Abstract
Objectives
To assess the feasibility of delivering and evaluating a weight management (WM) programme for overweight patients with a family history (FH) of breast cancer (BC) or colorectal cancer (CRC).
Study design
A two-arm (intervention vs usual care) randomised controlled trial.
Setting
National Health Service (NHS) Tayside and NHS Grampian.
Participants
People with a FH of BC or CRC aged≥18 years and body mass index of ≥25 kg/m2 referred to NHS genetic services.
Intervention
Participants were randomised to a control (lifestyle booklet) or 12-week intervention arm where they were given one face-to-face counselling session, four telephone consultations and web-based support. A goal of 5% reduction in body weight was set, and a personalised diet and physical activity (PA) programme was provided. Behavioural change techniques (motivational interviewing, action and coping plans and implementation intentions) were used.
Primary outcome
Feasibility measures: recruitment, programme implementation, fidelity measures, achieved measurements and retention, participant satisfaction assessed by questionnaire and qualitative interviews.
Secondary outcomes
Measured changes in weight and PA and reported diet and psychosocial measures between baseline and 12-week follow-up.
Results
Of 480 patients approached, 196 (41%) expressed interest in the study, and of those, 78 (40%) patients were randomised. Implementation of the programme was challenging within the time allotted and fidelity to the intervention modest (62%). Qualitative findings indicated the programme was well received. Questionnaires and anthropometric data were completed by >98%. Accelerometer data were attained by 84% and 54% at baseline and follow-up, respectively. Retention at 12 weeks was 76%. Overall, 36% of the intervention group (vs 0% in control) achieved 5% weight loss. Favourable increases in PA and reduction in dietary fat were also reported.
Conclusions
A lifestyle programme for people with a family history of cancer is feasible to conduct and acceptable to participants, and indicative results suggest favourable outcomes.
Trial registration number
ISRCTN13123470; Pre-results.
Keywords: cancer genetics, nutritional support, preventive medicine, public health
Strengths and limitations of this study.
This feasibility study is the first attempt to offer and assess a structured, comprehensive lifestyle programme (diet, alcohol, physical activity and body weight) for people referred to family history clinics for colorectal and breast cancer risk assessment.
The study design is a randomised, two-centred, lifestyle intervention study with subjective and objective assessment measures.
Participants were all attendees at the National Health Service Family History clinics due to a family history of breast cancer (BC) or colorectal cancer (CRC) and are not representative of the general population.
The lifestyle intervention was not fully implemented, recruitment was lower than anticipated and indicative findings suggest favourable effects of the intervention on physiological measures.
The primary and secondary outcome data provide sufficient information to inform a definitive trial.
Introduction
It is recognised that cancer arises from an interaction between genetic and environmental factors (nature and nurture), although there may be more emphasis given to genetics and family history in the National Health Service (NHS) rather than health behaviour profiles. Clearly, it is desirable that people who are at greater risk of cancer due to a family history of the disease (which may reflect shared genetic and behavioural profiles) are supported to follow recommendations for cancer surveillance and lifestyle. NHS genetics centres in Scotland offer early detection and counselling for people with a family history (FH) of breast cancer (BC) and colorectal cancer (CRC),1 but there is little evidence of lifestyle advice.
Current estimates for the role of lifestyle in postmenopausal BC suggest that around 38% of the disease could be prevented by increased physical activity and decreases in alcohol intake and excess body weight.2 A number of studies show that BC risk is lowered with intentional weight loss3 4 and recent data from bariatric surgery5 show that weight loss is correlated with significant decreases in the incidence of cancers at several sites—notably postmenopausal breast, colon, endometrium and pancreas. Gramling et al6 reported from the Women’s Health Initiative Observational study that participating in healthy lifestyles (greater physical activity, lower alcohol intake and lower body mass index (BMI)) led to risk reduction in postmenopausal women, and the degree of this benefit was the same for women with and without an FH of BC. A recent review by Pettapiece-Phillips et al7 reports evidence of a protective role of a healthy body size and regular activity among mutation carriers notably in adolescence and early adulthood.
It is estimated that around 45% of CRC could be prevented by diet and other lifestyle behaviours (dietary fibre intake and physical activity and low intakes of red and processed meat, alcohol and body fatness).2 For CRC, people with an FH may be more susceptible to lifestyle-related risk. For example, in a pooled analysis, Cho et al8 reported that the relative risks of CRC with alcohol consumption of ≥30 g/d were 1.23 (95% CI 0.96 to 1.57; Not Significant) among those with no FH and 2.02 (95% CI 1.30 to 3.13) among those with an FH of CRC. The importance of excess weight and increased risk of developing CRC has been highlighted by Movahedi et al,9 who reported that in patients with Lynch syndrome, CRC risk was increased by 7% for each 1 kg/m2 increase in BMI. A recent review of alcohol, obesity and other nutritional factors in people with familial CRC by Fardet et al10 present a clear case for why modifiable risk factors could offer new perspectives on prevention.
Akhtar et al11 reported, in a UK survey of relatives of patients diagnosed with CRC, that most (88%) said they were prepared to make lifestyle changes if given enough information. In the BeWEL12 study (lifestyle intervention for people at higher CRC risk due to an adenoma), around half (49%) of 997 patients showed an interest in finding out more about the lifestyle (weight loss) trial.
It is also noteworthy that, for patients subsequently diagnosed with BC or CRC, obesity is associated with poorer prognosis, increases in disease recurrence and overall mortality.13 14
Identifying increased risk of developing a disease is unlikely to be sufficient to change behaviour,15 and knowledge of FH per se is unlikely to be sufficient to initiate sustained weight management behaviours. In a questionnaire study of 237 (49%) people attending family history clinics in the East of Scotland, Anderson et al16 reported that while smoking rates were modest (11%), most respondents had a BMI ≥25 kg/m2, low levels of physical activity and high alcohol consumption. Interview data in a subgroup of these respondents highlighted doubts about the link between lifestyle and cancer, and few were familiar with the current evidence. While lifestyle advice was considered interesting in general, there was little appetite for non-tailored guidance.
Marteau and Lerman17 argues that motivation to change behaviour may be achieved by increasing beliefs that changing behaviour can reduce risks by increasing beliefs in the individual’s ability to change. However, it is more likely that self-efficacy and self-regulatory techniques (eg, goal setting) play a greater role in achieving effective behaviour change.18 19 National Institute for Health and Care Excellence guidelines on familial BC recommend that standard written information for people with concerns about familial BC risk should include ‘lifestyle, including diet, alcohol etc’,20 but the reasons for this choice of communication is unclear. Genetic counsellors (GCs) receive little if any training in promoting healthy lifestyles, and there is little evidence that advice is routinely provided.
This study aimed to assess the feasibility and acceptability of delivering a 12-week lifestyle intervention programme (LivingWELL) for people with an FH of BC and CRC initiated within the FH setting in order to inform the design of a definitive randomised controlled trial (RCT) to assess the clinical and cost-effectiveness of this intervention.
Specific objectives were to estimate recruitment rate for a full RCT, assess the feasibility of data collection procedures and protocol adherence, explore participant experience and establish a power calculation required for a full-scale study
Methods
Study design and setting
This study was a two-arm, two-centre, parallel, randomised-controlled, feasibility study integrated with qualitative interviews conducted with people referred to NHS genetic services in Tayside and Grampian from August 2015 to March 2016. This study is reported in accordance with the Consolidated Standards of Reporting Trials 2010 statement: extension to randomised pilot and feasibility trials.21
Sample size
We aimed to recruit 120 (60 intervention and 60 control) participants in order to be able to assess feasibility objectives allowing for a loss to follow-up of 10% with precision of ±1% at 95% CI.22
Recruitment and randomisation
New attendees at genetics clinic were informed about the study by the GCs and given a brief information sheet, clinician endorsement letter, non-participation questionnaire and reply slip with stamped addressed envelope. They were also offered the opportunity to discuss the study with the research team. Where low-risk patients did not attend the clinics in person, postal versions of the study materials were sent. Low initial recruitment after 3 months resulted in a protocol amendment to allow study information to be passed to established patients returning for mammographic or endoscopic procedures in one centre (not considered feasible in the other centre).
GCs/clinical staff recorded the numbers of patients attending clinics and informed patients with an FH of BC or CRC about the study. Staff noted when they felt it inappropriate to provide information about the study and recorded any reasons that patients gave for non-participation. Interested patients were given a brief information sheet and an endorsement letter from the relevant lead clinician. They were asked to provide their contact details. An opt-out slip and non-participant feedback form were included for those who decided not to participate.
Research nurses made contact with interested patients to assess eligibility and provide a full information sheet. All those written to (or given packs) could also respond by telephone/postal reply slip. Eligible participants were adults, age ≥18 years, with an FH of BC or CRC and measured BMI ≥25 kg/m2. Exclusion criteria were severe cognitive impairment, or conditions where physical activity was contraindicated, pregnancy, breast feeding or currently undergoing active treatment for cancer.
Once continued interest and eligibility were established, an appointment was made for a baseline measurement visit where informed consent was obtained. Postbaseline visit, participants were allocated (by the study administrator who informed the participant and lifestyle coach (LC) of the outcome) into usual care (control) or intervention groups 1:1 using a computer-generated random allocation list. The research nurse was blinded to the randomisation.
Intervention
The 12-week intervention, ‘LivingWELL’, aimed to help participants increase physical activity, modify diet, achieve weight loss towards 5% of body weight and avoid weight gain. Personalised advice was given on a 600 kcal deficit dietary intake as recommended by the Scottish Intercollegiate Guidelines Network (SIGN)23 and a graduated approach aimed at increasing activity to 225–300 min per week by 12 weeks. The theoretical basis for the intervention draws on Leventhal’s Self-Regulatory Theory24 (which highlights the importance of the individual’s beliefs regarding illness cause, identity, control/cure, timeline and consequences), Social Cognitive Theory,25 which emphasises the importance of self-efficacy, and the Health Action Process Approach,26 which emphasises the importance of action and coping planning.
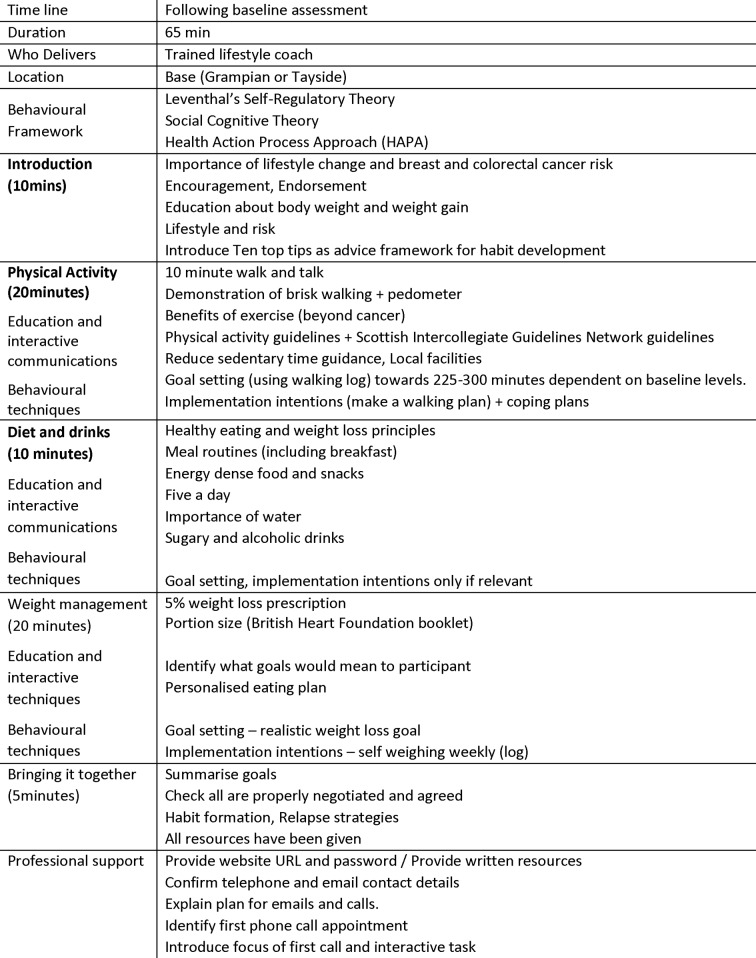
The ‘LivingWELL’ programme was delivered by LCs personnel (with a nursing background) who received bespoke training on the delivery of the intervention programme. Intervention participants received one face-to-face session plus (up to) four telephone consultations over the 12-week programme, a ‘LivingWELL’ information pack, pedometer and walking programme. Participants were also offered a web-based support programme. The face-to-face session was designed to be interactive and included a 10 min ‘walk and talk’ session during which pedometer use and walking goals were discussed, self-identification of BMI category and a portion weight estimate task.
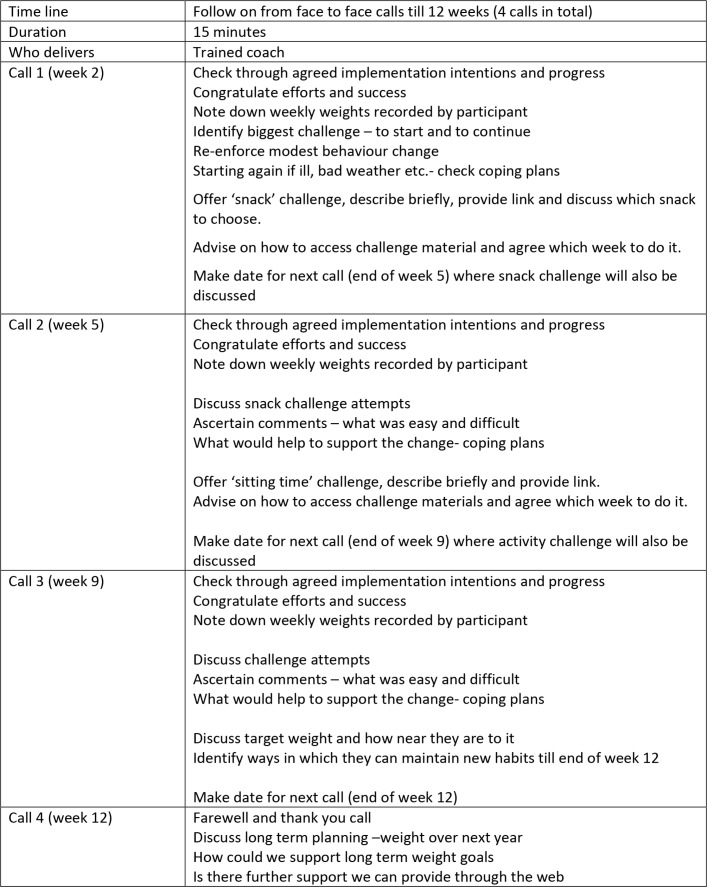
Participants received a personalised energy deficit diet, a personalised graduated walking plan and guidance on setting personal goals, how to make changes habitual and relapse prevention strategies for times of deviation.
Motivational interviewing techniques were used to explore self-assessed confidence to change and self-perceived benefits. Behavioural change techniques (goal setting, action and coping plans and implementation intentions) were also used. The importance of self-monitoring pedometer data, diet and drink logs and weekly body weight was emphasised. These data formed the basis for the 15 min phone consultations, which checked well-being, self-monitoring behaviours, actions and coping plans. Details of the intervention are described in figures 1 and 2.
Figure 1.
Intervention components (face-to-face visits). BHF, British Heart Foundation.
Figure 2.
Intervention components (telephone calls).
Primary outcome measure
Data were collected on numbers of people told about the study, those expressing an interest, numbers meeting eligibility criterion, rates of recruitment, achieved measurements and retention. Reasons for non-participation and withdrawal were collected where possible.
Intervention contact time was recorded by LCs, and the ease of implementing the counselling sessions and perceived engagement and motivation of participants was recorded using a 5-point Likert scale (scored −2 to +2). A random sample of face-to-face visits and telephone calls were recorded and independently analysed to assess fidelity to the detailed intervention protocol.
All participants who completed the study were asked to fill out an anonymous patient satisfaction questionnaire (to rate programme visits, measurements and intervention) that was distributed after the follow-up visit. Qualitative interviews (n=20) explored intervention participants’ views on study acceptability (recruitment, assessment and intervention programme) and factors influencing adherence. Interviews were audio recorded, transcribed and analysed using a thematic framework approach.
Secondary outcome measures
Follow-up procedures took place after the 12-week intervention period. Both baseline and follow-up measures were undertaken by research nurses blinded to the group allocation, and participants were asked not to share their group allocation with the researchers.
Research nurses recorded sociodemographic information and measured height at the baseline visit. At both baseline and follow-up, physiological measures (body weight, waist circumference and blood pressure) were taken, and BMI was assessed.
Physical activity was subjectively assessed using the International Physical Activity Questionnaire Short Form27 (for low/medium and high activity grading and to get insight into activity modes) and objectively measured by Sensewear (BodyMedia, Pittsburgh, Pennsylvania, USA) physical activity monitors, which provided detailed analysis of sedentary time, moderate and vigorous activity and step counts. Eating habits were assessed by the Dietary Instrument for Nutrition Education questionnaire28 and alcohol intake by a 7-day alcohol record.29 Smoking was assessed by questions on current and historic tobacco use.
Psychosocial variables measured included beliefs about cancer cause and risk reduction (modified brief illness perception questionnaire30) and quality of life using the Euro-Qol-5D questionnaire.31 These measures were undertaken primarily to assess participant burden for planning a full-scale trial.
Statistical analysis
For all quantitative outcome measures, the main aim of this study was principally to assess feasibility of intervention delivery to inform the design of a main trial, thus the indicative outcomes are underpowered for statistical interpretation.
Quantitative data analysis was undertaken using STATA V.14. Descriptive statistics were used to characterise the cohort. Within group changes between baseline and follow-up outcomes were estimated for intervention and control groups using a paired t-test. Between group differences in the outcome change from baseline and follow-up adjusted for baseline values were estimated using linear regression methods. Both within and between group differences are presented as means and 95% CIs.
Results
Primary outcomes (feasibility)
Patients informed about the study
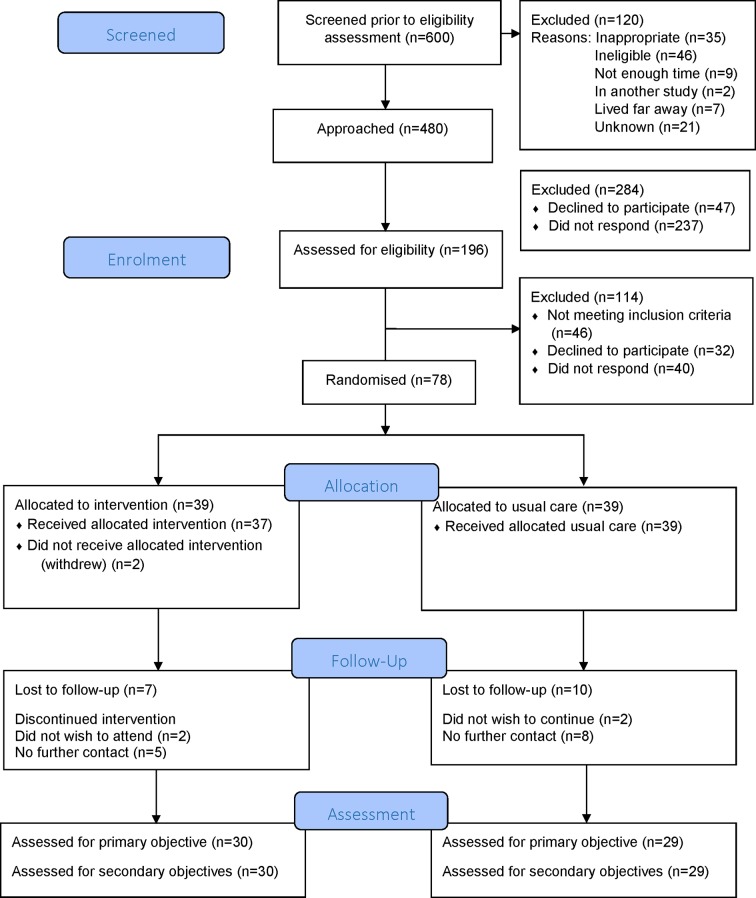
In total, 600 patients were identified as potentially eligible for the study (364 BC and 236 CRC FH) over the 8-month recruitment period (figure 3). Of those, 312 (52%) were new patients and 288 (48%) review patients (4 month period only). Records kept by clinical and study staff show a total of 480 patients (282 BC and 198 CRC FH) were given study information (169 in person and 311 by invitation letters). Within the clinics, 120 people were not approached where GCs considered it inappropriate (35), the person was thought to be ineligible (46), there was not enough time (9), patients were in another study (2), staff noted they lived far from the hospital (7) or for reasons unknown (21).
Patients interested in the study
A total of 196 (41%) people (132 BC and 64 CRC FH) agreed to be contacted. Greatest interest in the study was expressed by new and review patients advised about the study in face-to-face clinic visits (70% and 93%, respectively, agreed to be contacted) compared with new and review patient advised by letter (7% and 27%, respectively). It is notable that despite the high interest expressed by review patients in face-to-face visits (93%), the numbers randomised dropped to 32%. No new patients contacted by letter went on to be randomised.
Eligibility and recruitment rate
Of the 196 who agreed to be contacted, 32 (16%) declined to take part, 40 (20%) did not respond to telephone approaches and 46 (23%) were ineligible (due to BMI). A total of 78 people (40% of those who agreed to be contacted; 39% of BC and 42% of CRC FH approached; 16% of those who were potentially eligible for the study) were randomised.
Baseline characteristics
The mean age was 27.1+12.8 years (range 18–72) and 88% were female (table 1). Most were in employment, had postschool professional or academic qualifications and belonged to the two least deprived quintiles of the Scottish Index of Multiple Deprivation (SIMD).
Table 1.
Demographic characteristics at baseline
| Intervention (n=39) |
Control (n=39) |
All (n=78) |
|
| Age (years) mean | 49.1±12.7 | 45.1±12.8 | 47.1±12.8 |
| Range | 22–72 | 18–71 | 18–72 |
| Gender | |||
| Male | 6 (15) | 3 (8) | 9 (12) |
| Female | 33 (85) | 36 (92) | 69 (88) |
| Marital status | |||
| Single | 3 (8) | 8 (20.5) | 11 (14) |
| Married/cohabiting | 32 (82) | 24 (61.5) | 56 (72) |
| Divorced/widowed/separated | 4 (10) | 7 (18) | 11 (14) |
| Ethnicity | |||
| White | 38 | 39 | 77 |
| Other | 1 | 0 | 1 |
| Highest educational qualification | |||
| Secondary school | 15 (38.5) | 11 (28) | 26 (33) |
| Other professional/technical qualification after school | 9 (23) | 15 (39) | 24 (31) |
| University/postgraduate degree | 15 (38.5) | 13 (33) | 28 (36) |
| Employment status | |||
| Retired | 9 (23) | 4 (10) | 13 (17) |
| Employed full-time | 20 (51) | 17 (44) | 37 (48) |
| Employed part-time | 5 (13) | 8 (20) | 13 (17) |
| Unemployed | 2 (5) | 3 (8) | 5 (6) |
| Other | 2 (5) | 3 (8) | 5 (6) |
| Student | 1 (3) | 4 (10) | 5 (6) |
| SIMD (quintiles) | |||
| 1 (most deprived) | 4 (11) | 8 (20.5) | 12 (15) |
| 2 | 6 (16) | 3 (8) | 9 (12) |
| 3 | 10 (26) | 7 (18) | 17 (22) |
| 4 | 11 (29) | 8 (20.5) | 19 (25) |
| 5 (least deprived) | 7 (18) | 13 (33) | 20 (26) |
| Missing | 1 | 0 | 1 |
| Type of cancer* | |||
| Breast | 24 (62) | 27 (69) | 51 (65) |
| Colorectal | 15 (38) | 12 (31) | 27 (35) |
*Cancer family history, which resulted in a genetics referral.
All results are n (%) unless stated otherwise.
SIMD, Scottish Index of Multiple Deprivation.
Achieved measurements
Evaluation measures were 99%–100% complete for all participants who attended baseline and follow-up appointments. Accelerometer data (for at least 4 days) was obtained for 67 (84%) participants at baseline and 42 (54%) at follow-up.
Retention
Of the 78 randomised, 59 (75%) completed the study (74% control and 77% intervention). Completion rates were similar by BC (72%) and CRC (81%) (figure 3). A greater proportion of people from SIMD quintiles 1 and 2 (high deprivation) were likely to withdraw compared with SIMD quintiles 3, 4 and 5 (38% vs 22%).
Figure 3.
CONSORT diagram ‘LivingWELL’ total recruitment. CONSORT, Consolidated Standards of Reporting Trials.
Non-participation
It is estimated that approximately 100 non-participation feedback forms were distributed at clinic appointments, and over 300 were posted with study information packs. A total of 28 questionnaires were returned. Most responders were women (86%), and the average age was 51.2 years (range 25–72). Of those, 21 people reported a weight and height: 9 had a normal BMI and 12 were overweight or obese. The majority of respondents were from areas of low deprivation (59%). The most common reasons for not taking part were that ‘their lifestyle was already good’ (29%) and ‘too little time to participate’ (14%).
Intervention contact and delivery
In the intervention group, 37 of the 39 randomised (two withdrew) received a face-to-face session with a LC. The mean time for the face-to-face visit was 81 min (range 70–130) compared with an estimated 65 min. The mean time for telephone calls at all time points was 15 min (range 5–30), the same as the estimated allowance.
The LCs estimated around 73% of the total programme was covered, while fidelity scores for nine face-to-face sessions and that 16 telephone calls rated that 62% of the intervention components were delivered as per protocol. LCs assessed perceived patient engagement, receptivity and motivation highly: 89%, 92%, and 89% respectively, in Likert-scaled questions.
Access to the ‘LivingWELL’ study website was given to all intervention participants and 10 (33%) logged on.
Participant feedback
Acceptability data were obtained from 47 (80%) anonymised patient satisfaction questionnaires (23 control and 24 intervention) and from the 20 participant interviews. Results from the questionnaire showed that most (89%) participants reported that they found study participation quite/very easy and 38 (81%) reported that they would recommend ‘LivingWELL’ to others. Most (80%) of the intervention group expressed a high degree of satisfaction with intervention components. The following suggestions for change were made: feedback of results, further contacts with coaches and longer term follow-up.
The qualitative interview data revealed that intervention staff were perceived by participants as professional, knowledgeable, helpful and approachable. Appointments at the centre with LCs were largely perceived as convenient in terms of duration, scheduling and travel incurred. The various measurements were not considered invasive or inappropriate. However, a few participants found the activity monitors heavy and uncomfortable. Overall, most participants were satisfied with the study and recommended few improvements. Participants reported the following barriers to change:
everyday routines
sedentary occupations
family commitments
poor physical or mental health
stressful events such as exams, bereavement or family illness
long-standing complex relationships with food.
In general, participants appeared to find it easier to change physical activity than diet.
Secondary outcomes
The aim of this study was to determine feasibility, not to evaluate statistically significant change. However, changes in physiological measures suggest a favourable effect of the intervention on the primary outcome of body weight loss and diastolic blood pressure (table 2). These findings are reflected in the proportions achieving 5% weight loss (37% in the intervention group compared with 0% in control group) and 10% weight loss (10% of the intervention group).
Table 2.
Changes in physiological measures
| Intervention | Control | Between group differences
Mean (95% CI) |
||||||
| n | Mean (SD) | Difference to baseline Mean (95% CI) |
n | Mean (SD) | Difference to baseline Mean (95% CI) |
|||
| Bodyweight (kg) | Baseline | 39 | 90.9 (17.0) | −3.2 (1.5 to 4.8) | 39 | 88.2 (15.9) | −0.3 (−0.4 to 1.0) | 2.8 (1.1 to 4.6) |
| 12 weeks | 30 | 85.9 (16.3) | 29 | 87.6 (15.6) | ||||
| Body mass index (kg/m2) | Baseline | 39 | 33.1 (6.3) | −1.1 (0.5 to 1.7) | 39 | 32.3 (5.2) | −0.1 (−0.1 to 0.3) | 1.0 (0.4 to 1.7) |
| 12 weeks | 30 | 31.6 (6.2) | 29 | 31.9 (5.0) | ||||
| Waist circumference (cm) | Baseline | 39 | 102.4 (12.9) | −5.0 (3.3 to 6.8) | 39 | 101.2 (13.8) | −0.8 (−1.0 to 2.5) | 4.2 (1.8 to 6.7) |
| 12 weeks | 30 | 96.3 (14.5) | 29 | 99.7 (14.2) | ||||
| Mean systolic blood pressure (mm Hg) | Baseline | 39 | 139.5 (18.9) | −10.6 (5.6 to 15.7) | 39 | 134.5 (13.6) | −4.5 (−0.1 to 9.1) | 4.5 (−1.0 to 10.1) |
| 12 weeks | 30 | 129.7 (11.8) | 29 | 132.6 (14.4) | ||||
| Mean diastolic blood pressure (mm Hg) | Baseline | 39 | 84.3 (10.4) | −2.9 (0.5 to 5.3) | 39 | 82.1 (9.4) | −0.7 (−1.6 to 3.0) | 1.9 (−1.0 to 4.8) |
| 12 weeks | 30 | 81.6 (8.0) | 29 | 82.8 (8.5) | ||||
Favourable increases in moderate physical activity (from objective accelerometer data) and decreases in dietary fat scores were also reported (tables 3 and 4). Little change was observed in reported alcohol intake.
Table 3.
Changes in physical activity
| Intervention | Control | Between group differences
Mean (95% CI) |
||||||
| n | Mean (SD) | Difference to baseline Mean (95% CI) |
n | Mean (SD) | Difference to baseline Mean (95% CI) |
|||
| Daily average time spent in moderate activity (min) | Baseline | 34 | 58.1 (49.5) | 20.3 (−36.2 to −4.4) | 38 | 60.3 (39.4) | 5.82 (−27.2 to 15.5) | −13.6 (39.0 to 11.7) |
| 12 weeks | 23 | 86.8 (62.0) | 23 | 73.2 (46.5) | ||||
| Daily average time spent in vigorous activity (min) | Baseline | 34 | 1.1 (2.8) | 0.1 (−1.2 to 1.0) | 38 | 1.5 (3.5) | −0.5 (−1.3 to 2.3) | 1.0 (−3.0 to 1.0) |
| 12 weeks | 23 | 1.0 (2.1) | 23 | 1.2 (3.3) | ||||
| Daily average step count | Baseline | 34 | 7544 (3738) | 1760 (−3111 to −408) | 38 | 7999 (3937) | 699 (−2629 to 1230) | 939 (−3187 to 1309) |
| 12 weeks | 23 | 10 315 (5551) | 23 | 9468 (3793) | ||||
Table 4.
Changes in dietary intake scores
| Intervention | Control | Between group differences
Mean (95% CI) |
||||||
| n | Mean (SD) | Difference to baseline Mean (95% CI) |
n | Mean (SD) | Difference to baseline Mean (95% CI) |
|||
| Fat consumption score | Baseline | 39 | 29.3 (9.0) | −7.8 (5.2 to 10.4) | 39 | 28.6 (9.3) | −1.2 (−1.5 to 4.0) | 6.6 (3.7 to 9.5) |
| 12 weeks | 30 | 20.5 (6.0) | 29 | 27.2 (7.6) | ||||
| Unsaturated fat score | Baseline | 39 | 8.3 (1.4) | −0.2 (−0.4 to 0.7) | 39 | 8.2 (1.2) | 0.2 (−0.7 to 0.4) | 0.3 (−0.4 to 1.0) |
| 12 weeks | 30 | 8.2 (1.7) | 29 | 8.4 (1.4) | ||||
| Fibre food consumption score | Baseline | 39 | 31.7 (11.5) | 0.6 (−4.4 to 3.2) | 39 | 29.6 (9.2) | −0.8 (−2.5 to 4.0) | 1.8 (−6.2 to 2.6) |
| 12 weeks | 30 | 32.5 (10.9) | 29 | 30.1 (9.4) | ||||
Using the data from the current study, where we observed a mean body weight of 89.5 kg (±SD 13.3), a total of 187 participants per group would be needed to detect a between group difference of 5% weightt change (4.47 kg) at follow-up, at 90% power and 5% alpha based on a two-tailed unpaired t-test. Allowing for an assumed 25% drop-out this would mean recruiting 250 participants per group.
Based on the current figures: to recruit 500 people, 1250 would need to express an interest (40% of those who expressed an interest were recruited). For 1250 to express an interest, 3048 would require to be approached (based on 41% of people approached were interested). If all clinics recruited similar numbers to the two in the current study (240 in an 8-month period/360 per year), then nine centres would be needed for a 12-month recruitment period.
Discussion
Main findings
Despite evidence for the importance of lifestyle in the aetiology of BC and CRC, counselling on diet, physical activity and weight management is not routinely provided for people with a family history of these conditions referred to NHS genetics clinics. While cancer preventability estimates suggest that healthful ways of life could significantly reduce cancer risk, the impact of lifestyle interventions in this patient group is unknown and randomised controlled trial data is needed to examine the cost, benefits and harms. The results from this pilot study show that it is feasible to recruit, randomise and retain patients and to implement and evaluate a weight management programme combining diet and physical activity advice with behavioural change techniques that is acceptable to patients. The results also indicate that implementation of this programme is associated with favourable outcomes. However, the current work has highlighted the importance of face-to-face approaches for study recruitment and the cautious approach taken by genetic clinic staff to introducing the study. The findings have also enabled the collection of data that allows a power calculation to be performed for the design of a definitive randomised controlled trial.
Strengths and weaknesses
The main strength of the study is that (to the best of our knowledge) the current work is the first attempt to offer a comprehensive lifestyle programme (diet, alcohol, physical activity and body weight) for people referred to FH clinics for CRC and BC risk assessment. The main weakness of the study is the sociodemographic profile of participants recruited who were predominantly Caucasian and >50% were from the two lowest deprivation quintiles (eg, higher socioeconomic status). Although this distribution reflects the general demographic for high-risk attendees for breast mammography clinics in this region32 (where there is little ethnic diversity), it highlights the difficulties in offering both surveillance and lifestyle interventions to affected people from more deprived areas.
The sample size was less than planned due to fewer patients being referred to the service than previous years (on which our estimate was based), fewer informed about the study than estimated (despite input from clinic staff in study design) and fewer being seen face to face than anticipated. The current results underline the need for feasibility work. Overall, study retention was high but further efforts to reduce loss to follow-up in people living in areas of higher deprivation should be explored. Attempts to increase recruitment from existing patients demonstrate that both new and returning patients have some interest in the topic, but in general a greater awareness of the relevance of lifestyle in cancer prevention would be beneficial to recruitment. Other weaknesses include the time allocation for the face-to-face meeting, which was insufficient for the intended content, resulting in the LCs deviating from the original protocol. In addition, retention was only assessed over a 12-week period, and a longer test period is likely to be associated with higher loss to follow-up. Independent assessment of fidelity also suggests scope for improvement in time allocation as only 62% of intervention components were delivered per protocol.
Other studies
No fully powered trials of lifestyle interventions in people with family history of cancer have yet reported and those that have been described in the literature focus primarily on physical activity33 34 in Breast Cancer, early onset (BRCA) mutation carriers. Ongoing trials of weight management to decrease BC risk are being undertaken in women already diagnosed with the disease35–38 but are not exclusive to people at increased genetic risk. Pilot and formative work on weight loss and reducing markers of BC has been undertaken39 (notably on the intermittent energy diet approach) in women with family history of BC, demonstrating significant changes in insulin sensitivity and body fat reduction, but no work has been reported for patients with a family history of CRC. Postintervention interviews with participants40 have highlighted the importance of providing a credible rationale for weight control and weight loss that underlines the need for health professionals working in this area to introduce and endorse the importance of lifestyle to aid recruitment.
Implications for clinicians and policymakers
This pilot study has highlighted a number of perceived challenges for NHS staff discussing lifestyle issues among patients with a family history of BC and CRC but has also demonstrated that there is an interest in being able to offer more help that could usefully be developed. Indicative results suggest the intervention had a favourable effect on body weight, physical activity and fat intake score with potential for clinically relevant benefits for both cancer risk reduction and long-term health (with or without a cancer diagnosis). While the qualitative data highlight the complexity and challenge of changing health behaviours, the measured changes in physical activity and weight loss suggest that small but significant changes can be achieved, at least in the short term. Support for habitual and sustained lifestyle change is essential as part of long-term intervention design.
Unanswered questions and future research
The generalisability of the current findings are unclear. However, work to date suggests that the intervention is feasible to deliver and evaluate and could be tested in a large RCT, subject to modifications. In a fully powered trial, the first stage would be to assess the magnitude of lifestyle change that can be achieved by this type of programme. In turn, this fully powered trial would act as a ‘pilot’ for a full trial of reduction in CRC markers. Adenomas (number and size) might be an appropriate end point depending on funding for the length of follow-up.
It would be desirable to increase patient recruitment, and the current findings suggest that overall uptake could be increased with better training, support and endorsement from the GCs and other clinical staff. This area of study was almost entirely new and met with scepticism from staff and indeed patients. Our earlier work suggests ambiguous attitudes about the importance of lifestyle with little evidence that these topics have been previously discussed with clinicians.18
The programme was received enthusiastically by participants, but further work is needed to refine intervention components, particularly dietary aspects that were less appreciated than the physical activity aspects and may need tailoring for ethnic groups. Measurements were successfully attained and appropriate for a full study protocol, although a review of accelerometry approaches is warranted. The numbers required for a full trial with weight change as a primary outcome would require a multicentre approach with a minimum recruitment period of at least 12 months. Overall, the main uncertainties required for planning a definitive randomised controlled trial have been addressed.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the valuable contribution given by Heather Gow (research nurse), Maria Nika and Catherine Savage (lifestyle coaches) and all centre staff involved in the study.
Footnotes
Contributors: ASA and RJCS had the original idea for the study and, with JD, MM, ZM, NM, REO, MS, SV and JB, designed the trial parameters and formed the investigator group who obtained the funding. ASA, JB, JD, ZM, REO, SG and MS were responsible for overseeing study implementation and data collection. MM, REO and RST carried out the analysis. ASA and MM drafted the manuscript, which was revised by all authors. All authors approved the final paper and were independent from funders.
Funding: This work was supported by Chief Scientist Office for Scotland, grant number CZH/4/1080.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethical approval for this study was provided by East of Scotland Research Ethics Service (REC reference: 15/ES/0055).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Technical appendix, statistical code and dataset available from the authors on request.
References
- 1.Genetic Alliance UK [Internet]. NHS Genetics services in the UK. 2017. http://www.geneticalliance.org.uk/information/services-and-testing/nhs-genetic-services-in-the-uk/ (cited Aug 2017).
- 2.World Cancer Research Fund [Internet]. Cancer Preventability Statistics. https://www.wcrf-uk.org/uk/preventing-cancer/cancer-preventability-statistics (cited 2017 Aug 7).
- 3.Harvie M, Howell A, Vierkant RA, et al. . Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev 2005;14:656–61. 10.1158/1055-9965.EPI-04-0001 [DOI] [PubMed] [Google Scholar]
- 4.Ahn J, Schatzkin A, Lacey JV, et al. . Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med 2007;167:2091–102. 10.1001/archinte.167.19.2091 [DOI] [PubMed] [Google Scholar]
- 5.Schauer DP, Feigelson HS, Koebnick C, et al. . Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg 2017. [Epub ahead of print 21 Sep 2017]. 10.1097/SLA.0000000000002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gramling R, Lash TL, Rothman KJ, et al. . Family history of later-onset breast cancer, breast healthy behavior and invasive breast cancer among postmenopausal women: a cohort study. Breast Cancer Res 2010;12:R82 10.1186/bcr2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettapiece-Phillips R, Narod SA, Kotsopoulos J. The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes Control 2015;26:333–44. 10.1007/s10552-014-0521-0 [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Lee JE, Rimm EB, et al. . Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. Am J Clin Nutr 2012;95:413–9. 10.3945/ajcn.111.022145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Movahedi M, Bishop DT, Macrae F, et al. . Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 study. J Clin Oncol 2015;33:3591–7. 10.1200/JCO.2014.58.9952 [DOI] [PubMed] [Google Scholar]
- 10.Fardet A, Druesne-Pecollo N, Touvier M, et al. . Do alcoholic beverages, obesity and other nutritional factors modify the risk of familial colorectal cancer? A systematic review. Crit Rev Oncol Hematol 2017;119:94–112. 10.1016/j.critrevonc.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 11.Akhtar S, Sinha S, McKenzie S, et al. . Awareness of risk factors amongst first degree relative patients with colorectal cancer. Colorectal Dis 2008;10:887–90. 10.1111/j.1463-1318.2008.01502.x [DOI] [PubMed] [Google Scholar]
- 12.Anderson AS, Craigie AM, Caswell S, et al. . The impact of a bodyweight and physical activity intervention (BeWEL) initiated through a national colorectal cancer screening programme: randomised controlled trial. BMJ 2014;348:g1823 10.1136/bmj.g1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Catalano PJ, Haller DG, et al. . Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer 2003;98:484–95. 10.1002/cncr.11544 [DOI] [PubMed] [Google Scholar]
- 14.Chan DS, Vieira AR, Aune D, et al. . Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14. 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French DP, Cameron E, Benton JS, et al. . Can communicating personalised disease risk promote healthy behaviour change? A systematic review of systematic reviews. Ann Behav Med 2017;51:718–29. 10.1007/s12160-017-9895-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AS, Caswell S, Macleod M, et al. . Health behaviors and their relationship with disease control in people attending genetic clinics with a family history of breast or colorectal cancer. J Genet Couns 2017;26:40–51. 10.1007/s10897-016-9977-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ 2001;322:1056–9. 10.1136/bmj.322.7293.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dombrowski SU, Sniehotta FF, Avenell A, et al. . Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: a systematic review. Health Psychol Rev 2012;6:7–32. 10.1080/17437199.2010.513298 [DOI] [Google Scholar]
- 19.Michie S, Richardson M, Johnston M, et al. . The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Clinical and Health Excellence. Familial breast cancer. NICE clinical guideline 164. 2017. https://www.nice.org.uk/guidance/cg164/chapter/Recommendations#clinical-significance-of-a-family-history-of-breast-cancer. [PubMed]
- 21.Eldridge SM, Chan CL, Campbell MJ, et al. . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2:64 10.1186/s40814-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301–8. 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 23.Scottish Intercollegiate Guideline Network (SIGN). Management of Obesity. Edinburgh: SIGN, 2010. [Google Scholar]
- 24.Leventhal H, Leventhal EA, Breland JY. Cognitive science speaks to the "common-sense" of chronic illness management. Ann Behav Med 2011;41:152–63. 10.1007/s12160-010-9246-9 [DOI] [PubMed] [Google Scholar]
- 25.Bandura A. Social Foundations of Thought and Action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall, 1986. [Google Scholar]
- 26.Schwarzer R. Modeling health behaviour change: how to predict and modify the adoption and maintenance of health behaviors. Appl Psychol 2008;57:1–29. [Google Scholar]
- 27.Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 28.Roe L, Strong C, Whiteside C, et al. . Dietary intervention in primary care: validity of the DINE method for diet assessment. Fam Pract 1994;11:375–81. 10.1093/fampra/11.4.375 [DOI] [PubMed] [Google Scholar]
- 29.Emslie C, Lewars H, Batty GD, et al. . Are there gender differences in levels of heavy, binge and problem drinking? Evidence from three generations in the west of Scotland. Public Health 2009;123:12–14. 10.1016/j.puhe.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broadbent E, Petrie KJ, Main J, et al. . The brief illness perception questionnaire. J Psychosom Res 2006;60:631–7. 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 31.EQ-5D 2017. Euroqol organisation [Internet]. https://euroqol.org/ (cited Aug 2017).
- 32.NHS Tayside. The Business Unit Information Team. Heraklion, Greece: The European Union Agency for Network and Information Security, 2014. [Google Scholar]
- 33.Pasanisi P, Bruno E, Manoukian S, et al. . A randomized controlled trial of diet and physical activity in BRCA mutation carriers. Fam Cancer 2014;13:181–7. 10.1007/s10689-013-9691-2 [DOI] [PubMed] [Google Scholar]
- 34.Kiechle M, Engel C, Berling A, et al. . Lifestyle intervention in BRCA1/2 mutation carriers: study protocol for a prospective, randomized, controlled clinical feasibility trial (LIBRE-1 study). Pilot Feasibility Stud 2016;2:74 10.1186/s40814-016-0114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pegington M, Adams JE, Bundred NJ, et al. . Recruitment to the “Breast-Activity and Healthy Eating After Diagnosis” (B-AHEAD) Randomized Controlled Trial. Integr Cancer Ther 2017;1:153473541668785 10.1177/1534735416687850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical Trials.Gov [Internet]. Breast Cancer WEight Loss Study (BWEL Study). https://clinicaltrials.gov/ct2/show/NCT02750826 (Cited 7 Aug 2017).
- 37.Clinical Trials.Gov [Internet]. Weight Gain Prevention for Breast Cancer Survivors. https://clinicaltrials.gov/ct2/show/NCT00533338 (Cited Aug 2017).
- 38.Gnagnarella P, Dragà D, Baggi F, et al. . Promoting weight loss through diet and exercise in overweight or obese breast cancer survivors (InForma): study protocol for a randomized controlled trial. Trials 2016;17:363 10.1186/s13063-016-1487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvie M, Wright C, Pegington M, et al. . The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 2013;110:1534–47. 10.1017/S0007114513000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright CE, Harvie M, Howell A, et al. . Beliefs about weight and breast cancer: an interview study with high risk women following a 12 month weight loss intervention. Hered Cancer Clin Pract 2015;13:1 10.1186/s13053-014-0023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.