Keywords: nerve regeneration, dexmedetomidine, sevoflurane, bispectral index, fast-track anesthesia, embolization of intracranial aneurysm, stress response, neuroprotection, neural regeneration
Abstract
Dexmedetomidine has sedative, anxiolytic, analgesic, anti-sympathetic, and anti-shivering effects. Dexmedetomidine might be effective in combination with sevoflurane for anesthesia, but prospective randomized controlled clinical trials with which to verify this hypothesis are lacking. In total, 120 patients who underwent embolization of an intracranial aneurysm were recruited from Anhui Provincial Hospital and Renmin Hospital of Wuhan University of China and randomly allocated to two groups. After intraoperative administration of 2% to 3% sevoflurane inhalation, one group of patients received pump-controlled intravenous injection of 1.0 μg/kg dexmedetomidine for 15 minutes followed by maintenance with 0.3 μg/kg/h until the end of surgery; the other group of patients only underwent pump-controlled infusion of saline. Bispectral index monitoring revealed that dexmedetomidine-assisted anesthesia can shorten the recovery time of spontaneous breathing, time to eye opening, and time to laryngeal mask removal. Before anesthetic induction and immediately after laryngeal mask airway removal, the glucose and lactate levels were low, the S100β and neuron-specific enolase levels were low, the perioperative blood pressure and heart rate were stable, and postoperative delirium was minimal. These findings indicate that dexmedetomidine can effectively assist sevoflurane for anesthesia during surgical embolization of intracranial aneurysms, shorten the time to consciousness and extubation, reduce the stress response and energy metabolism, stabilize hemodynamic parameters, and reduce adverse reactions, thereby reducing the damage to the central nervous system. This trial was registered at the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) (registration number: ChiCTR-IPR-16008113).
Introduction
Rupture of an intracranial aneurysm may cause subarachnoid hemorrhage or intraparenchymal hemorrhage accompanied by severe headache, vomiting, and changes in consciousness (Brisman et al., 2006; Korja et al., 2016). Embolization of intracranial aneurysms is highly promising; it causes fewer traumas and bleeding than other currently available methods and does not require craniotomy. In recent years, increasing evidence regarding the greater efficacy of interventional embolization than aneurysm clipping has been reported (Taki et al., 1998; Wu et al., 2010). However, during embolization of intracranial aneurysms, hemodynamic and intracranial pressure fluctuations, especially during emergence from general anesthesia, can increase the transmural pressure and stress on the blood vessel wall, increasing the risk of aneurysm rupture (Fukuda et al., 2015; Zhang et al., 2016). Therefore, careful selection of the anesthetic agent and method is needed.
Fast-track anesthetic techniques result in rapid recovery from general anesthesia, which is important for timely management of adverse events. Despite the usefulness of these fast-track techniques, they lack uniform standards. Fast-track anesthesia can be achieved using a balanced anesthesia technique and a combination of drugs given at the minimum dosage required to obtain the best effect (Ali Hassan, 2015). Fast-track anesthesia is preferred for embolization of intracranial aneurysms because of the low demand for muscular relaxation. Sevoflurane, a new, safe, and effective inhalable anesthetic, is characterized by rapid absorption/discharge and good controllability along with neuroprotective effects such as a reduced brain metabolic rate and little effect on the cerebral blood flow. Sevoflurane is commonly used for aneurysm embolization (Sakai et al., 2005). However, sevoflurane can also elevate the intracranial pressure and trigger respiratory depression (Holmström and Akeson, 2004). Therefore, a low dose of sevoflurane might reduce the likelihood of adverse reactions during aneurysm embolization. Dexmedetomidine (DEX), a novel, highly selective α2 adrenergic receptor agonist that has a short elimination half-life and is non-addictive, produces dose-dependent sedative, anxiolytic, and analgesic effects. Moreover, DEX has anti-sympathetic and anti-shivering properties. DEX can also be used for semi-arousable sedation and cooperative sedation, acting as an auxiliary analgesic to enhance sedative and analgesic effects; can maintain hemodynamic stability; and can reduce the necessary dosage of other analgesic agents (Tang et al., 2015b; Prontera et al., 2017). Bispectral index monitoring, highly associated with sedation, consciousness, and memory, helps to determine the depth of anesthesia, adjust the amount of anesthetic agents administered, and reduce the recovery time from general anesthesia (Kim et al., 2014).
While the effects of DEX have been retrospectively examined in many studies (Cheung et al., 2015; Tang et al., 2015b, 2017; Tang and Li, 2016), its clinical applications as an auxiliary analgesic with sevoflurane anesthesia for embolization of intracranial aneurysms have not been adequately investigated. Therefore, we conducted this prospective, randomized, double-blind study in two tertiary-care hospitals in Hefei City and Wuhan City of China to explore the use of fast-track anesthesia utilizing DEX combined with sevoflurane for the embolization of intracranial aneurysms by monitoring the bispectral index.
Subjects and Methods
Study design
This prospective, randomized, double-blind clinical trial was approved by the Clinical Research Ethics Committees of Anhui Provincial Hospital of Anhui Medical University and Renmin Hospital of Wuhan University of China (approval Nos. 2015[31] and 2016K-C007) and registered at the Chinese Clinical Trial Registry (ChiCTR) (registration number: ChiCTR-IPR-16008113).
Study population
Patients were recruited by attending physicians at Anhui Provincial Hospital and Renmin Hospital of Wuhan University of China. After providing written informed consent, patients were screened according to the inclusion and exclusion criteria.
Inclusion criteria
Patients of either sex presenting with all of the following criteria were considered for study inclusion: an American Society of Anesthesiologists physical status of I to IV (Tang et al., 2016), age of 18 to 70 years, Glasgow coma scale score of > 11 (Teasdale and Jennett, 1974), Hunt-Hess grade of I to III (Hunt and Hess, 1968), and embolization of intracranial aneurysms.
Exclusion criteria
Patients with one or more of the following conditions were excluded from this study: coagulation dysfunction, history of drug allergy to DEX or sevoflurane, severe hypertension or cardiovascular disease, liver or kidney dysfunction, use of sedatives within 2 days prior to surgery, sinus bradycardia, known history of second- or third-degree heart block, and ischemic heart disease.
Randomization
Patients were enrolled and randomly assigned to two groups by a blinded statistician: the NS group (sevoflurane anesthesia with normal saline) and DS group (sevoflurane anesthesia with DEX) (n = 60).
Anesthesia procedure
Patients were sent to the operating room with no premedication. The standard monitoring consisted of five-lead electrocardiography and measurement of oxygen saturation and noninvasive blood pressure. One anesthesia nurse who was not involved in the study prepared a 50-mL syringe containing DEX (4 μg/mL) or normal saline. A 20-gauge intravenous cannula was inserted into the dorsum of each patient's left hand. A central venous catheter was then inserted into the jugular vein under local anesthesia. The bispectral index was monitored. A loading dose of DEX (1.0 μg/kg) or normal saline was administered for 15 minutes and then changed to a continuous infusion at 0.3 μg/kg/h.
Before induction, 100% oxygen was administered through a face mask for at least 3 minutes. General anesthesia was induced after infusion of the loading dose of the drug: 1.5 to 2.0 mg/kg of propofol (AstraZeneca, London, UK), 0.2 μg/kg of sufentanil (Yichang Renfu Pharmaceutical Co., Ltd., China), and 0.1 to 0.15 mg/kg of cisatracurium (Shanghai Hengrui Pharmaceutical Co., Ltd., China). Manual face mask ventilation was continued for at least 3 minutes until the jaw was relaxed and the bispectral index was < 50 to allow insertion of an I-gel laryngeal mask airway (LMA) (Intersurgical Ltd., Wokingham, Berkshire, UK) (size 3 and 4 were used for patients weighing 30–60 and 50–90 kg, respectively). Successful LMA insertion was characterized by up-and-down movement of the thorax on both sides, symmetrical respiratory sounds upon auscultation, no respiratory sound with an airway pressure of > 20 cmH2O, a normal end-tidal carbon dioxide (ETCO2) waveform, and easy insertion of the gastric tube. The patients were subsequently connected to a Datex-Ohmeda S/5 Avance Anesthesia Machine (Datex-Ohmeda Inc., Madison, WI, USA), followed by mechanical ventilation with a fraction of inspired oxygen of 60%, tidal volume of 6 to 8 mL/kg, and respiratory rate of 10 to 12 breaths/min to maintain the ETCO2 in the normal range. Sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) was administered to maintain the bispectral index at 40 to 60. Variations in blood pressure and heart rate were monitored so that they did not exceed 20% of the baseline values in both groups. Cisatracurium was used if required. Hypotension (a decrease of > 20% of the baseline values) was treated with intravenous ephedrine (5 mg) or phenylephrine (40 μg), while bradycardia was treated with intravenous atropine (0.5 mg). The same surgical team of three neurosurgeons performed all operations. The evaporator was closed and the infusion of DEX was stopped approximately 10 minutes before the end of surgery, and the flow rate of fresh gas was reduced to 0.3 to 0.5 L/min. Tramadol (1.0 mg/kg) and azasetron (10 mg) were then administered intravenously. The flow rate of fresh gas was increased to 4 L/min, and anesthesia was terminated when the femoral artery was sutured using a vascular closure device (Abbott, Santa Clara, CA, USA). The patient's I-gel LMA was removed in accordance with the following standard extubation criteria (Tang et al., 2015a): (1) recovery of consciousness, normal muscle tension, and strong clenching of fist when instructed; (2) steady spontaneous breathing, ETCO2 of < 45 mmHg, and tidal volume of > 7 mL/kg; (3) oxygen saturation of > 97% after stopping to receive oxygen for 5 minutes; (4) spontaneous breathing rate of < 24 breaths/min; and (5) recovery of cough and swallowing reflex. The modified observer's assessment of alertness/sedation (MOAA/S) score was 3 when assessed according to the following criteria: 0 (asleep) = no response to painful trapezius squeeze; 1 = response only after painful trapezius squeeze; 2 = response only after mild prodding or shaking; 3 = response only after name is called loudly, repeatedly, or both; 4 = lethargic response to name spoken in a normal tone; 5 (alert) = immediate response to name spoken in normal tone (Tang et al., 2015a).
After I-gel LMA removal, the patients were transferred for computed tomography and then to the neurosurgery intensive care unit for monitoring and routine treatments, including lowering of the intracranial pressure, dehydration, and continuous intravenous infusion of 0.15% nimodipine (Bayer, Leverkusen, Germany) to prevent postoperative cerebral vasospasm.
Outcome measures
The anesthesiologists recording the details were unaware of the type of infusion the patients received. Systolic blood pressure, diastolic blood pressure, and heart rate were recorded at different time points: before DEX infusion (baseline, T0), induction of anesthesia (T1), immediately after I-gel LMA placement (T2), immediately after femoral artery intubation (T3), end of operation (T4), immediately after I-gel LMA removal (T5), 10 minutes after I-gel LMA removal (T6), and 1 hour after the operation (T7). The end-tidal sevoflurane concentration was recorded at T2, T3, and T4, and the total usage of sevoflurane was automatically calculated and displayed in mL by the Datex Ohmeda Avance S5 system (S/5; Datex Ohmeda, Helsinki, Finland). Recovery from anesthesia was measured from the time point at which the femoral artery was sutured. The time required for spontaneous breathing recovery, eye opening, and I-gel LMA removal, and later, the MOAA/S score after I-gel LMA removal, were also observed. Changes in physiological indicators and the incidence of adverse events when inserting or removing the LMA (e.g., cough, restlessness, and chills), if any, were also recorded. The Modified Confusion Assessment Method-S score was used to diagnose postoperative delirium, which was categorized into four types: normal, mild, moderate, and severe; this was assessed 24 hours after the operation (Inouye et al., 2014b).
Blood processing and analyses
Two milliliters of venous blood was collected from the central venous catheter at T0, T1, and T5 into tubes without an anticoagulant and kept perfectly still until the serum separated. The serum was centrifuged at 4,000 r/min at 4°C for 10 minutes. The supernatant was then aspirated and placed at –80°C in a cryogenic refrigerator prior to evaluation of neuron-specific enolase (NSE) and S100β, which were measured using an immulite automated chemiluminometer (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Meanwhile, 2 mL of arterial blood was collected for arterial blood gas analysis with an automatic blood gas system (cobas b 123; Roche, Basel, Switzerland).
Sample size
The primary outcome measures were differences in the spontaneous breathing recovery time, eye opening time, and I-gel removal time. The power calculation for the study was based on the time from anesthesia termination until the recovery of spontaneous breathing. As previously reported (Tang et al., 2016), the time to recovery of spontaneous breathing from sevoflurane anesthesia alone was approximately 6.5 minutes, while that from sevoflurane anesthesia with DEX infusion was expected to be 5.0 minutes with a standard deviation of 2.3 minutes. To compensate for the possibility of a dropout rate of 20%, 50 patients per group were needed at a power of 90% and two-sided significance level of 0.05.
Statistical analysis
All data were statistically analyzed by statisticians using the SPSS 22.0 statistical software package (IBM Corp., Armonk, NY, USA) in line with the intention-to-treat principle. All measurement indexes were expressed as the mean ± standard deviation/standard error of the mean or count (%), followed by a normal distribution analysis. Normally distributed data were compared using the independent sample t-test, and non-normally distributed data were compared using the Wilcoxon test. The Mann-Whitney U test was employed for intergroup comparison, and the Wilcoxon signed-rank test was used for comparison between different time points within the same group. The chi-square test was used to analyze categorical variables between the groups. A P value of < 0.05 was considered statistically significant.
Results
Quantitative analysis of patients
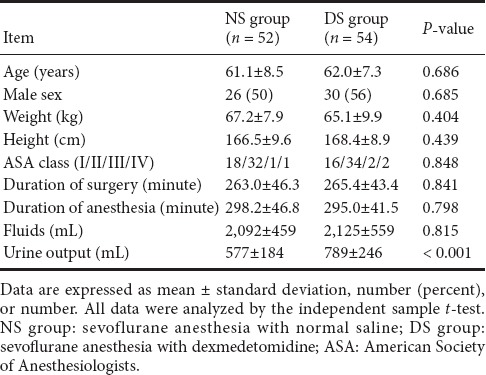
One hundred twenty patients were recruited from March to December 2016. Two patients in the DS group discontinued the study protocol: one required conversion from interventional embolization to aneurysm clipping, and the other developed postoperative complications. Four patients in the NS group discontinued the study protocol: two required conversion from interventional embolization to aneurysm clipping, and two developed postoperative complications. One hundred six patients completed the study: 54 in the DS group and 52 in the NS group (Figure 1). There were no significant differences in demographic data, surgical characteristics, or intraoperative variables between the two groups except urine output (Table 1).
Figure 1.
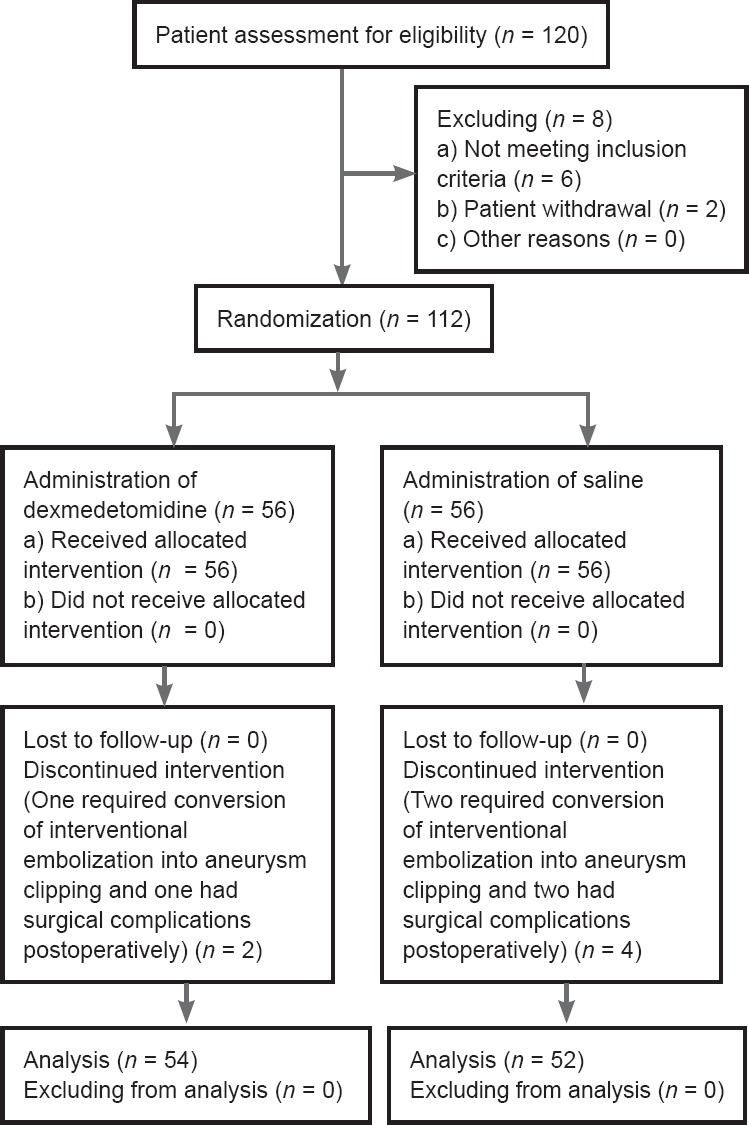
Flow diagram of the study.
Table 1.
Patient characteristics and intraoperative data
Hemodynamic variables
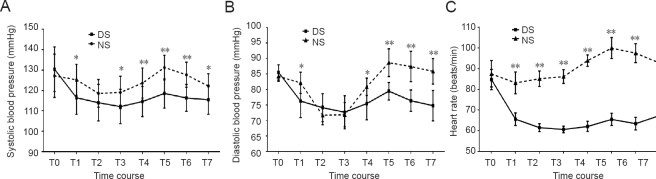
There were no differences in the systolic blood pressure, diastolic blood pressure, or heart rate at baseline between the two groups. Patients in the DS group showed a greater decrease in the blood pressure and heart rate than those in the NS group during the observation period. In the NS group, the blood pressure and heart rate were significantly higher upon emergence from anesthesia than at baseline (P < 0.0001). In the intergroup comparison of the blood pressure and heart rate at similar time intervals during the intraoperative period, the DS group had a steadier blood pressure and heart rate (P < 0.05), although no significant difference was present at the time of I-gel LMA placement. Similarly, the blood pressure and heart rate upon emergence from anesthesia were stable in the DS group, while they were elevated in the NS group (P < 0.0001) (Figure 2).
Figure 2.
Changes in mean hemodynamic variables in patients receiving sevoflurane anesthesia with saline or sevoflurane anesthesia with dexmedetomidine.
(A) Systolic blood pressure, (B) diastolic blood pressure, (C) heart rate. Data are expressed as the mean ± standard deviation and were analyzed by two-way analysis of variance. *P < 0.05, **P < 0.0001 vs. DS group. NS group (n = 52): Sevoflurane anesthesia with normal saline; DS group (n = 54): sevoflurane anesthesia with DEX. T0 (baseline): Before DEX infusion; T1: induction of anesthesia; T2: immediately after I-gel LMA placement; T3: immediately after femoral artery intubation; T4: end of operation; T5: immediately after I-gel LMA removal; T6: 10 minutes after I-gel LMA removal; T7: 1 hour after the operation. DEX: dexmedetomidine; LMA: laryngeal mask airway.
PaO2 and PaCO2
PaO2 and PaCO2 were comparable and within normal limits at baseline in both groups. Although they tended to be more optimal in the NS group from the time of drug infusion until the induction of anesthesia, the differences were not significant (P > 0.05). Immediately after I-gel LMA removal, the PaCO2 was 39.80 ± 0.74 mmHg in the DS group and 41.60 ± 2.39 mmHg in the NS group, with a significant difference (P = 0.003) (Figure 3B).
Figure 3.
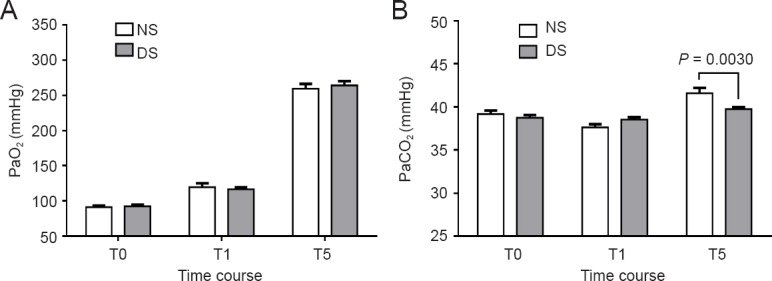
Changes in (A) PaO2 and (B) PaCO2 in patients receiving dexmedetomidine or saline intraoperatively.
Data are expressed as the mean ± standard error of the mean and were analyzed by two-way analysis of variance. NS group (n = 52): Sevoflurane anesthesia with normal saline; DS group (n = 54): sevoflurane anesthesia with DEX. T0 (baseline): Before DEX infusion; T1: induction of anesthesia; T5: immediately after I-gel laryngeal mask airway removal. DEX: Dexmedetomidine.
Glucose and lactate levels
The glucose level was lower after DEX infusion than at baseline in the DS group (P < 0.05). The glucose level was lower in the DS group than NS group at induction of anesthesia and immediately after I-gel LMA removal (P = 0.0046 and P = 0.0138, respectively) (Figure 4A). The lactate level was lower after DEX infusion than at baseline in the DS group (P < 0.05). The lactate level began to slowly increase approximately at the time of induction of anesthesia and upon emergence from anesthesia, but the differences were not significant (P > 0.05). However, a significant difference was noted in the NS group (P < 0.05). On intergroup comparison, the DS group had lower lactate levels before the induction of anesthesia and immediately after I-gel LMA removal (P = 0.0182 and P = 0.0002, respectively) (Figure 4B).
Figure 4.
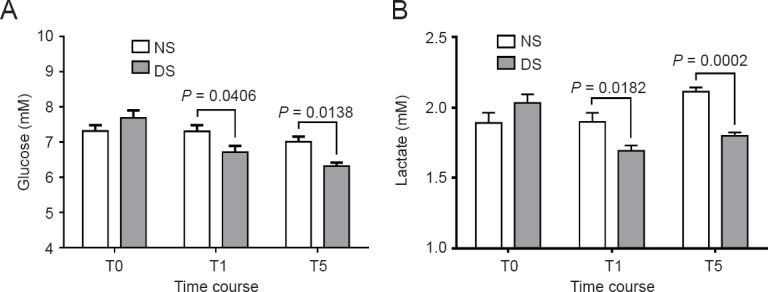
Changes in glucose and lactate levels in patients receiving dexmedetomidine or saline intraoperatively.
NS group (n = 52): Sevoflurane anesthesia with normal saline; DS group (n = 54): sevoflurane anesthesia with DEX. T0 (baseline): Before DEX infusion; T1: induction of anesthesia; T5: immediately after I-gel laryngeal mask airway removal. Data are expressed as the mean ± standard error of the mean and were analyzed by two-way analysis of variance. DEX: Dexmedetomidine.
S100β and NSE plasma concentrations
The plasma concentration of S100β began to fall from the baseline value after DEX infusion in the DS group, and the difference was significant before the induction of anesthesia (P < 0.05). The S100β concentration was lower before the induction of anesthesia and immediately after I-gel LMA removal than in the NS group (P = 0.0170 and P = 0.0002, respectively) (Figure 5A). The plasma concentration of NSE immediately after I-gel LMA removal was higher than that at baseline in the NS group (P < 0.0001). The NSE concentration was higher in the NS group than in the DS group immediately after I-gel LMA removal (P = 0.0003) (Figure 5B).
Figure 5.
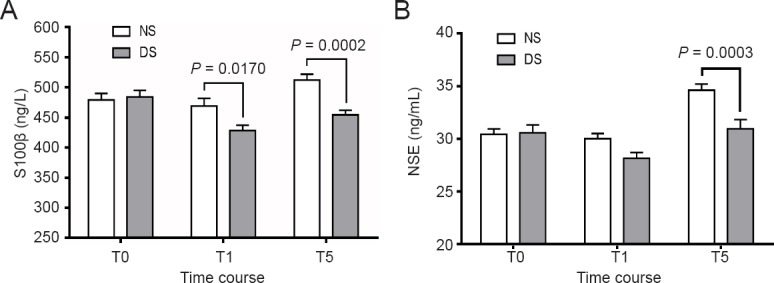
Changes in S100β (A) and NSE (B) plasma concentrations in patients receiving dexmedetomidine or saline intraoperatively (chemiluminescence immunoassay).
Data are expressed as the mean ± standard error of the mean and were analyzed by two-way analysis of variance. NS group (n = 52): Sevoflurane anesthesia with normal saline; DS group (n = 54): sevoflurane anesthesia with DEX. T0 (baseline): Before DEX infusion; T1: induction of anesthesia; T5: immediately after I-gel laryngeal mask airway removal. DEX: Dexmedetomidine; NSE: neuron-specific enolase.
End-tidal sevoflurane concentration and cumulative sevoflurane consumption
Patients in the DS group had a significantly lower end-tidal sevoflurane concentration than those in the NS group at all time points (P < 0.0001, P < 0.0001, and P = 0.0023). This indicates a decrease in the end-tidal sevoflurane concentration of > 40% (Figure 6A). The overall dose of sevoflurane consumed in the DS group was less than that in the NS group, suggesting a statistically significant reduction in > 27% (Figure 6B).
Figure 6.
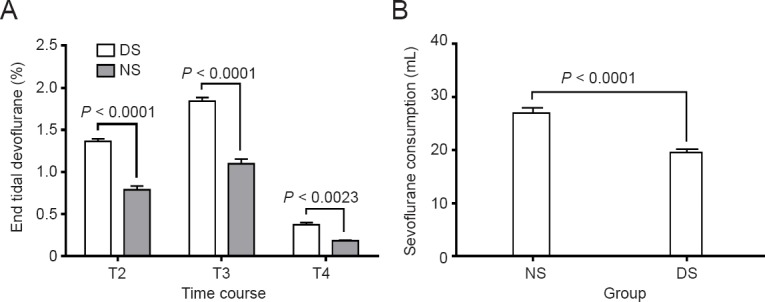
End-tidal sevoflurane concentration (A) and cumulative sevoflurane consumption (B) in patients receiving dexmedetomidine or saline.
Data are expressed as the mean ± standard error of the mean and were analyzed by two-way analysis of variance and the independent-sample t-test. NS group (n = 52): Sevoflurane anesthesia with normal saline; DS group (n = 54): sevoflurane anesthesia with dexmedetomidine. T2: Immediately after I-gel laryngeal mask airway placement; T3: immediately after femoral artery intubation; T4: end of operation.
Anesthesia recovery
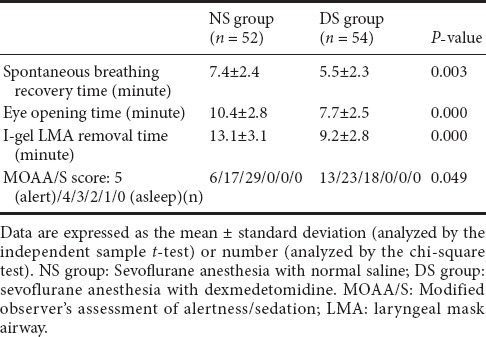
As shown in Table 2, the spontaneous breathing recovery time, eye opening time, and I-gel removal time were shorter in the DS group than NS group (P = 0.003, P = 0.000, and P = 0.000, respectively). Moreover, the MOAA/S score was significantly higher in the DS group at the time of I-gel removal (P = 0.049).
Table 2.
Spontaneous breathing recovery time, eye opening time, I-gel removal time, and MOAA/S score in patients receiving dexmedetomidine or saline
Postoperative delirium categories
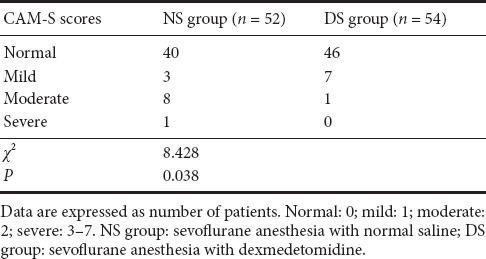
The postoperative delirium categories based on the Confusion Assessment Method-S scores are shown in Table 3. More patients from the NS than DS group had serious delirium. Furthermore, postoperative delirium occurred in 12 (23%) of 52 patients in the NS group and in 8 (15%) of 54 patients in the DS group (P = 0.038).
Table 3.
Postoperative delirium categories based on the Confusion Assessment Method (CAM)-S scores in patients receiving dexmedetomidine or saline
Discussion
This randomized, double-blinded, comparative study was performed to evaluate the effects of intravenous DEX during sevoflurane anesthesia for embolization of intracranial aneurysms on anesthetic recovery, the stress response, neuroprotective effects, postoperative delirium, and sevoflurane requirements. Our principal findings suggest that the use of intravenous DEX during sevoflurane anesthesia results in more stable hemodynamics and rapid recovery during emergence from general anesthesia as indicated by lower cumulative sevoflurane consumption, a lower stress response, better neuroprotection, a decreased incidence of postoperative delirium, and improved recovery.
Some studies have shown that bispectral index monitoring during anesthesia helps to reduce the sevoflurane requirements and results in more rapid emergence (Song et al., 1997; Mudakanagoudar and Santhosh, 2016). Others have also observed that DEX infusion during sevoflurane anesthesia significantly reduced the end-tidal sevoflurane concentration by approximately 33% while maintaining stable hemodynamic conditions, although others found a varying 17% to 58% reduction in the minimum alveolar concentration of sevoflurane (Magalhães et al., 2004). Our study revealed a > 27% decrease in sevoflurane consumption. Appropriate management of systemic and cerebral hemodynamic variables is the cornerstone of neuroanesthesia. The use of DEX results in hemodynamic stability even during peak surgical stress (Dilek et al., 2011). When used as an adjuvant to general anesthesia, DEX allows easy extubation without affecting the recovery time (Turan et al., 2008; Aksu et al., 2009; Chavan et al., 2016). During the observation period, hemodynamic parameters were significantly lower in patients receiving DEX but remained within hemodynamically stable limits. In the current study, all patients receiving DEX exhibited rapid emergence, including a short time to spontaneous breathing recovery, eye opening, and I-gel LMA removal, while the sedation levels were better; the analgesic action rather than the sedative effect was more likely responsible for the reduced sevoflurane requirements with the use of DEX.
Surgical stress can excite the hypothalamus, resulting in the secretion of adrenocorticotrophic hormone, which in turn initiates a sudden increase in the cortisol level. Cortisol mobilizes amino acids and fat from the body stores and makes them available for synthesis of glucose, thus causing hyperglycemia. Although the blood glucose level is only the tip of the iceberg with respect to showcasing the stress response to surgery, the blood glucose level can reflect the metabolic stress response to surgery and the effect of DEX in blunting this stress response. Lactic acid is an important energy material in brain tissue, playing an important role in the protection of the brain in certain pathological conditions. Moreover, lactic acid is a marker of ischemic hypoxia and central nervous system damage (Smith et al., 2003; Dienel, 2014; Bergersen, 2015). Low blood pressure, hypoxemia, and hypocapnia can lead to increased lactic acid levels. Mildly increased lactic acid was generated and analyzed by arterial blood gas analysis in neurosurgery patients with hypotension and hyperventilation (Karlsson et al., 1995). The blood glucose and lactic acid levels can also reflect the metabolic stress response to surgery and cerebral oxygen supply as well as the effect of DEX in this respect. Our results may indicate a significant decrease in the glucose and lactic concentrations after DEX infusion, supporting the ability of DEX to effectively blunt the stress response to surgery, improve brain tissue perfusion, and reduce the cerebral oxygen metabolism rate generated by anaerobic glycolysis and lactic acid in brain tissue. PaO2 and PaCO2 were comparable and within normal limits in both groups. Immediately after I-gel LMA removal, PaCO2 was 39.80 ± 0.74 mmHg in the DS group and 41.60 ± 2.39 mmHg in the NS group; although this small difference was statistically significant, it may not be clinically significant. The cerebral oxygen supply fully explains why there was no accumulation of lactic acid during the study.
A large amount of NSE can rapidly leak from damaged neurons and traverse the damaged blood-brain barrier into the cerebrospinal fluid. The rise of the NSE level in the blood and cerebrospinal fluid is consistent with the extent of neuronal damage. S100β mainly exists in glial cells and Schwann cells, playing an important role in glial cell growth, proliferation, and differentiation in the central nervous system as well as maintaining calcium homeostasis, learning, and memory. S100β may ooze from damaged central nervous system cells into the cerebrospinal fluid and subsequently traverse the blood-brain barrier into the blood. Therefore, the NSE and S100β levels can be used as biochemical and diagnostic indicators reflecting the degree of central nervous system damage (Büttner et al., 1997). Our results may indicate a slight decrease in the plasma concentrations of S100β and NSE after DEX infusion compared with those at baseline; these concentrations were also lower immediately after I-gel LMA removal in patients receiving DEX than in those receiving saline. Some studies have also shown that the administration of DEX during the perioperative period in patients undergoing brain surgery can significantly reduce S100β and NSE generation with certain neuroprotective effects (Luo et al., 2016; Zhao and Zhou, 2016).
Postoperative delirium, characterized by dysfunction in consciousness, attention, and cognition, is always regarded as a reversible cognitive dysfunction syndrome (McCusker et al., 2014; Zaal et al., 2015). Excessive and prolonged postoperative delirium may influence the patient's recovery (Cropsey et al., 2015; Whalin et al., 2015). In the present study, the Confusion Assessment Method-S scores, a rigorous assessment system to diagnose postoperative delirium, were used. Furthermore, postoperative delirium occurred in 12 (23%) of 52 patients receiving saline, similar to previous studies (Vasilevskis et al., 2012; Inouye et al., 2014a; Su et al., 2016), and in 8 (15%) of 54 patients receiving DEX. Although the incidence of postoperative delirium was not significantly different, patients receiving DEX had lower moderate and severe categories of postoperative delirium than those receiving saline (1 and 0 vs. 8 and 1, respectively). The physiopathologic mechanism of the decrease in delirium by DEX remains unknown; however, the anesthetic-sparing and anti-inflammatory effects of this drug may play a role (Sobbi and van den Boogaard, 2014; Tasker and Menon, 2016). Our main results regarding the decreased NSE and S100β levels after DEX infusion could suggest another mechanism. One possible reason for the decreased delirium in our study may be that DEX intervention was initiated as soon as patients were transferred to the interventional radiotherapy room, preventing a high prevalence of stress response in the early hours.
There were several limitations in our study. This was a multicenter trial, and two different surgical teams conducted the surgery; this conferred a margin of procedure variability. Standard anesthetic management was used, while the surgical procedure was discussed and decided based on consensus between the two operative teams to minimize the inter-institutional gap. Although the study had a sufficient sample size to show significant differences in the endpoints between the two groups, it was not large enough to detect the effects of DEX on postoperative delirium. Furthermore, Confusion Assessment Method-S might not be as sensitive as other tools for delirium assessment, such as the 3D-Confusion Assessment Method, especially in other ethnic groups (van Eijk et al., 2011; Nishimura et al., 2016). Thus, further studies with larger sample sizes and more sensitive delirium assessment tools are needed to evaluate the effects of DEX on postoperative delirium. The NSE and S100β levels were only measured intraoperatively. Our study may serve as a starting point for future studies in which the postoperative NSE and S100β levels are measured for several days to explore the effect of DEX on regulating the NSE and S100β levels and postoperative delirium.
In conclusion, intravenous DEX during sevoflurane anesthesia could yield a stable hemodynamic condition, lower stress response, and rapid recovery during emergence of general anesthesia as indicated by the low cumulative sevoflurane consumption, better neuroprotective effect, and decreased incidence of delirium after surgery seen in the present study, thereby improving patients' recovery. Fast-track anesthesia using sevoflurane combined with DEX, with measurement-based control of surgical stress, neuroprotection, and rapid recovery during emergence, would improve the postoperative outcome of embolization of intracranial aneurysms.45
Acknowledgments:
The authors are grateful for the enthusiastic support of the nurses from the Neurosurgery Intensive Care Unit at Renmin Hospital of Wuhan University and Southern District of Anhui Provincial Hospital of China.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81671891.
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81671891.Funders had no involvement in the study design; data collection, management, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Research ethics: The study protocol was approved by the Clinical Research Ethics Committees of Anhui Provincial Hospital of Anhui Medical University and Renmin Hospital of Wuhan University of (approval No. 2015[31], and 2016K-C007). The study followed international and national regulations in accordance with the Declaration of Helsinki and relevant ethical principles.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form, patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
(Copyedited by Morben A, Wysong S, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Aksu R, Akin A, Biçer C, Esmaoğlu A, Tosun Z, Boyaci A. Comparison of the effects of dexmedetomidine versus fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: A double-blind, randomized, controlled study. Curr Ther Res Clin Exp. 2009;70:209–220. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali Hassan HI. Comparison between two different selective spinal anesthesia techniques in ambulatory knee arthroscopy as fast-track anesthesia. Anesth Essays Res. 2015;9:21–27. doi: 10.4103/0259-1162.150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Büttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961–1965. doi: 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- 4.Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. 2015;35:176–185. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355:928–939. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 6.Chavan SG, Shinde GP, Adivarekar SP, Gujar SH, Mandhyan S. Effects of dexmedetomidine on perioperative monitoring parameters and recovery in patients undergoing laparoscopic cholecystectomy. Anesth Essays Res. 2016;10:278–283. doi: 10.4103/0259-1162.171460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CW, Qiu Q, Liu J, Chu KM, Irwin MG. Intranasal dexmedetomidine in combination with patient-controlled sedation during upper gastrointestinal endoscopy: a randomised trial. Acta Anaesthesiol Scand. 2015;59:215–223. doi: 10.1111/aas.12445. [DOI] [PubMed] [Google Scholar]
- 8.Cropsey C, Kennedy J, Han J, Pandharipande P. Cognitive dysfunction, delirium, and stroke in cardiac surgery patients. Semin Cardiothorac Vasc Anesth. 2015;19:309–317. doi: 10.1177/1089253215570062. [DOI] [PubMed] [Google Scholar]
- 9.Dienel GA. Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab. 2014;34:1736–1748. doi: 10.1038/jcbfm.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilek O, Yasemin G, Atci M. Preliminary experience with dexmedetomidine in neonatal anesthesia. J Anaesthesiol Clin Pharmacol. 2011;27:17–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda H, Handa A, Koyanagi M, Yoshida K, Lo BW, Yamagata S. Endovascular therapy for ruptured cerebral aneurysms in the elderly: poor accessibility of the guiding catheter and use of local anesthesia as the predictors of procedure-related rupture. Neurosurgery. 2015;77:544–552. doi: 10.1227/NEU.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 12.Holmström A, Akeson J. Desflurane increases intracranial pressure more and sevoflurane less than isoflurane in pigs subjected to intracranial hypertension. J Neurosurg Anesthesiol. 2004;16:136–143. doi: 10.1097/00008506-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014a;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Jones RN. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014b;160:526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson T, Stjernstrom EL, Stjernstrom H, Wiklund L, Essen-Gustavsson B, Jorfeldt L. Lactate metabolism and hypocarbic hyperventilation. An experimental study in piglets. Acta Anaesthesiol Scand. 1995;39:109–117. doi: 10.1111/j.1399-6576.1995.tb05601.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Kim DK, Lee MJ. Relationship of bispectral index to minimum alveolar concentration during isoflurane, sevoflurane or desflurane anaesthesia. J Int Med Res. 2014;42:130–137. doi: 10.1177/0300060513505525. [DOI] [PubMed] [Google Scholar]
- 18.Korja M, Kivisaari R, Rezai Jahromi B, Lehto H. Size and location of ruptured intracranial aneurysms: consecutive series of 1993 hospital-admitted patients. J Neurosurg. 2016:1–6. doi: 10.3171/2016.9.JNS161085. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Zheng X, Huang H. Protective effects of dexmedetomidine on brain function of glioma patients undergoing craniotomy resection and its underlying mechanism. Clin Neurol Neurosurg. 2016;146:105–108. doi: 10.1016/j.clineuro.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Magalhães E, Govêia CS, Ladeira LC, Espíndola BV. Relationship between dexmedetomidine continuous infusion and end-tidal sevoflurane concentration, monitored by bispectral analysis. Rev Bras Anestesiol. 2004;54:303–310. doi: 10.1590/s0034-70942004000300003. [DOI] [PubMed] [Google Scholar]
- 21.McCusker J, Cole MG, Voyer P, Monette J, Champoux N, Ciampi A, Vu M, Belzile E. Six-month outcomes of co-occurring delirium, depression, and dementia in long-term care. J Am Geriatr Soc. 2014;62:2296–2302. doi: 10.1111/jgs.13159. [DOI] [PubMed] [Google Scholar]
- 22.Mudakanagoudar MS, Santhosh MC. Comparison of sevoflurane concentration for insertion of proseal laryngeal mask airway and tracheal intubation in children (correlation with BIS) Braz J Anesthesiol. 2016;66:24–28. doi: 10.1016/j.bjane.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Yokoyama K, Yamauchi N, Koizumi M, Harasawa N, Yasuda T, Mimura C, Igita H, Suzuki E, Uchiide Y, Seino Y, Nomura M, Yamazaki K, Ishigooka J, TMAD investigators Sensitivity and specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for detecting post-cardiac surgery delirium: A single-center study in Japan. Heart Lung. 2016;45:15–20. doi: 10.1016/j.hrtlng.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Prontera A, Baroni S, Marudi A, Valzania F, Feletti A, Benuzzi F, Bertellini E, Pavesi G. Awake craniotomy anesthetic management using dexmedetomidine, propofol, and remifentanil. Drug Des Devel Ther. 2017;11:593–598. doi: 10.2147/DDDT.S124736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai EM, Connolly LA, Klauck JA. Inhalation anesthesiology and volatile liquid anesthetics: focus on isoflurane, desflurane, and sevoflurane. Pharmacotherapy. 2005;25:1773–1788. doi: 10.1592/phco.2005.25.12.1773. [DOI] [PubMed] [Google Scholar]
- 26.Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;23:658–664. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- 27.Sobbi SC, van den Boogaard M. Inflammation biomarkers and delirium in critically ill patients: new insights? Crit Care. 2014;18:153. doi: 10.1186/cc13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology. 1997;87:842–848. doi: 10.1097/00000542-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 30.Taki W, Nakahara I, Sakai N, Irie K, Murao K, Ohkata N, Tanaka M, Kikuchi H. Large and giant middle to lower basilar trunk aneurysms treated by surgical and interventional neuroradiological methods. Neurol Med Chir (Tokyo) 1998;38:826–834. doi: 10.2176/nmc.38.826. [DOI] [PubMed] [Google Scholar]
- 31.Tang C, Chai X, Kang F, Huang X, Hou T, Tang F, Li J. I-gel laryngeal mask airway combined with tracheal intubation attenuate systemic stress response in patients undergoing posterior fossa surgery. Mediators Inflamm. 2015a;2015:965925. doi: 10.1155/2015/965925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang C, Huang X, Kang F, Chai X, Wang S, Yin G, Wang H, Li J. Intranasal dexmedetomidine on stress hormones, inflammatory markers, and postoperative analgesia after functional endoscopic sinus surgery. Mediators Inflamm. 2015b;2015:939431. doi: 10.1155/2015/939431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang CL, Li J. Postoperative sedation by intranasal dexmedetomidine in patients with hypertensive cerebral hemorrhage: study protocol for a randomized parallel-cohort controlled trial. Asia Pac Clin Transl Nerv Syst Dis. 2016;1:6–11. [Google Scholar]
- 34.Tang CL, Li J, Zhao B, Xia ZY. Bispectral index-guided fast track anesthesia by sevoflurane infusion combined with dexmedetomidine for intracranial aneurysm embolization: study protocol for a multi-center parallel randomized controlled trial. Asia Pac Clin Transl Nerv Syst Dis. 2016;1:177–185. [Google Scholar]
- 35.Tang CL, Li J, Zhao B, Shi S, Shen H, Xia ZY. Effects of dexmedetomidine combined with sodium creatine phosphate on inflammation, oxidative stress, and neurological function recovery in patients undergoing intracranial hematoma evacuation: study protocol for a multi-center, prospective randomized parallel-cohort controlled trial. Asia Pac J Clin Trials Nerv Syst Dis. 2017;2:1–8. [Google Scholar]
- 36.Tasker RC, Menon DK. Critical care and the brain. JAMA. 2016;315:749–750. doi: 10.1001/jama.2016.0701. [DOI] [PubMed] [Google Scholar]
- 37.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 38.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–820. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 39.van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A, Karakus A, Klijn IA, Kuiper MA, de Leeuw FE, de Man T, van der Mast RC, Osse RJ, de Rooij SE, Spronk PE, van der Voort PH, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184:340–344. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 40.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whalin MK, Kreuzer M, Halenda KM, Garcia PS. Missed opportunities for intervention in a patient with prolonged postoperative delirium. Clin Ther. 2015;37:2706–2710. doi: 10.1016/j.clinthera.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Wu C, Xu BN, Li BM, Jiang JL, Wang J, Cao XY, Yu XG, Zhou DB. The analysis of intracranial aneurysms undergoing surgical treatment. Zhonghua Wai Ke Za Zhi. 2010;48:1496–1499. [PubMed] [Google Scholar]
- 43.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43:40–47. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Jing L, Zhang Y, Liu J, Yang X. Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: case report and literature review. BMC Neurol. 2016;16:231. doi: 10.1186/s12883-016-0759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, Zhou C. The protective and hemodynamic effects of dexmedetomidine on hypertensive cerebral hemorrhage patients in the perioperative period. Exp Ther Med. 2016;12:2903–2908. doi: 10.3892/etm.2016.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]