods
Population and data source
We performed our analysis using the MarketScan® Commercial Claims Databases from 2005–2014 (Truven Health Analytics, Ann Arbor, MI). MarketScan® collects de-identified individual data from commercial health insurance claims and has a wide geographical representation.4,5 Because no birthdate is available, we used as a proxy the diagnosis-related group (DRG) codes 789 through 795 for newborn hospitalization to identify subjects and included individuals who were enrolled in their insurance plan continuously from within a month of the birth hospitalization through 15 months. We defined birthdate as the date of admission for the newborn hospitalization. We evaluated children who had received at least three doses of DTaP prior to age 16 months to limit the possibility of bias attributable to factors related to under-vaccination.6 We excluded children enrolled in capitated insurance plans because of the lack of incentive among their providers to report individual claims for immunization services. We also excluded children who were immunosuppressed or had other contraindications for receiving vaccines. [See supplementary table for International Classification of Diseases ninth revision (ICD-9) codes] Furthermore, because influenza vaccination was low or nonexistent in Denmark7 during the period of the Sorup study3, we excluded children who had ever received an influenza vaccination (i.e., anytime up until 25 months).
Vaccine status information
Types of vaccines received were identified using individual Current Procedural Terminology (CPT) codes. Inactivated vaccines included DTaP, Hib, IPV, inactivated influenza vaccine (IIV), pneumococcal conjugate vaccine (PCV), HAV, and HBV. Live vaccines included MMR, varicella, and rotavirus, and live attenuated influenza vaccine (LAIV, licensed for ages 2–49 years). Because certain vaccines (e.g., inactivated: HAV and DTaP) may still be administered between the ages of 16 and 24 months, vaccination status was treated as a time-dependent exposure. The children’s vaccination status was defined as follows: 1) inactivated and live vaccines received on the same day; 2) live vaccines only; and 3) inactivated vaccines only. The first occurrence of vaccination status was the last vaccine received prior to 16 months, and if a vaccine was received between 16 months and 24 months, their vaccination status changed accordingly on the date of vaccination.
Outcomes
For comparability, we assessed the same infectious conditions/outcomes evaluated by Sorup and colleagues3 (Supplementary Table). We evaluated up to 15 secondary discharge diagnoses, in addition to the primary diagnosis, for hospitalizations in all eligible children from age 16 through 24 months.
Statistical Analyses
To assess unadjusted rates of admissions per 100,000 person-years we counted the number of hospitalizations and number of person years according to most recent vaccine and use these numbers to calculate the unadjusted rates. For the adjusted analyses, we used extended Cox regression (which allows for time-dependent covariates and interactions with time for variables that do not meet the proportional hazards assumption) to estimate the hazard rate ratios (HRs), and 95% confidence intervals (CI). Age was the underlying time scale. In separate models, we assessed live only versus inactivated only, live only versus concurrent (live and inactivated), and concurrent versus inactivated only. We stratified by admission date for the newborn hospitalization (to control for age, seasonality and year) and adjusted for region, urban or rural metropolitan statistical area, mother’s age, low birth weight or prematurity (ICD-9 765.x for child, 644.20 and 644.21 for mother), number of previous hospitalizations prior to age 16 months (excluding hospitalization for birth), chronic conditions of the child (Supplementary Table 2),8 any previous hospitalization for NTI before 16 months of age, and number of outpatient visits before age 16 months. Based on previous studies,9 we evaluated sex as an effect modifier. Additionally, we assessed if any other factors modified the effect of type of vaccine received on risk of hospitalization. We assessed the risk of hospitalization until the child’s first hospital admission. Children were followed from the day they turned 16 months of age to hospitalization, or censorship due to disenrollment or turning 25 months of age.
We conducted sensitivity analyses which excluded vaccine preventable diseases (VPDs), such as pneumonia (potentially vaccine-preventable pneumonias, not all pneumonias), hepatitis, pertussis, sepsis due to Haemophilus influenzae, and measles complicated by encephalitis or meningitis.
We also conducted secondary analyses for four groups of infections: upper respiratory infections (URI), lower respiratory infections (LRI), gastrointestinal infections (GI), and other infections.
Similar to the Danish study,3 we also evaluated emergency department visits for unintentional injuries (ICD-9 codes E800–E869 and E880–E929) as a control outcome using the same model as for the main analyses. We chose this event as a control outcome because it should not be causally associated with last type of vaccine received.10
The validity of the assumption of proportional hazards for all covariates was evaluated using Schoenfeld residuals. Violations of the proportionality assumption were identified for different variables across models. To account for these violations, we included interaction terms with time (where p < 0.05) for these terms in the models. Only vaccination status was modeled as a time-dependent variable in all the models. Joint multicollinearity was assessed using eigenvalues. This analysis involved only existing claims data, therefore Institutional Review Board review was not required.
Results
We identified a total of 687,022 infants with the code for newborn hospitalization who were enrolled within 31 days of birth and were continuously enrolled through 15 months of age, after excluding those on capitated plans (n=1,917). [Figure 1] After excluding those with immunosuppressive conditions (n=5,938) and those who received an influenza vaccine any time before 25 months of age (n=271,145) the sample included 409,939 children. Of these, 342,659 (84%) received at least three doses of DTaP by 16 months and we could link 311,663 (91%) to their mother’s information.
Figure 1.
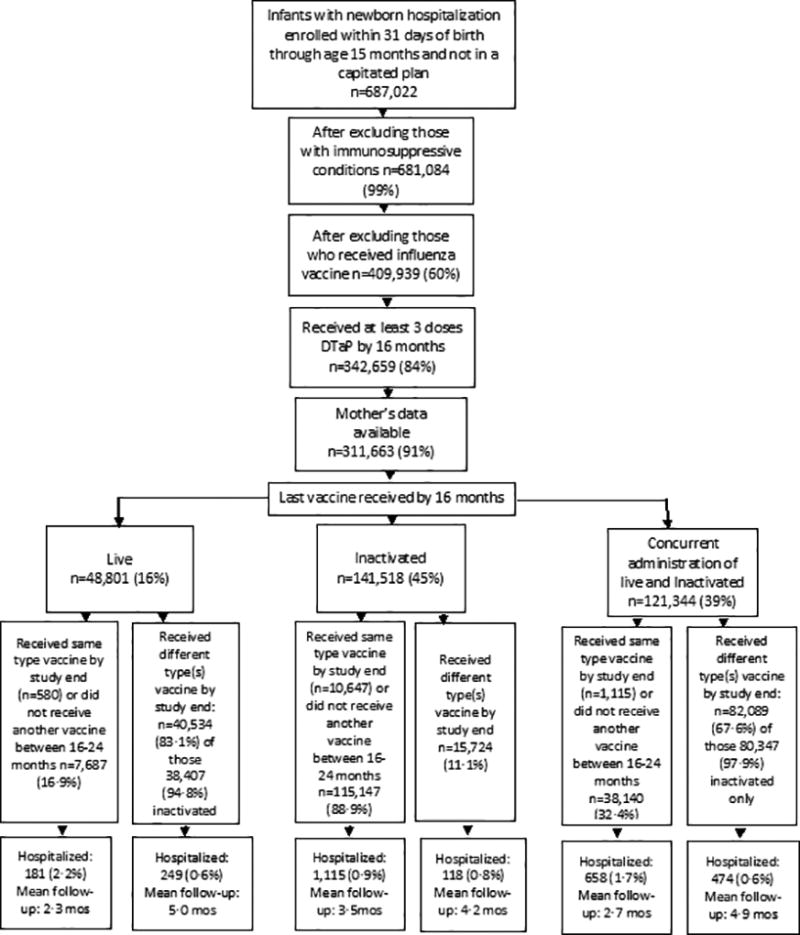
Flowchart of Study Inclusion
Of the 311,663 children included in the analyses, 51.3% were male, and 45% had claims indicating the last vaccine received prior to 16 months was inactivated only; for 16% the last vaccine received was live only; and 39% of the children received inactivated and live vaccines on the same day; 44.4% had a change in their vaccination status due to receipt of additional vaccines between 16 through 24 months of age [Figure 1]. Of those whose vaccine was live by 16 months, 88% received MMR-containing (i.e., MMR and MMRV) vaccines. Among those whose last vaccination status by 25 months was inactivated vaccine only, the majority (91.9%) had previously received MMR and/or varicella vaccine (81% received during the recommended age [i.e.12–15 months] and 19% after age 15 months). See supplement table A.
Children whose last vaccination status by 16 months was live vaccine only differed statistically from children whose last vaccination status was inactivated only or concurrent (live and inactivated) in that their mothers were older when they were born, they had more outpatient visits before 16 months, were more likely to be from the Northeastern US than the South or the West, and to live in urban areas (Table 1). Notably, there were no significant differences in last vaccination status among children with chronic conditions or those who had low birthweight or were premature.
Table 1.
Descriptive characteristics of the Marketscan® population at baseline, age 16 months, 2005–2014
| Type last vaccine received by 16 months | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | Concurrent | Live | Inactivated | |||||
| n | % | N | % | n | % | n | % | |
| Sex of Patient | ||||||||
| Male | 159,892 | 51·3 | 62,022 | 51·1 | 25,042 | 51·3 | 72,828 | 51·5 |
| Female | 151,771 | 48·7 | 59,322 | 48·9 | 23,759 | 48·7 | 68,690 | 48·5 |
| Mother’s age at child’s birtha | ||||||||
| 14–24y | 23,018 | 7·4 | 10,357 | 8·5 | 2,843 | 5·8 | 9,818 | 6·9 |
| 25–29y | 94,834 | 30·4 | 38,317 | 31·6 | 13,442 | 27·6 | 43,075 | 30·4 |
| 30–34y | 118,926 | 38·2 | 45,244 | 37·3 | 19,149 | 39·2 | 54,533 | 38·5 |
| ≥35y | 74,885 | 24·0 | 27,426 | 22·6 | 13,367 | 27·4 | 34,092 | 24·1 |
| Metropolitan statistical areaa | ||||||||
| Rural | 32,641 | 10·5 | 15,461 | 12·7 | 3,586 | 7·4 | 13,594 | 9·6 |
| Urban | 279,022 | 89·5 | 105,883 | 87·3 | 45,215 | 92·6 | 127,924 | 90·4 |
| Premature or low birth weight | ||||||||
| No | 287,314 | 92·2 | 111,835 | 92·2 | 45,028 | 92·3 | 130,451 | 92·2 |
| Yes | 24,349 | 7·8 | 9,509 | 7·8 | 3,773 | 7·7 | 11,067 | 7·8 |
| Pre-existing chronic disease | ||||||||
| No | 292,918 | 94·0 | 114,129 | 94·1 | 45,904 | 94·1 | 132,885 | 93·9 |
| Yes | 18,745 | 6·0 | 7,215 | 5·9 | 2,897 | 5·9 | 8,633 | 6·1 |
| Any hospitalization prior to 16 months other than birtha | ||||||||
| No | 294,988 | 94·6 | 114,665 | 94·5 | 46,232 | 94·7 | 134,091 | 94·8 |
| Yes | 16,675 | 5·4 | 6,679 | 5·5 | 2,569 | 5·3 | 7,427 | 5·2 |
| NTI hospitalization prior to 16 monthsa | ||||||||
| No | 301,438 | 96·7 | 117,198 | 96·6 | 47,267 | 96·9 | 136,973 | 96·8 |
| Yes | 10,225 | 3·3 | 4,146 | 3·4 | 1,534 | 3·1 | 4,545 | 3·2 |
| Number of outpatient visits prior to 16 months of agea | ||||||||
| <9 | 23,802 | 7·6 | 11,913 | 9·8 | 2,745 | 5·6 | 9,144 | 6·5 |
| 9–15 | 144,489 | 46·4 | 57,839 | 47·7 | 22,076 | 45·3 | 64,574 | 45·6 |
| ≥16 | 143,372 | 46·0 | 51,592 | 42·5 | 23,980 | 49·1 | 67,800 | 47·9 |
| Regiona | ||||||||
| Northeast | 55,746 | 17·9 | 16,500 | 13·6 | 14,503 | 29·7 | 24,743 | 17·5 |
| North Central | 96,370 | 30·9 | 37,196 | 30.7 | 15,015 | 30·8 | 44,159 | 31·2 |
| South | 112,889 | 36·2 | 46,880 | 38·6 | 13,698 | 28·1 | 52,311 | 37·0 |
| West | 45,535 | 14·6 | 20,299 | 16·7 | 5,427 | 11·1 | 19,809 | 14·0 |
| Unknown | 1,123 | 0·4 | 469 | 0·4 | 158 | 0·3 | 496 | 0·4 |
Indicates statistically significant difference using χ2 test, p<0.05 for last vaccine received by 16 months
Infectious Disease Hospital Admission Risk
The most common NTI disease hospitalizations included pneumonia (33.0%) and ear infections (17.8%) (Supplemental Table B). Overall, the crude rate of hospital admissions for any NTI among children aged 16 through 24 months was 1,398 per 100,000 person-years (Table 2). The crude rate was higher among boys than girls. The overall crude risk of hospitalization for a NTI was 1,506 per 100,000 among those whose last vaccine type was live only compared with 1,317 per 100,000 among inactivated only and 1,599 per 100,000 among concurrent receipt.
Table 2.
Unadjusted and Adjusted Results for any Non-targeted Infectious Disease Hospitalization, between 16 and 24 months
| Time-dependent vaccination status: Most recent vaccination |
Children No.a |
Events | Person-Years | Unadjusted Infectious Disease Admissions per 100,000 |
Adjusted* Hazard Ratio (95% CI) |
p-value |
|---|---|---|---|---|---|---|
| Overall | 311,663 | 2,795 | 199,875 | 1,398 | ||
| Inactivated | 244,548 | 1,807 | 137,156 | 1,317 | ref | |
| Live | 15,908 | 241 | 16,002 | 1,506 | 0.50 (0.43, 0.57) | <0.0001 |
| Concurrent administration of live and inactivated | 51,207 | 747 | 46,716 | 1,599 | ref | |
| Live | 15,908 | 241 | 16,002 | 1,506 | 0.78 (0.67, 0.91) | 0.0011 |
| Inactivated | 244,548 | 1,807 | 137,156 | 1,317 | ref | |
| Concurrent administration of live and inactivated | 51,207 | 747 | 46,716 | 1,599 | 0.64 (0.58, 0.70) | <0.0001 |
| Sex Specific: | ||||||
| Boys: | 159,892 | 1,545 | 102,442 | 1,508 | ||
| Inactivated | 125,068 | 985 | 70,208 | 1,403 | ref | |
| Live | 8,332 | 136 | 8,272 | 1,644 | 0.51 (0.42, 0.62) | <0.0001 |
| Concurrent administration of live and inactivated | 26,492 | 424 | 23,961 | 1,770 | ref | |
| Live | 8,332 | 136 | 8,272 | 1,644 | 0.77 (0.63, 0.94) | 0.0090 |
| Inactivated | 125,068 | 985 | 70,208 | 1,403 | ref | |
| Concurrent administration of live and inactivated | 26,492 | 424 | 23,961 | 1,770 | 0.66 (0.58, 0.75) | <0.0001 |
| Girls: | 151,771 | 1,250 | 97,429 | 1,283 | ||
| Inactivated | 119,480 | 822 | 66,947 | 1,228 | ref | |
| Live | 7,576 | 105 | 7729 | 1,359 | 0.49 (0.40, 0.61) | <0.0001 |
| Concurrent administration of live and inactivated | 24,715 | 323 | 22,753 | 1,420 | ref | |
| Live | 7,576 | 105 | 7729 | 1,359 | 0.79 (0.63, 0.99) | 0.0409 |
| Inactivated | 119,480 | 822 | 66,947 | 1,228 | ref | |
| Concurrent administration of live and inactivated | 24,715 | 323 | 22,753 | 1,420 | 0.62 (0.54, 0.72) | <0.0001 |
Number of children by vaccine type at the end of the study
Live vaccines include: MMR, V, and MMRV
Inactivated vaccines include: DTaP, IPV, Hib, PCV, HBV, and HAV (and combined vaccines with these antigens)
Adjusted for chronic conditions, sex, low birth weight, premature, number of hospitalizations prior to 16 months, number of outpatient visits prior to age 16 months, region, urban/rural, and mother’s age; all HR are stratified by birthdate to control for age, seasonality and year, and interactions between time with previous hospitalizations and number of outpatient visits prior to age 16 months were included to account for the violations of the proportional hazards assumption
In the adjusted analyses, we found statistically significant decreases in the risk of hospitalization for NTI diseases when the last type of vaccine received included a live vaccine. In the overall Cox regression model, compared with those whose last vaccine was inactivated only, the hazard ratio (HR) for those who received a live vaccine was 0.50; 95% CI: 0.43, 0.57. (Table 2) This finding was also consistent for boys and for girls. Similarly, for those whose last vaccine was live only compared with concurrent receipt of live and inactivated vaccines the overall HR was 0.78 (95% CI: 0.67, 0.91), and was also significantly reduced among boys and girls. The adjusted analyses found statistically significant reduced risks of NTI hospitalization for concurrent vaccines compared with inactivated. No factors were found to have statistically significant interaction with last vaccine type received.
Sensitivity analyses and control outcome
In the secondary analyses that excluded VPDs (Table 3), the adjusted risk of hospitalization scarcely changed from the results of the models including VPDs. In the four models that evaluated NTI hospitalization risks for different categories of infections, the adjusted risk for upper and lower respiratory infections was lower for those whose last vaccine type included a live vaccine compared with inactivated only (Table 4). Risk of hospitalization was lower for other viral and bacterial infections among those whose last vaccine type received was live only compared with inactivated only (Table 5). We also performed the analyses to assess variation between regions but did not find an association. (Supplemental Table C).
Table 3.
Sensitivity analysis: Unadjusted and Adjusted Results for any Non-Targeted Infectious Disease Hospitalizations, excluding vaccine preventable diseases between 16 and 24 months
| Time-dependent vaccination status: Most recent vaccination |
Children No.a |
Events | Person-Years | Unadjusted Infectious Disease Admissions per 100,000 |
Adjusted* Hazard Ratio (95% CI) |
p-value |
|---|---|---|---|---|---|---|
| Overall | 311,519 | 2,651 | 199,827 | 1,327 | ||
| Inactivated | 244,458 | 1,717 | 137,129 | 1,252 | ref | |
| Live | 15,886 | 219 | 15,994 | 1,369 | 0.48 (0.41, 0.55) | <0.0001 |
| Concurrent administration of live and inactivated | 51,175 | 715 | 46,703 | 1,531 | ref | |
| Live | 15,886 | 219 | 15,994 | 1,369 | 0.74 (0.63, 0.86) | 0.0001 |
| Inactivated | 244,458 | 1,717 | 137,129 | 1,252 | ref | |
| Concurrent administration of live and inactivated | 51,175 | 715 | 46,703 | 1,531 | 0.64 (0.59, 0.71) | <0.0001 |
| Sex Specific: | ||||||
| Boys: | 159,806 | 1,459 | 102,416 | 1,425 | ||
| Inactivated | 125,014 | 931 | 70,193 | 1,326 | ref | |
| Live | 8,318 | 122 | 8,267 | 1,476 | 0.48 (0.39, 0.59) | <0.0001 |
| Concurrent administration of live and inactivated | 26,474 | 406 | 23,955 | 1,695 | ref | |
| Live | 8,318 | 122 | 8,267 | 1,476 | 0.71 (0.58, 0.88) | 0.0015 |
| Inactivated | 125,014 | 931 | 70,193 | 1,326 | ref | |
| Concurrent administration of live and inactivated | 26,474 | 406 | 23,955 | 1,695 | 0.67 (0.59, 0.77) | <0.0001 |
| Girls: | 151,713 | 1,192 | 97411 | 1,224 | ||
| Inactivated | 119,444 | 786 | 66935 | 1,174 | ref | |
| Live | 7,568 | 97 | 7727 | 1,255 | 0.48 (0.38, 0.60) | <0.0001 |
| Concurrent administration of live and inactivated | 24,701 | 309 | 22749 | 1,358 | ref | |
| Live | 7,568 | 97 | 7727 | 1,255 | 0.76 (0.60, 0.97) | 0.0247 |
| Inactivated | 119,444 | 786 | 66935 | 1,174 | ref | |
| Concurrent administration of live and inactivated | 24,701 | 309 | 22749 | 1,358 | 0.63 (0.54, 0.72) | <0.0001 |
Number of children by vaccine type at the end of the study
Live vaccines include: MMR, V, and MMRV
Inactivated vaccines include: DTaP, IPV, Hib, HBV, HAV, and MCV (and combined vaccines with these antigens)
See Supplementary table for infections included
Adjusted for chronic conditions, sex, low birth weight, premature, number of hospitalizations prior to 16 months, number of outpatient visits prior to age 16 months, region, urban/rural, birthdate and mother’s age; all HR are stratified by birthdate to control for age, seasonality and year, and interactions between time with number of hospitalizations prior to 16 months, chronic conditions, and number of outpatient visits prior to age 16 months were included to account for the violations of the proportional hazards assumption
Table 4.
Unadjusted and Adjusted Results Upper and Lower Respiratory Infections
| Upper Respiratory Infections | Lower Respiratory Infections | |||||||
|---|---|---|---|---|---|---|---|---|
| Time-dependent vaccination status: Most recent vaccination |
Events | Person- years |
Admissions per 100,000 Person-Years |
Adjusted* Hazard Ratio (95% CI) |
Events | Person- years |
Admissions per 100,000 Person- Years |
Adjusted* Hazard Ratio (95% CI) |
| Overall | 1,041 | 200,730 | 519 | 1,476 | 200,515 | 736 | ||
| Inactivated | 665 | 137,650 | 483 | ref | 958 | 137,519 | 697 | ref |
| Live | 103 | 16,093 | 640 | 0.41 (0.32, 0.51) | 108 | 16,086 | 671 | 0.45 (0.36, 0.56) |
| Concurrent administration of live and inactivated | 273 | 46,987 | 581 | Ref | 410 | 46,910 | 874 | Ref |
| Live | 103 | 16,093 | 640 | 0.75 (0.59, 0.94) | 108 | 16,086 | 671 | 0.63 (0.50, 0.78) |
| Inactivated | 665 | 137,650 | 483 | ref | 958 | 137,519 | 697 | ref |
| Concurrent administration of live and inactivated | 273 | 46,987 | 581 | 0.54 (0.46, 0.64) | 410 | 46,910 | 874 | 0.71 (0.63, 0.81) |
CI: Confidence interval
Adjusted for sex, chronic conditions, low birth weight, premature, number of hospitalizations prior to 16 months, number of outpatient visits prior to age 16 months, region, urban/rural, ever hospitalized previously for nontargeted infections, birthdate and mother’s age; all HR are stratified by birthdate to control for age, seasonality and year, and interactions between time with sex and number of outpatient visits before age 16 months were included to account for the violations of the proportional hazards assumption in the URI model and interactions between time with urban/rural and number of hospitalizations before age 16 months were included to account for the violations of the proportional hazards assumption in the LRI model
Table 5.
Unadjusted and Adjusted Results for Gastrointestinal and Other Infection
| Gastrointestinal Infections | Other Infections | |||||||
|---|---|---|---|---|---|---|---|---|
| Time-dependent vaccination status: Most recent vaccination |
Events | Person- years |
Admissions per 100,000 Person- Years |
Adjusted* Hazard Ratio (95% CI) |
Events | Person- years |
Admissions per 100,000 Person- Years |
Adjusted* Hazard Ratio (95% CI) |
| Overall | 224 | 201,143 | 111 | 698 | 200,909 | 347 | ||
| Inactivated | 146 | 137,884 | 106 | ref | 444 | 137,752 | 322 | ref |
| Live | 28 | 16,138 | 174 | 0.92 (0.59, 1.42) | 51 | 16,124 | 316 | 0.85 (0.63, 1.14) |
| Concurrent administration of live and inactivated | 50 | 47,121 | 106 | Ref | 203 | 47,034 | 432 | Ref |
| Live | 28 | 16,138 | 173 | 1.37 (0.85, 2.22) | 51 | 16,124 | 316 | 0.72 (0.53, 0.98) |
| Inactivated | 146 | 137,884 | 106 | ref | 444 | 137,752 | 322 | ref |
| Concurrent administration of live and inactivated | 50 | 47,121 | 106 | 0.67 (0.47, 0.94) | 203 | 47,034 | 432 | 1.18 (0.99, 1.40) |
CI: Confidence interval
Adjusted for chronic conditions, low birth weight, premature, number of hospitalizations prior to 16 months, number of outpatient visits prior to age 16 months, region, urban/rural, ever hospitalized previously for nontargeted infections, birthdate and mother’s age; all HR are stratified by birthdate to control for age, seasonality and year, and an interaction between time with urban/rural was included to account for the violation of the proportional hazards assumption in the gastrointestinal infections model.
Results of the control outcome, unintentional injuries, were not significantly associated with type of last vaccine received. The risk of an emergency room visit for an unintentional injury from 16 through 24 months of age was not different if the type of last vaccine received was live only compared with inactivated only (HR: 1.16, 95% CI: 0.90, 1.48) or compared with concurrent vaccines (HR: 1.09 95% CI: 0.83, 1.43). No difference was found comparing concurrent receipt with inactivated vaccine.
Discussion
We found a significantly lower risk of NTI hospitalization for children aged 16 through 24 months if the last type of vaccine they received was live only compared with receipt of inactivated only vaccines. A similar but less pronounced decreased risk was found if the type of last vaccine received was concurrent (live and inactivated) vaccines compared with inactivated only vaccine. These findings were similar for boys and girls. The results of our secondary analyses that excluded hospitalizations for vaccine preventable diseases were not materially different. Thus, our study tends to support the overall findings of the Danish study3. Additionally, we found that the reduced risk of NTI hospitalization when the last type vaccine was live only was strongest for lower and upper respiratory infections.
Like the Danish study3, we also assessed the risk of hospitalization including VPDs and their complications in the outcomes. Since their inclusion mixes non-specific with specific (targeted) outcomes and could result in apparently stronger associations through the prevention of targeted outcomes, we excluded VPDs from secondary analyses. However, our results changed only minimally.
Importantly, our study extends the earlier findings of Sørup and colleagues because we evaluated the more comprehensive US childhood vaccination schedule, which includes several vaccines not included in the Danish childhood vaccination schedule. For example, the Danish study specifically evaluated MMR vs. DTaP-IPV-Hib as the last vaccine received, whereas we assessed receipt of any live vaccine (i.e., MMR or Varicella) and any inactivated vaccine (DTaP, Hib, IPV, PCV, HAV, and HBV) so our analyses extend support for non-specific effects by type of vaccine rather than only by specific vaccines. Also, since children who received influenza vaccine (IIV or LAIV) may be at different risk for respiratory infections, to be consistent with the Danish study, we excluded children who ever received an influenza vaccine during the study.
The 2014 Danish study3 did not assess concurrent receipt of live and inactivated vaccines, but another study by the same authors published in 2016 found simultaneous administration of MMR and DTaP-IPV-Hib compared with MMR alone may increase the rate of hospital admissions related to lower respiratory tract infections.11 Similarly, we found that receipt of live vaccine alone had a lower risk compared with inactivated alone or concurrent and that concurrent had a lower risk compared with inactivated. This suggests that the decreased risk found when a live vaccine was received alone was diluted but still present when concurrent (live and inactivated) vaccines were received compared with inactivated vaccine only.
A limitation of our study is that data on potential confounders such as the children’s race and/or ethnicity and socio-economic status were not available. Although race and/or ethnicity could not be inferred from Marketscan®, socio-economic status could be partially inferred because all the children in the database were covered by commercial health insurance plans in which the family’s health insurance was provided through an employer of a family member. Also, our analysis was limited in that we were unable to conduct medical chart reviews to ensure the validity of the ICD-9 codes specified in the hospitalization inpatient claims and the CPT codes for vaccinations in the outpatient claims.
Distinctly, the children included in our analyses had received at least 3 doses of DTaP, an indication that they were a vaccinated group, so we were not comparing vaccinated to unvaccinated, and therefore they differed only on the type(s) of vaccine received last. The vast majority (91.9%) of the children whose last vaccine received was inactivated by the end of the study had previously received the MMR and/or varicella vaccines; most received the vaccine when recommended, yet 19% received the vaccine after the recommended age of 15 months but before they received an inactivated vaccine just prior to study-end; therefore it may be that our results are affected by selection bias if the healthiest children received MMR and/or varicella vaccine by 15 months, and received the last dose of DTaP later12. However, if that were the case the results should be the same for all infections, yet, like the Danish study,3 our results were strongest for respiratory infections. Also, there was no significant difference in the proportion of children with chronic conditions, low birth weight or prematurity receiving live or inactivated vaccines last. Moreover, in the sensitivity analyses, receipt of live vaccine last was not associated with a lower risk of unintentional injury, which implies that our results are not an effect of health-seeking bias. Lastly, another potential concern is misclassification bias if the 8.1% of children who did not have claims for MMR and/or varicella vaccines actually did receive one or both of them; for example, through an immunization provider not participating in MarketScan®. There is no way to know the direction of the potential misclassification with respect to the outcome.
Notably, the hazard ratios in our analyses were less than 1 yet some of the unadjusted rates of NTI hospitalizations per 100,000 person-years were in the opposite direction. The confounding variables that impacted the adjusted results included mother’s age, region, and number of outpatient visits. Inclusion of number of outpatient visits had the strongest effect on the adjusted hazard ratios. It is not unexpected that number of prior outpatient visits would influence the risk of NTI hospitalization, but we do not know why number of outpatient visits would also be strongly associated with receiving a live vaccine only as the last vaccine.
Possible biologic mechanisms to support our findings have not been identified, but could include the concept of ‘heterologous immunity.’ That is, each person has a unique history of infections and vaccinations and every exposure leaves an imprint on the immune system that can affect future immune (innate and adaptive) responses to pathogens.13 Additionally, studies have shown that innate immune responses have adaptive traits that have the potential to provide protection against unrelated infections, a process called ‘trained immunity.’14 A review by IOM in 2002 concluded that “…there is strong evidence for the existence of biological mechanisms by which multiple immunizations under the current U.S. infant immunization schedule could possibly influence an individual’s risk for heterologous infections.”15
Our study addresses a topic of current interest as evidenced by the review being conducted by the World Health Organization Strategic Advisory Group of Experts who are currently reviewing all available evidence on nonspecific vaccine effects to determine if immunization policy adjustments are needed.16,17,18
Along with other recent studies, our study raises the possibility that the order in which vaccines are administered may carry benefits in addition to the prevention of the targeted infections. But the interpretation of our results should be tempered because the extent of potential biases from confounding and selection bias is unknown. To further improve the quality of the evidence on this topic, future studies should include chart reviews to ensure the validity of the ICD codes for the outcomes of interest and to use well-validated databases or registries to ensure correct identification of vaccination status. Ideally, randomized control trials would best control for confounding and avoid selection bias.
Supplementary Material
Summary.
We found a significantly lower risk of non-targeted infectious disease hospitalization for children aged 16 through 24 months if the last type of vaccine they received prior to hospitalization was live only compared with receipt of inactivated only vaccines.
Acknowledgments
Funding
This work was done as routine work of the Immunization Safety Office at the Centers for Disease Control and Prevention.
The authors would like to thank the members of the CDC’s Immunization Safety Office and the Vaccine Safety Datalink Group for their valuable critiques of this study. This work was funded by the Department of Health and Human Services, Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Contributors
BHB, FD, and MMM conceived and designed the study. BHB, FD, and MMM drafted the manuscript. BHB obtained the data. All authors revised the manuscript for important intellectual content and contributed to the literature search. BHB did statistical analyses. MMM and FD provided administrative, technical, and material support.
Declaration of interests
All authors declare no competing interests.
References
- 1.Aaby P, Martins CL, Garly ML, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biering-Sorensen S, Aaby P, Napirna BM, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. The Pediatric infectious disease journal. 2012;31:306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 3.Sorup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. Jama. 2014;311:826–835. doi: 10.1001/jama.2014.470. [DOI] [PubMed] [Google Scholar]
- 4.Truven Health Analytics. Marketscan Research. 2016 http://marketscan.truvenhealth.com/marketscanportal.
- 5.Hansen L, Chang S. White Paper Health Research Data for the Real World: The MarketScan Databases. 2016 http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf, 2016.
- 6.Glanz JM, Newcomer SR, Narwaney KJ, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA pediatrics. 2013;167:274–281. doi: 10.1001/jamapediatrics.2013.502. [DOI] [PubMed] [Google Scholar]
- 7.Danish Health and Medicines Authority. Denmark's Childhood Vaccination Programme 2012. 7. Denmark: Danish Health and Medicines Authority; 2013. https://sundhedsstyrelsen.dk/~/media/1782C0C1B63A4F239C2908A98B3E9456.ashx. [Google Scholar]
- 8.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133:e1647–1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Children’s Safety Network Economics & Data Analysis Resource Center. From E to VWXY Cause of Injury Codes. 2005 http://www.cippp.org/pubs/vwxy-no-h.pdf.
- 11.Sorup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Simultaneous vaccination with MMR and DTaP-IPV-Hib and rate of hospital admissions with any infections: A nationwide register based cohort study. Vaccine. 2016;34:6172–80. doi: 10.1016/j.vaccine.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrington CP, Firth MJ, Moulton LH, et al. Epidemiological studies of the non-specific effects of vaccines: II--methodological issues in the design and analysis of cohort studies. Trop Med Int Health. 2009;14:977–985. doi: 10.1111/j.1365-3156.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodridge HS, Ahmed SS, Curtis N, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. [Accessed 04/13/2016]; Immunization Safety Review: Multiple Immunizations and Immune Dysfunction. 2002 http://iom.nationalacademies.org/reports/2002/immunization-safety-review-multiple-immunizations-and-immune-dysfunction.aspx. [PubMed]
- 16.World Health Organization. [Accessed 07/06/2016];SAGE Working Group on Non-specific Effects of Vaccines (March 2013 – June 2013) 2013 http://www.who.int/immunization/sage/sage_wg_non_specific_effects_vaccines_march2013/en.
- 17.Higgins JP, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 - conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:221–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


