Abstract
Unesterified cholesterol controls the fluidity, permeability and electrical properties of eukaryotic cell membranes. Consequently, cholesterol levels in the retina and the brain are tightly regulated whereas depletion or oversupply caused by diet or heredity contribute to neurodegenerative diseases and vision loss. Astroglia play a central role in the biosynthesis, uptake and transport of cholesterol and also drive inflammatory signaling under hypercholesterolemic conditions associated with high-fat diet (diabetes) and neurodegenerative disease. A growing body of evidence shows that unesterified membrane cholesterol modulates the ability of glia to sense and transduce ambient information. Cholesterol-dependence of Müller glia - which function as retinal sentinels for metabolic, mechanical, osmotic and inflammatory signals - is mediated in part by transient receptor potential V4 (TRPV4) channels. Cholesterol supplementation facilitates, whereas depletion suppresses, TRPV4-mediated transduction of temperature and lipid agonists in Müller cells. Acute effects of cholesterol supplementation/depletion on plasma membrane ion channels and calcium homeostasis differ markedly from the effects of chronic dyslipidemia, possibly due to differential modulation of modality-dependent energy barriers associated with the functionality of polymodal channels embedded within lipid rafts. Understanding of cholesterol-dependence of TRP channels is thus providing insight into dyslipidemic pathologies associated with diabetic retinopathy, glaucoma and macular degeneration.
Keywords: dyslipidemia, cholesterol, Müller glia, transient receptor potential V4, cyclodextrin, calcium, retina
Cholesterol Imbalance Linked to a Range of Diseases
Cholesterol regulates the permeability, fluidity and bending rigidity of eukaryotic plasma membranes, serves as a precursor for steroid synthesis and regulates the function of numerous membrane proteins via specialized cholesterol-enriched membrane microdomains (lipid rafts) (Dietschy, 2009). Its levels in healthy organs and blood are tightly controlled whereas abnormal accumulation or deficiency may lead to fatal outcomes in animal models of dyslipidemia and patients with cardiovascular and neurodegenerative diseases that include Huntington's, Alzheimer's, Parkinson's diseases and glaucoma (Fliesler and Bretillon, 2010; Omarova et al., 2012; Martín et al., 2014; Gambert et al., 2017). Cholesterol-enriched diets damage the central nervous system (CNS) in part through upregulation of inflammatory signaling mediated by astrocytes. This large class of functionally diverse cells maintain the blood-retina barrier, provide trophic and metabolic support to neurons, and also express specialized sterol carriers (adenosine triphosphate-binding cassette transporters ABCA1 and ABCG1, lecithin-cholesterol acyltransferase and the sterol regulatory element-binding protein 2) are represent the principal source of brain/retinal cholesterol biosynthesis (Dietschy and Turley, 2004; Marquer et al., 2011; Hammer and Busik, 2017). Dysregulation of systemic or local cholesterol metabolism and transport represent particular risks for developing visual dysfunction. For example, altered cholesterol levels underlie debilitating blinding diseases such as Smith-Lemli-Opitz and Niemann-Pick Syndromes, diabetic retinopathy, glaucoma and macular degenerations whereas animals fed cholesterol-deprived or cholesterol-enriched diets show loss of neurons (Fliesler et al., 2007; Fliesler and Bretillon, 2010; Di Paolo and Kim, 2011; Omarova et al., 2012; Gambert et al., 2017).
Cholesterol, which represents > 98% of total sterols in the vertebrate retina, is required for neuronal function, glia-dependent synapse formation and visual signaling (Fliesler et al., 2007; Martín et al., 2014). Systemic cholesterol is delivered to the retina via the low-density lipoprotein (LDL) receptor mediated pathway in the retinal pigment epithelium (RPE) and retinal microvasculature, respectively. While the retina expresses many genes that have been linked to cholesterol homeostasis in other parts of the body, the principal hub for de novo production and transport of cholesterol are Müller glia, radial cells that serve as sentinels for metabolic, osmotic, mechanical and inflammatory signals (Fliesler and Bretillon, 2010; Jo et al., 2015; Newman, 2015). Their unique access to retinal ganglion cells, astrocytes, pericytes and endothelial cells that form the neurogliovascular unit allows Müller cells to control the transport of ions, water, lipids and protein across the inner blood-retina barrier (Reichenbach and Bringmann, 2010). Extravasation of LDL-cholesterol into the Müller glial interstitium exacerbates inflammatory signaling in animals and patients (Hammer and Busik, 2017) and suggests that Müller cells function as sentinels for cholesterol-dependent retinal phenotypes. However, the molecular mechanisms that link lipid dysregulation to glial activation in retinopathy are relatively unclear. For example, it remains to be seen whether proinflammatory glial activation in dyslipidemic retinas results from glial susceptibility to local cholesterol or simply represents a secondary consequence of neuronal viability loss.
Cholesterol Levels Influence the Sentinel and Physiological Properties of Müller Glia
In the majority of retinal neurodegenerative diseases Müller cells adopt an inflammatory reactive phenotype that is associated with increased release of cytokines/chemokines (vascular endothelial growth factor, tumor necrosis factor-α, monocyte chemotactic protein 1, interleukelin 6, C-X-C motif chemokine ligand 11, etc.) and decreased capacity for water and ionic homeostasis, neurotransmitter recycling and antioxidant support (Vázquez-Chona and Geisert, 2012; Coughlin et al., 2017). Reactive activation of Müller glia can also be induced experimentally by either depletion or oversupply of cholesterol. Interestingly, cholesterol-lowering statin drugs suppress Müller glial activation in hypercholesterolemic rabbits (Fernández-Navarro et al., 2016), suggesting that membrane cholesterol levels participate in abnormal function. This hypothesis was explored in the recent study by Lakk et al. (2017) who wondered whether cholesterol levels might alter the ability of Müller glia to sense inputs from the ambient milieu. The authors employed cyclodextrins (hydrophobic molecules that extract cholesterol from membranes and can also be used as cholesterol delivery vehicles) to supplement cells with extra cholesterol or deplete their free membrane cholesterol levels. The consequence was a profound effect on Müller glial transduction of osmotic, temperature and lipid agonist information. Specifically, cholesterol depletion suppressed activation of TRPV4 (transient receptor potential vanilloid isoform 4), a nonselective cation channel enriched in cell types that constitute the retinal gliovascular unit: Müller glia (Jo et al., 2015), retinal ganglion cells (RGCs; Ryskamp et al., 2011) and microvascular endothelial cells (Phuong et al., 2017). This suggests an intriguing potential for cholesterol to modulate inflammatory signaling at the blood-retina barrier, vascular permeability and tone.
A New Target in Dyslipidemia Pathology: Polymodal Sensory Transduction
Lakk et al. (2017) found that near-total depletion of free membrane cholesterol dissolves the lipid raft domains in Müller cells without affecting Trpv4 gene expression, trafficking and localization in adult Müller cells. The absence of effects of cholesterol depletion on gene expression and TRPV4 trafficking was in contrast to its pronounced modulation of TRPV4 activation, as indicated by the effects of cyclodextrins on the amplitude of agonist- and temperature-evoked calcium signals and transmembrane cation currents. Cholesterol depletion reduced whereas supplementation increased, the amplitudes of agonist- and temperature-evoked signals. In contrast, swelling-induced signals that are also mediated by TRPV4 channels (Ryskamp et al., 2014; Jo et al., 2015) were resistant to cholesterol modulation. This suggests that cholesterol differentially modulates the polymodality of ion channel signaling. The molecular mechanism of its modulatory action on TRPV4 channels is unclear but might include binding to conserved cholesterol-binding CRAC/CARC/YIYF motifs, changes in the function of prohibitin-domain-containing proteins (flotillin, stomatin, prohibitin) and/or modulation of annular lipids that constitute the lipoprotein microdomains that surround the channel (Anishkin et al., 2014; Kumari et al., 2015). Another possibility is that cholesterol regulates the open probability of mode-dependent channel activation via its effects on the overall macroscopic rigidity of the membrane (Levitan and Barrantes, 2012).
Cholesterol also modulated the centrifugal propagation of TRPV4-dependent Ca2+ waves across Müller cells. Such waves can be triggered in multiple ways that include mechanical forces, purinergic receptors and TRP channels. They involve two components: an initial influx mediated by Ca2+ influx through TRPV4, transient receptor potential C1 (TRPC1) and/or Orai-containing signaling complexes and regenerative activation of store release and local gliotransmitter release associated with the relase from type 2 inositol 1,4,5-triphosphate receptor (InsP3R2) and ryanodine receptor (RyR)-containing stores, activation of stromal interaction molecule 1 (STIM1) proteins and auto-feedback mediated through P2Y1 receptors (Molnár et al., 2016; Phuong et al., 2017). Cholesterol depletion suppressed wave propagation in Müller glia, suggesting that statins could be employed to mitigate excessive glial activation.
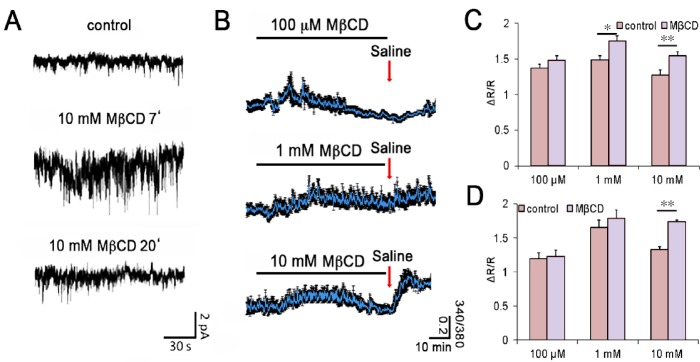
While Lakk et al. (2017) focused on the effects of chronic cholesterol depletion, we also wondered how retinal glia respond to acute extraction of membrane cholesterol. As a preliminary step towards addressing this question, we recorded transmembrane currents and [Ca2+]i in dissociated Müller cells loaded with the calcium indicator dye Fura-2 AM (5–10 μM). Cholesterol depletion with methyl-beta-cyclodextrin (MβCD; 0.1–10 mM) resulted in an immediate increase in the frequency and amplitude of spontaneous transmembrane events (Figure 1A; ion channel openings) together with an elevation in baseline [Ca2+]i (Figure 1B–D). This effect was time-dependent, as the amplitude/frequency of MβCD-induced currents and [Ca2+]i returned to roughly pre-stimulation levels in the continued presence of the MβCD. The identity of ion channels activated by the reduction in free membrane cholesterol remains to be determined but the sterol has been shown to lower the thresholds for activation of many voltage-dependent K+ and Ca2+ conductances, volume-activated Cl− channels as well as TRPM3/8 channels (Levitan and Barrantes, 2012). The acute glial excitation phenotype might also involve cholesterol-dependent modulation of Ca2+ clearance mechanisms and/or calcium release from internal stores. These data suggest that both acute and chronic membrane cholesterol levels affect ion channel signaling but the effects are opposite. That is, an initial increase in excitability caused by cholesterol lowering is followed by long-term suppression of membrane signaling and inhibition of TRPV4-dependent temperature and lipid ligand sensing. Another intriguing feature of glial cholesterol signaling is the increase in [Ca2+]i that was observed during re-introduction of control saline (red arrows in Figure 1B). The magnitude of this effect was a function of the MβCD concentration and reminiscent of store-operated signaling in Müller cells (Molnár et al., 2016).
Figure 1.
Acute cholesterol depletion transiently increases the frequency of spontaneous transmembrane currents (SMC) and the amplitude of [Ca2+]i in intact Müller cells.
(A) Whole cell recording from a representative voltage-clamped mouse Müller cell in the retinal wholemount preparation. Exposure to methyl-beta-cyclodextrin (MβCD) increases the SMC frequency at 7 minutes (middle panel) but the effect subsides at 20 minutes of cholesterol depletion (bottom panel). (B) Dissociated Müller cells loaded with Fura-2 AM. Exposure to 0.1 mM (top), 1 mM (middle) and 10 mM (bottom panel) MβCD transiently elevates [Ca2+]i. (C) Summary of the results shown in panel B shows significant increase in calcium levels induced by 10 mM MβCD. (D) Summary of results for baseline [Ca2+]i induced by return to control saline in B (red arrows). ΔR/R = ([Ca2+]i response peak – baseline) / baseline. The voltage clamp and optical imaging experiments were performed as previously described (Lakk et al., 2017). Statistical comparisons were performed using one-way analysis of variance test followed by post-hoc Tukey's multiple comparison of means. *P < 0.05, **P < 0.01.
Conclusions
Recent studies demonstrate that modulation of the lipid microenvironment induces acute and chronic changes in glial ion channel activity, Ca2+ homeostasis and reactive (proinflammatory) activation. Phylogenetically conserved interactions between cholesterol and TRPV4 signaling pathways point at polymodal signal integration as a likely target of cholesterol modulation (Kumari et al., 2015). Figure 2 expands the classical ‘lipid-ligand gating’ concept to suggest that cholesterol-dependent modulation represents a platform for the regulation of cellular coincidence detection and sensing of ambient information. Through its regulation of membrane stiffness and TRPV4 gating, cholesterol could modulate intracellular signaling and reactive gliosis and thereby influence baseine and/or activity-dependent release of gliotransmitters and cytokines/chemokines. An interesting aspect of cholesterol-dependent chronic facilitation of TRPV4 involves the effect on polyunsaturated fatty acid metabolism downstream from the phospholipase A2 which is required for Müller cell TRPV4 activation (Ryskamp et al., 2014) (Figure 2). It is also important to note that Müller cells are not the sole retinal target of systemic and local dyslipidemia, and that shifts in cholesterol availability modulate signaling in many cell types including the retinal pigment epithelium, photoreceptors, microvascular endothelial cells, trabecular meshwork cells, microglia and RGCs (Fliesler and Bretillon, 2010; Gambert et al., 2017; Hammer and Busik, 2017). It remains to be determined whether manipulation of free membrane cholesterol similarly modulates channel activation in these TRPV4-expressing cells (Jo et al., 2015; Ryskamp et al., 2016; Phuong et al., 2017). Among other likely mechanotransduction targets of hypercholesterolemia are the excitation-contraction coupling mechanisms that regulate blood flow, cell volume regulation and pressure sensing in the cardiovascular system (White et al., 2016). In any case, the striking effects of cholesterol supplementation and depletion on retinal glial ability to sense ambient information identify targeting of retinal TRPV4 channels as a potential treatment of inflammatory dyslipidemic pathologies that involve pathological sensing of ambient milieu by glia. In the retina, such diseases might include diabetic retinopathy, glaucoma and macular degeneration.
Figure 2.
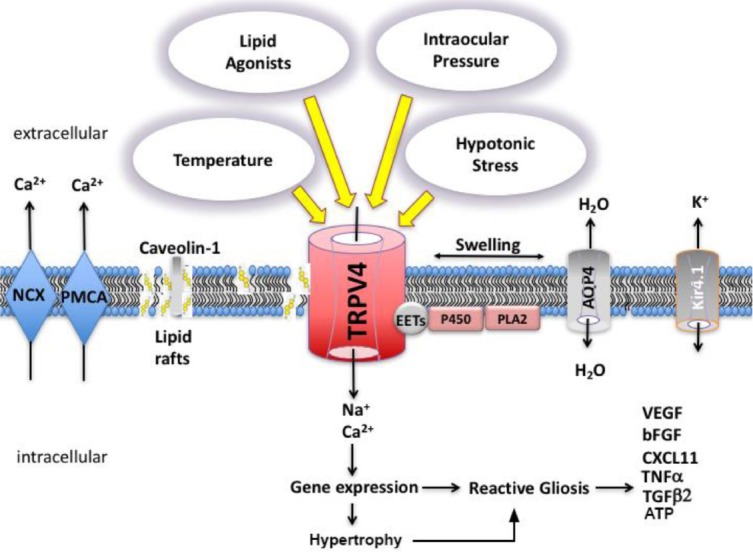
Model of cholesterol-dependent modulation of transient receptor potential V4 (TRPV4) polymodality in astroglia.
The docked sterol enriches Cav-1-containing membrane microdomains (lipid rafts) and organizes the partitioning of CARC-CRAC domain-containing proteins such as TRPV4 into the amphiphilic microenvironment of the membrane. TRPV4 activation modulates glial excitability via changes in intracellular [Ca2+]i which in turn may downstream processes such as hypertrophy, proliferation, gliosis and release of gliotransmitters and chemokines. TRPV4 gating requires biosynthesis of eicosatrienoic acids (EETs) through concomitant activation of phospholipase A2 (PLA2) and cytochrome P450 (CYP450). The figure is adapted from Ryskamp et al., 2015; Iuso and Križaj, 2016. NCX: Sodium-calcium exchanger; PMCA: plasma membrane calcium ATPase; AQP4: aquaporin 4 water channel; Kir4.1: inwardly rectifying potassium channel isoform 4.1.
Footnotes
Funding: This study was supported by the NIH (R01EY022076, R01EY027920; P30EY014800), the Willard Eccles Foundation, Glaucoma Research Foundation, the Diabetes and Metabolism Center at the University of Utah and unrestricted support from Research to Prevent Blindness to the Moran Eye Institute at the University of Utah, USA.
Conflicts of interest: None declared.
Financial support: This study was supported by the NIH (R01EY022076, R01EY027920; P30EY014800), the Willard Eccles Foundation, Glaucoma Research Foundation, the Diabetes and Metabolism Center at the University of Utah and unrestricted support from Research to Prevent Blindness to the Moran Eye Institute at the University of Utah, USA.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vision Res. 2017;139:93–100. doi: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Paolo G, Kim TW. Linking lipids to Alzheimer's disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009;390:287–293. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Navarro J, Aldea P, de Hoz R, Salazar JJ, Ramírez AI, Rojas B, Gallego BI, Triviño A, Tejerina T, Ramírez JM. Neuroprotective effects of low-dose statins in the retinal ultrastructure of hypercholesterolemic rabbits. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154800. e0154800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fliesler SJ, Bretillon L. The ins and outs of cholesterol in the vertebrate retina. J Lipid Res. 2010;51:3399–3413. doi: 10.1194/jlr.R010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliesler SJ, Vaughan DK, Jenewein EC, Richards MJ, Nagel BA, Peachey NS. Partial rescue of retinal function and sterol steady-state in a rat model of Smith-Lemli-Opitz syndrome. Pediatr Res. 2007;61:273–278. doi: 10.1203/pdr.0b013e318030d1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambert S, Gabrielle PH, Masson E, Leger-Charnay E, Ferrerro A, Vannier A, Gendrault C, Lachot M, Creuzot-Garcher C, Bron A, Gregoire S, Leclere L, Martine L, Lucchi G, Truntzer C, Pecqueur D, Bretillon L. Cholesterol metabolism and glaucoma: Modulation of Müller cell membrane organization by 24S-hydroxycholesterol. Chem Phys Lipids. 2017;207:179–191. doi: 10.1016/j.chemphyslip.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Hammer SS, Busik JV. The role of dyslipidemia in diabetic retinopathy. Vision Res. 2017;139:228–236. doi: 10.1016/j.visres.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iuso A, Križaj D. TRPV4-AQP4 interactions ‘turbocharge’ astroglial sensitivity to small osmotic gradients. Channels (Austin) 2016;10:172–174. doi: 10.1080/19336950.2016.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N, Križaj D. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neurosci. 2015;35:13525–13537. doi: 10.1523/JNEUROSCI.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumari S, Kumar A, Sardar P, Yadav M, Majhi RK, Kumar A, Goswami C. Influence of membrane cholesterol in the molecular evolution and functional regulation of TRPV4. Biochem Biophys Res Commun. 2015;456:312–319. doi: 10.1016/j.bbrc.2014.11.077. [DOI] [PubMed] [Google Scholar]
- 14.Lakk M, Yarishkin O, Baumann JM, Iuso A, Krizaj D. Cholesterol regulates polymodal sensory transduction in Müller glia. Glia. 2017;65:2038–2050. doi: 10.1002/glia.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitan I, Barrantes FJ. Cholesterol Regulation of Ion Channels and Receptors. Hoboken, NJ: John Wiley & Sons, Inc; 2012. [Google Scholar]
- 16.Marquer C, Devauges V, Cossec JC, Liot G, Lecart S, Saudou F, Duyckaerts C, Leveque-Fort S, Potier MC. Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 2011;25:1295–1305. doi: 10.1096/fj.10-168633. [DOI] [PubMed] [Google Scholar]
- 17.Martín MG, Pfrieger F, Dotti CG. Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 2014;15:1036–1052. doi: 10.15252/embr.201439225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molnár T, Yarishkin O, Iuso A, Barabas P, Jones B, Marc RE, Phuong TTT, Križaj D. Store-operated calcium entry in Müller glia is controlled by synergistic activation of TRPC and orai channels. J Neurosci. 2016;36:3184–3198. doi: 10.1523/JNEUROSCI.4069-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140195. doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omarova S, Charvet CD, Reem RE, Mast N, Zheng W, Huang S, Peachey NS, Pikuleva IA. Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. J Clin Invest. 2012;122:3012–3023. doi: 10.1172/JCI63816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phuong TTT, Redmon SN, Yarishkin O, Winter JM, Li DY, Križaj D. Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. J Physiol. 2017;595:6869–6885. doi: 10.1113/JP275052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichenbach A, Bringmann A. Müller Cells in the Healthy and Diseased Retina. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 23.Ryskamp DA, Frye AM, Phuong TT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY, Krizaj D. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep. 2016;6:30583. doi: 10.1038/srep30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryskamp DA, Iuso A, Križaj D. TRPV4 links inflammatory signaling and neuroglial swelling. Channels (Austin) 2015;9:70–72. doi: 10.1080/19336950.2015.1017998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, Križaj D. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci. 2014;34:15689–15700. doi: 10.1523/JNEUROSCI.2540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Rentería RC, Liedtke W, Krizaj D. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31:7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vázquez-Chona FR, Geisert EE. Networks modulating the retinal response to injury: insights from microarrays, expression genetics, and bioinformatics. Adv Exp Med Biol. 2012;723:649–656. doi: 10.1007/978-1-4614-0631-0_82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: molecular conductor of a diverse orchestra. Physiol Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]