Abstract
Blockade of immune checkpoint molecules to reverse cancer-induced immune suppression can improve anti-tumor immune responses in cancer patients. Monoclonal antibodies targeting two such molecules, Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) have shown clinical benefit in the treatment of advanced malignancies, including metastatic melanoma. Adverse effects of these immune checkpoint inhibitors include immune-related adverse events (irAE), of which one of the most common is autoimmune thyroiditis. Though thyroiditis is increasingly recognized, there are no reports of the pathological findings that occur in immunotherapy-induced thyroiditis. We present a case of immunotherapy-induced thyroiditis demonstrating its unique cytopathologic features. A 51-year-old woman with metastatic melanoma was found to have a suppressed TSH and elevated free thyroxine concentration 14 days after starting treatment with nivolumab (PD-1 antagonist) plus ipilimumab (CTLA-4 antagonist) therapy. A thyroid biopsy was performed based on ultrasound findings and cytopathology revealed unique features including abundant clusters of necrotic cells, lymphocytes and CD163-positive histiocytes. This case reports cytopathologic features found in immune checkpoint inhibitor related thyroiditis. These appear to be unique findings and may help inform future research regarding the pathophysiology and mechanisms of this condition.
Keywords: Thyroiditis, PD-1, CTLA-4, Ipilimumab, Nivolumab, Cytology
Introduction
Cancer-induced immune suppression prevents effective anti-tumor immune responses. Blockade of immune checkpoint molecules by monoclonal antibodies represents one form of immunotherapy to reverse this immune suppression in cancer patients. Antibodies targeting two such molecules, Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4), have shown clinical benefit in several cancer types including metastatic melanoma.1, 2 Adverse effects of these immune checkpoint inhibitors include immune-related adverse events (irAE), in which there is induction of new autoimmunity against tissues in the body. Dysfunction of endocrine glands are among the most common irAE, of which pituitary hypophysitis and autoimmune thyroiditis are likely the most common.3, 4, 5, 6 Unique features of thyroiditis occurring during treatment with checkpoint inhibitor therapy have been recognized, including frequent lack of thyroperoxide (TPO) antibody that normally characterizes autoimmune thyroid disease.7 Despite increasing recognition of thyroiditis as a common adverse effect, there are no reports of the associated pathological findings that occur. Here, we present a case of immunotherapy-induced thyroiditis demonstrating unique cytopathologic features.
Case discussion
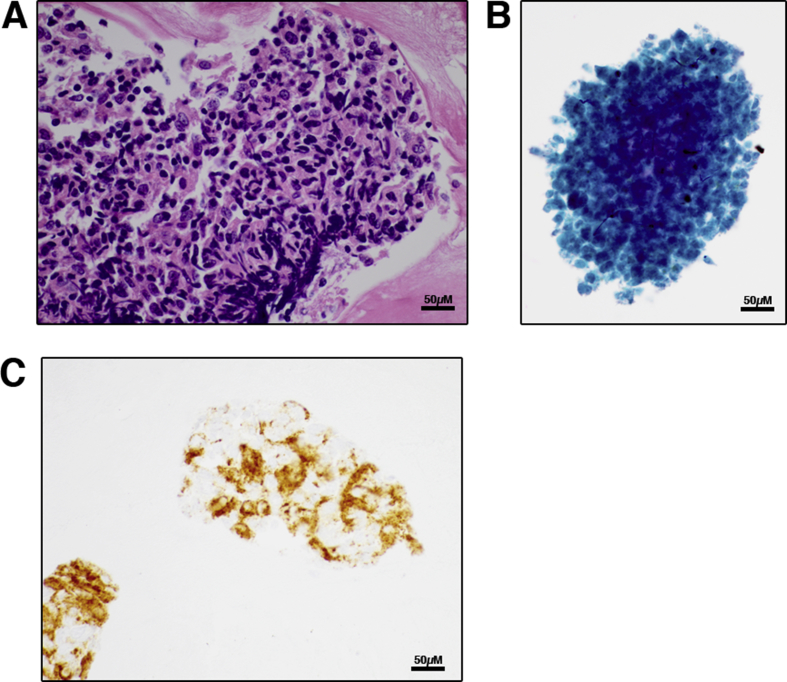
A 51-year-old woman with metastatic melanoma was found to have a suppressed TSH and elevated free thyroxine concentration 14 days after starting treatment with nivolumab (PD-1 antagonist) plus ipilimumab (CTLA-4 antagonist) therapy. Of note, she had thyrotoxicosis with spontaneous resolution five years prior, but thyroid testing was normal at initiation of immunotherapy. An Iodine-123 uptake was 0.3% at 24 h and hypothyroidism subsequently developed requiring levothyroxine replacement, consistent with a thyroiditis. Restaging imaging showed a possible right thyroid nodule and ultrasound was obtained showing diffuse heterogeneity and hypervascularity, with a right 1.0 cm hypoechoic area. Fine needle aspiration biopsy was performed and cytopathology revealed a few lymphohistiocytic aggregates typical of Hashimoto thyroiditis (Fig. 1A), and a unique finding: abundant clusters of necrotic cells (Fig. 1B). Typical thyroid follicular cells were rare to absent. Immunostaining confirmed the presence of few AE1/AE3 keratin-positive thyroid follicular epithelial cells clustered with lymphocytes and CD163-positive histiocytes (Fig. 1C).
Figure 1.
Fine needle aspiration biopsy in thyroiditis induced by immune checkpoint therapy. (A) Hematoxylin and eosin stained cell block, showing lymphohistiocytic aggregates (600×). (B) Papanicolaou stained ThinPrep showing necrotic cells with thyroid follicular cells rare to absent (600×). (C) Immunostain for CD163 (cell block) showing epithelial cells clustered with CD163+ histiocytes (600×).
The development of thyroiditis occurs commonly in patients treated with immune checkpoint therapy. Recognizing the pathologic features of this entity may help in understanding its mechanisms, which remain to be fully elucidated. In this case, cytopathology showed necrotic cells, together with lymphocytes and CD163+ histiocytes. CD163 is a hemoglobin scavenger receptor present on macrophages,8 typically of M2 phenotype involved in tissue repair. Such CD163+ macrophages have been observed in the tumor microenvironment of cancers and their abundance may increase after immune checkpoint therapy. While CD163+ cells may be found in patients with autoimmune thyroid disease, necrotic cells are not present in this context, making this a unique cytologic specimen. Our report is the first to show the possible cytologic findings indicative of thyroiditis associated with immune checkpoint therapy.
Disclosures
No authors have conflicts of interest to declare.
Funding
NIH K08 HD070957 (PI: Le Min, Mentor: Ursula Kaiser)
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Hodi F.S., O'Day S.J., McDermott D.F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlov S., Salari F., Kashat L., Walfish P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100:1738–1741. doi: 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]
- 4.Villa N.M., Farahmand A., Du L. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf) 2017 Sep 23 doi: 10.1111/cen.13483. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barroso-Sousa R., Barry W.T., Garrido-Castro A.C. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2017 Sep 28 doi: 10.1001/jamaoncol.2017.3064. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H., Hodi F.S., Giobbie-Hurder A. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res. 2017 Oct 27 doi: 10.1158/2326-6066.CIR-17-0208. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delivanis D.A., Gustafson M.P., Bornschlegl S. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017 Aug 1;102:2770–2780. doi: 10.1210/jc.2017-00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaer D.J., Schaer C.A., Buehler P.W. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]