Abstract
Compared with imaging in the visible (400 – 650 nm) and near-infrared window I (NIR-I, 650 – 900 nm) regions, imaging in near-infrared window II (NIR-II, 1,000–1,700 nm) is a highly promising in vivo imaging modality with improved resolution and deeper tissue penetration. In this work, a small molecule NIR-II dye,5,5'-(1H,5H-benzo[1,2-c:4,5-c'] bis[1,2,5]thiadiazole)-4,8-diyl)bis(N,N-bis(4-(3-((tert-butyldimethylsilyl)oxy)propyl)phenyl) thiophen-2-amine), has been successfully encapsulated into phospholipid vesicles to prepare a probe CQS1000. Then this novel NIR-II probe has been studied for in vivo multifunctional biological imaging. Our results indicate that the NIR-II vesicle CQS1000 can noninvasively and dynamically visualize and monitor many physiological and pathological conditions of circulatory systems, including lymphatic drainage and routing, angiogenesis of tumor and vascular deformity such as arterial thrombus formation and ischemia with high spatial and temporal resolution. More importantly, by virtue of the favorable half-life of blood circulation of CQS1000, NIR-II imaging is capable of aiding us to accomplish precise resection of tumor such as osteosarcoma, and to accelerate the process of lymph nodes dissection to complete sentinel lymph node biopsy for better decision-making during the tumor surgery. Overall, CQS1000 is a highly promising NIR-II probe for multifunctional biomedical imaging in physiological and pathological conditions, surpassing traditional NIR-I imaging modality and pathologic assessments for clinical diagnosis and treatment.
Keywords: NIR-II, fluorescence imaging, tumor, sentinel lymph node, vascular and lymphatic system
Graphical abstract
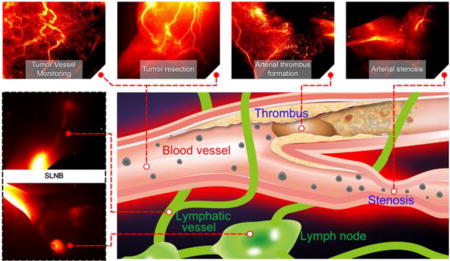
This study unambiguously shows that NIR-II CQS1000 vesicles can not only visualize and monitor circulatory system noninvasively and dynamically, but also aid us to accomplish more precise resection of tumor and accelerate the process of SLNB for better decision-making. Deep tissue penetration ability, desirable chemical and optical properties render NIR-II CQS1000 a highly promise probe for future clinical applications.
1. Introduction
Currently, fluorescence-based optical imaging is highly attractive for observing and assessing biological processes in living subjects because of its high sensitivity, high spatial and temporal resolution, non-ionizing radiation, ease of use, low cost, etc.[1] Particularly, fluorescence imaging in the near-infrared (NIR) window (650–1,700 nm) has shown to be superior to traditional visible imaging. This novel imaging technique has already been used in numerous clinic fields, such as evaluation of hepatic function,[2] monitoring the treatment response in inflammatory arthritis,[3] non-specific tumor targeting,[4] guiding tumor surgery,[5] and intra-operative tumor imaging.[6] However, fluorescence imaging at the first NIR window region (NIR-I; 650–900 nm) still has limited capability to obtain detailed information of many physiological or pathological processes including vascular and lymphatic system malfunction, because of scattering and high auto-fluorescence of normal tissue in the NIR-I region,[7] leading to the failure of precise observation.[8] Consequently, fluorescence imaging at the NIR-II window (1,000–1,700 nm) has attracted significant interests recently owing to its inherent advantages of reduced scattering (inverse wavelength dependence of photon scattering as the photons travel through subcutaneous tissue and skin[9]) and negligible auto-fluorescence of normal tissue, thus allowing much deeper tissue penetration[10] with high resolution of anatomic characteristics[11] compared with NIR-I imaging.[11–12]
At present, single-walled carbon nanotubes (SWNTs),[11, 13] Ag2S quantum dots (QDs),[14] rare-earth doped nanoparticles[15] have shown to be useful for NIR-II imaging in small animal models. But these nanoprobes suffer from low fluorescence quantum yield,[9, 16] long retention in vivo and potential toxicity,[14b, 14c, 17] which dramatically hinder their further biomedical applications, especially clinical translation. Therefore, it is highly desirable to develop new fluorescent probes with improved properties for in vivo NIR-II imaging. To achieve such a goal, small organic molecule-based fluorophores have been investigated as promising platforms, because of their favorable characteristics such as high quantum yield,[18] high biocompatibility, and rapid excretion from living subject.[19] Recently, we have successfully developed a small-molecule organic NIR-II dye, CH1055, which shows high aqueous solubility, renal clearance capability, and biocompatibility. It can be used for in vivo passive or targeted tumor imaging with much higher imaging resolution and contrast than conventional NIR-I dyes.[10] Further work on newly designed NIR-II fluorophores with a different core structure from CH1055 also highlights the promise and advantages of using NIR-II small molecule probes for in vivo imaging.[20] However, the imaging properties of small molecule based NIR-II probes need to be further improved, and the versatility of NIR-II probes to visualize and evaluate biological processes and anatomical structure precisely also remains unclear. Furthermore, many potential important applications of small molecule NIR-II fluorophores for aiding physicians and surgeons to overcome the obstacles in clinical treatment such as real-time assessment of circulatory system and lymphatic routing have not been fully explored. Herein, we encapsulate a NIR-II dye, 5,5'-(1H,5H-benzo[1,2-c:4,5-c'] bis[1,2,5]thiadiazole)-4,8-diyl)bis(N,N-bis(4-(3-((tert-butyldimethylsilyl)oxy)propyl)phenyl) thiophen-2-amine), into phospholipids vesicles to prepare the probe CQS1000 and evaluate its’ usefulness for providing information of physiological and pathological processes in vivo (Figure 1a). Specifically, the in vivo NIR-II imaging using CQS1000 has been explored, including dynamic observation of blood circulation with ordinary and pathological processes and angiogenesis development in tumor, lymphatic system and its response under pathological conditions. We have further used CQS1000 to realize the image-guided surgery for sentinel lymph node mapping and biopsy, and to perform precise and complete resection of tumor by distinguishing its blood supply and lymphatic drainage for better survival and reduced relapse.
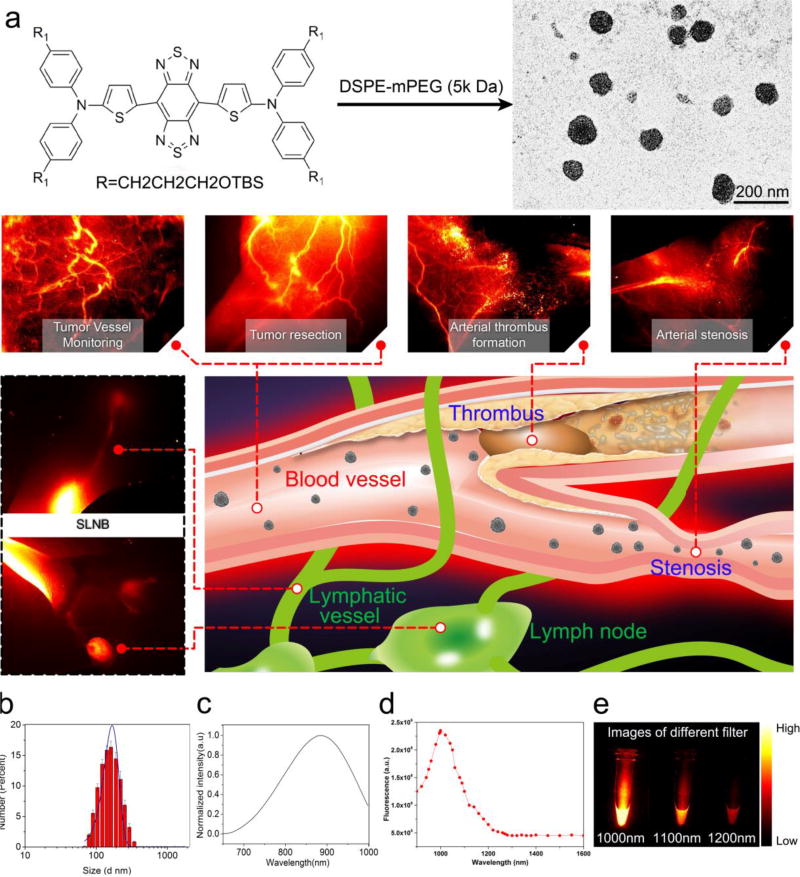
Figure 1. Schematic illustration of biomedical applications using CQS1000 and characterization of CQS1000.
a. Schematic illustration of the biological application using CQS1000. b. DLS of CQS1000. c and d. Absorbance and fluorescent emission of CQS1000, demonstrating an absorbance peak at 830 nm and an emission peak at 1,000 nm. The fluorescent emission spectrum was obtained with an 808 nm excitation laser. e. NIR-II signals of CQS1000 with sequential long-pass filters (1,000–1,200 nm).
2. Results
2.1. Characterization of CQS1000
The schematic structure of 5,5'-(1H,5H-benzo[1,2-c:4,5-c'] bis[1,2,5]thiadiazole)-4,8-diyl)bis(N,N-bis(4-(3-((tert-butyldimethylsilyl)oxy)propyl)phenyl)thiophen-2-amine) was shown in Figure 1a, and it was synthesized according to the previously reported method[20]. CQS1000 vesicles with NIR-II fluorescent emission were then prepared. The hydrodynamic size of CQS1000 was determined as 167 ± 52 nm by DLS analysis (Figure 1b). The UV/Vis/NIR absorption spectrum of the CQS1000 vesicles showed an absorption band at 800–900 nm, with maximum absorption at 830 nm (Figure 1c). The fluorescence emission spectrum of CQS1000 showed a peak emission wavelength at 1000 nm (Figure 1d), indicating a Stokes shift 170 nm. A typical TEM image showed that CQS1000 vesicles were monodispersed with an average diameter of 100.2 ± 13.3 (Figure 1a). Lastly, the NIR-II fluorescence signals of CQS1000 under different LP filters (1,000–1,200 nm) showed that the fluorescence signals of CQS1000 were distinct at 1,000nm filter and the signals above 1,100nm were also apparent, demonstrating that CQS1000 can be used for NIR-II imaging (Figure 1E).
2.2. Cytotoxicity assay and biodistribution
The potential cell toxicity of CQS1000 to the mouse embryonic fibroblast cell line NIH 3T3 and the glioblastoma cell line U87MG was evaluated. It was found that CQS1000 showed no apparent cytotoxicity to both cell lines even at the concentration up to 16 µM, demonstrating its good biocompatibility (Figure S1a). Ex vivo biodistribution studies performed at 24 h post-injection of the CQS1000 vesicles displayed that CQS1000 vesicles mainly accumulated in the liver and spleen, and a small fraction of probe signal was also detected in kidneys (Figure S1b and S1c). Lastly, H&E staining of vital organs including heart, liver, spleen, lung and kidney showed that no obvious tissue damages or lesions were observed in the tissue slices on 7 days after CQS1000 vesicles injection compared with normal tissues (Figure S1d).
2.3. NIR-II imaging for evaluation of circulatory system
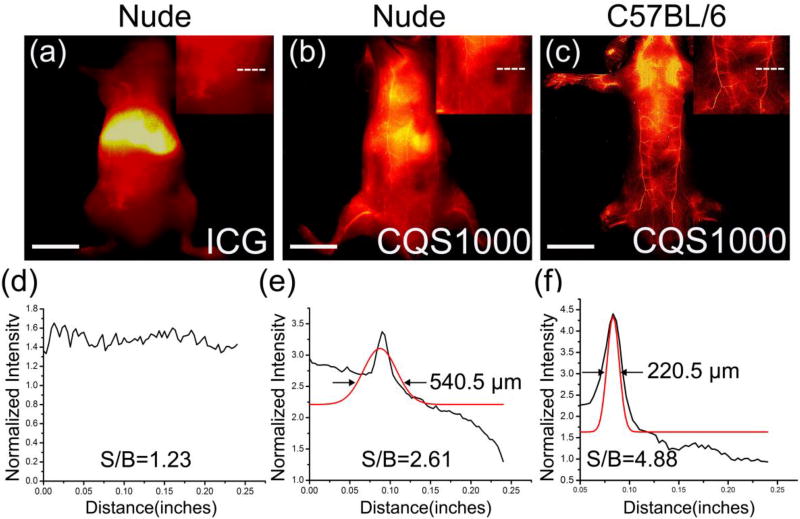
To explore the potential of CQS1000 as a novel NIR-II probe for reliable assessment of the circulatory system in living subject, CQS1000 vesicles were injected intravenously into mice. After 10 min, NIR-II images showed that the circulatory system was clearly visualized in nude mice and C57BL/6 mice (n=3), while blurry images were observed in nude mice injected with NIR-I dye, ICG (Figure 2a–c). The signal-to-background ratios (SBRs) were measured by plotting cross-sectional intensity profiles of the same vessel(superior epigastric artery, marked by a white dash line, Figure 2a–c) imaged in the NIR-I window with ICG and NIR-II window with CQS1000. The SBR obtained by imaging in NIR-II window was proved to be much higher than that of in NIR-I window (4.88 ± 0.09 vs.1.23 ± 0.15; P<0.01). Moreover, using the Gaussian-fitted full width at half maximum (FWHM) of the cross-sectional intensity profiles of the blood vessels, we observed that the FWHM of the distinct feature imaged in the NIR-II window was 220.5µm (Figure 2f), while the FWHM of the same blood vessel imaged by ICG was un-achievable (Figure 2d), suggesting that CQS1000 was capable of providing us much higher resolution and more accurate information of deep tissues compared with ICG because of its long circulation time, reduced photon scattering, and low auto-fluorescence of normal tissue (Figure 2).
Figure 2. In vivo fluorescence images of CQS1000(NIR-II) and ICG (NIR-I).
(a)–(c) ICG and CQS1000 were injected intravenously into nude mice and C57BL/6 mice, and then NIR images were obtained at 10 minutes after injection, with the vessel FWHM width (white lines in a, b, c) and SBR (white lines in a, b, c) analysis shown in (c) (failed to acquire because of the broadened peak of signal), (d) and (e), respectively, basing on the cross-sectional intensity profiles. Scale bar: 1.5 cm.
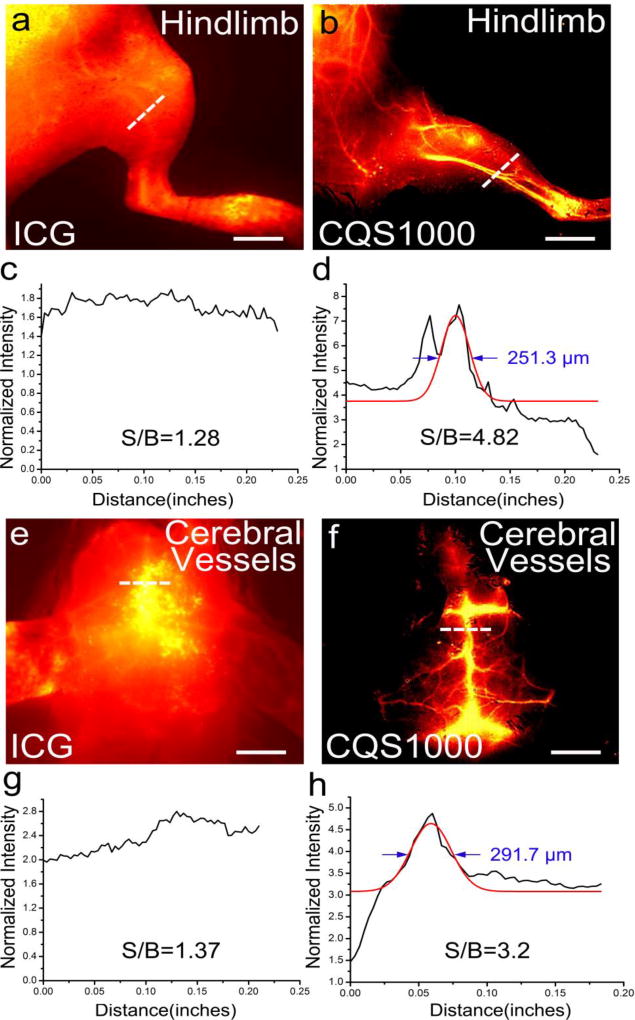
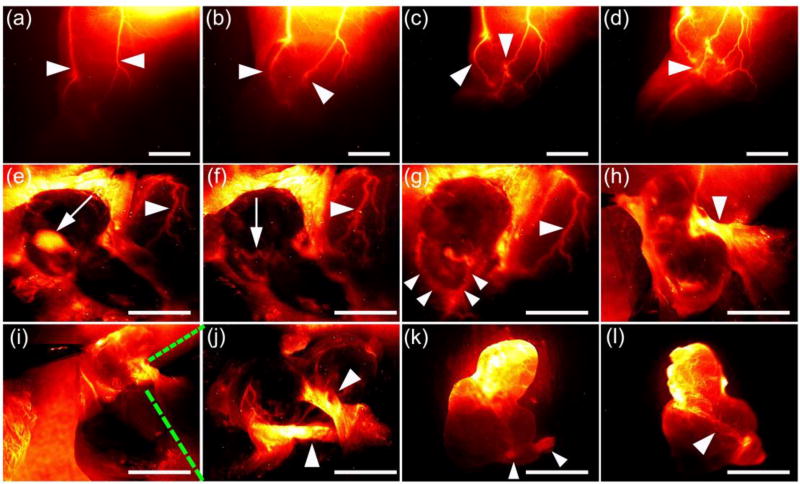
We further observed high-magnification vascular system imaging in mice (n=3) hind limb and cerebrovascular imaging using ICG and CQS1000. The vessels imaged by CQS1000 exhibited improved sharpness feature, and higher SBR for femoral artery of 4.82 ± 0.08 was obtained for CQS1000 compared to 1.28 ± 0.11 for ICG (Figure 3a–d). Similarly, significant higher SBR for superior sagittal sinus of 3.22± 0.12 was obtained for CQS1000 than 1.37 ± 0.12 for ICG (Figure 3e–h). FWHM of the cross-sectional intensity profiles of the hind limb and cerebral blood vessels were measured as 251.3 µm± 2.6 and 291.7 µm± 6.1, respectively, whereas the Gaussian fit was failed in the NIR-I image because of the poor contrast and resolution of ICG imaging (Figure 3a and 3e).
Figure 3. Fluorescence images of the vasculature of hind limb and brain in C57BL/6 mice using CQS1000(NIR-II) and ICG (NIR-I).
In vivo fluorescence vascular image showing hind limb(femoral artery) and cerebrovascular vessels in the NIR-I (ICG) and NIR-II (CQS1000) regions in C57BL/6 mice shown in (a, b, e, f), with the vessel FWHM width and SBR (white lines in a, b, e, f) analysis shown in (c, g) (failed to acquire because of the broadened peak of signal), (d) and (h), respectively, basing on the cross-sectional intensity profiles. Scale bar: 1.5 cm.
2.4. NIR-II imaging for assessment of angiogenesis of tumor
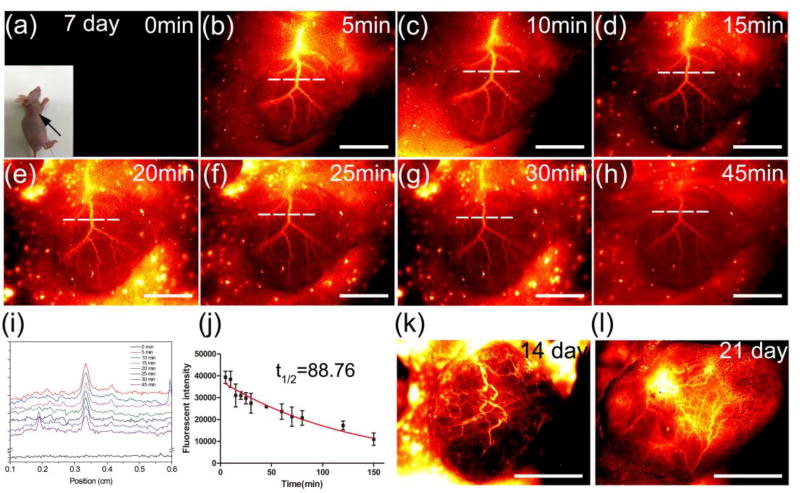
To visualize the vascular network in tumor, NIR-II images were captured at every 5 minutes during a 45 minutes imaging period after injection of 100µL of CQS1000 (200 nmol) into the tail vein of nude mice on day 7 after 143B tumor inoculation subcutaneously. The major blood vessel supplying the tumor accompanied with the branches and capillary network to feed the tumor were unambiguously observed (Figure 4a–h). Fluorescent intensities were also determined by plotting the cross-sectional intensity profiles of the same vessel (major trunk) imaged in the tumor area at different time points (Figure 4i). It showed that the blood-circulation half-life of the CQS1000 was determined as 88.76 ±2.90 min based on a Gaussian fit to the fluorescent intensity with different time after QCS1000 injection (Figure 4j). Furthermore, the angiogenesis development and evolution were explored using CQS1000 in the same tumor at 14 and 21 days after 143B inoculation, respectively. A vascular network with gradually complicated pattern was clearly observed in NIR-II images (Figure 4k and l).
Figure 4. In vivo dynamic assessment of angiogenesis development of tumor.
NIR-II fluorescence images were captured on the 143B osteosarcoma tumor-bearing mouse every 5 minutes after the CQS1000 injection intravenously (a)–(h). (i). A series analysis of cross-sectional intensity profile measured along the white-dashed line in (a)–(h). (J). A fluorescent intensity analysis representing the intersection point with white-dashed line and the major artery supporting the tumor in (a)–(h), and the half - life of blood circulation was calculated as 88.76 min. (k) and (l). Fluorescent images captured on 14 and 21 days after the 143B osteosarcoma inoculation on nude mice, respectively, indicating the disorganized and tortuous pattern of blood vessel in tumor. Scale bar: 4mm.
2.5. NIR-II imaging for precise resection of tumor
The potential of CQS1000 for intra-operative assessment of the vascular supply for precise tumor resection was evaluated. First, pre-evaluation of the blood supply of the tumor was shown in Figure 5a–d. Notably, there were two separate blood vessels to support the tumor and the convergence point was clearly visualized (Figure 5c). Next, the fluorescent image-guided surgery to dissect the tumor under NIR-II setup was successfully accomplished (Figure 5e–l). During this process, the sentinel lymph node (iliac lymph node) and the concomitant artery were easily exposed and identified (Figure 5e and f, respectively). After the major artery to support tumor were surgically exposed and isolated, the precise resection of the tumor was achieved (Figure 5k and l). As the ex vivo image shown in Figure 6k, the major blood artery and vein of tumor were successfully ligated.
Figure 5. Intra-operative assessment of the vascular supply using NIR-II CQS1000 for precise tumor resection.
(a)–(d). Pre-evaluation of the blood supply of the tumor. White arrowheads indicate the major blood vessels to supply the tumor. (e) and (f) Intra-operative fluorescent images, indicating that the sentinel lymph node(iliac lymph node) and the concomitant artery were easily exposed and identified (white arrows), respectively. White arrowheads indicate the major blood vessels to supply the tumor. (g) and (h). Major blood vessels supporting the tumor were dissected and exposed (white arrowheads). Major blood vessels were further dissected surgically into two small branches (i) and magnified as (j). As the ex vivo image shown in (k), the major blood artery and vein of tumor were successfully ligated (white arrowheads). Scale bar: 4mm.
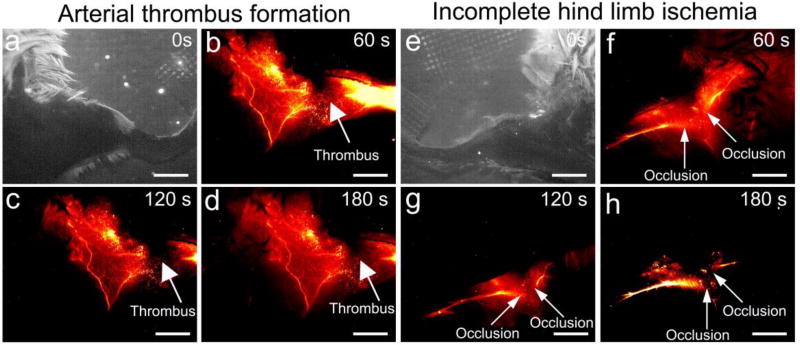
Figure 6. In vivo NIR-II images for arterial thrombus formation and incomplete hind limb ischemia.
NIR-II images of the left hind limb were captured at 0s (a), 60s (b), 120s (c), 180s (d) after CQS1000 injection intravenously into the tail vein of C57BL/6 mice. White arrows indicate the thrombus formation in femoral artery. NIR-II images of the left hind limb were captured at 0s (e), 60s (f), 120s (g), 180s (h) after CQS1000 injection intravenously into the tail vein of C57BL/6 mice. White arrows indicate the incomplete ischemia (occlusion) in femoral artery. Scale bar: 4mm.
2.6. NIR-II imaging for arterial thrombus formation and incomplete hind limb ischemia
To observe the arterial thrombus formation in real-time, a reported method was used to generate the complete thrombus in femoral artery.[22] As observed in Figure 6(a–d), a major coloboma-like signal was clearly observed from 60 second post-injection of the probe right at the induction area of arterial thrombus. Moreover, a modified method was also used to induce incomplete hind limb ischemia at femoral artery and its major branch. Not surprisingly, the occlusion site was also confirmed by CQS1000 NIR-II imaging, which precisely matched with our suture knots made during the surgery (Figure 6e–h).
2.7. NIR-II imaging for lymphatic drainage
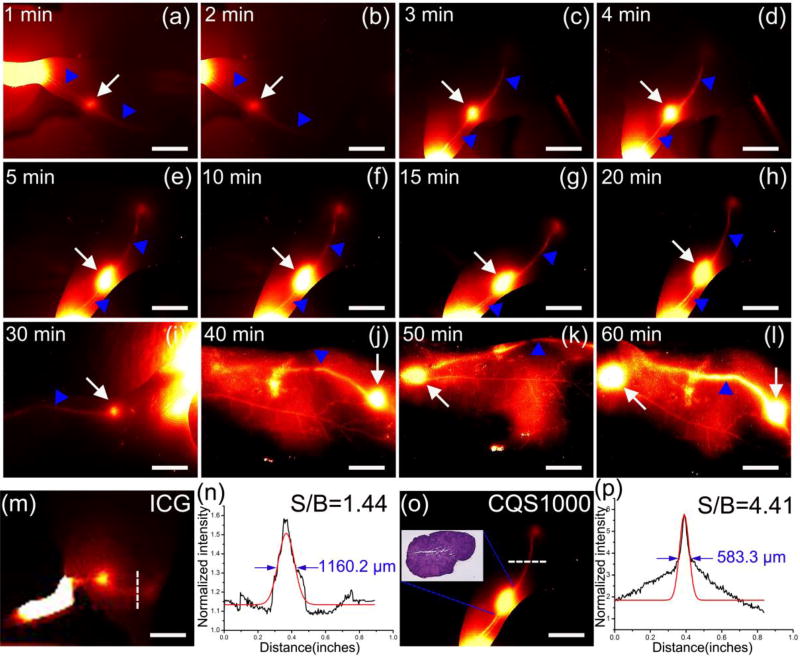
To identify the lymphatic system including lymphatic vessels and lymph nodes under in vivo NIR-II imaging, CQS1000 vesicles were injected intradermally at rear paw of nude mice. The popliteal lymph nodes were easily identified at 1 minute post-injection (Figure 7a). Then the inguinal and axillary lymph nodes were sequentially and clearly observed during the next 55 minutes after CQS1000 injection (Figure 7b–l). The lymphatic vessels connecting the injection site with the sentinel lymph node as well as the afferent and efferent lymphatic vessels were also unambiguously distinguished, providing us a comprehensive understanding of the real time lymphatic drainage in living subjects. We also compared the resolution of lymphatic vessel and lymph node imaged by ICG and CQS1000 using the Gaussian-fitted FWHM. As shown in Figure 7n and 7p, compared to NIR-I imaging, the lymphatic vessel visualized in the NIR-II window demonstrated significant enhanced feature sharpness (583.3±11.30 µm vs.1160.2 ±27.41 µm) and increased SBR (4.41 ± 0.27 µm vs.1.44 ± 0.15 µm). In addition, the acquisition time of lymph node mapping using ICG (more than 15 min) is too long to satisfy the requirement of sentinel lymph node biopsy during the tumor surgery (Figure S2). Moreover, the identification of inguinal lymph node was failed during the ICG mapping process due to the excessive fat and soft tissue coverage. These results clearly demonstrated the superiority of NIR-II imaging compared to NIR-I imaging.
Figure 7. In vivo NIR-II imaging for lymphatic drainage.
The popliteal lymph nodes (white arrowhead) could be identified at 1 minute post-injection (a). Then, the inguinal and axillary lymph nodes (white arrowheads) were sequentially and clearly observed during the next 55 minutes after CQS1000 injection (b)–(l). The lymphatic vessels connecting the injection site with the sentinel lymph node (white arrows) as well as the afferent and efferent lymphatic vessels (blue arrowheads) were also unambiguously distinguished. ICG and CQS1000 (40µL) were injected in the left rear footpad of a mouse, respectively. As shown in (m) and (o), the lymph nodes and vessel anatomy identified with CQS1000 are much sharper in comparison with those of ICG, with the FWHM width and SBR (white lines in m, o) analysis shown in (n), and (p), respectively, basing on the cross-sectional intensity profiles. Scale bar: 4mm.
2.8. NIR-II imaging-guided sentinel lymph node mapping and biopsy
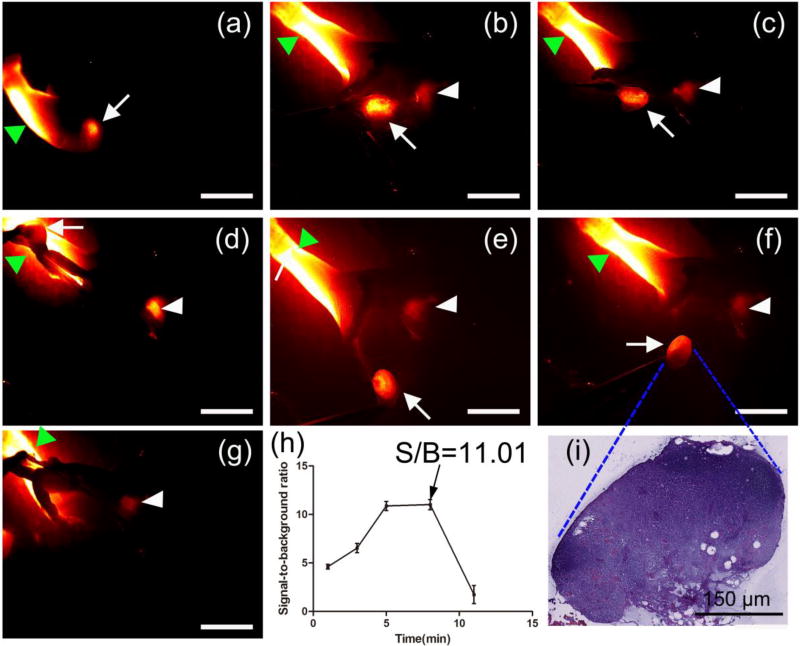
Because of the excellent photochemical and physical properties of CQS1000, lymphatic flow was successfully traced toward the sentinel lymph node from the injection site in real time with high sensitivity, and then the node was dissected at its precise position in a short time, mimicking the standard SLNB procedure as in clinical cancer surgery (Figure 8a–i). It should be emphasized that not only the afferent lymphatic vessel but also the axillary artery was clearly luminous in our study (Figure 8d and 8e). After the lymph node was resected, the operative field was clearly identified with NIR-II imaging, proving no lymph node remained except the axillary artery (Figure 8g). Because of the high contrast property of CQS1000, SBR in the sentinel lymph node biopsy was achieved as high as 11.01± 0.14 (Figure 8h).
Figure 8. In vivo NIR-II imaging-guided sentinel lymph node mapping and biopsy.
The axillary lymph node(white arrow) was clearly identified at 10mins after CQS1000 injection (a). Then, the lymphatic flow toward the sentinel lymph node from the injection site was traced successfully in real time, and then the lymph node (white arrows) was dissected at its precise position in a short time (b)–(i), mimicking the standard SLNB procedure of in clinical cancer surgery. The axillary artery (white arrowhead) was clearly luminous in (e). After the lymph node was resected, the operative field was clearly identified with NIR-II imaging, proving no lymph node was remained except the axillary artery (white arrowhead) (g). SBR was determined with different time after CQS1000 injection (h) and the dissected lymph node was further confirmed by histological analysis (i). Scale bar: 4mm.
To evaluate the potential application of NIR-II imaging using CQS1000 for image-guided sentinel lymph node mapping and biopsy under the pathological status, CQS1000 was injected intradermally at rear raw of C57BL/6 mice bearing with B16F10 mouse melanoma (Figure S3a). Five minutes later, popliteal lymph node was notably identified (Figure S3c). It was remarkable that the afferent lymphatic vessel became visible and distinguishable at 10 minutes post-injection and it exhibited increased fluorescent signal over time (Figure S3d–f). With the help of the NIR-II imaging set up and the outstanding optical property such as high optical intensity of CQS1000, we successfully performed the SLN biopsy under a high contrast (Figure S3g–i).
3. Discussion
Compared to traditional radiative imaging modalities such as positron emission tomography (PET), single photon emission computed tomography (SPECT) and X-ray computed tomography (CT) scan, fluorescence-based optical imaging techniques benefits from its high safety without radiation, fast imaging capability because of its high temporal resolution.[23] But optical imaging in the visible (400–650 nm) and traditional NIR-I region (650–900 nm) is still limited by the poor tissue penetration ability because of tissue absorption and scattering of photons.[12b] Recently, NIR-II imaging (1,000–1,700 nm) has drawn high interest because of its’ improved tissue penetration and high-resolution imaging capability in living subjects, and several studies has made important progress on demonstrating the potential use of NIR-II imaging techniques in a couple of significant clinical implications such as cerebrovascular imaging in physiological and pathological processes.[12a, 12c] In this report, we has developed a novel NIR-II dye labeled organic vesicles CQS1000 and further demonstrate that several complicated clinical procedures using conventional CT angiography (CTA), ultrasonography or magnetic resonance imaging (MRI) can be successfully accomplished by our simple and safe NIR-II imaging setup and new NIR-II probe.
Providing detailed anatomic information of circulatory system with high spatial and temporal resolution is of great clinical significance and may facilitate development of new therapies for peripheral arterial diseases (PADs).[24] However, current methods for imaging vascular structures such as CT and MRI are limited by long scanning and post-analyzing times, resulting in poor temporal resolution.[25] Similarly, color doppler ultrasonography possessing with high temporal resolution is limited with poor spatial resolution.[26] In our study, we have shown that the circulatory system both in cerebrovascular and peripheral region can be distinctly visualized by NIR-II imaging setup using CQS1000 vesicles, and the SBR and imaging resolution of CQS1000 are much higher than those of ICG, indicating that CQS1000 is a promising molecular probe for future clinical procedures such as vascular deformity (Figure 2 and 3). Particularly, the signal of CQS1000 distributed in the blood vessels of mice demonstrates the long blood circulation time of CQS1000(t1/2 = 88.76±2.90 min). On the contrary, the signal of ICG in blood vessels is hard to be observed and ICG was mainly accumulated in the liver. For pathological condition, as the solid tumor with a diameter >1 mm requires an adequate blood supply for growth and metastasis,[27] therefore it is of great clinical significance to have effective methods for monitoring the tumor angiogenesis as well as microvascular density and branching patterns in a noninvasive manner, which is crucial for early detection of tumor and screening of anti-angiogenic drugs. In this study, with the help of high spatial and temporal resolution as well as the appreciable long blood circulation time of CQS1000, the angiogenesis and the vascular evolution of tumor have been clearly observed and evaluated in the desired time course (Figure 4), indicating the disorganized and tortuous pattern of blood vessel in tumor.[28] This finding leads us to conclude that CS1000 NIR-II imaging can serve as a promising strategy for evaluation of new early anti-angiogenesis therapy of tumor.
It has been believed that patterns and characteristics of blood vessel supporting tumor are significant factors to predict tumor aggressiveness and prognosis of cancer patients.[29] For example, it has been found that the density and the pattern of the microvascular loops and networks revealed by standard immunohistochemistry (IHC) in ocular melanoma vessels are closely correlated to the decreased survival of patients.[30] However, it is always difficult to obtain intravital information of the blood vessel supporting the tumor before the surgery.[31] Although CTA is the gold standard method to perform preoperative blood supply mapping, it has significant limitations due to the risk of radiation exposure, cost and the side effect of the CT contrast agents.[32] Fisher et al. has reported the optical observation of living human tumors with high spatial resolution using intravital microscopic setup and fluorescence in 400ms exposure time,[29] but this setup needs to stop the respirations temporarily for 30s to facilitate stabilization of the images. In our study, we have successfully performed in vivo real-time direct observation of vessels for pre-surgery evaluation and intra-operative resection of tumor with high temporal resolution of 200ms and no need to stop the respirations (Figure 5). With the help of the NIR-II imaging setup and CQS1000, interestingly, it has found that the junction of two blood supplies can be clearly observed and distinguished, suggesting that the intervention therapy such as occlusion of tumor vessel can be achieved using this powerful technique. In addition, attributing to the desirable optical property of CQS1000, the tumor, sentinel lymph node coupled with the major blood supply have been easily visualized and then completely resected (Figure 5), highlighting that the NIR-II fluorescent image-guided surgery using CQS1000 may introduce a new strategy to reduce the possibility of tumor dissemination and metastasis (e.g. by circulatory tumor cells).
Currently, how to non-invasively monitor the lymphatic drainage dynamically remains a challenge to physicians. Moreover, since the initial introduction of SLN biopsy (SLNB) in 1992,[33] it became a gold-standard for cancer management and has been widely used in tumor surgery. NIR imaging has recently emerged as a promising tool to visualize lymphatic system for monitoring lymphatic system and SLNB in a non-invasive manner, because the current used non-invasive imaging techniques for lymphatic system visualization including lymphoscintigraphy, PET, and MRI are all suffer from certain limitations such as poor spatial resolution or temporal resolution, high cost for equipment or the need for radiotracers,[34] thus precluding their applications in the dynamic visualization of lymphatic vessels and lymph nodes in real-time.[35] Nowadays, ICG is the fluorophore approved by the Food and Drug Administration (FDA),[36] and ICG based fluorescence imaging has been widely used for investigating biological systems in clinic.[32, 37] However, ICG is not an ideal probe for NIR lymphatic imaging because of its relatively poor long term stability in vivo.[38] Even worse, it tends to aggregates and self-quenches in aqueous solution due to its amphiphilic property. All of these drawbacks hinder the broad applications of ICG, especially for the longitudinal quantitative lymphatic imaging.[39] In our study, we found that the identification of sentinel lymph node using ICG is relative time-consuming and hard to distinguish the lymph node with the ambient tissue because of auto-fluorescence and scattering of skin in NIR-I region (Figure S2). In contrast, we successfully performed SLNB with favorable temporal and spatial resolution using NIR-II imaging and confirmed the effectiveness by the histological analysis (Figure 8). Our finding suggests that SLNB procedure under NIR-II imaging using CQS1000 with high SBR (11.01) is an effective tool to guide the surgeon's scalpel without any unnecessary interference or injury to the vital organs and tissues, and it can minimize inaccurate incision and dissection as much as possible, thus permitting surgeons to ensure completeness of the resection intra-operatively. The maximum SBR reached to 11.01 at 8 min post-injection, and when the lymph node was dissected and removed from the soft tissue in the axillary region, it dropped to 1.7, indicating that the sentinel lymph node was thoroughly dissected. More importantly, as shown in Figure 8f, there is barely normal tissue remained on the dissected lymph node, implying that the SLN can be easily identified by pathologist after resection. This technique leads to a reduced time of examination, which may aid the surgeon to make better decision during the surgery. In comparison, Sungjeeet al. has demonstrated that using QDs allows a successful cancer surgery, sentinel lymph node mapping and biopsy.[8b] However, the tendency of phagocytosis and potential toxicity because of long term retention of QDs in LNs hinder their potential for further clinical translation.[40] In comparison, our findings indicate that CQS1000 is a promising NIR-II fluorescent probe for biopsy of SLN and evaluation other lymphatic diseases.
Lastly but not leastly, it should be noted that although it is generally acknowledged that a large number of nanomedicine are not ready to be translated into clinic, some nanoparticles based therapeutics have been used or evaluated in the clinical application.[42] For example, Doxil (Doxorubicin encapsulated into PEG-liposome) was approved for the treatment of AIDS-related Kaposi’s sarcoma and other cancers such as ovarian cancer and multiple myeloma;[43] Abraxane (Nanoparticle albumin-bound-paclitaxel) has been tested in phase II clinical trial in patients with metastatic breast cancer[44]). The success of these nanomedicine demonstrates the promise of developing small organic molecules based nanoparticles for clinical translation. Considering the high in vitro and in vivo biocompatibility of CQS1000 as demonstrated in this study, our future work will focus on thoroughly investigating the long term toxicity of the NIR-II nano-vesicles and eventually translating the NIR-II probe into clinical applications.
4. Conclusion
In summary, this study unambiguously shows that NIR-II CQS1000 vesicles can visualize and monitor circulatory system noninvasively and dynamically, including lymphatic drainage and routing, angiogenesis of tumor and vascular deformity such as arterial thrombus formation and ischemia. More importantly, NIR-II imaging using CQS1000 is capable to aid us to accomplish more precise resection of tumor for reduced metastasis and relapse, and it can accelerate the process of lymph nodes dissection to complete SLNB for better decision-making during the tumor surgery. Deep tissue penetration ability, desirable chemical and optical properties as well as long circulation time render NIR-IICQS1000 a highly promise probe for future clinical applications, surpassing traditional NIR-I imaging modality and pathologic examinations for clinical diagnosis and treatment.
5. Experimental Section
Materials
The small molecule NIR-II dye, 5,5'-(1H,5H-benzo[1,2-c:4,5-c'] bis[1,2,5]thiadiazole)-4,8-diyl)bis(N,N-bis(4-(3-((tert-butyldimethylsilyl)oxy)propyl)phenyl)thiophen-2-amine), was synthesized as described previously.[20] It was dissolved in tetrahydrofuran (THF) at a concentration of 100 µg/mL and mixed with an aqueous solution of 1,2-Distearoyl-phosphatidylethanolamine-methyl- polyethyleneglycol conjugate (DSPE-mPEG, 5 kDa) at a concentration of 1 mg/mL with 1 : 9 volume ratio and then stirred at room temperature for 4 h. Next, the mixture was dialysed against water to remove THF and make a THF-free, clear aqueous solution of phospholipids vesicles, CQS1000. To remove aggregates formed during dialysis, the suspension was ultra-centrifuged for 30 min at 300,000 g and only the supernatant was retained. Free unbound surfactant in the solution was removed through 30 kDa centrifugal filters (Amicon) without causing any instability to the phospholipids vesicles.
Characterization of CQS1000
The sizes of the CQS1000 phospholipids vesicles were measured by a dynamic light scattering (DLS) instrument (Malvern Instruments Ltd, Southborough, Massachusetts). Ultraviolet-visible (UV-vis) absorption spectroscopy of the probe was recorded on an Agilent 8453 UV spectrophotometer. Fluorescence was recorded on a Fluoromax-3 spectrophotometer (JobinYvon). Samples were deposited and dried on copper grids covered with a carbon support film, and then the morphologies of the probe were obtained under on a JEOL 2010 transmission electronic microscopy (TEM) at an accelerating voltage at 100 kV. The fluorescence emission of phantoms was measured under various filters from 1,000–1,200nm.
Cells and animal models
B16F10 (mouse melanoma), NIH 3T3 (mouse embryonic fibroblast cell) U87MG (human glioblastoma) and 143B (human osteosarcoma) were cultured in Dulbecco’s modified Eaglemedium (DMEM) containing high glucose (Gibco), all of which were supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. The cells were expanded in tissue culture dishes and kept in a humidified atmosphere of 5% CO2 at 37 °C. The medium was changed every other day. A confluent monolayer was detached with 0.5% trypsin, and dissociated into a single-cell suspension for inoculation.
C57BL/6, Balb/c and nude mice (Charles River Laboratories, USA) were maintained in pathogen free conditions until imaging. All animal experiments were performed under the approval of Stanford University’s Administrative Panel on Laboratory Animal Care. The animal model including various tumor inoculations were established (for details please see the supporting information).
Cytotoxicity assay
The potential cytotoxicity of CQS1000 on NIH3T3 cells and U87MG was examined by employing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, USA) assay. U87MG or NIH 3T3 cells with a density of 5 × 103 cells per well were seeded in 96-well plates. After 24 h, the medium of 96-well plates was replaced with 100µL of medium containing different concentration of CQS1050 and incubated followed by 48 h. After that, MTT (10 µL, 0.5 mg/mL) solution was added to each well and incubated for 4 h at 37 °C. The supernatant was removed and the residues were lysed with 200 µL of dimethyl sulfoxide (DMSO). The absorbance value was recorded at 490 nm using a microplate reader. The absorbance of the untreated cells was used as a control, and its absorbance was as the reference value for calculating 100% cellular viability.
Ex vivo biodistribution
Ex vivo biodistribution studies were further performed at 24 h post-injection of CQS1000 (200nmol) through tail vein to evaluate its’ distribution in vital organs including heart, liver, spleen, lung, kidney, brain, bone, intestine, skin and lymph node. The organs were harvested and imaged under NIR-II imaging at 1,000nm LP filter with 200ms exposure time. In addition, the major organs including heart, liver, spleen, lung, and kidney were harvested on 7 days after CQS1000 injection (200nmol) through tail vein and analyzed with histological assay to evaluate the potential in vivo toxicity of CQS1000.
NIR-II imaging of vascular system
NIR-II imaging setup was described in the supporting information. Before the imaging, the hairs of the brain and lower limb region of four to six-week-old C57BL/6 mice (n=3) were removed by using depilatory gel. Next, mice were mounted on an imaging stage beneath the laser, then 100 µL of CQS1000 (200nmol) was injected through tail vein. To compare the wavelength-dependent vascular imaging quality of brain or lower limb, different emission filters were used to select the collection range for NIR-I (850 nm long-pass filter) and NIR-II (1,000 nm long-pass filters), respectively. A 2.5× (high magnification) was performed. Brain or hind limb NIR-II fluorescence images were obtained with an exposure time of 200 ms. For assessment of blood vessel development in tumor, mice (n=3) bearing with subcutaneous U87MG glioblastoma or 143B osteosarcoma were imaged using the NIR-II setup. Images were captured at different time points (7,14,21 days) post inoculation of tumor. To explore the intra-operative assessment of blood supply, mice (n=3) were placed on a stage connected with an electric heating pad to maintain the temperature of the animal. Surgery was performed by a skilled surgeon according to the NIR-II imaging screening results at 1,000nm LP filter with 200ms exposure time. During the whole process of imaging, the mice was kept anaesthetized by a nose cone delivering 2 L/min O2 gas mixed with 3% isoflurane.
NIR-II imaging of arterial thrombus formation and ischemia
Animal models was prepared based on a method reported previously with a minor modification for NIR-II imaging of thrombus formation in artery.[21] Briefly, being anaesthetized in a rodent anesthesia station with 2 L/min O2 gas mixed with 3% isoflurane, the left femoral artery of C57BL/6 mice (n=3) was exposed under a microscope, and then vascular injuries were generated at the central part of femoral artery by wrapping with a Waterman filter paper saturated with 20% FeCl3 for 15 min (3M Waterman filter, 3 mm length and 1 mm width). Subsequently, CQS1000 (100 µL, 200 nmol) was injected through tail vein, and then NIR-II images were recorded immediately after injection and at 5,10 and 15min post-injection at 1,000nm LP filter with 500ms exposure time.
Induction of unilateral incomplete hind limb ischemia was modified according to the previous report.[22] Briefly, after the right femoral artery of mice (n=3) was dissected, two strands of 7-0 silk suture were passed underneath the proximal end of the femoral artery and the beginning point of superficial epigastric artery, respectively. Another two strands were performed distal than the previous ones. Then the proximal femoral artery was occluded using double knots mentioned above. Ten minutes later, the mice were placed on the a stage connected with an electric heating pad to maintain the temperature of the animal and CQS1000(100 µL, 200 nmol) was injected through tail vein, and then NIR-II images were recorded immediately after injection and at 5,10 and 15min post-injection at 1,000nm LP filter with 500ms exposure time.
NIR imaging of lymphatic drainage
Four to six-week-old nude mice (n=3) were anaesthetized in a rodent anesthesia station with 2 L/min O2 gas mixed with 3% isoflurane. For visualization of lower limb collecting lymphatic vessels and drainage of rodent lymphatic system, CQS1000 (40 µL, 80 nmol) was injected intradermally into the dorsal skin of the rear paw. Fifteen minutes later, mice were placed and kept anaesthetized by a nose cone delivering 2 L/min O2 gas mixed with 3% isoflurane on a stage connected with an electric heating pad to maintain the temperature of the animal. Images were recorded at 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 50, and 60 min post-injection to visualize the dynamic drainage from lower limb to upper limb through flank trunk. For comparison, ICG (50 µL,1 mg/mL) was injected subcutaneously into left rear footpad and then the fluorescent images were performed using IVIS 200 Imaging System (Xenogen Corporation, Hopkinton, MA) with at an excitation and emission wavelength of 745 nm and 810nm. Optical images were acquired at 15, 60, 90, 120 min after injection. All the luminous lymph nodes were resected and embedded by optimal cutting temperature (OCT) compound (Tissue-Tek®, Sakura Finetek, USA), and then cut into 6 µm sections in the cryostat at −20°C with a microtome and transferred onto microscope slides for hematoxylin and eosin (H&E) using Shandon™ rapid Chrome kit (Thermo Scientific, USA).
NIR-II image-guided lymph node mapping and resection
Four to six-week-old nude mice (n=3) were used for lymph node mapping experiment. Before imaging, all mice were anaesthetized in a rodent anesthesia station with 2 L/min O2 gas mixed with 3% isoflurane. For visualization of axillary lymph node, CQS1000 (30 µL, 60 nmol) was injected intradermally into the dorsal skin of the forepaw. With the help of NIR-II imaging, the sentinel lymph node (axillary) was visualized and resected precisely.
To explore the NIR-II image-guided sentinel lymph node mapping and biopsy, we further performed the surgery on the tumor-bearing mice. Before the imaging, the hairs of the lower limbs and inguinal region of C57BL/6mice (n=3) bearing with melanoma were removed by using depilatory gel. Being anaesthetized in a rodent anesthesia station with 2 L/min O2 gas mixed with 3% isoflurane, mice were placed on a stage under the NIR-II imaging setup. CQS1000 (30 µL, 60 nmol) was injected into forepaw near the melanoma located at the left hind limb. Sequential NIR-II images were recorded every 5 minutes during a period of 30 min. After the imaging experiments, the popliteal lymph nodes were resected and embedded by OCT, and then cut into 6 µm sections in the cryostat at −20 °C with a microtome and transferred onto microscope slides for H&E using Shandon™ rapid Chrome kit.
Statistical analysis
The fluorescence measurement was performed to quantitate NIR fluorescence signal intensity through the Image J 1.45× software (National Institutes of Health, Bethesda, MD). Data are given as mean ± SD (standard deviation). Statistical analysis was performed using a two-tailed student t-test. Statistical significance was assigned for P-value < 0.05.
Supplementary Material
Acknowledgments
This work was partially supported by the Office of Science (BER), U.S. Department of Energy (DE-SC0008397) (Z.C.), NCI of Cancer Nanotechnology Excellence Grant CCNE-TR U54 CA119367, Huanghe Talents Project of Wuhan City(2014) (A.Y.), China Scholarship Council (No. 2011627067, to KQ. S.), and NSFC (81572163, 81301160, 61571239).
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of interest
No conflict of interest.
References
- 1.a) Zhu Q, DeFusco PA, Ricci A, Jr, Cronin EB, Hegde PU, Kane M, Tavakoli B, Xu Y, Hart J, Tannenbaum SH. Radiology. 2013;266:433. doi: 10.1148/radiol.12112415. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Golijanin J, Amin A, Moshnikova A, Brito JM, Tran TY, Adochite R-C, Andreev GO, Crawford T, Engelman DM, Andreev OA. Proceedings of the National Academy of Sciences. 2016;113:11829. doi: 10.1073/pnas.1610472113. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sun Y, Ma X, Cheng K, Wu B, Duan J, Chen H, Bu L, Zhang R, Hu X, Deng Z. Angewandte Chemie International Edition. 2015;54:5981. doi: 10.1002/anie.201500941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okochi O, Kaneko T, Sugimoto H, Inoue S, Takeda S, Nakao A. Journal of Surgical Research. 2002;103:109. doi: 10.1006/jsre.2001.6328. [DOI] [PubMed] [Google Scholar]
- 3.Meier R, Thuermel K, Noël PB, Moog P, Sievert M, Ahari C, Nasirudin RA, Golovko D, Haller B, Ganter C. Radiology. 2014;270:176. doi: 10.1148/radiol.13130039. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Braun GB, Zhong H, Hall DJ, Han W, Qin M, Zhao C, Wang M, She ZG, Cao C. Advanced functional materials. 2016;26:267. doi: 10.1002/adfm.201503453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Bu L, Shen B, Cheng Z. Advanced drug delivery reviews. 2014;76:21. doi: 10.1016/j.addr.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Su X, Cheng K, Wang C, Xing L, Wu H, Cheng Z. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2013;5:219. doi: 10.1002/wnan.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M, Kokudo N. Cancer. 2009;115:2491. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]; b) Ishizawa T, Bandai Y, Harada N, Muraoka A, Ijichi M, Kusaka K, Shibasaki M, Kokudo N. Asian Journal of Endoscopic Surgery. 2010;3:42. [Google Scholar]
- 7.Chen G, Tian F, Li C, Zhang Y, Weng Z, Zhang Y, Peng R, Wang Q. Biomaterials. 2015;53:265. doi: 10.1016/j.biomaterials.2015.02.090. [DOI] [PubMed] [Google Scholar]
- 8.a) Cheng Z, Yan X, Sun X, Shen B, Gambhir SS. Engineering. 2016;2:132. [Google Scholar]; b) Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM. Nature biotechnology. 2004;22:93. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang X, Gu L, Qi J, Correa S, Zhang G, Belcher AM, Hammond PT. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1521175113. 201521175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B. Nature materials. 2015 doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- 11.Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, Cooke JP, Dai H. Nature medicine. 2012;18:1841. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Zhang XD, Wang H, Antaris AL, Li L, Diao S, Ma R, Nguyen A, Hong G, Ma Z, Wang J. Advanced materials. 2016;28:6872. doi: 10.1002/adma.201600706. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Diao S, Blackburn JL, Hong G, Antaris AL, Chang J, Wu JZ, Zhang B, Cheng K, Kuo CJ, Dai H. Angewandte Chemie. 2015;127:14971. doi: 10.1002/anie.201507473. [DOI] [PubMed] [Google Scholar]; c) Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI. Nature Photonics. 2014;8:723. doi: 10.1038/nphoton.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hong G, Zou Y, Antaris AL, Diao S, Wu D, Cheng K, Zhang X, Chen C, Liu B, He Y. Nature communications. 2014;5 doi: 10.1038/ncomms5206. [DOI] [PubMed] [Google Scholar]
- 13.Hong G, Wu JZ, Robinson JT, Wang H, Zhang B, Dai H. Nature communications. 2012;3:700. doi: 10.1038/ncomms1698. [DOI] [PubMed] [Google Scholar]
- 14.a) Hong G, Robinson JT, Zhang Y, Diao S, Antaris AL, Wang Q, Dai H. Angewandte Chemie. 2012;124:9956. doi: 10.1002/anie.201206059. [DOI] [PubMed] [Google Scholar]; b) Dong B, Li C, Chen G, Zhang Y, Zhang Y, Deng M, Wang Q. Chemistry of Materials. 2013;25:2503. [Google Scholar]; c) Zhang Y, Zhang Y, Hong G, He W, Zhou K, Yang K, Li F, Chen G, Liu Z, Dai H. Biomaterials. 2013;34:3639. doi: 10.1016/j.biomaterials.2013.01.089. [DOI] [PubMed] [Google Scholar]; d) Wang Q. Nanomedicine: Nanotechnology, Biology and Medicine. 2016;12:464. doi: 10.1016/j.nano.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Naczynski D, Tan M, Zevon M, Wall B, Kohl J, Kulesa A, Chen S, Roth C, Riman R, Moghe P. Nature communications. 2013;4 doi: 10.1038/ncomms3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C. Science. 2002;297:593. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 17.a) Zhu C-N, Jiang P, Zhang Z-L, Zhu D-L, Tian Z-Q, Pang D-W. ACS applied materials & interfaces. 2013;5:1186. doi: 10.1021/am303110x. [DOI] [PubMed] [Google Scholar]; b) Li C, Cao L, Zhang Y, Yi P, Wang M, Tan B, Deng Z, Wu D, Wang Q. Small (Weinheim an der Bergstrasse, Germany) 2015;11:4517. doi: 10.1002/smll.201500997. [DOI] [PubMed] [Google Scholar]
- 18.Casalboni M, De Matteis F, Prosposito P, Quatela A, Sarcinelli F. Chemical physics letters. 2003;373:372. [Google Scholar]
- 19.Vahrmeijer AL, Hutteman M, Van Der Vorst JR, Van De Velde CJ, Frangioni JV. Nature reviews Clinical oncology. 2013;10:507. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Qu C, Chen H, He M, Tang C, Shou K, Hong S, Yang M, Jiang Y, Ding B. Chemical Science. 2016;7:6203. doi: 10.1039/c6sc01561a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin P, Gachet C. Journal of Thrombosis and Haemostasis. 2011;9:779. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 22.Niiyama H, Huang NF, Rollins MD, Cooke JP. JoVE (Journal of Visualized Experiments) 2009:e1035. doi: 10.3791/1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Wu H-i. New York. 2007 [Google Scholar]
- 24.O'leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr New England Journal of Medicine. 1999;340:14. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 25.a) Saba L, Sanfilippo R, Montisci R, Mallarini G. Neuroradiology. 2010;52:75. doi: 10.1007/s00234-009-0589-5. [DOI] [PubMed] [Google Scholar]; b) LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Circulation. 1996;94:932. doi: 10.1161/01.cir.94.5.932. [DOI] [PubMed] [Google Scholar]
- 26.Greco A, Mancini M, Gargiulo S, Gramanzini M, Claudio P, Brunetti A, Salvatore M. BioMed research international. 2011;2012 doi: 10.1155/2012/519238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Proceedings of the National Academy of Sciences. 2013;110:12474. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Folkman J. New england journal of medicine. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Cancer research. 2005;65:4739. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 29.Fisher DT, Muhitch JB, Kim M, Doyen KC, Bogner PN, Evans SS, Skitzki JJ. Nature communications. 2016;7 doi: 10.1038/ncomms10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mäkitie T, Summanen P, Tarkkanen A, Kivelä T. Journal of the National Cancer Institute. 1999;91:359. doi: 10.1093/jnci/91.4.359. [DOI] [PubMed] [Google Scholar]
- 31.a) Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You W-K, Chapman HA, Christensen JG. Cancer discovery. 2012;2:270. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT. Cancer research. 2012;72:402. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJ, Wen PY, Ivy P, Batchelor TT, Rosen BR. Nature medicine. 2013;19:1178. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pestana IA, Zenn MR. Annals of plastic surgery. 2014;72:S144. doi: 10.1097/SAP.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 33.Morton DL, Wen D-R, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Archives of surgery. 1992;127:392. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 34.a) Tsopelas C. Journal of Nuclear Medicine. 2001;42:460. [PubMed] [Google Scholar]; b) Wilhelm AJ, Mijnhout GS, Franssen EJ. European journal of nuclear medicine. 1999;26 doi: 10.1007/pl00014793. [DOI] [PubMed] [Google Scholar]
- 35.a) Mumprecht V, Honer M, Vigl B, Proulx ST, Trachsel E, Kaspar M, Banziger-Tobler NE, Schibli R, Neri D, Detmar M. Cancer research. 2010;70:8842. doi: 10.1158/0008-5472.CAN-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Proulx ST, Kwok E, You Z, Beck CA, Shealy DJ, Ritchlin CT, Boyce BF, Xing L, Schwarz EM. Annals of the New York Academy of Sciences. 2007;1117:106. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 36.Wishart G, Loh S-W, Jones L, Benson J. European Journal of Surgical Oncology (EJSO) 2012;38:651. doi: 10.1016/j.ejso.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Mohajerani P, Meier R, Noël PB, Rummeny EJ, Ntziachristos V. Journal of biomedical optics. 2013;18:097004. doi: 10.1117/1.JBO.18.9.097004. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen JC, Tan I-C, Marshall MV, Fife CE, Sevick-Muraca EM. Current opinion in biotechnology. 2009;20:74. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gashev AA, Nagai T, Bridenbaugh EA. Lymphatic research and biology. 2010;8:127. doi: 10.1089/lrb.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosaka N, Ogawa M, Sato N, Choyke PL, Kobayashi H. Journal of Investigative Dermatology. 2009;129:2818. doi: 10.1038/jid.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J, Li Y, Orza A, Lu Q, Guo P, Wang L, Yang L, Mao H. Advanced functional materials. 2016 doi: 10.1002/adfm.201504185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis ME, Shin DM. Nature reviews Drug discovery. 2008;7:771. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 43.Allen TM, Cullis PR. Advanced drug delivery reviews. 2013;65:36. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Roy V, LaPlant BR, Gross GG, Bane CL, Palmieri FM. Annals of oncology : official journal of the European Society for Medical Oncology. 2009;20:449. doi: 10.1093/annonc/mdn661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.