Abstract
Objectives:
cardiopulmonary bypass (CPB) can be complicated by vasoplegia that is refractory to vasopressors. Methylene blue (MB) represents an alternative in such cases.
Patients and Methods:
Retrospective observational historical control-matched study. From 2010 to 2015, all patients who received MB for vasoplegia post-CPB were included in this study. Historical controls from the period of 2004 to 2009 were matched. End-points were the time till improvement of vasoplegia (Ti), 30-day mortality, cardiac surgical Intensive Care Unit (CSICU) morbidity, and length of stay (LOS).
Results:
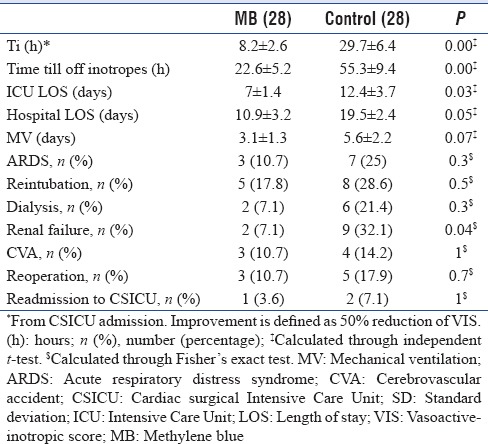
Twenty-eight patients were matched in both groups. There were no statistically significant differences between the two groups in demographic, laboratory data on admission, or hemodynamic profile before use of MB. Ti and time to complete discontinuation of vasopressors were statistically significant less in MB group (8.2 ± 2.6 vs. 29.7 ± 6.4, P = 0.00 and 22.6 ± 5.2 vs. 55.3 ± 9.4, P = 0.00) respectively. Mortality at day 30 was significantly higher in controls compared to MB (1 patient [3.6%] vs. 6 patients [21.4%], long rank P = 0.04). CSICU, hospital LOS, and incidence of renal failure was significantly higher in control group (12.4 ± 3.7 vs. 7 ± 1.4, P = 0.03), (19.5 ± 2.4 vs. 10.9 ± 3.2, P = 0.05) and (9 patients [32.1%] vs. 2 patients [7.1%], P = 0.04), respectively. Duration of mechanical ventilation was less in MB patients; however, did not reach statistical significance.
Conclusions:
the use of MB for vasoplegia postcardiac surgery was associated with rapid recovery of hemodynamics, shorter need for vasopressors, less ICU mortality, less incidence of renal failure, and shorter LOS.
Keywords: Cardiac surgery, methylene blue, vasoplegia
INTRODUCTION
Vasoplegia is a term that describes excessive loss of vascular muscle tone leading to a distributive shock status. Vasoplegic syndrome (VS) after cardiac surgery is a condition characterized by hypotension increased cardiac index (CI), low systemic vascular resistance (SVR), normal filling pressures, and increased vasopressor and fluid requirements.[1] The syndrome is reported with an incidence ranging between 5% and 25%.[2] The exact mechanism of VS is unknown; it is suggested to be multifactorial, hemodilution, baroreceptor reflexes, and complement activation are involved. However, VS is associated with a systemic inflammatory response that results in endothelial dysfunction and production of nitric oxide (NO) from L-arginine by polymorphonuclear blood cells in response to the enzyme inducible NO synthase. NO in turn stimulates the production of cyclic guanosine monophosphate (cGMP) which is the final messenger of the NO pathway responsible for vasoplegia. cGMP formation is dependent on the enzyme guanylate cyclase (GC). Excessive formation of NO and cGMP is related to profound vasodilatation, myocardial depression, and decreased response to catecholamines.[3]
Traditional treatment for VS would be fluid resuscitation and the use of vasopressor agents, such as phenylephrine, norepinephrine, and vasopressin. As an alternative, methylene blue (MB) was proposed because it antagonizes the effect of NO and other vasodilators in the endothelium and vascular smooth muscle by binding to GC and blocking cGMP action in vascular smooth muscle.[4] Using MB to counteract excessive vasodilatation has been described in different settings: septic shock,[5,6,7] endocarditis,[8] transplant,[9] and protamine reaction.[10] The treatment of vasoplegic manifestation after cardiopulmonary bypass (CPB) using MB administration has been suggested in both adult[11,12,13,14,15] and pediatric cardiac surgery.[16,17]
The aim of this study is to evaluate the effectiveness and outcome of MB therapy in VS after cardiac surgery in adults.
PATIENTS AND METHODS
After approval by Institutional research center and ethics committees, the study was conducted in cardiac surgical Intensive Care Unit (CSICU), (24 beds postcardiac ICU), King Faisal Heart Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia. Being retrospective chart review study, consent was waived by the ethical committee.
Inclusion criteria
All adult patients who had cardiac surgery between January 2010 and May 2015 and received MB for VS during their stay in CSICU were included in the study.
VS was defined by the presence of all the following criteria:
Hypotension (mean arterial pressure [MAP] <50 mm Hg)
Low filling pressure ([central venous pressure] CVP <5 mm Hg and pulmonary capillary wedge pressure (PCWP) <10 mm Hg)
CI >2.5 L/min/m2
Low resistance: SVR <800 dyn. s/cm5, or SVR index (SVRI) <1500 dyn. s/cm5/m2
Vasoactive inotropic score (VIS)[18] ≥50.
VIS = Dopamine dose (μg/kg/min) + Dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 100 × Norepinephrine dose (μg/kg/min) + 10,000 × Vasopressin dose (U/kg/min) + 10 × Milrinone dose (μg/kg/min).
Exclusion criteria
Study design
Retrospectively, the medical records of all patients who had symptoms and signs suggestive of VS were reviewed. Before 2010, the use of MB for VS was not popular in our institution. However, since then many intensivist and anesthesiologists starts to use it until it became almost the gold standard for all patients who had VS after cardiac surgery and failed to respond to vasopressors. All patients who received MB from January 2010 to May 2015 were included in this study. Another group containing the patients who suffered from the signs and symptoms of VS during the period of 2004 till 2009 were included and acted as historical control. To reduce the confounding effects, a propensity score was created and both groups were matched accordingly.
Postoperative management in cardiac surgical intensive care
If VS is suspected despite adequate fluid resuscitation and adequate doses of vasopressors, a thermodilution PAC was inserted (if not present) to confirm the diagnosis and help further management. Once VS is confirmed, norepinephrine infusion was started (if not yet) and adjusted according to the cardiac output (CO) and SVR. Failure to respond to norepinephrine alone will usually initiate the use of other vasopressors as vasopressin/epinephrine or both. Increasing vasopressor support to a VIS is ≥50, initiated the use of MB. A bolus dose of 2 mg/kg of MB over 30 min was started and followed by 0.5–1 mg/kg/h if needed. Invasive MAP, mean pulmonary arterial pressure, CVP, PCWP, CO, CI, SVR, SVRI, heart rate, arterial oxygen saturation, respiratory rate (RR), core temperature, and electrocardiography were recorded. IV MB infusion was discontinued once the patient had improvement in hemodynamic parameters.
Data collection
Data collected included demographic (age, sex, weight, body mass index [BMI]); premorbid conditions and risk factors (diabetes mellitus [DM], hypertension [HTN], smoking, renal function including creatinine level, dialysis and cerebrovascular accidents [CVA]); the preoperative cardiac conditions (LVEF and ischemic heart disease [IHD]); preoperative medications (angiotensin converting enzyme inhibitor [ACEI], beta blockers, anticoagulant, and antiplatelets); admission laboratory values [hemoglobin, prothrombin time (PT), activated partial thromboplastin time, (INR), electrolyte levels, bilirubin level, white blood counts, and platelet counts]. Information needed to calculate European System for Cardiac Operative Risk Evaluation II (Euro-SCORE II)[21] was obtained for each patient. Operative data included previous cardiac surgery, priority of the cardiac surgery, CPB time, cross-clamp time (X clamp) time, type of the procedure, need and type of mechanical circulatory support. Postoperatively, maximum VIS, arterial blood gases, urine output and chest tube bleeding were recorded hourly. The data collected was entered into an Excel database designed for the study.
End-points
To compare the two groups regarding:
Time to improvement of vasoplegia (Ti) defined as: Time from admission till the improvement of hemodynamic parameters (MAP, CVP, PCWP, CI, and SVRI) and decrease in VIS by 50% or more from the maximum value
-
Postoperative Complications: Renal failure,[22] need for dialysis, respiratory complications (ARDS),[23] duration of mechanical ventilation (MV), reintubation, CVA,[24] duration of inotropic support, readmission to CSICU, and reoperation.
- CSICU, hospital length of stay (LOS) and
- Mortality at 30 days.
Statistical analysis
Descriptive statistics for the continuous variables are reported as mean ± standard deviation, and categorical variables are summarized as frequencies and percentages. Continuous variables are compared by Student's independent t-test; while categorical variables are compared by Chi-square or Fisher's exact test. Demographic date (age, sex, and BMI), preoperative comorbidities (DM, HTN, smoking, IHD, dialysis, and CVA), operative risks (LVEF, Euro-SCORE II, CPB, and X clamp), and postoperative hemodynamic status (MAP, CVP, PCWP, CO, CI, and VIS) were used in generation of propensity score through logistic regression. The level of significance was set at 0.25. Then patients were matched 1:1 with the nearest neighbor according to propensity scores. Then, all analyses were performed in this matched cohort. Kaplan–Meir survival curve was estimated, and comparison between overall survivals in the two groups was done using log-rank test. The level of significance is set at P < 0.05. Statistical analyses of data were done using the software package IBM Statistical Package for the Social Science (SPSS), Chicago, Illinois, USA version 23 for Microsoft Window.
RESULTS
All adult patients who had cardiac surgery between 1st of January 2010 and 31st of May 2015 were screened for those who had symptoms and signs suggestive of VS. Fifty-one patients identified, three were excluded due to confirmed septic shock association, one due to failure to insert PAC secondary to superior vena cava thrombosis, 12 patients responded to vasopressor/inotropes, and 35 received MB for unresponsive VS. These 12 patients who responded to vasopressors could have acted as controls for the treatment group. However, although fulfilled the inclusion criteria of VS according to definition used in this study, they had a statistically significant better hemodynamic parameters and could not be matched to MB patients. We thought that this will imply effects on the interpretation of the results. For this, we preferred to have a historical control group from an equal period of time (2004 till 2009) where the use of MB was not familiar in our CSICU. Revising the medical charts, 57 patients were identified to have VS. Twenty-eight patients in MB group were successfully matched to patients in the control group in 1:1 ratio using the propensity score according to Figure 1.
Figure 1.
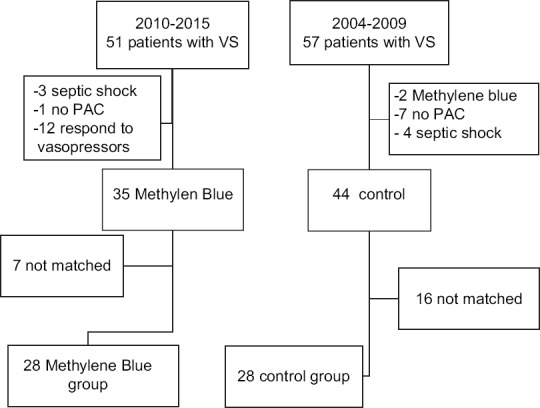
Patients included in the study
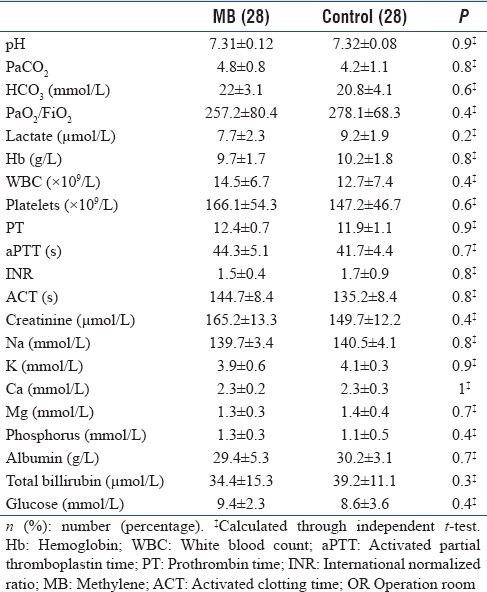
There was no statistical significant difference between the two groups regarding demographic, preoperative risks, preoperative cardiac condition, preoperative medications, operative data, admission laboratory results, hemodynamics, and VIS before start of MB [Tables 1–3]. The mean time from admission to start the MB was 4.3 ± 1.4 h. Five patients received only bolus dose of MB while the other 23 patients received both bolus and IV infusion. The mean time for MB IV infusion was 4.7 ± 0.9 h.
Table 1.
Descriptive data of both groups
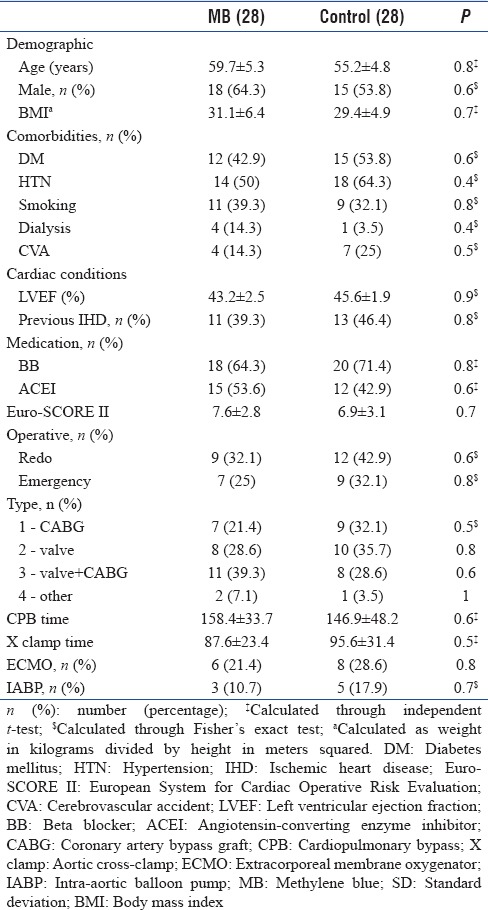
Table 3.
Hemodynamics before methylene blue
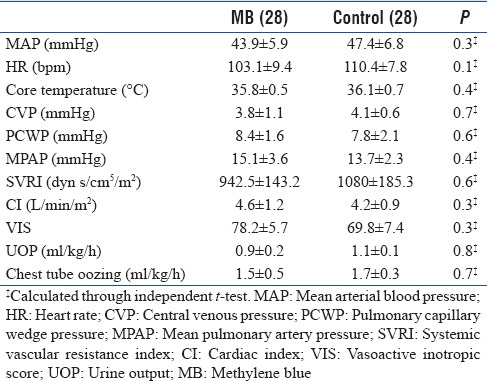
Table 2.
Laboratory results on admission from OR to cardiac surgical Intensive Care Unit
The Ti and time till complete discontinuation of vasopressors were statistically significant less in MB group compared to controls (8.2 ± 2.6 vs. 29.7 ± 6.4, P = 0.00 and 22.6 ± 5.2 vs. 55.3 ± 9.4, P = 0.00) respectively [Table 4].
Table 4.
End-points
The evolution of hemodynamic parameters to improvement was rapid in MB group compared to controls . There was a statistical significant difference in CI, MAP, SVRI, lactate, and VIS between MB group and control group as rapid as 8–12 h after admission which equals almost 4–8 h after the start of MB. On the other hand, the control group took between 24 and 36 h to show adequate improvement to the degree of insignificance compared to MB group [Figure 2a–e].
Figure 2.
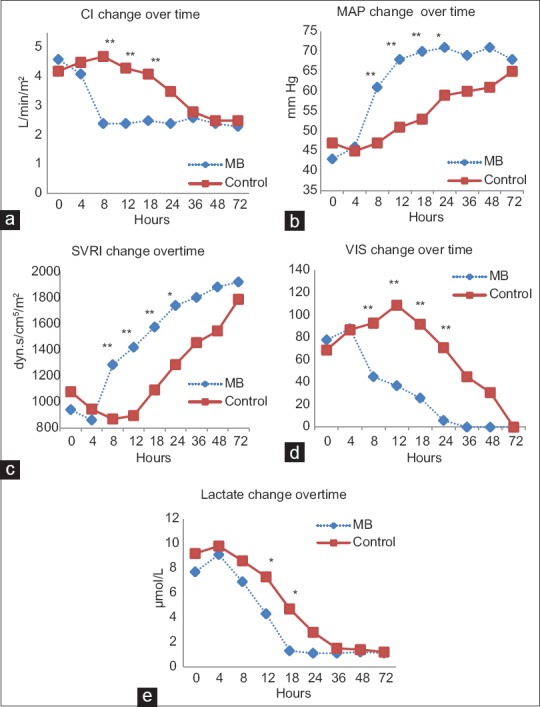
Evolution of hemodynamics over time in both groups. a: CI, cardiac index; b:MAP, mean arterial pressure; c: SVRI, systemic vascular resistance index; d: VIS, vasoactive inotropic score; e: lactate. *P < 0.05, **P < 0.01
Mortality at day 30 was statistically significant higher in controls compared to MB (1 patient [3.6%] vs. 6 patients [21.4%], long rank P = 0.04) [Figure 3].
Figure 3.
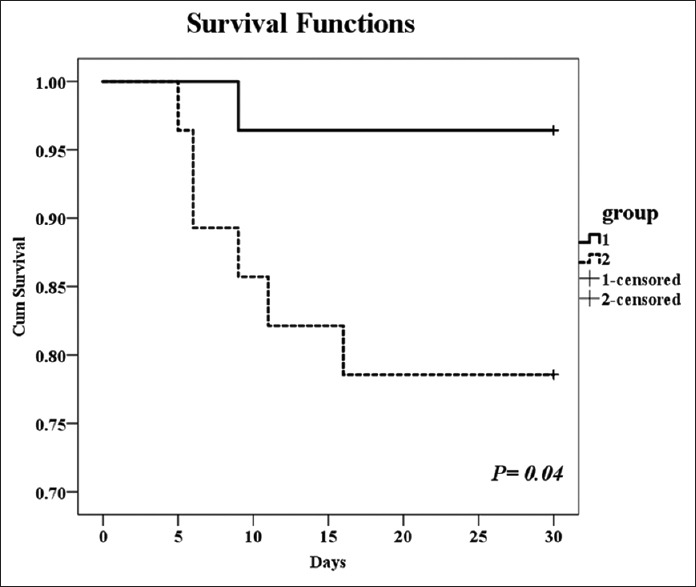
Kaplan–Meier curve for survival in two groups
ICU, hospital LOS, and incidence of new renal failure not requiring dialysis were statistically significant higher in control grouped compared to MB [Table 4]. Duration of MV was less in MB patients, however, did not reach statistical significance. Other ICU acquired complications showed no statistical significant different between patients.
DISCUSSION
VS is a well-known complication following cardiac surgery.[1] In this study, we have demonstrated that the treatment of VS after CPB with intravenous MB showed a significantly quicker recovery to normal hemodynamics, shorter duration of vasopressor infusions, less incidence of renal complications, shorter LOS and less 30-days mortality.
Duration of VS is another factor that adds to the poor outcome. Persistence of the VS for more than 48 h is associated with worse prognosis or even death.[25] Mehaffey et al.[26] showed that the early administration of MB improves survival and reduces the risk-adjusted rate of major adverse events for VS after cardiac operation. In this review, the time between the admission and the start of MB was around 4 h and the Ti was 4–5 h. Even time till complete discontinuation of vasopressors in MB group was less than Ti in the control group (22.6 ± 5.2 vs. 29.7 ± 6.4). We think that this has resulted in a less LOS, renal impairment, and mortality in MB-treated patients.
The favorable effect of MB on SVR and CI in shock situations has been established. Earlier, Leyh et al.[11] assessed the clinical effect of MB therapy for norepinephrine-refractory vasoplegia after CPB showing an increase in SVR and a decrease in norepinephrine dosage. However, unlike Leyh et al.[11] this work shows the clear improvement over time of different hemodynamic parameters, lactate (as a marker of restoration of peripheral circulation), and inotropic support compared to a control group.
Levin et al.[12] confirmed the effectiveness of MB treatment in reducing postoperative morbidity and mortality and showed no incidence of complications in VS patients treated with MB in 2 h. Similarly, the current study shows a significant decrease in mortality after 30 days (3.6% in MB group vs. 21.4% in control), length of ICU (7 ± 1.4 in MB group vs. 12.4 ± 3.7 in control), and in length of hospital stay (10.9 ± 3.2 in MB group vs. 19.5 ± 2.4 in control). This also goes in agreement with results of MB prophylactic administration in the preoperative period for patients at risk of having vasoplegia with CPB.[13] In contrast, Weiner et al.[15] in their retrospective study, demonstrated an increase in mortality in patients receiving MB. However, patients who received MB were sicker than those who did not.[15] Moreover, those patients[15] were sicker than those of other studies.[13,12] They had higher CBP times (194 min), and higher Euro-score II (11) compared to an average of 7.6 in the current study. The time when MB was used[15] was variable in relation to the proposed window of opportunity[27,28] and even after matching patients, this can still lead to difference in morbidity. Weiner et al.[15] also suggested that MB improves macrocirculation while having a detrimental effect on microcirculation; however, studying lactate levels in our patients, showed a significant drop in the MB group after 12 h from admission, and a normal lactate levels 18 h after admission onward [Figure 2e]. This goes in agreement with results of Maslow et al.[14] in their prospective study of MB effect when given during CPB in patients receiving ACEI.
Higher MAP at the time of drug administration and surgery with deep hypothermic circulatory arrest have been suggested by Mazzeffi et al.[29] as a predictor of greater MB response. This was not the case in our MB-treated patients who had a lower MAP on admission compared to the control group, (43.9 ± 5.9 vs. 47.4 ± 6.8, P = 0.3); however, they had a better outcomes.
As for the incidence of postoperative ICU complications in the current study, there was no advantage between two groups except for a significant decrease in renal complications within MB group. Complications related to MB administration have been described. This includes decreased mesenteric perfusion with high dose (7 mg/kg),[30] slight increase in serum glutamic pyruvic transaminase has been observed and found to be quickly resolving.[13] Increase in pulmonary vascular pressure/resistance, and deterioration in gas exchange can occur following MB in large doses.[31] Our results shows a slight nonsignificant increase in pulmonary artery pressure associated with a slight decrease in PaO2/FiO2 ratio; however, MB patients had lower ventilation duration, less ARDS incidence, and lower incidence of reintubation.
The current study has limitations. First, the retrospective nature of the study means that findings may be affected by unmeasured confounders and mostly should be viewed as associative. Second, the control group was chosen from an older time window which could have added more confounding factors; however, propensity score matching controlled this issue. Third, no clear definition for hemodynamic improvement, we could not use crude target values for MAP, CVP, CI, and SVRI to represent improvement nor percentage of improvement, mainly because differences between the patients, for example, rise of MAP from 40 to 48 is 20% improvement but is it considered a target improvement and discontinue MB? Hence, we based mainly on the reduction of VIS to <50% of the maximum as an indicator of improvement of VS and hence starting discontinuation of MB.
CONCLUSIONS
MB for treatment of VS postcardiac surgery showed rapid restoration of hemodynamics, decreased need for vasopressors, significantly less mortality, renal impairment, and LOS. The relatively early utilization of MB might have influenced our outcomes. The recent controversies regarding morbidity and mortality with MB require more large randomized controlled trials to explore the impact on outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gomes WJ, Carvalho AC, Palma JH, Gonçalves I, Jr, Buffolo E. Vasoplegic syndrome: A new dilemma. J Thorac Cardiovasc Surg. 1994;107:942–3. [PubMed] [Google Scholar]
- 2.Fischer GW, Levin MA. Vasoplegia during cardiac surgery: Current concepts and management. Semin Thorac Cardiovasc Surg. 2010;22:140–4. doi: 10.1053/j.semtcvs.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Evora PR. Should methylene blue be the drug of choice to treat vasoplegias caused by cardiopulmonary bypass and anaphylactic shock? J Thorac Cardiovasc Surg. 2000;119:632–4. doi: 10.1016/s0022-5223(00)70152-8. [DOI] [PubMed] [Google Scholar]
- 4.Evora PR, Levin RL. Methylene blue as drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2004;127:895–6. doi: 10.1016/j.jtcvs.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Andresen M, Dougnac A, Díaz O, Hernández G, Castillo L, Bugedo G, et al. Use of methylene blue in patients with refractory septic shock: Impact on hemodynamics and gas exchange. J Crit Care. 1998;13:164–8. doi: 10.1016/s0883-9441(98)90001-6. [DOI] [PubMed] [Google Scholar]
- 6.Donati A, Conti G, Loggi S, Münch C, Coltrinari R, Pelaia P, et al. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med. 2002;30:2271–7. doi: 10.1097/00003246-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–15. doi: 10.1592/phco.30.7.702. [DOI] [PubMed] [Google Scholar]
- 8.Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg. 2003;125:426–7. doi: 10.1067/mtc.2003.140. [DOI] [PubMed] [Google Scholar]
- 9.Flynn BC, Sladen RN. The use of methylene blue for vasodilatory shock in a pediatric lung transplant patient. J Cardiothorac Vasc Anesth. 2009;23:529–30. doi: 10.1053/j.jvca.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Del Duca D, Sheth SS, Clarke AE, Lachapelle KJ, Ergina PL. Use of methylene blue for catecholamine-refractory vasoplegia from protamine and aprotinin. Ann Thorac Surg. 2009;87:640–2. doi: 10.1016/j.athoracsur.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, et al. Methylene blue: The drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003;125:1426–31. doi: 10.1016/s0022-5223(02)73284-4. [DOI] [PubMed] [Google Scholar]
- 12.Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. 2004;77:496–9. doi: 10.1016/S0003-4975(03)01510-8. [DOI] [PubMed] [Google Scholar]
- 13.Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kücükarslan N, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79:1615–9. doi: 10.1016/j.athoracsur.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Maslow AD, Stearns G, Butala P, Schwartz CS, Gough J, Singh AK, et al. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg. 2006;103:2–8. doi: 10.1213/01.ane.0000221261.25310.fe. [DOI] [PubMed] [Google Scholar]
- 15.Weiner MM, Lin HM, Danforth D, Rao S, Hosseinian L, Fischer GW, et al. Methylene blue is associated with poor outcomes in vasoplegic shock. J Cardiothorac Vasc Anesth. 2013;27:1233–8. doi: 10.1053/j.jvca.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla T, Sawardekar A, Russell H, Tobias JD. The role of methylene blue in the pediatric patient with vasoplegic syndrome. World J Pediatr Congenit Heart Surg. 2011;2:652–5. doi: 10.1177/2150135111410992. [DOI] [PubMed] [Google Scholar]
- 17.Abdelazim R, Salah D, Labib H, El Midany A. Methylene blue compared to norepinephrine in the management of vasoplegic syndrome in pediatric patients after cardiopulmonary bypass: A randomized controlled study Egyptian. J Anaesth. 2016;32:269–75. [Google Scholar]
- 18.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR. VasoplegiPostoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds HR, Hochman JS. Cardiogenic shock: Current concepts and improving outcomes. Circulation. 2008;117:686–97. doi: 10.1161/CIRCULATIONAHA.106.613596. [DOI] [PubMed] [Google Scholar]
- 20.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–44. doi: 10.1093/ejcts/ezs043. [DOI] [PubMed] [Google Scholar]
- 22.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A, et al. A comparison of the RIFLE and acute kidney injury network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–6. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 23.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: The berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gando S, Kameue T, Nanzaki S, Hayakawa T, Nakanishi Y. Participation of tissue factor and thrombin in posttraumatic systemic inflammatory syndrome. Crit Care Med. 1997;25:1820–6. doi: 10.1097/00003246-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Mehaffey JH, Johnston LE, Hawkins RB, Charles EJ, Yarboro L, Kern JA, et al. Methylene blue for vasoplegic syndrome after cardiac operation: Early administration improves survival. Ann Thorac Surg. 2017;104:36–41. doi: 10.1016/j.athoracsur.2017.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evora PR, José Rodrigues A, Celotto AC. “Methylene blue should be relegated to rescue use and not as first-line therapy” cannot become a paradigm. J Cardiothorac Vasc Anesth. 2014;28:e11–2. doi: 10.1053/j.jvca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Landoni G, Pasin L, Di Prima AL, Dossi R, Taddeo D, Zangrillo A, et al. Methylene blue: Between scylla (meta-analysis) and charybdis (propensity) J Cardiothorac Vasc Anesth. 2014;28:e12–3. doi: 10.1053/j.jvca.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Mazzeffi M, Hammer B, Chen E, Caridi-Scheible M, Ramsay J, Paciullo C, et al. Methylene blue for postcardiopulmonary bypass vasoplegic syndrome: A cohort study. Ann Card Anaesth. 2017;20:178–81. doi: 10.4103/aca.ACA_237_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juffermans NP, Vervloet MG, Daemen-Gubbels CR, Binnekade JM, de Jong M, Groeneveld AB, et al. Adose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide. 2010;22:275–80. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Evora PR, Alves Junior L, Ferreira CA, Menardi AC, Bassetto S, Rodrigues AJ, et al. Twenty years of vasoplegic syndrome treatment in heart surgery. Methylene blue revised. Rev Bras Cir Cardiovasc. 2015;30:84–92. doi: 10.5935/1678-9741.20140115. [DOI] [PMC free article] [PubMed] [Google Scholar]


