Keywords: nerve regeneration, suspended moxibustion, middle cerebral artery occlusion, cerebral ischemia/reperfusion injury, infarct volume, apoptosis, Bcl-2, Bax, caspase-9, caspase-3, neural regeneration, traditional Chinese medical therapy
Abstract
Heat-sensitive suspended moxibustion has a neuroprotective effect against focal cerebral ischemia/reperfusion injury, but the underlying mechanisms remain unclear. The duration of heat-sensitive suspended moxibustion (usually from 30 minutes to 1 hour) is longer than traditional suspended moxibustion (usually 15 minutes). However, the effects of 15- and 35-minute suspended moxibustion in rats with cerebral ischemia/reperfusion injury are poorly understood. In this study, we performed 15- or 35-minute suspended moxibustion at acupoint Dazhui (GV14) in an adult rat model of focal cerebral ischemia/reperfusion injury. Infarct volume was evaluated with the 2,3,5-triphenyltetrazolium chloride assay. Histopathological changes and neuronal apoptosis at the injury site were assessed by hematoxylin-eosin staining and terminal deoxynucleotidyl transferase dUTP nick end labeling assay. Caspase-9 and caspase-3 expression at the injury site was detected using immunofluorescent staining. Bax and Bcl-2 expression at the injury site was assessed using western blot assay. In the 35-minute moxibustion group, infarct volume was decreased, neuronal apoptosis was reduced, caspase-9, caspase-3 and Bax expression was lower, and Bcl-2 expression was increased, compared with the 15-minute moxibustion group. Our findings show that 35-minute moxibustion has a greater anti-apoptotic effect than 15-minute moxibustion after focal cerebral ischemia/reperfusion injury.
Introduction
Moxibustion is a traditional Chinese medical therapy using the burning of a preparation of dried mugwort (moxa). Similar to other Chinese herbal medicines (Cui et al., 2008; Gu et al., 2014), moxibustion is widely used in China for the treatment of various chronic diseases and symptoms of “deficient conditions” (weakness), including stroke. Indirect moxibustion involves placing a lit cigar-like stick of dried mugwort either near a particular acupuncture point (acupoint) to heat the skin, or on an acupuncture needle inserted into the skin to heat the needle. Suspended moxibustion is an indirect form of moxibustion in which the dried mugwort is placed at an acupoint without skin contact to stimulate circulation by warming the acupoints and inducing smoother flow of blood and qi, to improve health and facilitate recovery from brain injury. The recent technique of heat-sensitive suspended moxibustion has been shown to be neuroprotective in stroke patients, with greater effectiveness than traditional suspended moxibustion (Zhang, 2006; Chen and Kang, 2007). In clinical practice, heat-sensitive suspended moxibustion improves recovery following cerebral infarction (Chen et al., 2009). Traditional moxibustion takes 15 minutes (Sun et al., 2008; Chen et al., 2011b). The duration of heat-sensitive suspended moxibustion treatment ranges from 30 minutes to 1 hour (Chen et al., 2011a, b; Xiao et al., 2013), which is longer than conventional moxibustion (approximately 15 minutes). Therefore, in the present study, we used a 35-minute treatment period for comparison with a 15-minute treatment period.
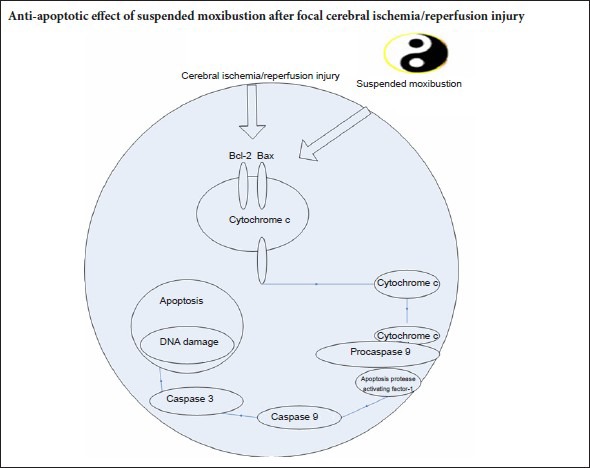
Neuronal cell death in the ischemic brain is mediated by activation of caspase-dependent apoptotic signaling pathways (Love, 2003; Park and Jonas, 2017). A 40-minute period of suspended moxibustion reduces apoptosis, as assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Chen et al., 2011a). Bax accelerates apoptotic processes, whereas Bcl-2 inhibits apoptotic cell death (Bredesen, 1995; Wang and Cui, 2013). In the ischemic region, the levels of Bax increase while the levels of Bcl-2 decrease (Won et al., 2006), leading to a release of cytochrome c from mitochondria. Cytochrome c initiates cell death following brain ischemia (Ouyang et al., 1999; Sugawara et al., 1999; Cao et al., 2004; Baydas et al., 2005; Hetz et al., 2005). Reducing Bax levels and/or increasing Bcl-2 levels suppress the release of cytochrome c (Li et al., 2012; Lan et al., 2014). Once cytochrome c is released into the cytosol, it binds to apoptosis protease activating factor-1 and procaspase-9 to form the apoptosome, thereby activating caspase-9 and caspase-3, and initiating apoptotic cell death (Green, 2000; Li et al., 2012; Lan et al., 2014). Caspase-3 is the major executioner caspase in neurons (Baydas et al., 2005), and its protein levels increase significantly following focal cerebral ischemic injury in rats (Li et al., 2012; Lan et al., 2014).
In this study, we compared the effects of suspended moxibustion of 15- and 35-minute periods on brain infarction, apoptosis and neuronal expression levels of caspase-9, caspase-3, Bax and Bcl-2 in a rat model of focal cerebral ischemia/reperfusion injury.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 220–280 g were purchased from the Experimental Animal Center of Jiangxi University of Traditional Chinese Medicine of China (SCXK (Gan) 2011-0003). Rats were housed under controlled conditions (lights from 07:00–19:00, 50–60% humidity, 25°C) with free access to water and food. The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1986). The study was approved by the Ethical Committee of Jiangxi University of Traditional Chinese Medicine (approval No. jzdwll20140013).
A total of 93 rats were randomly divided into the following five groups: normal (n = 3), sham surgery (n = 15), ischemia/reperfusion (I/R) (n = 25; middle cerebral artery occlusion (MCAO) only), 15-minute moxibustion (n = 25; MCAO + 15-minute moxibustion) and 35-minute moxibustion (n = 25; MCAO + 35-minute moxibustion) groups. The experimental protocol is shown in Figure 1.
Figure 1.
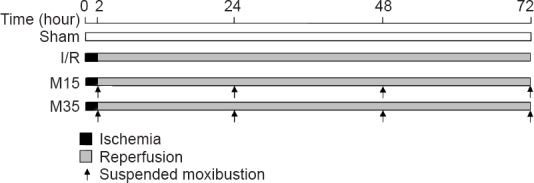
Diagram of the experimental timeframe.
Sham group: Rats (n = 15) underwent general anesthesia and sham surgical procedure. Ischemia/reperfusion (I/R) group: rats (n = 25) underwent 2 hours of ischemia, followed by 3 days of reperfusion. M15 group (n = 25): I/R rats given 15 minutes of suspended moxibustion at 2, 24, 48 and 72 hours after surgery (one treatment session per time point). M35 group (n = 25): I/R rats given 35 minutes of suspended moxibustion at 2, 24, 48 and 72 hours after surgery (one treatment session per time point).
Preparation of rat focal cerebral I/R injury model
The rat model of focal cerebral I/R injury was prepared by performing 2 hours of MCAO followed by 3 days of reperfusion in spontaneously breathing rats. All rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (3% w/v) at a dose of 40 mg/kg. Body temperature was maintained at 37.0 ± 0.5°C using a heat lamp and heating pad. MCAO was performed using the intraluminal filament method, as described previously (Sun et al., 2006, 2007, 2008; Chen et al., 2011b). Briefly, a midline neck incision was made, and the left common carotid, internal carotid and external carotid arteries were isolated. The external carotid artery was ligated with 4-0 silk suture distal from the carotid bifurcation, and the common carotid artery was ligated with 4-0 silk suture at the proximal end. Another 4-0 silk suture was tied loosely around the common carotid artery close to the carotid bifurcation. A piece of fishing line (Simago Fishing Tackle Company, Hangzhou, Zhejiang Province, China), 0.237 mm in diameter and 5 cm in length with a rounded tip, was introduced into a small incision in the common carotid artery and gently advanced to the origin of the middle cerebral artery (20–22 mm from the carotid bifurcation). The silk suture around the common carotid artery stump was tied tightly to prevent bleeding and to secure the fishing line. After 2 hours of occlusion, the fishing line was withdrawn to allow for reperfusion. Sham-operated rats were handled in the same way, but the middle cerebral artery was not occluded.
Suspended moxibustion treatment
Dazhui points (GV14, in the depression of the spinous process of the seventh cervical vertebra, on the posterior median line), considered to be important for brain function (Luo, 2000), were heated by suspended moxibustion using a moxibustion cigar produced from mugwort (custom-made for use with animals, length 12 cm, diameter 0.6 cm, made in the Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China) at a height of approximately 3 cm over a hairless area of the skin once a day for 4 days (i.e. at 2, 24, 48 and 72 hours after surgery).
Infarct volume assessment
After 3 days of reperfusion, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital 3% (weight/volume) at a dose of 40 mg/kg, and the brains were removed and cut into 2-mm-thick coronal slices at the optic chiasm level, i.e., the coronal level at which the largest ischemic infarct was observed (Bederson et al., 1986; Simpkins et al., 1997; Sun et al., 2008). These slices were incubated for 30 minutes in 1% (weight/volume) 2,3,5-triphenyltetrazolium chloride (TTC) and fixed in 10% (v/v) formalin. The stained slices were photographed using a COOLPIX L1 camera (Nikon, Tokyo, Japan). The ischemic lesion in each slice was measured using Infinity Analyze software (Lumenera Corporation, Ottawa, ON, Canada). The size of the ischemic lesion (expressed as a percentage) was calculated as the ratio of the area of the infarct to that of the whole slice.
Brain histological structures
After TTC staining and lesion area analysis, the slices were embedded in paraffin. A series of sequential 5 μm-thick sections were cut in the coronal plane and stained with hematoxylin and eosin. These sections were photographed with the use of an image analysis system (Leica 2000 microscope, LAS V3.7 software, Leica, Watzlar, Germany).
TUNEL assay
After 3 days of reperfusion, the brain was perfused with sodium chloride and 4% formaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. Apoptotic cells were then detected with the TUNEL assay using the KeyGEN In Situ Apoptosis Detection Kit, according to the manufacturer's instructions (KGA7022, KeyGEN Biotech, Nanjing, China). Apoptotic nuclei were stained brown using this method. The area of TUNEL-positive cells was calculated as follows: the area of apoptotic cells (%) = The area of apoptotic cells/the total area of the image in a high-power field (200×) × 100%.
Immunofluorescent staining
After 3 days of reperfusion, rats were transcardially perfused with 4% paraformaldehyde after an intraperitoneal injection of sodium pentobarbital 3% (w/v) at a dose of 40 mg/kg. The brains were removed, and the cortices were separated and sectioned (35 μm thickness) on a freezing sliding microtome (Leica CM 1950; Leica). All sections were cryoprotected in an antifreeze solution for storage at –20°C. After rinsing with phosphate buffered saline (PBS), cortical sections were blocked with 10% goat serum (0.3% Triton X-100 in PBS) for 2 hours at 37°C, incubated at 4°C overnight with rabbit polyclonal antibody against cleaved caspase-9 (Asp353) (#9507, Cell Signaling Technology; 1:400) and rabbit polyclonal antibody against cleaved caspase-3 (Asp175) (#9661, Cell Signaling Technology; 1:400). After washing with PBS, sections were incubated for 1 hour at 37°C with rhodamine-labeled affinity purified antibody to rabbit IgG (H+L) produced in goat (No. 03-15-06, 1:200; KPL, Milford, MA, USA). Following rinsing with PBS (10 minutes × 3), sections were mounted on slides. The slides were imaged with a Leica DM 2000 microscope and Leica microscope software (LAS V3.7). Under the microscope, positive cells were counted in five random high-power fields (200×).
Reverse transcription-polymerase chain reaction
At 3 days of reperfusion, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital 3% (w/v) at a dose of 40 mg/kg. The brains were removed and the cortices were dissected. Total RNA was isolated using TRIZOL reagent. cDNA was synthesized from each RNA using a random primer and RevertAid™ M-MuLV reverse transcriptase (Fermentas), according to the manufacturer's instructions. The obtained cDNA was used to determine the amount of Bcl-2 and Bax mRNA using PCR with Taq DNA polymerase (Fermentas). The primers used for amplification of the Bcl-2 and Bax transcripts were as follows: Bcl-2 forward, 5′-CCG GGA GAT CGT GAT GAA GT-3′ and reverse, 5′-ATC CCA GCC TCC GTT ATC CT-3′, product size 600 bp; Bax forward, 5′-CCA AGA AGC TGA GCG AGT GTC-3′ and reverse, 5′-TGA GGA CTCCAG CCA CAA AGA-3′; product size was 400 bp. PCR was performed in a final volume of 25 μL, containing 12.5 μL 2 × PCR master mix, 2 μL cDNA, 1 μL forward primer, 1 μL reverse primer, and 8.5 μL sterilized DEPC water. PCR was conducted in a Bio-Rad S1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA), with the amplification condition of 94°C for 45 seconds, followed by 55°C for 60 seconds and then 72°C for 45 seconds, for 30 cycles. The amplification products were analyzed with 1.6% agarose gel electrophoresis and observed with a gel imaging system (Bio-Rad).
Western blot assay
After 3 days of reperfusion, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (3% w/v; 40 mg/kg). The brains were removed, and the cortices were dissected and homogenized in RIPA buffer containing PMSF (2% w/v). The sample was centrifuged at 12,000 r/min for 20 minutes at 4°C, and an aliquot of the supernatant was taken for protein concentration estimation using the BCA assay. Western blot assay was performed with 30 μg total protein extract separated on 10–12% SDS-PAGE gels that were subsequently transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Blocking of membranes (5% nonfat dry milk or 5% bovine serum albumin), washes (Tris-buffered saline) and secondary antibody (goat anti-rabbit, 1:4,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) incubations were all performed at room temperature for 1 hour, whereas primary antibodies were allowed to incubate overnight at 4°C (rabbit anti-Bcl-2, 1:1,000; rabbit anti-Bax, 1:1,000; rabbit anti-β-actin, 1:1,000). These primary antibodies were all from Cell Signaling Technology. Signals were detected using the SuperSignal West Pico Chemiluminescence Substrate system (Thermo Scientific, Waltham, MA, USA) by exposure to X-ray film.
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Quantitative data are expressed as the mean ± SEM and analyzed using one-way analyses of variance followed by Dunnett's test. A value of P < 0.05 was considered statistically significant.
Results
35-minute suspended moxibustion reduced brain infarct volume
To investigate the effect of a 35-minute period of suspended moxibustion, infarct size was measured in the sham surgery and I/R groups treated with or without moxibustion (Figure 2A). Brain slices were stained with TTC after 2 hours of MCAO followed by 3 days of reperfusion. As shown in Figure 2B, the infarct volume was significantly reduced in the 35-minute moxibustion group (19.74%; n = 8) compared with the I/R group (44.94%; n = 9) (P < 0.05). Rats in the 15-minute moxibustion group also showed a reduction in infarct size (27.31%; n = 7) compared with the I/R group, but the difference was not statistically significant (P > 0.05).
Figure 2.
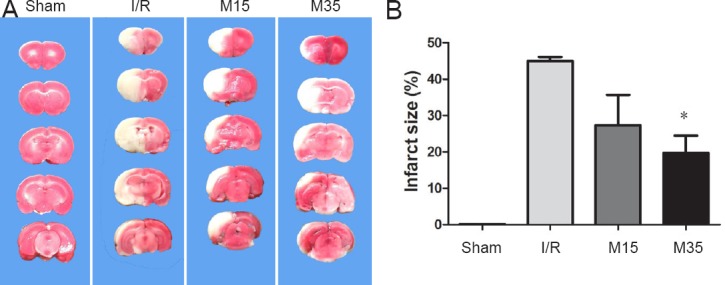
Infarct volume in the injured brain.
(A) Brain coronal sections were stained with 2,3,5-triphenyltetrazolium chloride after 2 hours of middle cerebral artery occlusion followed by 3 days of reperfusion. The ischemic area remained white, while the intact area stained red. (B) Analysis of brain infarct volumes in the sham surgery, I/R and moxibustion groups. The data are presented as the mean ± SEM and were analyzed using one-way analysis of variance followed by Dunnett's test. *P < 0.05, vs. I/R group. Sham: Sham surgery group; I/R: I/R group; M15: 15-minute moxibustion group; M35: 35-minute moxibustion group.
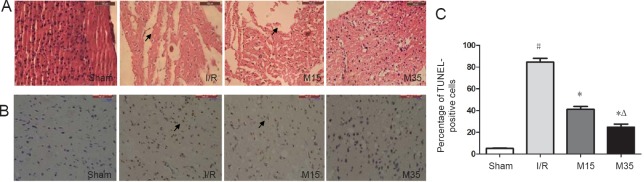
35-minute suspended moxibustion reduced pathological changes and neuronal loss
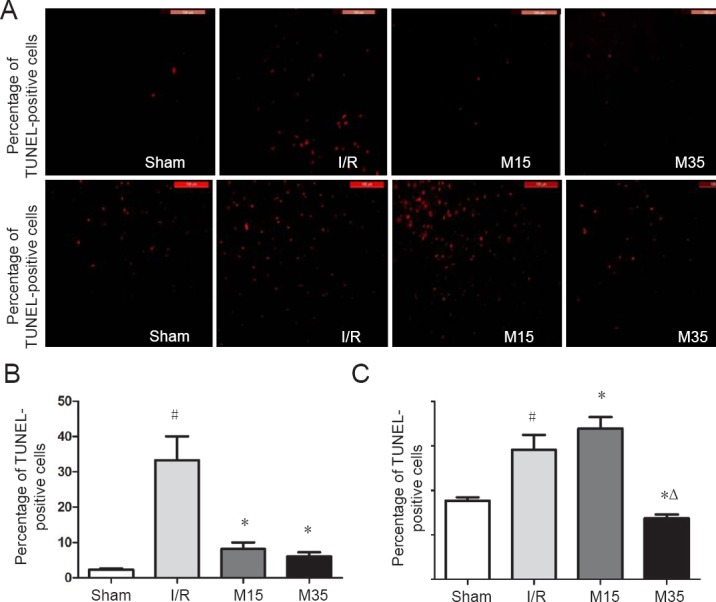
We further examined morphological changes and neuronal loss in the rat brain after 2 hours of MCAO followed by 3 days of reperfusion to investigate the effects of a 35-minute period of suspended moxibustion. As shown by hematoxylin-eosin staining of the ipsilateral cerebral cortex (Figure 3A), extensive brain damage characterized by severe neuronal cell loss was observed in both the I/R and 15-minute moxibustion groups, compared with the normal and 35-minute moxibustion groups. Apoptotic cells in the ipsilateral cortex were assessed by TUNEL assay (Figure 3B). The total area of TUNEL-positive cells was increased significantly in the I/R group compared with the sham surgery group (P < 0.05). MCAO induced a significant increase in the total area of TUNEL-positive cells, while suspended moxibustion for 15 minutes (P < 0.05) and 35 minutes (P < 0.05) decreased the total area of these apoptotic cells (Figure 3C). The anti-apoptotic effect of 35-minute suspended moxibustion was greater than that of 15-minute suspended moxibustion.
Figure 3.
Pathological changes and apoptotic cells in the injured brain.
(A) Photographs of brain sections stained with hematoxylin and eosin 3 days after reperfusion. Suspended moxibustion for 35 minutes alleviated the pathological changes. The arrows indicate cell loss. Scale bars: 100 μm. (B) Photographs of apoptotic cells (arrows) detected by the TUNEL assay 3 days after reperfusion in rats. The 35-minute moxibustion treatment decreased the total area of TUNEL-positive cells compared with the I/R and 15-minute moxibustion groups. Scale bars: 100 μm. (C) Quantitative analysis of the percentage of TUNEL-positive cells, expressed as the mean ± SEM (n = 3) and analyzed by one-way analysis of variance followed by Dunnett's test. #P < 0.05, vs. sham group; *P < 0.05, vs. I/R group; ΔP < 0.05, vs. M15 group. Sham: Sham surgery group; I/R: I/R group; M15: 15-minute moxibustion group; M35: 35-minute moxibustion group. I/R: ischemia/reperfusion; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
35-minute suspended moxibustion decreased the expression of caspase-9 and caspase-3
Caspase-9 and caspase-3 are the major effectors of apoptosis (Green, 2000; Li et al., 2012; Lan et al., 2014). We compared the numbers of caspase-9- and caspase-3-positive cells in ipsilateral cortical regions after 3 days of reperfusion among the treated and normal groups to clarify whether the neuroprotective effects of suspended moxibustion involve the suppression of apoptosis. As shown by immunofluorescence labeling (Figure 4), the total number of caspase-9-positive cells was significantly greater in the I/R group compared with the sham surgery group (P < 0.05). Suspended moxibustion treatment for 15 or 35 minutes caused a significant reduction in the number of caspase-9-positive cells (P < 0.05). The number of caspase-3-positive cells was significantly greater in the I/R group than in the sham surgery group (P < 0.05). The 35-minute suspended moxibustion treatment caused a statistically significant reduction in the number of caspase-3-positive cells (P < 0.05), compared with the I/R group. In comparison, the numbers of caspase-3-positive cells were similar in the 15-minute suspended moxibustion and I/R groups.
Figure 4.
Immunofluorescence labeling for caspase-9 and caspase-3 in the injured rat cortex.
Brain slices were prepared 3 days after sham surgery or after I/R (2 hours of middle cerebral artery occlusion followed by 3 days of reperfusion). (A) Immunofluorescence images of caspase-9 and caspase-3 labeling in the cortex 3 days after I/R. Caspase-positive cells were stained red. Scale bars: 100 μm. Quantitative analysis of the number of caspase-9-positive cells (B) and caspase-3-positive cells (C). The numbers of caspase-positive cells were counted in five random high-power fields (200×) and are presented as the mean ± SEM (n = 3) and analyzed using one-way analysis of variance followed by Dunnett's test. #P < 0.05, vs. sham group; *P < 0.05, vs. I/R group; ΔP < 0.05, vs. M15 group. Sham: Sham surgery group; I/R: I/R group; M15: 15-minute moxibustion group; M35: 35-minute moxibustion group.
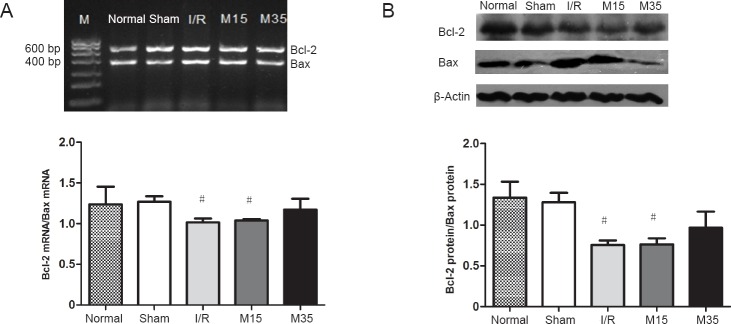
35-minute suspended moxibustion increased Bcl-2 expression and decreased Bax expression
Bcl-2 and Bax are anti-apoptotic and pro-apoptotic factors, respectively (Oltvai et al., 1993). We therefore compared the mRNA and protein levels of Bcl-2 and Bax in ipsilateral cortical brain samples among the groups using RT-PCR and western blot assay (Figure 5). The ratio of Bcl-2 to Bax mRNA levels was significantly decreased in the I/R and 15-minute suspended moxibustion groups, compared with the sham surgery group (both P < 0.05). The ratio of Bcl-2 to Bax mRNA was similar to the sham control level in rats given the 35-minute suspended moxibustion treatment. Consistent with the changes in mRNA levels, the ratio of Bcl-2 to Bax protein was also significantly lower in the I/R and 15-minute suspended moxibustion groups, compared with the sham surgery or 35-minute suspended moxibustion groups (all P < 0.05).
Figure 5.
Gene and protein expression levels of Bcl-2 and Bax in the injured rat cortex.
The cortical tissues were prepared on day 3 after sham surgery or after I/R (2 hours of middle cerebral artery occlusion followed by 3 days of reperfusion). (A) Representative image and quantitative analysis of the densitometric values of Bcl-2 and Bax mRNA levels in the rat cortex detected by reverse transcription-polymerase chain reaction after 3 days of reperfusion. (B) Representative western blots and quantitative analysis of the densitometric value of the protein levels of Bcl-2 and Bax (β-actin, control protein) in the rat cortex after 3 days of reperfusion. Data are presented as the mean ± SEM (n = 3) and analyzed using one-way analysis of variance followed by Dunnett's test. #P < 0.05, vs. sham group. M: DNA marker. Normal: normal group; Sham: sham surgery group; I/R: I/R group; M15: 15-minute moxibustion group; M35: 35-minute moxibustion group.
Discussion
In this study, we found that the duration of suspended moxibustion at acupoint Dazhui (GV14) on the dorsomedial neck is critical for optimal neuroprotection. Suspended moxibustion for 35 minutes significantly reduced infarct volume and neuronal loss after focal cerebral I/R injury. In addition, suspended moxibustion for 35 minutes altered levels of apoptosis-related proteins—it decreased caspase levels, and it increased Bcl-2 and reduced Bax levels in the cortices of rats after the focal cerebral I/R injury. Our findings collectively suggest that the neuroprotective effects of 35-minute suspended moxibustion are superior to those of 15-minute suspended moxibustion.
Previous controlled trials have demonstrated an enhancement of recovery in stroke patients given 15-minute traditional suspended moxibustion on the fixed acupoints (Zhang et al., 2005; Rao et al., 2006); however, some other studies have shown contrasting results (Lee et al., 2010). The newly developed technique of heat-sensitive suspended moxibustion has a substantially improved therapeutic effect compared with traditional suspended moxibustion (Chen and Kang, 2007). The amount of moxibustion is critical when applying heat-sensitive suspended moxibustion. When other factors are constant, the duration of suspended moxibustion is a key factor in optimizing efficacy (Zhang, 2006; Chen and Kang, 2007; Chen et al., 2009). In clinical practice, the duration of heat-sensitive suspended moxibustion ranges from 30 minutes to 1 hour, which is usually longer than conventional moxibustion (usually around 15 minutes). Prolonged application of moxibustion above certain acupoints can induce not only internal heat-sensation, but also physically detectable elevated temperature at locations distant to the suspended moxibustion acupoint. The acupoint, according to ancient Chinese medical theory, represents the region of the body surface that can be sensitized under pathological conditions. The locations of acupoints are arranged along the meridians. Moxibustion at an acupoint penetrates deeply into the body along the meridians, which can conduct the heat to distant regions. When such distant heat occurs, the efficacy of moxibustion increases (Chen et al., 2010a).
Generally, distant heat first appears in the patient at approximately 15 minutes into suspended moxibustion treatment (Chen et al., 2010b). After 15 minutes, a rapid increase in distant heat is exhibited in the patients and maintained until the end of the treatment, usually 30 minutes to one hour. A 40-minute suspended moxibustion treatment causes a measurable increase in tail temperature in a rat model of MCAO, and this effect is not observed in rats given a 15-minute treatment (Chen et al., 2011b). Similarly, suspended moxibustion for 35 minutes has neuroprotective effects in a rat stroke model (Xiao et al., 2013). Consistent with these previous reports, we found here that suspended moxibustion for 35 minutes, but not 15 minutes, over a period of 3 days (four sessions in total) markedly reduced infarct volume and neuronal loss in rats subjected to I/R injury. Our findings suggest that prolonged moxibustion is capable of stimulating the heat-sensitive acupoints and gradually transfers heat to distant areas. According to traditional Chinese medicine, moxibustion functions by restoring the balance and flow of “vital energy” through sensitized acupoints, although there is currently no clear physiological correlate of this theory. In addition, it remains unknown how the moxibustion heat stimulus exerts its therapeutic effects. However, clinical observations suggest that moxibustion improves health by regulating homeostasis. We conjecture that acupoints might function as switches that control the complex internal regulatory system. Under pathological conditions, the acupoints become heat-sensitive. Prolonged moxibustion at the heat-sensitive acupoints might activate the regulatory system and enhance the internal ability to correct the imbalance by inducing rapid, precise and optimal responses to harmful stimuli.
Apoptosis, an energy-requiring process resulting in nuclear fragmentation and cytoplasmic condensation, contributes to cell death and cerebral I/R injury (Chomova et al., 2012; Ye et al., 2012; Zhang et al., 2012; Sanderson et al., 2013; Talha et al., 2013). Apoptosis involves the induction of intrinsic and extrinsic caspase-dependent signaling pathways (Love, 2003). Apoptosis is also regulated by the Bcl-2 family of proteins, including the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax (Oltvai et al., 1993; Bredesen, 1995; Wang and Cui, 2013). The balance between Bcl-2 and Bax determines the mitochondrial response to apoptotic stimuli, which induces activation of caspase-9, an intrinsic pathway in I/R injury (Fliss and Gattinger, 1996; Murriel et al., 2004; Lan et al., 2014). Mitochondria play major roles in normal cellular functions. It is the main site of energy production, and participates in intracellular Ca2+ cycling and the synthesis of many intracellular signaling molecules (Saraste, 1999; Halestrap, 2006; Feissner et al., 2009). Mitochondria are central in ischemic injury, and are therefore key targets of therapeutic strategies (Thaveau et al., 2007; Charles et al., 2011). The disruption of mitochondrial membrane potential is believed to be an early event in apoptosis. Bax, which is inactivated by Bcl-2, can cause a disruption of the mitochondrial membrane potential, leading to the release of pro-apoptotic factors, such as cytochrome c, and the subsequent activation of caspase-9 and caspase-3. A change in the ratio of Bcl-2 to Bax is a critical determinant of cell fate (Oltvai et al., 1993). Our results demonstrate that a 35-minute period of suspended moxibustion is needed to prevent the ischemia-induced reduction in the Bcl-2 to Bax ratio, suggesting that prolonged moxibustion is required to mitigate ischemic cell death. The I/R-induced up-regulation of caspase-9 was largely prevented by the 15-minute and 35-minute treatment period, suggesting high sensitivity of this caspase to moxibustion. In contrast, suppression of caspase-3 expression was only seen following the 35-minute treatment.
Caspase-9 participates in the intrinsic apoptotic pathway (Wang and Cui, 2013), whereas caspase-3 participates in both the intrinsic and extrinsic (caspase-8 dependent) pathways (Fliss and Gattinger, 1996; Ouyang et al., 1999; Love, 2003; Zhang, 2006; Li et al., 2009). Our data suggest that suspended moxibustion predominantly affects the mitochondria-dependent intrinsic pathway. However, it remains unknown how the heat delivered by moxibustion regulates the apoptotic process. Nonetheless, acupoints appear to be sensitized during I/R insults, suggesting that an imbalance in the internal force causes not only physical symptoms, but also molecular and cellular perturbations. Persistent application of moxibustion for 35 minutes during this sensitized state is required to correct the homeostatic imbalance.
In conclusion, the neuroprotective effects of heat-sensitive suspended moxibustion after I/R injury involve modulation of the mitochondrial apoptotic pathway. The longer 35-minute moxibustion treatment suppressed this apoptotic pathway more robustly than the shorter 15-minute session. Additional studies are needed to identify the specific molecular targets of this modulation.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81060305 & 81660819; the Natural Science Foundation of Jiangxi Province of China, No. 2015BAB205068; Key Program for Science and Technology Cooperation Projects of Jiangxi Province of China, No. 20161BBH80053; a grant from the Key Project of Health Commission of Jiangxi Province of China, No. 2014Z003; and the Natural Science Foundation of Jiangxi University of Traditional Chinese Medicine of China, No. 2014ZR018 & 2015jzzdxk024.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81060305 & 81660819; the Natural Science Foundation of Jiangxi Province of China, No. 2015BAB205068; Key Program for Science and Technology Cooperation Projects of Jiangxi Province of China, No. 20161BBH80053; a grant from the Key Project of Health Commission of Jiangxi Province of China, No. 2014Z003; and the Natural Science Foundation of Jiangxi University of Traditional Chinese Medicine of China, No. 2014ZR018 & 2015jzzdxk024. Funders had no involvement in the study design; data collection, management, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics: The study protocol was approved by the Ethics Committee of Jiangxi University of Traditional Chinese Medicine (approval number: jzdwll20140013). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Patel B, Wysong S, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Baydas G, Reiter RJ, Akbulut M, Tuzcu M, Tamer S. Melatonin inhibits neural apoptosis induced by homocysteine in hippocampus of rats via inhibition of cytochrome c translocation and caspase-3 activation and by regulating pro- and anti-apoptotic protein levels. Neuroscience. 2005;135:879–886. doi: 10.1016/j.neuroscience.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2 3, 5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 3.Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 4.Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, Graham SH, Simon RP, Chen J. Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24:6189–6201. doi: 10.1523/JNEUROSCI.1426-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles AL, Guilbert AS, Bouitbir J, Goette-Di Marco P, Enache I, Zoll J, Piquard F, Geny B. Effect of postconditioning on mitochondrial dysfunction in experimental aortic cross-clamping. Br J Surg. 2011;98:511–516. doi: 10.1002/bjs.7384. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Chen M, Kang M. Practical Reading of Heat-sensitization Moxibustion. Beijing: People's Medical Publishing House; 2009. [Google Scholar]
- 7.Chen R, Chen M, Xiong J, Yi F, Chi Z, Zhang B. Comparison of heat-sensitive moxibustion versus fluticasone/salmeterol (seretide) combination in the treatment of chronic persistent asthma: design of a multicenter randomized controlled trial. Trials. 2010a;11:121. doi: 10.1186/1745-6215-11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R, Chen M, Kang M, Xiong J, Chi Z, Zhang B, Fu Y. The design and protocol of heat-sensitive moxibustion for knee osteoarthritis: a multicenter randomized controlled trial on the rules of selecting moxibustion location. BMC Complement Altern Med. 2010b;10:32. doi: 10.1186/1472-6882-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen RX, Kang MF. Clinical application of acupoint heat-sensitization. Zhongguo Zhen Jiu. 2007;27:199–202. [PubMed] [Google Scholar]
- 10.Chen RX, Lv ZM, Chen MR. Neuronal apoptosis and inflammatory reaction in rat models of focal cerebral ischemia following 40-minute suspended moxibustion. Neural Regen Res. 2011a;6:1180–1184. [Google Scholar]
- 11.Chen RX, Lv ZM, Chen MR, Yi F, An X, Xie DY. Stroke treatment in rats with tail temperature increase by 40-min moxibustion. Neurosci Lett. 2011b;503:131–135. doi: 10.1016/j.neulet.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Chomova M, Tatarkova Z, Dobrota D, Racay P. Ischemia-induced inhibition of mitochondrial complex I in rat brain: effect of permeabilization method and electron acceptor. Neurochem Res. 2012;37:965–976. doi: 10.1007/s11064-011-0689-6. [DOI] [PubMed] [Google Scholar]
- 13.Cui YH, Zhou S, Wu HG, Wang XM, Tan LY, Liu HR. Regulatory effect of herbs-partition moxibustion on cycloxygenase and prostaglandin E2 in ulcerative colitis rats at apoptosis directions. Zhongguo Zuzhi Gongcheng Yanjiu. 2008;12:4680–4684. [Google Scholar]
- 14.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 16.Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Chen J, Shen J. Herbal medicines for ischemic stroke: combating inflammation as therapeutic targets. J Neuroimmune Pharmacol. 2014;9:313–339. doi: 10.1007/s11481-014-9525-5. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 19.Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC, Antonsson B. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- 20.Lan R, Zhang Y, Xiang J, Zhang W, Wang GH, Li WW, Xu LL, Cai DF. Xiao-Xu-Ming decoction preserves mitochondrial integrity and reduces apoptosis after focal cerebral ischemia and reperfusion via the mitochondrial p53 pathway. J Ethnopharmacol. 2014;151:307–316. doi: 10.1016/j.jep.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Lee MS, Shin BC, Kim JI, Han CH, Ernst E. Moxibustion for stroke rehabilitation: systematic review. Stroke. 2010;41:817–820. doi: 10.1161/STROKEAHA.109.566851. [DOI] [PubMed] [Google Scholar]
- 22.Li GF, Luo HK, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Wang WW, Zhang JX. Dual effects of hydrogen sulphide on focal cerebral ischaemic injury via modulation of oxidative stress-induced apoptosis. Clin Exp Pharmacol Physiol. 2012;39:765–771. doi: 10.1111/j.1440-1681.2012.05731.x. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 2009;121:412–418. doi: 10.1016/j.jep.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 24.Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y. Subject of Acupoint. Shanghai: Shanghai Science and Technology Publishing House; 2000. [Google Scholar]
- 26.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 27.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjo BK, Hu BR. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. J Cereb Blood Flow Metab. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Park HA, Jonas EA. ΔN-Bcl-xL, a therapeutic target for neuroprotection. Neural Regen Res. 2017;2:1791–1794. doi: 10.4103/1673-5374.219033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao P, Zhou L, Mao M, Bai Y, Wen TM, Tang YH, Guo WL. A randomized controlled trial of acupuncture treatment of acute ischemic stroke. Zhongguo Zhen Jiu. 2006;26:694–696. [PubMed] [Google Scholar]
- 31.Sanderson TH, Mahapatra G, Pecina P, Ji Q, Yu K, Sinkler C, Varughese A, Kumar R, Bukowski MJ, Tousignant RN, Salomon AR, Lee I, Hüttemann M. Cytochrome C is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS One. 2013;8:e78627. doi: 10.1371/journal.pone.0078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 33.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:Rc39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun HS, Feng ZP, Miki T, Seino S, French RJ. Enhanced neuronal damage after ischemic insults in mice lacking Kir6.2-containing ATP-sensitive K+ channels. J Neurophysiol. 2006;95:2590–2601. doi: 10.1152/jn.00970.2005. [DOI] [PubMed] [Google Scholar]
- 36.Sun HS, Feng ZP, Barber PA, Buchan AM, French RJ. Kir6, 2-containing ATP-sensitive potassium channels protect cortical neurons from ischemic/anoxic injury in vitro and in vivo. Neuroscience. 2007;144:1509–1515. doi: 10.1016/j.neuroscience.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, Ryan CL, Bernard PB, Lau A, Forder JP, Salter MW, Wang YT, Tasker RA, Tymianski M. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–2553. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- 38.Talha S, Bouitbir J, Charles AL, Zoll J, Goette-Di Marco P, Meziani F, Piquard F, Geny B. Pretreatment with brain natriuretic peptide reduces skeletal muscle mitochondrial dysfunction and oxidative stress after ischemia-reperfusion. J Appl Physiol (1985) 2013;114:172–179. doi: 10.1152/japplphysiol.00239.2012. [DOI] [PubMed] [Google Scholar]
- 39.Thaveau F, Zoll J, Rouyer O, Chafke N, Kretz JG, Piquard F, Geny B. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. J Vasc Surg. 2007;46:541–547. doi: 10.1016/j.jvs.2007.04.075. discussion 547. [DOI] [PubMed] [Google Scholar]
- 40.Wang JJ, Cui P. Neohesperidin attenuates cerebral ischemia-reperfusion injury via inhibiting the apoptotic pathway and activating the Akt/Nrf2/HO-1 pathway. J Asian Nat Prod Res. 2013;15:1023–1037. doi: 10.1080/10286020.2013.827176. [DOI] [PubMed] [Google Scholar]
- 41.Won CK, Kim MO, Koh PO. Estrogen modulates Bcl-2 family proteins in ischemic brain injury. J Vet Med Sci. 2006;68:277–280. doi: 10.1292/jvms.68.277. [DOI] [PubMed] [Google Scholar]
- 42.Xiao AJ, Kang MF, hen RX, Tan SH. Effect of heat-sensitive moxibustion on tail-flick latency in rat models of focal cerebral ischemia-reperfusion injury. Shizhen Guoyi Guoyao. 2013;24:228–230. [Google Scholar]
- 43.Ye Z, Wang N, Xia P, Wang E, Yuan Y, Guo Q. Delayed administration of parecoxib, a specific COX-2 inhibitor, attenuated postischemic neuronal apoptosis by phosphorylation Akt and GSK-3beta. Neurochem Res. 2012;37:321–329. doi: 10.1007/s11064-011-0615-y. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B. Unblocking main and collateral channels originated by Chen RX. Jiangxi Zhongyiyao. 2006;37:7–8. [Google Scholar]
- 45.Zhang SH, Liu M, Asplund K, Li L. Acupuncture for acute stroke. Cochrane Database Syst Rev. 2005:CD003317. doi: 10.1002/14651858.CD003317.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang TL, Fu JL, Geng Z, Yang JJ, Sun XJ. The neuroprotective effect of losartan through inhibiting AT1/ASK1/MKK4/JNK3 pathway following cerebral I/R in rat hippocampal CA1 region. CNS Neurosci Ther. 2012;18:981–987. doi: 10.1111/cns.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]