Abstract
Smoke inhalation injury is a major determinant of morbidity and mortality in fire victims. It is a complex multifaceted injury affecting initially the airway; however, in short time, it can become a complex life-threatening systemic disease affecting every organ in the body. In this review, we provide a summary of the underlying pathophysiology of organ dysfunction and provide an up-to-date survey of the various critical care modalities that have been found beneficial in caring for these patients. Major pathophysiological change is development of edema in the respiratory tract. The tracheobronchial tree is injured by steam and toxic chemicals, leading to bronchoconstriction. Lung parenchyma is damaged by the release of proteolytic elastases, leading to release of inflammatory mediators, increase in transvascular flux of fluids, and development of pulmonary edema and atelectasis. Decreased levels of surfactant and immunomodulators such as interleukins and tumor-necrosis-factor-α accentuate the injury. A primary survey is conducted at the site of fire, to ensure adequate airway, breathing, and circulation. A good intravenous access is obtained for the administration of resuscitation fluids. Early intubation, preferably with fiberoptic bronchoscope, is prudent before development of airway edema. Bronchial hygiene is maintained, which involves therapeutic coughing, chest physiotherapy, deep breathing exercises, and early ambulation. Pharmacological agents such as beta-2 agonists, racemic epinephrine, N-acetyl cysteine, and aerosolized heparin are used for improving oxygenation of lungs. Newer agents being tested are perfluorohexane, porcine pulmonary surfactant, and ClearMate. Early diagnosis and treatment of smoke inhalation injury are the keys for better outcome.
Keywords: Burns, lung injury, smoke inhalation injury
INTRODUCTION
In major fire disasters such as 9/11 bombing of the World Trade Centre in New York, smoke inhalation injury was an important cause of mortality.[1] Smoke inhalation injury increases the mortality by 24 times in fire-related injuries[2] and is highlighted to be one of the most important risk factors increasing the morbidity and mortality.[2]
The objective of this article is to review the etiopathogenesis, early diagnosis, and latest management guidelines of smoke inhalation injury. This knowledge is important for anesthesiologists, emergency physicians, and critical care specialists, who are frequently involved in the care of these patients.
PATHOPHYSIOLOGY
Smoke inhalation injury is caused by the inspiration of steam, superheated gases, or toxic, often incomplete products of combustion. The heating capacity of steam is 4000 times that of hot dry air and thus causes tissue damage, even with momentary contact.[3] The various toxic compounds present in smoke are carbon monoxide (CO), hydrogen cyanide (HCN), phosgene, ammonia, sulfur dioxide, hydrogen sulfide (H2S), formaldehyde, and acrylonitriles.[3] Some of these superheated products of combustion are inhaled, causing thermal burns to the airway mucosa.[4] Particles larger than 10 μ in size are retained in the nasopharynx, but 1–2 μ sized particles can pass into the alveoli.[5]
Smoke inhalation results in three physiological types of injury: (a) thermal injury predominantly to the upper airway; (b) chemical injury to the upper and lower respiratory tract; and (c) systemic effects of the toxic gases such as CO and CN. Early deaths in fire are predominantly due to hypoxia, which results from a lethal synergistic effect of low oxygen (O2) levels (due to massive O2 consumption during combustion) and inhalation of high concentrations of CO and CN (resulting in inability to use O2 at tissue level).[6] The major pathophysiological change is edema formation in the respiratory system (airway and lungs).
The anatomical areas affected by the smoke inhalation are as follows.
Supraglottic area/upper airway
The oropharyngeal mucosal structures are damaged primarily by the heat transfer and secondarily via the release of chemical mediators [Figure 1].[4,7] Thermal injury releases substance P, calcitonin gene-related peptide, eicosanoids, neural endopeptidase, and interleukin-8 (IL-8), which attract polymorphonuclear cells and release proteases, thus further aggravating the inflammation.[7] There is marked swelling of the tongue, epiglottis, and glottis. This leads to increased edema in the upper airway and can lead to airway compromise. Thermal injury rarely occurs below the vocal cords because high blood flow in the upper airway cools it efficiently.[4,8]
Figure 1.
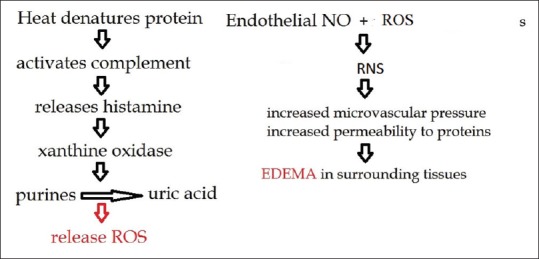
Pathophysiology of smoke inhalation injury in the upper airway. NO: Nitric oxide; ROS: Reactive oxygen species
Tracheobronchial tree/lower airway and lungs
The chemicals present in smoke lead to inflammation and edema of the tracheobronchial tree.[3,7,8] Within 3 h of smoke inhalation injury, the bronchial blood circulation is increased by 10–20 times due to increased cardiac output and hypermetabolic response.[9] The upper and lower airways become characteristically hyperemic.[8,9] The blood flow is increased by 4–6 times in the lungs.[9] This increase in bronchial blood flow leads to edema of the airway, fluid exudation, and flux of inflammatory mediators. Ligation of bronchial circulation attenuates the lung edema after smoke inhalation injury in ovine models.[10] The circulation of inflammatory mediators increases the permeability of the bronchial vasculature and pulmonary transvascular fluid flux exacerbating the pulmonary edema.[10]
All the variables of Starling equation favoring pulmonary edema are activated.[11] The microvascular hydrostatic pressure is increased, interstitial hydrostatic pressure is decreased, interstitial oncotic pressure is increased, and the reflection coefficient is decreased, leading to pulmonary edema.[11] There is disruption of the ciliary mucosa and sloughing of the bronchial columnar epithelium, which leads to obstruction of the airway and alveoli [Figure 2].[9,12] The leakage of plasma-rich fluid contains procoagulants that accentuate solidification of the cast. This bronchial occlusion increases the risk of infection in lungs. The toxic chemicals present in smoke decrease the surfactant levels, resulting in widespread micro-atelectasis and ventilation–perfusion mismatch.[13,14,15]
Figure 2.

The mechanism of cast formation in tracheobronchial tree
The pathophysiological details of airway and lung injury are shown in Figure 3. The irritants present in smoke stimulate sensory nerve endings of the tracheobronchial tree, releasing neuropeptides which cause bronchoconstriction and activate nitric oxide synthase (NOS).[13,14] The arginine levels are decreased in the burn victim, which cause NOS to produce reactive oxygen species (ROS) [Figure 3].[15] ROS react with NO to form reactive nitrogen species (RNS).[13,14] ROS and RNS increase the capillary permeability and hydrostatic pressure of the bronchial microvasculature [Figure 3].[13,14] There is an increase in neutrophil infiltration, resulting in pulmonary interstitial edema and marked reduction in lung compliance.[13,14] Neutrophils are activated to release the proteolytic enzymes (such as elastase), which damage lung parenchyma.[13,14] The biomarkers of acute lung injury (such as IL-6, IL-8, IL-10, and tumor-necrosis-factor [TNF]-alpha) are increased in most of the victims of smoke inhalation injury.[13,14] There is increased risk of developing acute respiratory distress syndrome, which subsequently leads to acute respiratory failure. The repair enzyme polyADP-ribose polymerase (PARP) is activated, depleting ATP, resulting in cellular dysfunction and apoptosis [Figure 3].[16] Increased production of NO inhibits the hypoxic pulmonary vasoconstriction,[17] further aggravating the hypoxia resulting from airspace obliteration.
Figure 3.
Pathophysiology of smoke inhalation injury – lungs. iNOS: Inducible nitric oxide synthase; NF: Nuclear factor; nNOS: Neuronal nitric oxide synthase; PARP: PolyADP-ribose polymerase; RNS: Reactive nitrogen species; ROS: Reactive oxygen species; PMN: Polymorphonucleocytes
SYSTEMIC CHANGES
Burn injury leads to cytokine-mediated inflammatory response at both the site of burn and even distant site. Sepsis, loss of proteins, and immunodeficiency result, which further aggravate the injury.[18] The CO and CN poisoning further compounds hypoxia by affecting the O2 transport or utilization.[6]
Carbon monoxide poisoning
Asphyxia from CO toxicity is the main cause of rapid death among the victims of fire.[6] As O2 is consumed, incomplete combustion predominates, generating CO.[19] CO and O2 compete for the O2-binding sites on hemoglobin (Hb) with an avidity of 250:1. The lower the partial pressure of O2 (pO2), the greater the success of CO in occupying the O2-binding sites. The binding of CO shifts the oxyhemoglobin (O2 Hb) dissociation curve to the left, increasing binding affinity of O2 to Hb; thus, requiring a greater degree of tissue hypoxia before O2 can be offloaded. Finally, CO binds to intracellular cytochromes (a and a3), resulting in an inability of the tissues to use oxidative energy pathways.[19] The net effect is widespread asphyxia, which is out of proportion to the pO2 measured by blood gases. The restricted O2 delivery predominantly affects the organs which have a high rate of O2 utilization, such as brain and heart.[6,19]
The clinical manifestations of CO toxicity often vary with the concentration of blood carboxyhemoglobin (COHb) [Table 1].[19] The survivors develop long-term neurologic and affective sequelae.[19] CO poisoning is diagnosed by a history of CO exposure, an increase in COHb levels, and ruling out the other causes of symptoms/signs. Pulse oximetry and arterial blood gases may be otherwise normal early in the course of treatment as they cannot differentiate between O2 Hb and COHb. Pulse oximetry gives falsely high readings. Newer generation pulse oximeters such as Masimo Rad 7 or Rainbows measure specifically HbCO (and also methemoglobin) and will therefore detect the CO poisoning early.[20]
Table 1.
Symptoms and signs of carbon monoxide toxicity at different concentrations of carboxyhemoglobin

Hydrogen cyanide poisoning
CN inhibits mitochondrial cytochrome C oxidase, a component of the respiratory chain, thus inhibiting oxidative phosphorylation and causing tissue hypoxia because of inability to use the delivered O2.[21,22] CN poisoning affects the central nervous system, respiratory system, and cardiovascular system, proportionate to the concentration of cyanide inhaled.[21,22] The symptoms of CN toxicity vary from tachycardia, tachypnea, dyspnea, drowsiness, and headache at low concentration to cardiac arrhythmias, hypotension, convulsions, paralysis, cardiorespiratory collapse, and coma at high concentration (>100 ppm).[21,22] Blood cyanide levels can be measured to confirm exposure to CN.[21,22]
COMPLICATIONS OF INHALATION INJURY
The victims of smoke inhalation injury can develop acute respiratory distress syndrome and respiratory failure needing ventilator support[23] and placing them at risk for ventilator-associated complications such as barotrauma and pneumonia.[24] Infectious complications such as tracheobronchitis, bronchiectasis, bronchiolitis obliterans, and pneumonia can develop in 38%–60% of the victims, after 3–10 days of smoke inhalation injury,[25] and are associated with a mortality of up to 60%.[25]
The airway may remain hyperreactive for up to 6 months after extubation. Damage to the larynx by inhaled toxins or prolonged intubation can cause persistent hoarseness or dysphonia.[26] The injury to epithelium of upper airway from initial injury can cause tracheoesophageal fistula, tracheomalacia, late subglottic stenosis, or tracheobronchial polyps.[26] Smoke inhalation injury leads to severe restrictive ventilatory dysfunction, mild obstructive ventilatory dysfunction, and reduced diffusing capacity,[27] which may persist for many months.
DIAGNOSIS OF SMOKE INHALATION INJURY
Smoke inhalation injury is diagnosed by a combination of history of exposure to fire and smoke, physical examination, fiberoptic bronchoscopy (FOB) (carbonaceous debris, erythema, ulceration), laboratory investigations, and radiological tests (chest computed tomographic [CT] scan).[28,29,30,31,32]
History
The history should be obtained regarding the source of combustion, duration of exposure to smoke, time elapsed since injury, and manner of exposure to smoke (open or enclosed space, such as house fires/industrial accidents).[28,29,30,31,32]
Signs/symptoms of inhalation injury
Shortly, after fire exposure, there may be only a few symptoms/signs of airway damage. This is not reassuring as life-threatening airway and lung impairment may follow quickly. Early clues of smoke inhalation injury are facial burns, hoarse voice, singed nasal hair, carbonaceous sputum, and soot/carbonaceous material in the oral cavity.[28,29,30,31,32] Facial/oral edema, stridor, wheezing, dyspnea, and cyanosis are the signs of advanced damage.[28,29,30,31,32]
Investigations
The baseline tests include complete blood count, serum electrolytes, creatinine, arterial blood gases, electrocardiogram, and chest X-ray. A toxicology screen for prescribed and street drugs should be supplemented with fire-specific toxins such as blood cyanide and COHb.[19,22] The chest radiograph appears normal initially, but later, it may reveal atelectasis, consolidation, and/or pulmonary edema.[28,29,30,31,32] Chest CT scan may reveal ground-glass opacities in peribronchial distribution, within a few hours of inhalation injury.[33] Chest CT scan is helpful to rule out smoke inhalation injury in case of any doubt in diagnosis and can identify the extent of injury to the distal airway, which is missed by FOB.[33]
Fiberoptic bronchoscopy
FOB can be used to identify soot/carbonaceous material and suction it out[34] and has a high positive and negative predictive value to diagnose smoke inhalation injury.[34] The patency of the airway can be assessed for risk of upper airway obstruction.[34] If indicated, FOB-guided endotracheal intubation can be performed.
Grading of severity
FOB is used to assess the severity of smoke inhalation injury, which correlates with clinical signs/symptoms.[35] Bronchial wall thickness measured by chest CT scan correlates with the severity of smoke inhalation injury.[36] Plasma COHb, airway neutrophilia, and cytokine release also correlate with the severity of smoke inhalation injury.[37] The PaO2/FiO2 ratio is a very good indicator of the prognosis and severity of smoke inhalation injury.[38]
MANAGEMENT
The victim should be immediately evacuated from the site of fire and decontaminated using full isolation precautions.[39] All clothing as well as rings, watch, and jewelry should be removed as soon as possible, as they may be contaminated by toxins (such as HCN), or may retain heat, and produce a tourniquet-like effect in the event of swelling.[39]
Advanced trauma guidelines should be followed, and a primary survey should be performed to identify any immediate life-threatening conditions (under “airway-breathing-circulation” scheme).[32] Definitive airway may be difficult to obtain in the field, depending on the skill level of the first responder and the degree of airway edema. Repeated failed attempts at intubation may result in upper airway trauma, exacerbation of edema, initiation of bleeding, and total loss of airway patency.[32] For victims without evidence of impending airway compromise at the scene, it would seem prudent to delay intervention until the patient arrives at the hospital and has access to advanced airway management tools and expertise. For victims with signs or symptoms of airway compromise from inhalation injury at the scene, the decision regarding airway management is more difficult. If necessary, bag-mask ventilation with humidified O2 may provide a bridge to deliver the patient to hospital, when the transport time is short. Intravenous (IV) access should be obtained and fluid infusion started at the scene.[39] Later, a secondary survey should be performed to obtain information regarding medical history, medications, allergies, and concomitant injuries.[31,32,39] A thorough physical examination, including neurological assessment, is advocated.[31,32,39]
Airway management
The patency of airway is at risk with time as is the maintenance of ventilation and oxygenation. The need for an advanced airway is difficult to predict as airway patency can decrease with even minimal appearing airway burn and small skin surface burns; the rate of encroachment of the airway may vary as well.[40,41] Progressive edema results from local trauma, skin burns, and large volumes of fluid used for resuscitation. Edema usually increases progressively in the first 72 h.[30,41] If endotracheal intubation is delayed, intubation is rendered difficult due to edematous tissue that swells the tongue and epiglottis, fills in aryepiglottic folds, and obscures arytenoid eminences and interarytenoid areas.[30,41]
There should be frequent reevaluation of the airway, and we need to keep a high index of suspicion for airway compromise. Regardless of initial symptoms, the best strategy is to have a low threshold for intubation.[30,40,41] It is best to intubate early when swelling is least and the patient is still physiologically intact. The indications of endotracheal intubation are airway obstruction, severe cognitive impairment (Glasgow coma scale ≤8), major cutaneous burn (≥40%), where there is a high risk of impending airway obstruction (such as moderate-to-severe facial burn, oropharyngeal burn, and airway injury seen on endoscopy).[40] Absence of history/signs of smoke inhalation injury on FOB, and burn site distant from the airway are reassuring to wait.
Awake FOB is preferred at our institute, as the patient stays awake and breathes spontaneously and the airway remains patent [Figure 4]. Use of videolaryngoscope for intubation is also emerging as a promising technique. Mask ventilation may also be difficult, resulting in “Can't Ventilate Can't Intubate” situation. All remaining alternatives such as supraglottic airways, cricothyrotomy, and tracheostomy are much more difficult in burn victims than in nonburn patients.[42] Safdarjung Hospital difficult airway algorithm in smoke inhalation injury is depicted in Figure 4.
Figure 4.
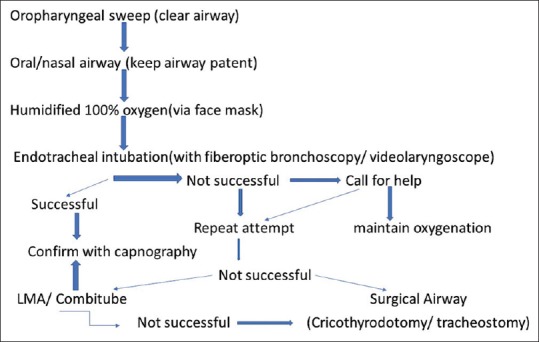
Safdarjung Hospital difficult airway algorithm in smoke inhalation injury
Fluid resuscitation and nutrition
Ringer lactate is the fluid of choice. Victims of smoke inhalation injury have a 25% higher fluid requirement, due to an increase in insensible loss of fluid through the injured lung.[43] In contrast, over-resuscitation may increase pulmonary microvascular hydrostatic pressure and accentuate edema formation. The goal of fluid resuscitation is to maintain an adequate urine output (adults 0.5–1 ml/kg/h; children 1–2 ml/kg/h).[31,32] The formulae of fluid resuscitation should be used only as a guide and titrated to clinical endpoints.
Bronchial hygiene therapy
Bronchial hygiene consists of therapeutic coughing, chest physiotherapy, deep breathing exercises, suctioning of the airway, and early ambulation.[28,29,30,44] The victim should be placed 30°–45° head-up to attenuate the upper airway edema and minimize the pressure of abdominal contents on the diaphragm. The management strategy targets decreasing airway edema/hyperemia, treatment of bronchospasm, attenuation of mucus secretion, and lysis of the casts in the airway.[28,29,30,44]
Therapeutic coughing
Cough impairment can lead to retained secretions, which can exacerbate bronchial obstruction, atelectasis, and pneumonia.[30] The goal of therapeutic coughing is to remove excess mucus or fibrin casts from the tracheobronchial tree. Repeating therapeutic coughing and deep breathing every 2 h effectively removes the secretions.[28,29,30,44]
Chest physiotherapy
Chest physiotherapy, in the form of gravity-assisted drainage of secretions from the bronchial tree, is achieved by chest percussion and vibration.[44] The victim should be turned from one side to another, every 4 h, to mobilize the secretions [Table 2].[28,29,30] Early ambulation is encouraged to prevent respiratory complications.[28,29,30,44]
Table 2.
Safdarjung Hospital Smoke Inhalation injury treatment protocol

PHARMACOLOGICAL AGENTS
Beta-2 agonists and muscarinic antagonist
The inhaled irritants in smoke cause severe bronchospasm, which is treated with inhaled beta-agonists such as albuterol, levalbuterol, or salbutamol.[45,46] Aerosolized sympathomimetics relax bronchial smooth muscles and stimulate mucociliary clearance.[45,46] This decreases the resistance of the airway and improves dynamic compliance of the lungs.[45,46] The β2-agonists also have an anti-inflammatory action, reflected by attenuation of inflammatory mediators, such as histamine, leukotrienes, and TNF-α.[45,46] Attenuation in pulmonary vascular permeability, pulmonary edema, and peak airway pressures, with improvement of oxygenation (PaO2/FiO2 ratio), has been reported with the use of nebulized albuterol (20 or 40 mg/h) in ovine model.[45] Tiotropium, a muscarinic receptor antagonist, has been reported to improve PaO2/FiO2 ratio and decrease the peak airway pressure after smoke inhalation injury.[47]
Racemic epinephrine
Epinephrine has a vasoconstrictive action, thus attenuating mucosal/submucosal edema in both upper and lower airway. It dilates terminal bronchioles, breaks the bonds in mucosal secretions, and attenuates the pulmonary flux of inflammatory mediators.[48] Administration of nebulized epinephrine causes significant improvement in oxygenation, dynamic compliance of lungs, and airway pressures and attenuates the airway hyperemia and pulmonary transvascular fluid flux in ovine model, preclinical studies,[48] and pediatric victims of smoke inhalation injury.[49]
N-Acetyl cysteine
N-Acetyl cysteine (NAC) has pulmonary antioxidant and mucolytic properties.[50] NAC releases sulfhydryl radical, which breaks the disulfide bonds in mucoprotein (mucus).[50] NAC stimulates production of glutathione, which protects cells from free radicals and ROS.[50] NAC is an irritant to respiratory tract and can cause bronchospasm; hence, we mix NAC (0.5 ml) with a bronchodilator (0.5 ml salbutamol) in 4 ml normal saline for nebulization.[50]
Aerosolized heparin and antithrombin
Smoke inhalation injury has procoagulant activity; therefore, aerosolized administration of anticoagulants is beneficial.[51] Aerosolized heparin prevents inspissation of secretions, improves microcirculation by breaking fibrin component of the bronchial cast,[52] and has been found to reduce mortality in preclinical and clinical studies of smoke inhalation injury.[53,54,55] Administration of nebulized heparin 5000 units (in 3 ml) and alternating it with 4 ml of 20% nebulized NAC every 4 h is beneficial [Table 2].[53,54,55] This combination has been reported to significantly improve pulmonary function, oxygenation, and survival rates in patients of smoke inhalation injury.[53,54,55]
Heparin exerts its anticoagulant effect by binding to antithrombin, and its effect is limited in antithrombin deficiency.[56] Antithrombin deficiency is common after burn injury and correlates significantly with increased mortality rate and longer hospital stay.[56,57] Antithrombin has anticoagulant and anti-inflammatory activity by inhibiting transduction of inflammatory signal.[57] In ovine model, combined nebulization of heparin and recombinant human antithrombin improved the pulmonary function after smoke inhalation injury by dissolving bronchial casts and decreasing the airway obstruction.[57] This improves lung oxygenation (PaO2:FiO2 ratio) and decreases the incidence of pneumonia.[58]
BRONCHOALVEOLAR LAVAGE
FOB-guided bronchoalveolar lavage is very useful for removal of secretions and casts, if chest physiotherapy and pharmacological agents fail.[28,29,30,54] In victims of smoke inhalation injury, removal of obstructing airway cast improves the oxygenation.[59]
The smoke inhalation injury treatment protocol being followed at Safdarjung Hospital is illustrated in Table 2.
Treatment of carbon monoxide poisoning
The half-time of elimination of COHb varies with concentration of O2 inhaled (320 min on room air; 74 min on 100% O2).[60] Hyperbaric O2 can reduce the half-life of COHb to 20 min.[19] A recent Cochrane review does not show any benefit of using hyperbaric O2 over normobaric O2 for the treatment of CO poisoning, thus leaving no highly effective treatment.[61]
The current limitation in hyperbaric O2 treatment is that hyperbaric chambers are not mobile, require considerable infrastructure, are very expensive to run and maintain, and are scarce, even in industrial countries (e.g., Canada has eight such facilities).[19] Even in institutions that house hyperbaric facilities, it takes from 90 min to 3 h after arrival of the patient to deploy. The latter is a severe limitation for treatment of CO poisoning. The greatest determinant of the reversibility of CO poisoning is not the high O2 partial pressure, but rather, how rapidly the elimination of CO can be implemented.[19]
A recent approach in eliminating CO is similar in concept to that of hemodialysis, cardiopulmonary bypass, and extracorporeal membrane oxygenation (ECMO).[62] In this approach, lung is used as the dialysis membrane and minute ventilation performs a function analogous to the flow of dialysate.[62,63] As in extracorporeal methods, the ventilatory dialysate can be conditioned by the addition to CO2 to prevent hypocapnia despite high minute ventilation. This isocapnic hyperpnea is implemented by a device that delivers 100% O2 via a self-inflating bag and passively administers CO2 in proportion to ventilation that exceeds baseline ventilation (ClearMate™ Thornhill Medical Inc., Toronto, Canada), thus maintaining normocapnia independent of the minute ventilation.[63] The reduction in COHb is a rectangular hyperbolic function of minute ventilation. This implies that initial increase in minute ventilation results in steep reduction in half-life of COHb, approaching that of hyperbaric O2 with as little ventilation as about 15 L/min.[62,63] The ClearMate™ is a portable device and takes minutes to set up and implement, making it available where there is no ready access to hyperbaric chambers, or during the preparation of the hyperbaric chamber, when available. Recent work has shown that such early intervention in severe CO poisoning results in three times faster recovery of consciousness, improved postrecovery quality of life, and reduction in the incidence of delayed neurological sequelae from 20% to 1%, independent of the use of hyperbaric O2.[63]
Treatment of cyanide poisoning
Administration of thiosulfate converts cyanide to thiocyanate, which is excreted by the kidneys. In a swine model of severe cyanide poisoning, sodium thiosulfate was found to be ineffective for CN-induced cardiac effects.[64] Hydroxocobalamin is a natural Vitamin B12 derivative, used as an antidote of CN poisoning.[65] It chelates CN to the nontoxic product cyanocobalamin (Vitamin B12).[65] Administration of 5 g hydroxocobalamin IV over 15 min in victims of smoke inhalation injury is associated with lower rate of pneumonia, faster weaning from the ventilator, and faster discharge from Intensive Care Unit and can be given empirically in victims of smoke inhalation injury.[65]
MECHANICAL VENTILATION
Indications
The indications of mechanical ventilation are presence of tachypnea, use of accessory muscles of respiration, upper airway edema, sternal retraction, respiratory rate >30/min, PaO2<65 mmHg, PaCO2>50 mmHg, and PaO2/FiO2 ratio <200.[30]
Goals
In smoke inhalation injury, increased resistance of the airway (due to edema) and decreased compliance of lungs may increase the airway pressures and predispose to barotrauma.[66] The goal of mechanical ventilation is to keep the alveoli patent and oxygenate the lungs, without causing overdistension of the alveoli and preventing barotrauma.[30,66]
Strategy
The lungs are ventilated using small tidal volume (6–8 ml/kg) and high respiratory rate.[67] The goal is to maintain peak airway pressure <35 mmHg, plateau airway pressure <30 mmHg, PaCO235–55 mmHg, and pH 7.25–7.45. FiO2 is adjusted to maintain PaO2 between 80 and 100 mmHg.[67] Optimal positive end-expiratory pressure may be administered to maintain the desired airway pressure, below which the alveoli collapse.[67]
Newer modes of ventilation
The newer modes of ventilation such as high-frequency percussive ventilation, high-frequency oscillatory ventilation, pressure control inverse ratio ventilation, and airway pressure release ventilation have proven to be beneficial in managing patients with smoke inhalation injury.[68,69] High-frequency percussive ventilation has been reported to improve O2 and CO2 tension (increased PaO2/FiO2 ratio), decrease lung inflammation/injury/infection, and improve the lung compliance and survival rate in victims of smoke inhalation injury.[69] Simple prone positioning has also been found to improve oxygenation (PaO2/FiO2 ratio).[70]
Future therapies
The use of ECMO is beneficial for the victims who develop severe respiratory failure after smoke inhalation injury.[71] Blood is circulated through an extracorporeal cardiopulmonary bypass circuit, which facilitates gas exchange through a semipermeable membrane.[71] Hence, low FiO2 and decreased ventilatory pressure can be used to ventilate the injured lungs and thus prevent barotrauma.
Newer agents such as perfluorohexane and porcine pulmonary surfactant are being investigated for their beneficial role in attenuating the release of inflammatory mediators in the airway and lungs.[72,73] In animal models, use of peroxynitrite decomposition catalyst, inducible NOS inhibitors, PARP inhibitors, Na2S (H2S donor), and inhalation of H2S are being studied for their role in attenuating the lung parenchymal injury.[74,75]
Limitations
The major limitation of this review is that many treatment strategies have been tested in animals but still need clinical trials on human subjects before implementation into clinical practice.
CONCLUSION
Early diagnosis and treatment of smoke inhalation injury are the keys for better outcome. Clinical signs/symptoms, FOB, and chest CT scan are helpful in early diagnosis. Initial treatment is early airway management, treatment of CO and CN toxicity, IV fluid resuscitation, and supportive respiratory care.
Financial support and sponsorship
The study was supported by the Department of Anesthesia and Critical Care, Vardhaman Mahavir Medical College and Safdarjung Hospital, New Delhi - 110 029, India.
Conflicts of interest
The ClearMate™ was developed by JF, his coworkers, and students. JF is a shareholder in Thornhill Research Inc., a spin-off company from the University Health Network.
REFERENCES
- 1.Yurt RW, Bessey PQ, Bauer GJ, Dembicki R, Laznick H, Alden N, et al. A regional burn center's response to a disaster: September 11, 2001, and the days beyond. J Burn Care Rehabil. 2005;26:117–24. doi: 10.1097/01.bcr.0000155543.46107.e6. [DOI] [PubMed] [Google Scholar]
- 2.You K, Yang HT, Kym D, Yoon J, HaejunYim, Cho YS, et al. Inhalation injury in burn patients: Establishing the link between diagnosis and prognosis. Burns. 2014;40:1470–5. doi: 10.1016/j.burns.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn IN. Physiological and toxicological aspects of smoke produced during the combustion of polymeric materials. Environ Health Perspect. 1975;11:163–89. doi: 10.1289/ehp.7511163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rong YH, Liu W, Wang C, Ning FG, Zhang GA. Temperature distribution in the upper airway after inhalation injury. Burns. 2011;37:1187–91. doi: 10.1016/j.burns.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Gamsu G, Weintraub RM, Nadel JA. Clearance of tantalum from airways of different caliber in man evaluated by a roentgenographic method. Am Rev Respir Dis. 1973;107:214–24. doi: 10.1164/arrd.1973.107.2.214. [DOI] [PubMed] [Google Scholar]
- 6.Moore SJ, Ho IK, Hume AS. Severe hypoxia produced by concomitant intoxication with sublethal doses of carbon monoxide and cyanide. Toxicol Appl Pharmacol. 1991;109:412–20. doi: 10.1016/0041-008x(91)90004-x. [DOI] [PubMed] [Google Scholar]
- 7.Cox RA, Burke AS, Jacob S, Oliveras G, Murakami K, Shimoda K, et al. Activated nuclear factor kappa B and airway inflammation after smoke inhalation and burn injury in sheep. J Burn Care Res. 2009;30:489–98. doi: 10.1097/BCR.0b013e3181a28e13. [DOI] [PubMed] [Google Scholar]
- 8.Baile EM, Dahlby RW, Wiggs BR, Paré PD. Role of tracheal and bronchial circulation in respiratory heat exchange. J Appl Physiol (1985) 1985;58:217–22. doi: 10.1152/jappl.1985.58.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Traber DL, Traber LD. Airway blood flow changes and airway obstruction following lung injury. Arch Physiol Biochem. 2003;111:297–300. doi: 10.3109/13813450312331337432. [DOI] [PubMed] [Google Scholar]
- 10.Hamahata A, Enkhbaatar P, Sakurai H, Nozaki M, Traber DL. Effect of ablated bronchial blood flow on survival rate and pulmonary function after burn and smoke inhalation in sheep. Burns. 2009;35:802–10. doi: 10.1016/j.burns.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund T. The 1999 everett idris evans memorial lecture. Edema generation following thermal injury: An update. J Burn Care Rehabil. 1999;20:445–52. doi: 10.1097/00004630-199920060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cox RA, Mlcak RP, Chinkes DL, Jacob S, Enkhbaatar P, Jaso J, et al. Upper airway mucus deposition in lung tissue of burn trauma victims. Shock. 2008;29:356–61. doi: 10.1097/shk.0b013e31814541dd. [DOI] [PubMed] [Google Scholar]
- 13.Maybauer MO, Rehberg S, Traber DL, Herndon DN, Maybauer DM. Pathophysiology of acute lung injury in severe burn and smoke inhalation injury. Anaesthesist. 2009;58:805–12. doi: 10.1007/s00101-009-1560-x. [DOI] [PubMed] [Google Scholar]
- 14.de Carvalho FO, Felipe FA, de Melo Costa AC, Teixeira LG, Silva ÉR, Nunes PS, et al. Inflammatory mediators and oxidative stress in animals subjected to smoke inhalation: A Systematic review. Lung. 2016;194:487–99. doi: 10.1007/s00408-016-9879-y. [DOI] [PubMed] [Google Scholar]
- 15.Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, et al. L-arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock. 2007;28:477–83. doi: 10.1097/shk.0b013e31804a59bd. [DOI] [PubMed] [Google Scholar]
- 16.Gerö D, Szabó C. Poly (ADP-ribose) polymerase: A new therapeutic target? Curr Opin Anaesthesiol. 2008;21:111–21. doi: 10.1097/ACO.0b013e3282f63c15. [DOI] [PubMed] [Google Scholar]
- 17.Westphal M, Cox RA, Traber LD, Morita N, Enkhbaatar P, Schmalstieg FC, et al. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med. 2006;34:1428–36. doi: 10.1097/01.CCM.0000215828.00289.B9. [DOI] [PubMed] [Google Scholar]
- 18.Davis CS, Albright JM, Carter SR, Ramirez L, Kim H, Gamelli RL, et al. Early pulmonary immune hyporesponsiveness is associated with mortality after burn and smoke inhalation injury. J Burn Care Res. 2012;33:26–35. doi: 10.1097/BCR.0b013e318234d903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, et al. Carbon monoxide poisoning: Pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. 2017;195:596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampson NB. Noninvasive pulse CO-oximetry expedites evaluation and management of patients with carbon monoxide poisoning. Am J Emerg Med. 2012;30:2021–4. doi: 10.1016/j.ajem.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmström P, et al. Cyanide poisoning by fire smoke inhalation: A European expert consensus. Eur J Emerg Med. 2013;20:2–9. doi: 10.1097/MEJ.0b013e328357170b. [DOI] [PubMed] [Google Scholar]
- 22.Lawson-Smith P, Jansen EC, Hyldegaard O. Cyanide intoxication as part of smoke inhalation – A review on diagnosis and treatment from the emergency perspective. Scand J Trauma Resusc Emerg Med. 2011;19:14. doi: 10.1186/1757-7241-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha SI, Kim CH, Lee JH, Park JY, Jung TH, Choi WI, et al. Isolated smoke inhalation injuries: Acute respiratory dysfunction, clinical outcomes, and short-term evolution of pulmonary functions with the effects of steroids. Burns. 2007;33:200–8. doi: 10.1016/j.burns.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Rue LW 3rd, Cioffi WG, Mason AD, Jr, McManus WF, Pruitt BA., Jr VasoplegiThe risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn Care Rehabil. 1995;16:262–8. doi: 10.1097/00004630-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Edelman DA, Khan N, Kempf K, White MT. Pneumonia after inhalation injury. J Burn Care Res. 2007;28:241–6. doi: 10.1097/BCR.0B013E318031D049. [DOI] [PubMed] [Google Scholar]
- 26.Fang-Gang N, Yang C, Yu-Xuan Q, Yan-Hua R, Wei-Li D, Cheng W, et al. Laryngeal morphologic changes and epidemiology in patients with inhalation injury: A retrospective study. Burns. 2015;41:1340–6. doi: 10.1016/j.burns.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Cao L, Zhang XG, Wang JG, Wang HB, Chen YB, Zhao DH, et al. Pulmonary function test findings in patients with acute inhalation injury caused by smoke bombs. J Thorac Dis. 2016;8:3160–7. doi: 10.21037/jtd.2016.11.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogura H, Sumi Y, Matsushima A, Tohma Y, Inoue Y, Tasaki O, et al. Smoke inhalation injury: Diagnosis and respiratory management. Nihon Geka Gakkai Zasshi. 2005;106:740–4. [PubMed] [Google Scholar]
- 29.Sheridan RL. Airway management and respiratory care of the burn patient. Int Anesthesiol Clin. 2000;38:129–45. doi: 10.1097/00004311-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2–13. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12:53–61. [PubMed] [Google Scholar]
- 32.Enkhbaatar P, Pruitt BA, Jr, Suman O, Mlcak R, Wolf SE, Sakurai H. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. Lancet. 2016;388:1437–46. doi: 10.1016/S0140-6736(16)31458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koljonen V, Maisniemi K, Virtanen K, Koivikko M. Multi-detector computed tomography demonstrates smoke inhalation injury at early stage. Emerg Radiol. 2007;14:113–6. doi: 10.1007/s10140-007-0579-z. [DOI] [PubMed] [Google Scholar]
- 34.Mosier MJ, Pham TN, Park DR, Simmons J, Klein MB, Gibran NS, et al. Predictive value of bronchoscopy in assessing the severity of inhalation injury. J Burn Care Res. 2012;33:65–73. doi: 10.1097/BCR.0b013e318234d92f. [DOI] [PubMed] [Google Scholar]
- 35.Suri JC, Sen MK. Respiratory tract injury in burns. Principles and Practice of Burn Care. In: Sarabahi S, editor. 1st ed. New Delhi: Jaypee Brothers Medical Publishers Ltd; 2010. pp. 129–30. [Google Scholar]
- 36.Yamamura H, Kaga S, Kaneda K, Mizobata Y. Chest computed tomography performed on admission helps predict the severity of smoke-inhalation injury. Crit Care. 2013;17:R95. doi: 10.1186/cc12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albright JM, Davis CS, Bird MD, Ramirez L, Kim H, Burnham EL, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med. 2012;40:1113–21. doi: 10.1097/CCM.0b013e3182374a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan Z, Wong JK, Bush J, Bayat A, Dunn KW. Assessing the severity of inhalation injuries in adults. Burns. 2010;36:212–6. doi: 10.1016/j.burns.2009.06.205. [DOI] [PubMed] [Google Scholar]
- 39.Wachtel TL. Major burns. What to do at the scene and en route to the hospital. Postgrad Med. 1989;85:178. doi: 10.1080/00325481.1989.11700545. [DOI] [PubMed] [Google Scholar]
- 40.Madnani DD, Steele NP, de Vries E. Factors that predict the need for intubation in patients with smoke inhalation injury. Ear Nose Throat J. 2006;85:278–80. [PubMed] [Google Scholar]
- 41.Haponik EF, Meyers DA, Munster AM, Smith PL, Britt EJ, Wise RA, et al. Acute upper airway injury in burn patients. Serial changes of flow-volume curves and nasopharyngoscopy. Am Rev Respir Dis. 1987;135:360–6. doi: 10.1164/arrd.1987.135.2.360. [DOI] [PubMed] [Google Scholar]
- 42.Purdue GF. To trach or not to trach. J Burn Care Res. 2009;30:192–3. doi: 10.1097/BCR.0b013e3181923ec6. [DOI] [PubMed] [Google Scholar]
- 43.Inoue T, Okabayashi K, Ohtani M, Yamanoue T, Wada S, Iida K, et al. Effect of smoke inhalation injury on fluid requirement in burn resuscitation. Hiroshima J Med Sci. 2002;51:1–5. [PubMed] [Google Scholar]
- 44.Silverberg R, Johnson J, Gorga D, Nagler W, Goodwin C. VasoplegiA survey of the prevalence and application of chest physical therapy in US Burn centers. J Burn Care Rehabil. 1995;16:154–9. doi: 10.1097/00004630-199503000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri TL, Enkhbaatar P, Bayliss R, Traber LD, Cox RA, Hawkins HK, et al. Continuous nebulized albuterol attenuates acute lung injury in an ovine model of combined burn and smoke inhalation. Crit Care Med. 2006;34:1719–24. doi: 10.1097/01.CCM.0000217215.82821.C5. [DOI] [PubMed] [Google Scholar]
- 46.Palmieri TL, Enkhbaatar P, Sheridan R, Traber DL, Greenhalgh DG. Studies of inhaled agents in inhalation injury. J Burn Care Res. 2009;30:169–71. doi: 10.1097/BCR.0b013e3181923c1d. [DOI] [PubMed] [Google Scholar]
- 47.Jonkam C, Zhu Y, Jacob S, Rehberg S, Kraft E, Hamahata A, et al. Muscarinic receptor antagonist therapy improves acute pulmonary dysfunction after smoke inhalation injury in sheep. Crit Care Med. 2010;38:2339–44. doi: 10.1097/CCM.0b013e3181f8557b. [DOI] [PubMed] [Google Scholar]
- 48.Lopez E, Fujiwara O, Lima-Lopez F, Suman OE, Mlcak RP, Hawkins HK, et al. Nebulized epinephrine limits pulmonary vascular hyperpermeability to water and protein in ovine with burn and smoke inhalation injury. Crit Care Med. 2016;44:e89–96. doi: 10.1097/CCM.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 49.Foncerrada G, Lima F, Clayton RP, Mlcak RP, Enkhbaatar P, Herndon DN, et al. Safety of nebulized epinephrine in smoke inhalation injury. J Burn Care Res. 2017;38:396–402. doi: 10.1097/BCR.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Csontos C, Rezman B, Foldi V, Bogar L, Drenkovics L, Röth E, et al. Effect of N-acetylcysteine treatment on oxidative stress and inflammation after severe burn. Burns. 2012;38:428–37. doi: 10.1016/j.burns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: A systematic review. Crit Care Med. 2014;42:413–9. doi: 10.1097/CCM.0b013e3182a645e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enkhbaatar P, Herndon DN, Traber DL. Use of nebulized heparin in the treatment of smoke inhalation injury. J Burn Care Res. 2009;30:159–62. doi: 10.1097/BCR.0b013e3181923bd3. [DOI] [PubMed] [Google Scholar]
- 53.Dries DJ, Endorf FW. Inhalation injury: Epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013;21:31. doi: 10.1186/1757-7241-21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCall JE, Cahill TJ. Respiratory care of the burn patient. J Burn Care Rehabil. 2005;26:200–6. [PubMed] [Google Scholar]
- 55.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998;19:210–2. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Niedermayr M, Schramm W, Kamolz L, Andel D, Römer W, Hoerauf K, et al. Antithrombin deficiency and its relationship to severe burns. Burns. 2007;33:173–8. doi: 10.1016/j.burns.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Murakami K, McGuire R, Cox RA, Jodoin JM, Schmalstieg FC, Traber LD, et al. Recombinant antithrombin attenuates pulmonary inflammation following smoke inhalation and pneumonia in sheep. Crit Care Med. 2003;31:577–83. doi: 10.1097/01.CCM.0000050444.52531.08. [DOI] [PubMed] [Google Scholar]
- 58.Kowal-Vern A, Orkin BA. Antithrombin in the treatment of burn trauma. World J Crit Care Med. 2016;5:17–26. doi: 10.5492/wjccm.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakae H, Tanaka H, Inaba H. Failure to clear casts and secretions following inhalation injury can be dangerous: Report of a case. Burns. 2001;27:189–91. doi: 10.1016/s0305-4179(00)00100-5. [DOI] [PubMed] [Google Scholar]
- 60.Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–8. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 61.Buckley NA, Juurlink DN, Isbister G, Bennett MH, Lavonas EJ. VasoplegiHyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2011;4:CD002041. doi: 10.1002/14651858.CD002041.pub3. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher JA, Iscoe S, Fedorko L, Duffin J. Rapid elimination of CO through the lungs: Coming full circle 100 years on. Exp Physiol. 2011;96:1262–9. doi: 10.1113/expphysiol.2011.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu W, Ma Y, Jiang F. A clinical study of treatment for delayed psychoneural sequela caused by acute CO poisoning with clear mate gas poisoning first aid ventilator. Chin J Hosp Pharm. 2016;36:425. [Google Scholar]
- 64.Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA, et al. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med. 2012;59:532–9. doi: 10.1016/j.annemergmed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen L, Afshari A, Kahn SA, McGrane S, Summitt B. Utility and outcomes of hydroxocobalamin use in smoke inhalation patients. Burns. 2017;43:107–13. doi: 10.1016/j.burns.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Mlcak R, Cortiella J, Desai M, Herndon D. Lung compliance, airway resistance, and work of breathing in children after inhalation injury. J Burn Care Rehabil. 1997;18:531–4. doi: 10.1097/00004630-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 67.Dries DJ. Key questions in ventilator management of the burn-injured patient (second of two parts) J Burn Care Res. 2009;30:211–20. doi: 10.1097/BCR.0b013e318198a33f. [DOI] [PubMed] [Google Scholar]
- 68.Reper P, van Looy K. Chest physiotherapy using intrapulmonary percussive ventilation to treat persistent atelectasis in hypoxic patients after smoke inhalation. Burns. 2013;39:192–3. doi: 10.1016/j.burns.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Miller AC, Ferrada PA, Kadri SS, Nataraj-Bhandari K, Vahedian-Azimi A, Quraishi SA, et al. High-frequency ventilation modalities as salvage therapy for smoke inhalation-associated acute lung injury: A Systematic review. J Intensive Care Med. 2017:1-11–885066617714770. doi: 10.1177/0885066617714770. doi: 10.1177/0885066617714770. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70.Hale DF, Cannon JW, Batchinsky AI, Cancio LC, Aden JK, White CE, et al. Prone positioning improves oxygenation in adult burn patients with severe acute respiratory distress syndrome. J Trauma Acute Care Surg. 2012;72:1634–9. doi: 10.1097/TA.0b013e318247cd4f. [DOI] [PubMed] [Google Scholar]
- 71.Thompson JT, Molnar JA, Hines MH, Chang MC, Pranikoff T. Successful management of adult smoke inhalation with extracorporeal membrane oxygenation. J Burn Care Rehabil. 2005;26:62–6. doi: 10.1097/01.bcr.0000150303.15345.79. [DOI] [PubMed] [Google Scholar]
- 72.Ding H, Lv Q, Wu S, Hou S, Liu Z, Landén NX, et al. Intratracheal instillation of perfluorohexane modulates the pulmonary immune microenvironment by attenuating early inflammatory factors in patients with smoke inhalation injury: A Randomized controlled clinical trial. J Burn Care Res. 2017;38:251–9. doi: 10.1097/BCR.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 73.Sun Y, Qiu X, Wu G, Wang J, Li J, Tang H, et al. The effects of porcine pulmonary surfactant on smoke inhalation injury. J Surg Res. 2015;198:200–7. doi: 10.1016/j.jss.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Hamahata A, Enkhbaatar P, Lange M, Yamaki T, Nakazawa H, Nozaki M, et al. Administration of a peroxynitrite decomposition catalyst into the bronchial artery attenuates pulmonary dysfunction after smoke inhalation and burn injury in sheep. Shock. 2012;38:543–8. doi: 10.1097/SHK.0b013e31826e9c54. [DOI] [PubMed] [Google Scholar]
- 75.Han ZH, Jiang YI, Duan YY, Wang XY, Huang Y, Fang TZ, et al. Protective effects of hydrogen sulfide inhalation on oxidative stress in rats with cotton smoke inhalation-induced lung injury. Exp Ther Med. 2015;10:164–8. doi: 10.3892/etm.2015.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]



